Abstract
Background: Previous studies have investigated the associations between polymorphisms of interleukin-10 (IL-10) gene and risk of ischemic stroke (IS). However, the results were inconsistent. The aim of this study was to clarify the relationship between IL-10 polymorphisms and IS risk by a meta-analysis approach. Methods: The meta-analysis was performed by searching PubMed and Wanfang databases. Odds ratio (OR) and corresponding 95% confidence interval (95% CI) as well as effect size were calculated by a fixed or random-effect model according to the I2 value. In total, five case-control studies for IL10-1082G/A and four studies for IL10-819C/T were included in this meta-analysis. Results: Combined analysis indicated that IL10-1082G/A polymorphism was associated with risk of IS (A/A vs. G/G+G/A: OR = 1.82, 95% CI = 1.21-2.74, P = 0.004; for A allele vs. G allele: OR = 1.55, 95% CI = 1.14-2.10, P = 0.006). However, there was no significant association between IL10-819C/T polymorphism and IS in any comparison model (C/C vs. T/C+T/T: OR = 0.96, 95% CI = 0.69-1.36, P = 0.84; C allele vs. T allele: OR = 1.00, 95% CI = 0.83-1.21, P = 0.97). Conclusions: Our results indicated that IL-10-1082G/A polymorphism, but not IL10-819C/T polymorphism was associated with the risk of IS.
Keywords: Interleukin-10 genes, polymorphisms, ischemic stroke, meta-analysis
Introduction
Stroke is one of the leading causes of morbidity and mortality worldwide [1-3]. The most common subtype is ischemic stroke, which caused by an occluding thrombus of a cerebral artery. There are sorts of risk factors for stroke, such as old age, high blood pressure, diabetes, high plasma cholesterol, and smoking [4]. Previous studies indicated that the inflammatory cytokines and the genetic polymorphism in the genes encoding the inflammatory mediators play a significant role in the development and progression of ischemic stroke (IS) [5,6]. Studies revealed that besides pro-inflammatory processes also anti-inflammatory play an important role in the pathogenesis of IS [7-10].
Interleukin-10, one of anti-inflammatory cytokines, is of a variety of functions involved in atherosclerosis of the arteries formation and inflammatory response, and there are multiple gene polymorphisms affecting its biological activity. IL-10, mainly secreted by lymphocytes and monocytes, can counterbalance the potentially harmful effects of tumor necrosis factor α (TNFα) and other pro-inflammatory [11]. IL-10 is a T helper 2 cytokine, on the other hand, by promoting B cell activation and antibody production, IL-10 is an immune stimulatory [12]. IL-10 also can regulate the expression of Th1 cytokines, MHC class II antigens, and co-stimulatory molecules on macrophages. Previous study showed that high serum IL-10 concentration was associated with IS [13]. In humans, the IL-10 gene has been mapped to chromosome 1, there were six different polymorphisms concluding -1082, -819, -652, -592, -127 and -41 position variants [14-16]. In the past years, increasing evidences indicated that IL-10 gene promoter polymorphisms (-1082A/G and -819C/T) were associated with IS. However, the conclusions were controversial. To draw a more reliable conclusion and further explore the associations between the two polymorphisms and IS risk, we performed the present meta-analysis including all published paper related to this topic.
Materials and methods
Search strategy
We searched eligible literatures published up to March 2014 in PubMed and Wanfang database using the following keywords: “interleukin-10” OR “IL-10” or “IL10” AND “stroke” OR “cerebral infarction” OR “cerebral thrombosis”. The association between IL-10 polymorphisms and stroke risk was assessed by odds ratio (OR) and its 95% confidence intervals (CI). The search was done without language limitation and article type.
Inclusion and exclusion criteria
Titles were scanned first and then abstracts were read, at last full articles were reviewed. The following inclusion criteria had to be met: (1) the study must explore the associations between IL-10 (including IL10-1082G/A, IL10 -819C/T) genetic polymorphisms and IS risk; (2) the study must be a case-control study, the stroke subjects must be ischemic stroke patients; and (3) the study must provide total number of cases and controls, and the number for each genotype; and (4) OR estimates and 95% CI were provided or could be calculated. The major reasons for exclusion were: (1) duplicated studies; (2) being a review or comment; (3) animal studies; (4) If more than one study was published using the same data series, the latest study was selected prior to the others.
Eligible study selection
Figure 1 showed how the eligible studies were selected. Finally, five studies [17-21] were included in this meta-analysis study, including 1150 cases and 856 controls for IL-10-1082G/A, and 670 patients and 386 controls for IL-10-819C/T.
Figure 1.
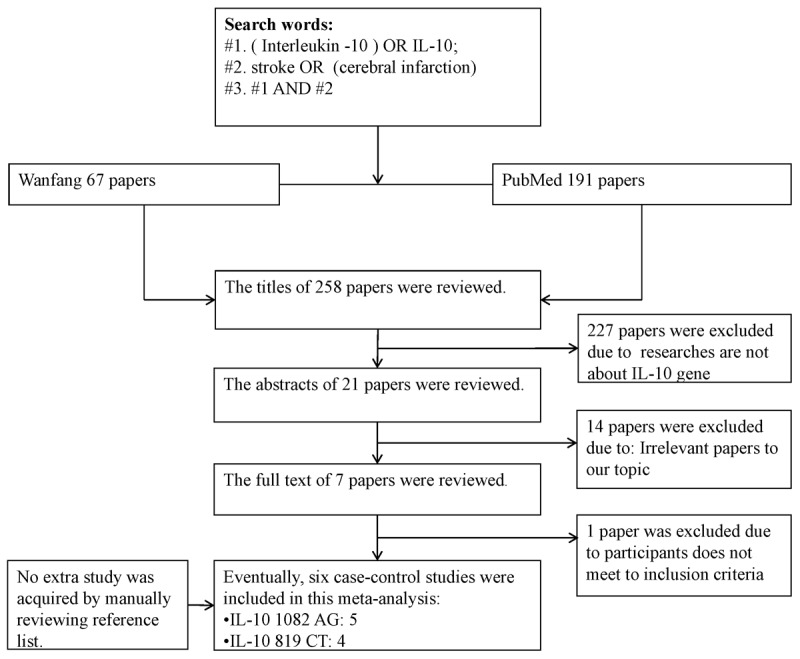
Flow chart shows study selection procedure.
Data extraction and quality assessment
Two investigators (Xiang Xie and Guo-Ting Yin) extracted data independently using a standard form, and reached a consensus on each item. Disagreements were solved by checking the data again and discussion with co-authors. The following data were extracted: the first author’s name, ethnicities and countries of origin, year of publication, genotypes, alleles and matching factors and number of case and control.
Statistical methods
Meta-analysis was performed using the Cochrane Collaboration RevMan 5.2 software. The association between the risk of IS and IL-10-1082G/A and -819C/T polymorphisms was estimated for each study using the OR and 95% CI. We first estimated this relationship with the dominant model (A/A vs. G/A+G/G), (C/C vs. C/T+T/T) and then with the allelic model (A allele vs. G allele), (C allele vs. T allele). We used Z test to analysis the merger statistics OR. A χ2-test-based Q statistic test was performed to examine the between-study heterogeneity [22,23]. We also quantified the effect of heterogeneity with an I2 test. If the Q test was significant (P < 0.05) or I2 > 50%, indicating heterogeneity across studies, we used the random effects model [24]. Otherwise, we used the fixed effects model [25]. We performed stratification analyses on ethnicities, sources of controls and sample sizes. Hardy-Weinberg equilibrium (HWE) in control group was calculated or extracted from the primary studies. Sensitivity analysis was performed, by limiting the meta-analysis to the studies conforming to HWE and the high quality studies, to evaluate the stability of the results. Every study was evaluated according to the Newcastle-Ottawa (NOS). Funnel plots were used to evaluate publication bias.
Results
Study characteristics
As shown in Tables 1 and 2, the characteristics of 5 case-control studies about IL-10-1082G/A and 4 studies about IL-10-819C/T extracted in this meta-analysis including publication year, ethnicity, total number of cases and controls, the number for each genotype and allele, OR estimates and 95% CI were present. Of all the studies, there are three studies from China, one from South India and one from Palermo.
Table 1.
Characteristics of included studies about IL-10-1082G/A
| Studies | Year | Ethnicity | Case | Control | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||
| N | Genotype (n) | allele | HWE | N | Genotype (n) | allele | HWE | NOS | |||||||||||
|
|
|
||||||||||||||||||
| AA | AG | GG | A | G | P | χ2 | AA | AG | GG | A | G | P | χ2 | ||||||
| Zhang et al. | 2006 | Chinese | 204 | 202 | 2 | 0 | 406 | 2 | 0.94 | 0.00 | 131 | 120 | 11 | 0 | 251 | 11 | 0.62 | 0.25 | 6 |
| Jin et al. | 2011 | Chinese | 189 | 161 | 27 | 1 | 349 | 29 | 0.91 | 0.01 | 92 | 78 | 12 | 2 | 168 | 16 | 0.09 | 2.93 | 5 |
| Lin et al. | 2009 | Chinese | 181 | 153 | 28 | 0 | 334 | 28 | 0.26 | 1.27 | 115 | 83 | 32 | 0 | 198 | 32 | 0.08 | 3.00 | 6 |
| Anjana et al. | 2010 | Indian | 480 | 92 | 241 | 147 | 425 | 535 | 0.70 | 1.47 | 470 | 63 | 218 | 189 | 344 | 596 | 0.99 | 0.00 | 6 |
| Antonino et al. | 2012 | Palermo | 96 | 58 | 14 | 24 | 130 | 62 | 0.00 | 42.65 | 48 | 20 | 17 | 11 | 57 | 39 | 0.07 | 3.39 | 5 |
Table 2.
Characteristics of included studies about IL-10-819C/T
| Studies | Year | Ethnicity | Case | Control | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||
| N | Genotype (n) | allele | HWE | N | Genotype (n) | allele | HWE | NOS | |||||||||||
|
|
|
||||||||||||||||||
| CC | CT | TT | C | T | P | χ2 | CC | CT | TT | C | T | P | χ2 | ||||||
| Zhang et al. | 2007 | Chinese | 204 | 28 | 90 | 86 | 146 | 262 | 0.57 | 0.33 | 131 | 27 | 48 | 56 | 102 | 160 | 0.01 | 6.89 | 6 |
| Jin et al. | 2011 | Chinese | 189 | 12 | 82 | 95 | 106 | 272 | 0.30 | 1.06 | 92 | 7 | 37 | 48 | 51 | 133 | 0.97 | 0.00 | 5 |
| Lin et al. | 2009 | Chinese | 181 | 32 | 73 | 76 | 137 | 225 | 0.05 | 3.69 | 115 | 18 | 44 | 53 | 80 | 150 | 0.09 | 2.82 | 6 |
| Antonino et al. | 2012 | Palermo | 96 | 63 | 14 | 19 | 140 | 52 | 0.00 | 38.20 | 48 | 26 | 17 | 5 | 69 | 27 | 0.39 | 0.74 | 5 |
Quantitative synthesis
The results of combined analyses for IL10-1082G/A polymorphism were showed in Figures 2, 3. The results indicated that IL10-1082G/A polymorphism was associated with risk of IS [OR (95% CI): 1.82 (1.21-2.74) for A/A vs. G/G+G/A and 1.55 (1.14-2.10) for A allele vs. G allele]. This study also showed that A allele might be one of the independent risk factors for stroke. Figures 4, 5 showed the results of combined analyses for IL10-819C/T polymorphism. Overall analyses indicated that no significant association was observed between IL0-819C/T polymorphism and IS risk [OR (95% CI): 0.96 (0.69-0.84) for C/C vs. T/T+C/T and OR (95% CI): 1.00 (0.83-1.21) for C allele vs. T allele].
Figure 2.

Forest plot for IL-10 gene -1082G/A polymorphism and IS risk in dominant model: A/A vs. A/G+G/G.
Figure 3.

Forest plot for IL-10 gene -1082G/A polymorphism and IS risk in allelic model: A allele vs. G allele.
Figure 4.

Forest plot for IL-10 gene -819C/T polymorphism and IS risk in dominant model: C/C vs. T/T+C/T.
Figure 5.
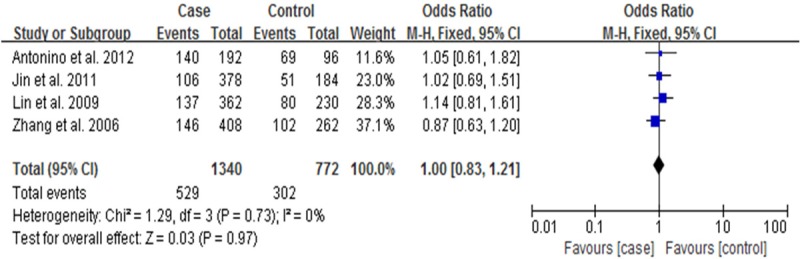
Forest plot for IL-10 gene -819C/T polymorphism and IS risk in allelic model: C allele vs. T allele.
Sensitivity analysis and publication bias
The sensitivity analyses were performed by limiting studies to those conforming to HWE. Then, removing one study at a time was performed to evaluate the stability of the results. We found that the pooled OR changed quite little, indicating that our results were statistically robust. In the present meta-analysis, funnel plot was used to assess the publication bias. No obvious asymmetry was observed in the dominant and allelic model according to the visual assessment of funnel plot (Figure 6A-D).
Figure 6.
Funnel plots for IL-10-1082A/G and IL-10-819C/T polymorphism and IS risk in different genetic models. A. (dominant model: A/A vs. A/G+G /G); B. (allelic model: A allele vs. G allele); C. (dominant model: C/C vs. T/T+C/T); D. (allelic model: C allele vs. T allele).
Discussion
A lot of epidemiological studies about genetic polymorphisms on the risks of diseases such as IS have been widely conducted [26,27]. Nowadays, meta-analysis has been widely used to gain more reliable evidence. To our knowledge, this is the first meta-analysis which comprehensively assessed the associations between IL-10-1082G/A and IL-10-819C/T polymorphism and IS risk. The main purpose of our meta-analysis was to review the published literature and attempt to clarify the role of IL-10-1082G/A and IL-10-819C/T polymorphism in IS. In this study, we found significant associations in the overall comparison between IL-10-1082G/A polymorphism and IS. However, IL-10 gene -819C/T polymorphism was not associated with developing IS.
IL-10, an anti-inflammatory cytokine, was showed association with diseases such as type 2 diabetes mellitus (T2DM), coronary heart disease, asthma, stroke and cancer. Consistently, there was also showed association between IL-10 gene polymorphisms and above-mentioned diseases [28-32]. Anjana et al found that, for IL-1082G/A gene polymorphism, there were statistically significant differences in the genotypic distribution and allelic frequency between the patients and healthy controls. The result also showed that A allele of IL-10-1082G/A gene was one of the most predictive independent risk factors for stroke. Similarly, Antonino et al reported, with regard of IL-10 1082G/A genotypes, a higher frequency of A/A genotype in stroke subjects, a higher frequency of G/A genotype in controls and no significant difference in G/G genotype frequency between stroke subjects and controls were observed. Regarding IL-10-819C/T polymorphism, the C/T genotype was significantly more frequent in controls, whereas T/T and C/C genotypes frequency between stroke subjects and controls was no significant difference. Lin et al found there was significant difference in A allele of IL-10-1082G/A gene frequency distribution between stroke and control subjects. But, with regard of IL-10-819C/T gene, there was no significant difference about the genotype and allele frequency distribution between stroke and control subjects. Jin et al found, for the IL-10-1082G/A and -819C/T polymorphisms, there was no significant difference about the genotype and allele frequency distribution between stroke and control subjects. Zhang et al reported that, for the IL-10-1082G/A, G/A genotype and A allele frequency distributions were different between stroke and control subjects. Consistently, for IL-10-819C/T gene, the result was same with the study of above-mentioned. While, Sultana S. et al found that, for the IL10-1082 variants between stroke and control subjects, the G/G genotype was significantly associated with stroke [33]. In our meta-analysis, we excluded the study by Sultana S. et al, because patients in their study might be not completely ischemic stroke. These inconsistent results may be attributed to differences in genetic backgrounds, ethnic difference, and other factors, such as small sample size or inadequate adjustment for confounding factors.
Our study had its limitations and encountered some potential bias. This situation also existed in other meta-analyses [34]. These limitations of our meta-analysis should be addressed. First, some studies with small sample sizes may not have enough statistical power to prove authentic associations. Second, the number of our studies is comparatively small, more studies are needed. Third, our meta-analyses were performed without adjustment, may cause serious confounding bias. Fourth, Significant heterogeneity was found in some models, which may lead to failure to confirm marginal associations.
The potential bias of this meta-analysis also should be addressed. First, because of the limitations of data bases, it was possible that some eligible studies were not included. Second, heterogeneity is a potential problem when interpreting the results of meta-analysis. Alternatively, lifestyle, environment and ethnicity may be sources of heterogeneity. Studies about IL-10-1082G/A, heterogeneity was found in the dominant model (A/A vs. G/A+G/G) and the allelic model (A allele vs. G allele), so the random effects model was used. For studies about IL-10-819C/T, there are no significant heterogeneity in the dominant model (C/C vs. C/T+T/T) and the allelic model (C allele vs. T allele), thus, the fixed model was used. Third, meta-analysis belonged to a retrospective study, and recall bias might exist. Given the limitations and potential bias above, our findings should be explained with caution, and be warranted by future studies.
In spite of these limitations and potential bias, several advantages are existed in our meta-analysis. First, according to previous experience, it was feasible to perform this study based on the current literatures. Second, the findings of our studies provided additional information. A substantial number of participants were pooled from different studies, which help us draw a more reliable conclusion. Third, no publication bias was detected suggesting that the whole pooled result is accurate. Additionally, to evaluate the stability of the results after removing one study at one time, we found that the estimated pooled odd ratio changed quite little, indicating that our results were statistically robust.
In summary, a significant association was identified between the IL-10-1082G/A polymorphism and the risk of IS. Especially, AA genotype may be closely related to the risk of IS. As to the IL10-819C/T polymorphisms, we could not get any evidence to support the associations between the polymorphisms and IS risk. And these results are not for other subtypes of stroke. Further large sample-sized studies were required, and studies should use standardized unbiased genotyping methods, examine IS patients and well matched controls, and include multiethnic groups.
Acknowledgements
This study was supported by Xinjiang Science and Technology Projects (201491181) and National Natural Science Foundation of China (81470014).
Disclosure of conflict of interest
None.
References
- 1.Flynn RW, MacWalter RS, Doney AS. The cost of cerebral ischaemia. Neuropharmacology. 2008;55:250–6. doi: 10.1016/j.neuropharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–8. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F. The global stroke initiative. Lancet Neurol. 2004;3:391–3. doi: 10.1016/S1474-4422(04)00800-2. [DOI] [PubMed] [Google Scholar]
- 4.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 5.Flex A, Gaetani E, Papaleo P, Straface G, Proia AS, Peconni G. Pro-inflammatory genetic profiles in subjects with history of ischemic stroke. Stroke. 2004;35:2270–5. doi: 10.1161/01.STR.0000140740.19421.fe. [DOI] [PubMed] [Google Scholar]
- 6.Hollegaard MV, Bidwell JL. Cytokine gene polymorphism in human diseases: on-line databases, Supplement 3. Genes Immun. 2006;7:269–76. doi: 10.1038/sj.gene.6364301. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P. Genes for ischaemic stroke: strategies for their detection. J Hypertens. 1996;14:277–85. doi: 10.1097/00004872-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Protti GG, Gagliardi RJ, Forte WC, Sprovieri SR. Interleukin-10 may protect against progressing injury during the acute phase of ischemic stroke. Arq Neuropsiquiatr. 2013;71:846–51. doi: 10.1590/0004-282X20130168. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. Atherosclerosis: an inflammatory disease. N Eng J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 10.Liu KJ, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med. 2005;39:71–80. doi: 10.1016/j.freeradbiomed.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Perini F, Morra M, Allecci M, Galloni E, Marchi M, Toso V. Temporal Profile of serum anti-inflammatory and pro-inflammatory interleukins in acute ischemic stroke patients. Neurol Sci. 2001;22:289–96. doi: 10.1007/s10072-001-8170-y. [DOI] [PubMed] [Google Scholar]
- 12.Fei GZ, Svenungsson E, Frostegård J, Padyukov L. The A-1087IL-10 allele is associated with cardiovascular disease in SLE. Atherosclerosis. 2004;177:409–14. doi: 10.1016/j.atherosclerosis.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Xie G, Myint PK, Zaman MJ, Li Y, Zhao L, Shi P, Ren F, Wu Y. Relationship of Serum Interleukin-10 and Its Genetic Variations with Ischemic Stroke in a Chinese General Population. PLoS One. 2013;8:e74126. doi: 10.1371/journal.pone.0074126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koss K, Fanning GC, Welsh KI, Jewell DP. Interleukin-10 gene promoter polymorphism in English and Polish healthy controls. Polymerase chain reaction haplotyping using 3’ mismatches in forward and reverse primers. Genes Immun. 2000;1:321–415. doi: 10.1038/sj.gene.6363669. [DOI] [PubMed] [Google Scholar]
- 15.Eskdale J, Kube D, Tesch H, Gallagher G. Mapping of the human IL10 gene and further characterization of the 5’ flanking sequence. Immunogenetics. 1997;46:120–8. doi: 10.1007/s002510050250. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz V, Yentur SP, Saruhan-Direskeneli G. IL-12 and IL-10 polymorphisms and their effects on cytokine production. Cytokine. 2005;30:188–194. doi: 10.1016/j.cyto.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Munshi A, Rajeshwar K, Kaul S, Al-Hazzani A, Alshatwi AA, Sai Babu M, Usha A, Jyothy A. Interleukin-10-1082 promoter polymorphism and ischemic stroke risk in a South Indian population. Cytokine. 2010;52:221–4. doi: 10.1016/j.cyto.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Tuttolomondo A, Di Raimondo D, Forte GI, Casuccio A, Vaccarino L, Scola L, Pecoraro R, Serio A, Clemente G, Arnao V, Palmeri M, Misiano G, Lio D, Pinto A, Licata G. Single nucleotide polymorphisms (SNPs) of pro-inflammatory/anti-inflammatory and thrombotic/fibrinolytic genes in patients with acute ischemic stroke in relation to TOAST subtype. Cytokine. 2012;58:398–405. doi: 10.1016/j.cyto.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Jin L, Ni PH, Wu JM, Fu Y, Ge HL. The correlation between gene polymorphism of IL-10-819C/T and-1082G/A and cerebral infarction. Lab Med. 2011;26:717–721. [Google Scholar]
- 20.Lin KX. Study on the relationship between Interleukin-10 gene polymorphisms and cerebral infarction. Fujian Medical University. 2009 [Google Scholar]
- 21.Zhang GZ, Pan SY, Du R, Lu BX, Li W. The relationship between interleukin-10 gene polymorphisms and cerebral infarction. Chin J Cere-brovase Dis. 2007;4:294–297. [Google Scholar]
- 22.Attia J, Thakkinstian A, D’Este C, Lau J, Ioannidis JP, Schmid CH. Quantitative Meta-analyses of molecular association studies: methodologic lessons for genetic epidemiology. J Clin Epidemiol. 2003;56:297–303. doi: 10.1016/s0895-4356(03)00011-8. [DOI] [PubMed] [Google Scholar]
- 23.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 26.Reiner AP, Wurfel MM, Lange LA, Carlson CS, Nord AS, Carty CL, Rieder MJ, Desmarais C, Jenny NS, Iribarren C, Walston JD, Williams OD, Nickerson DA, Jarvik GP. Polymorphisms of the IL1-receptor antagonist gene (IL1RN) are associated with multiple markers of systemic inflammation. Arterioscler Thromb Vasc Biol. 2008;28:1407–12. doi: 10.1161/ATVBAHA.108.167437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezaii AA, Hoseinipanah SM, Hajilooi M, Rafiei AR, Shikh N, Haidari M. Interleukin-1 receptor antagonist gene polymorphism and susceptibility to ischemic stroke. Immunol Invest. 2009;38:220–30. doi: 10.1080/08820130902745146. [DOI] [PubMed] [Google Scholar]
- 28.Hyun MH, Lee CH, Kang MH, Park BK, Lee YH. Interleukin-10 Promoter Gene Polymorphisms and Susceptibility to Asthma: A Meta Analysis. PLoS One. 2013;8:e53758. doi: 10.1371/journal.pone.0053758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua Y, Shen J, Song Y, Xing Y, Ye X. Interleukin-102592C/A, 2819C/T and 21082A/G Polymorphisms with Risk of Type 2 Diabetes Mellitus: A HuGE Review and Meta-analysis. PLoS One. 2013;8:e66568. doi: 10.1371/journal.pone.0066568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bis JC, Heckbert SR, Smith NL, Reiner AP, Rice K, Lumley T, Hindorff LA, Marciante KD, Enquobahrie DA, Monks SA, Psaty BM. Variation in inflammation-related genes and risk of incident nonfatal myocardial infarction or ischemic stroke. Atherosclerosis. 2008;198:166–73. doi: 10.1016/j.atherosclerosis.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marousi S, Ellul J, Antonacopoulou A, Gogos C, Papathanasopoulos P, Karakantza M. Functional polymorphisms of interleukin 4 and interleukin 10 may predict evolution and functional outcome of an ischaemic stroke. Eur J Neurol. 2011;18:637–43. doi: 10.1111/j.1468-1331.2010.03228.x. [DOI] [PubMed] [Google Scholar]
- 32.Xue H, Wang YC, Lin B, An J, Chen L, Chen J, Fang JY. A meta-analysis of interleukin-10 -592 promoter polymorphism associated with gastric cancer risk. PLoS One. 2012;7:e39868. doi: 10.1371/journal.pone.0039868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sultana S, Kolla VK, Jeedigunta Y, Penagaluru PK, Joshi S, Rani PU, Reddy PP. Tumour necrosis factor alpha and interleukin 10 gene polymorphisms and the risk of ischemic stroke in south Indian population. J Genet. 2011;90:361–4. doi: 10.1007/s12041-011-0079-5. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Liu L, Zeng F, Wang K, Huang J, Xin L, Zhu PQ. Meta-analysis of the association between VEGF-634 G>C and risk of malignancy based on 23 case-control studies. J Cancer Res Clin Oncol. 2011;137:1027–36. doi: 10.1007/s00432-010-0966-9. [DOI] [PubMed] [Google Scholar]



