Abstract
Actinomycin D (ActD), a well known transcription inhibitors, has been widely reported to induce cell apoptosis in several types of tumor cells by inhibiting the anti-apoptotic gene transcriptions. However, how ActD affects osteosarcoma cells survival and its molecular mechanism is currently unclear. In the present study, results of proliferation assays and Hoechst stainings suggested that MG63 human osteosarcoma cells showed impaired cell proliferations and significant apoptosis after ActD treatment. Moreover, biochemical results showed that cleaved caspase-3 is gradually increased with the increasing ActD concentrations and treated times. Importantly, results of western blots indicated that protein levels of cyclin factors, such as cyclin A, D1 and E, were all reduced after ActD treatment. And ActD treatments may inhibit mRNA transcription levels of these cyclin factors, which may finally lead to cell cycle arrest and consequently apoptosis. The present study have revealed a novel mechanism by which ActD inhibits osteosarcoma cell proliferations and induces apoptosis, and will provide an useful clue to chemotherapy in future treatment of osteosarcoma.
Keywords: Actinomycin D, proliferation, apoptosis, cyclins, osteosarcoma cell
Introduction
Osteosarcoma, occurs predominantly in children and young adults, is the most common form of primary bone tumor with high rate of metastasis [1-3]. Recent studies have reported significant research advances in adjuvant chemotherapy of osteosarcoma [4,5]. However, the rapid development of metastatic lesions and resistance to chemotherapy remain major mechanisms responsible for the failure of treatments and the poor survival rate for patients [1]. Considerting that osteosarcoma cells develop high rate of proliferation and metastasis [6,7], drugs targeted at cell replication and mobility may have great potentials for osteosarcoma therapy by disrupting tumorous hyperplasia.
During osteosarcoma development, cell populations are firstly expanded caused by activated cell proliferations. One current model of the cell cycle envisages transitions between different cell cycle states by passage through checkpoints. Passage through the restriction point is promoted by a group of cyclin proteins, which includes cyclin A, D type cyclins, and cyclin E [8,9]. Therefore, cyclins are key components of the core cell cycle machinery, which constitute regulatory subunits that bind, activate, and provide substrate specificity for their associated cyclin-dependent kinases (Cdks) [10]. Interestingly, aberrant cyclin expressions have been reported in many human cancers. For example, both cyclin A and cyclin E are overexpressed in lung carcinoma cells [11], and cyclin D1 gene amplification occurs in breast, esophageal, bladder, lung and squamous cell carcinomas [12-14]. Thus, inhibitors of cyclins or Cdks are thought to be of particular importance for the arrest of cell proliferation in tumor development.
Actinomycin D (ActD), is an anti-neoplastic agent that inhibits RNA synthesis by binding to guanine residues and inhibiting DNA-dependent RNA polymerase [15,16]. ActD is a known DNA-interacting transcription blocker with anti-cancer activity [17,18], working as a cytotoxic inducer of apoptosis against tumor cells [16]. For example, ActD is one of the chemotherapy drugs which have been used in treatment of a variety of cancers and is a component of the highly successful combination treatment of Wilms tumor [19]. Moreover, ActD has been reported to be an antineoplastic antibiotic that inhibits cell proliferation, by forming a stable complex with double-stranded DNA, thus inhibiting DNA-primed RNA synthesis [20-22]. It also causes single-strand breaks in DNA. Nevertheless, ActD exhibits antitumor activity and induces tumor cell apoptosis.
Despite these reports, the effects of ActD on osteosarcoma cells have not been extensively investigated. Therefore, in this study, we characterized the actions of ActD on MG63 human osteosarcoma cells. We now report that ActD inhibits cell proliferation and subsequently induces apoptosis in osteosarcoma cells. Furthermore, the effects of ActD on osteosarcoma cells were in a dose- and time-dependent manner. Moreover, ActD treatment reduces cyclins (e.g. cyclin A, D1 and E) gene transcriptions and translations to exert its proliferative inhibitory effects. All these findings have established that ActD is a potential drug for improving osteosarcoma therapy clinically.
Experimental
Materials
Actinomycin D was purchased from Calbiochem (San Diego, CA, USA). Dulbecco’s Modified Essential Medium (DMEM) and supplemented Fetal Bovine Serum (FBS) were purchased from Gibco Invitrogen Corporation (Carlsbad, CA, USA). The Hoechst kit was from Beyotime Biotechnology Co. (Haimen, Jiangsu, China). As for antibodies, the cyclin A, D1 ane E antibodie were from Abcam (Cambridge, UK). The cleaved-caspase 3 antibody was from Cell Signaling Technology (Danvers, MA, USA). And anti-beta actin was from Millipore (Billerica, MA, USA). Other chemicals were of the highest purity available.
Cell culture
Human osteosarcoma cell line MG63 was obtained from Shanghai Institute of Cell Biology (Introduced from American Type Culture Collection). The cells were cultured in DMEM supplemented with 10% FBS and antibiotics in 5% CO2 at 37°C. For the experiments, MG63 cells were plated in 6-well plates at 1.0×105 cells/mL for Hoechst staining, and 1.0×106 cells/mL for Western blots and real-time PCR assay.
Pharmacological manipulations
For pharmacological treatment of Actinomycin D in MG63 cells, the final concentrations (0.1, 0.5, 1 and 5 μM) of Actinomycin D were applied to these cells, and then incubated by 0, 2, 6 and 24 hours for subsequent examinations. Equivalent ethanol was used as internal controls.
Cell proliferation assay
Cell proliferation was measured using the sulphorhodamine B (SRB) colorimetric assay. Briefly, MG63 cells were seeded at 1×103 cells/well in a 96-well microtiter plate with Actinomycin D treatment. At various times, cells were fixed in 10% trichloro-acetic acid for 1 h at 4°C, rinsed and subsequently stained for 30 min at room temperature with 0.2% SRB dissolved in 1% acetic acid, followed by air drying. Bound dye was solubilized in 100 μL of 10 mM unbuffered Tris base for 30 min and the OD was read at 490 nm in an ELISA plate reader.
Hoechst staining and MTT assay
For the preparation of Hoechst staining, MG63 cells were plated with 1.0×105 cells/mL in 6-well plates. After pharmacological manipulations, cells were directly stained with Hoechst kit from Beyotime. Cell counting was carried out through National Institutes of Health software ImageJ. As for cell viability assay (MTT assay), 1×103 cells were seeded in 96-well plate for 24 h. On the next day, cells were incubated with Actinomycin D respectively. Then the viable cells were stained by adding 20 μl MTT solution (5 mg/ml) per 100 μl of growth medium. After incubating for 4 h at 37°C, the media were removed and 150 μl DMSO was added to dissolve the formazan. The absorbance of each well was measured by microplate reader and viable cells were presented as a percent of the control.
Western blots
To extract proteins, cultured MG63 cells were sonicated with lysis buffer (PBS with 1% Triton X-100 and protease inhibitors). The cell lysate supernatants were harvested by centrifugation at l0, 000 rpm for 10 min at 4°C. Protein concentrations of the cell supernatants were evaluated, and measured by BCA Protein Assay kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Equal amount of the proteins from each extract were separated in a SDS-polyacrylamide gel (12.6%) with 5% stacking gel in SDS-Tris-glycine running buffer. The proteins were then transferred electrophoretically using a PVDF membrane, and followed with standard Western blots manipulations. Finally, proteins were detected by Super Signal® enhanced chemiluminescence development (ECL) (Thermo Scientific Pierce) reagent and exposed to films (Kodak). The protein level quantification was carried out by ImageJ.
Real-time PCR assay
Total RNAs were extracted from cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA was subjected to reverse transcription with reverse transcriptase as Manufacturer’s instructions (Fermentas Inc., Hanover, Md., USA). Quantitative real-time PCR was perfor-med using the Bio-Rad iQ5 system using Bio-Rad proprietary iQ5 software (Hercules, CA, USA), and the relative gene expression was normalized to internal control as beta-actin. Primer sequences for SYBR Green probes of target genes were as follows, Cyclin A: AGCAGAAGAGACTCAGAA AGG and GATAGTCAAGAGGTGTCAGTGG; Cylin D1: CCGTCCATGCGGAAGA C and ATCCAGCGGGAAGAC; Cyclin E: GGAAGAGGAAGGCAAACGTGA and T CGATTTTGGCCATTTCTTCAT; β-actin: GAGACCTTCAACACCCCAGC and ATGT CACGCACGATTTCCC.
Statistical analysis
All statistical analysis was performed by Image software. Quantitative data were showed in X̅ ± s using ANOVA tests for comparisons. The value 0.05 (*), 0.01 (**) and 0.001 (***) was assumed as the level of significance for the statistic tests carried out.
Results
Actinomycin D inhibits proliferation of MG63 human osteosarcoma cells
Actinomycin D (ActD) is reported to produce anti-cancer activity by binding to guanine residues and inhibiting DNA-dependent RNA polymerase [23]. However, the toxic effects of ActD on osteosarcoma cells are not fully elucidated. To characterize the anti-cancer activity of ActD on osteosarcoma cells, we examined the ActD-mediated cell alternations, such as cell proliferation. To determine whether ActD affects cell proliferations in osteosarcoma cells, we quantified cell proliferation in optimal growth conditions over a 24-hour period using the sulphorhodamine B (SRB) colorimetric assay. By statistical analysis, we found that ActD exhibited inhibitory effect on cell replications at 1 μM concentration from 2 hours to 24 hours. And higher concentrations of ActD by 5 μM showed much stronger inhibitory effect on cell replications, while lower concentrations of 0.1 and 0.5 μM seemed not to alter cell proliferations (Figure 1). Thus, our results suggest that ActD may arrest cell proliferations in MG63 human osteosarcoma cells in a time- and dose-dependent manner.
Figure 1.
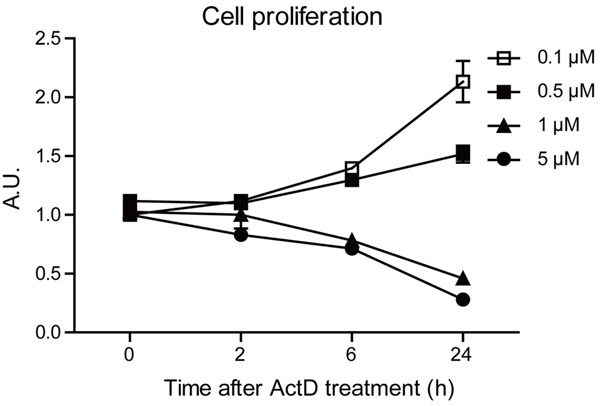
Actinomycin D inhibits proliferation of MG63 human osteosarcoma cells. Histograms showing the MG63 cell proliferation is impaired after Actinomycin D treatment (0.1, 0.5, 1 and 5 μM for 24 hours), by SRB colorimetric assay. Results are averages of three independent experiments.
Actinomycin D induces apoptosis of MG63 cells
We have shown that ActD may effectively affect cell proliferations in MG63 human osteosarcoma cells. Considering that non-replicated cells may develop cell apoptosis, we next examined whether ActD induced apoptosis in MG63 cells. We applied Hoechst staining to MG63 cells treated by ActD (5 μM) for different time points. The results howed that ActD could induce cell apoptosis from 2 hours (cell apoptosis by 23.2%) to 24 hours (cell apoptosis by 55.5%) (Figure 2A and 2B). To further determine the effect of ActD on cell apoptosis in MG63 cells, we next examined the cell viability of MG63 cells treated by ActD. Our results suggest that percentages of cell viability decrease to 89.0% (2 h), 72.7% (6 h) and 43.3% (24 h) after ActD treatment (Figure 2C).
Figure 2.
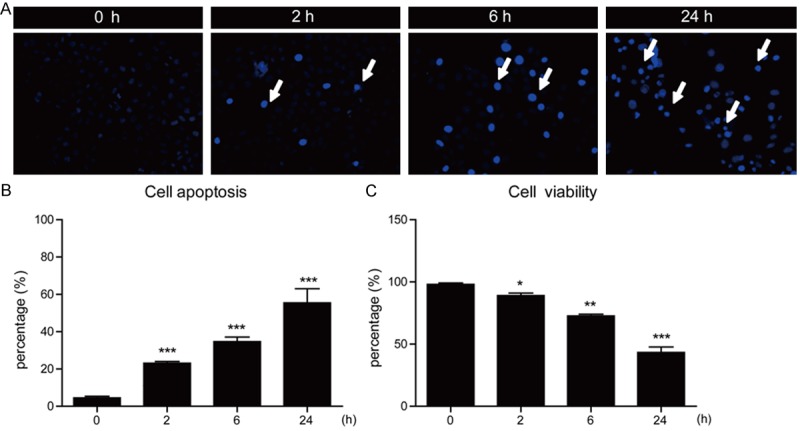
Actinomycin D induces apoptosis of MG63 cells in a time-dependent manner. (A) Hoechst stainings and (B) histograms showing the increased cell death (%) after Actinomycin D treatment (5 μM for 0, 2, 6 and 24 hours) in MG63 cells. (C) Histograms showing the reduced cell viability (%) after Actinomycin D treatment (5 μM for 24 hours) in MG63 cells. Results are averages of three independent experiments. Data represent mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001.
Since the ActD may induce apoptosis in MG63 cells in a time-dependent manner, we next would like to study whether the kill effect of ActD on MG63 cells was in a dose-dependent manner. Similarly, Hoechst staining results showed that percentages of cell apoptosis were enhanced as ActD concentrations increased (Figure 3A and 3B). Moreover, it’s also showed that cell viability decreased after ActD treatment (Figure 3C). Taken together, all these results support the notion that ActD would induce cell apoptosis in MG63 cells in a time- and dose-dependent manner.
Figure 3.
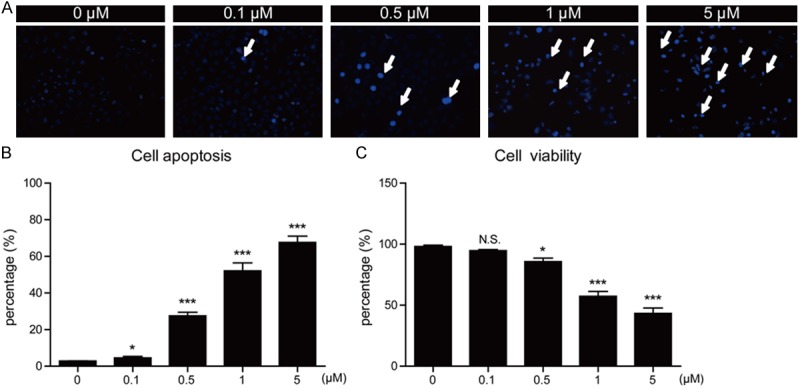
Actinomycin D induces apoptosis of MG63 cells in a dose-dependent manner. (A) Hoechst stainings and (B) histograms showing the increased cell death (%) after Actinomycin D treatment (0, 0.1, 0.5, 1 and 5 μM for 24 hours) in MG63 cells. (C) Histograms showing the reduced cell viability (%) after Actinomycin D treatment (0, 0.1, 0.5, 1 and 5 μM for 24 hours) in MG63 cells. Results are averages of three independent experiments. Data represent mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, and N.S, Not Statistically Significant.
To confirm the ActD-mediated cell apoptosis in MG63 cells, we assessed the apoptotic markers in MG63 cells by gradient ActD treatment. The results showed that ActD treatment indeed activated apoptotic marker cleaved caspase-3 in MG63 cells by the folds of 5.87 (1 μM for 24 h) and 8.74 (5 μM for 24 h) (Figure 4A and 4B). Thus, ActD may enhance apoptosis in MG63 human osteosarcoma cells.
Figure 4.
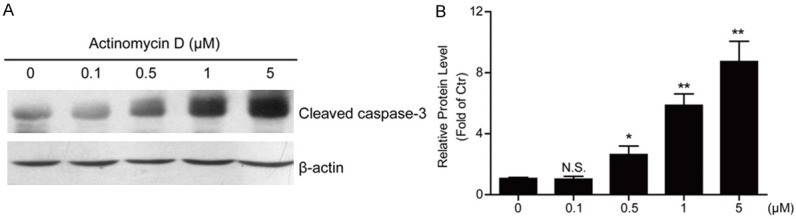
Actinomycin D induces caspase 3 cleavage in MG63 cells. A, B. Western blots and histograms showing that the cleaved caspase 3 protein level is decreased in MG63 cells by Actinomycin D treatment (0, 0.1, 0.5, 1 and 5 μM for 24 hours). Results are averages of three independent experiments. Data represent mean ± SEM. *P<0.05, **P<0.01, and N.S, Not Statistically Significant.
Actinomycin D decreases cyclins expressions in MG63 cells
To study the cellular mechanisms of how ActD inhibits cell proliferations and induces apoptosis in MG63 cells, we focused on the cell cycle factors. We assumed that ActD treatment may impair the cyclin proteins expressions in MG63 cells. To test this hypothesis, we examined the protein levels of cyclin proteins, such as cyclinA, cyclin D1 and cyclin E. The biochemical results showed that the protein levels of cyclinA, cyclin D1 and cyclin E were all reduced dramatically by ActD treatment, espeically for cyclin D1 (Figure 5A and 5B).
Figure 5.
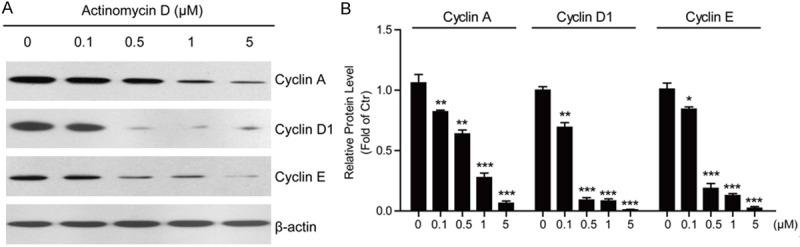
Actinomycin D decreases cyclins protein levels in MG63 cells. A, B. Western blots and histograms showing that the protein levels of Cyclin A, Cyclin D1 and Cyclin E are decreased in MG63 cells by Actinomycin D treatment (0, 0.1, 0.5, 1 and 5 μM for 24 hours). Results are averages of three independent experiments. Data represent mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001.
As for ActD is a well-known transcription blocker, we propose that ActD may affect cyclin protein levels via inhibiting their gene transcriptions. Indeed, the mRNA levels of cyclins after ActD treatment were analyzed using real-time PCR. The results suggested that the mRNA levels of cyclinA, cyclin D1 and cyclin E were also down-regulated by ActD treatment. It’s of note that the changes of transcription levels were highly correlated with the quantitative changes of protein levels assayed in MG63 cells (Figure 6A). Taken together, all these results indicate that ActD may decrease cyclin protein expressions at least partly by transcriptional regulation, finally leading to cell cycle arrest and cell apoptosis.
Figure 6.
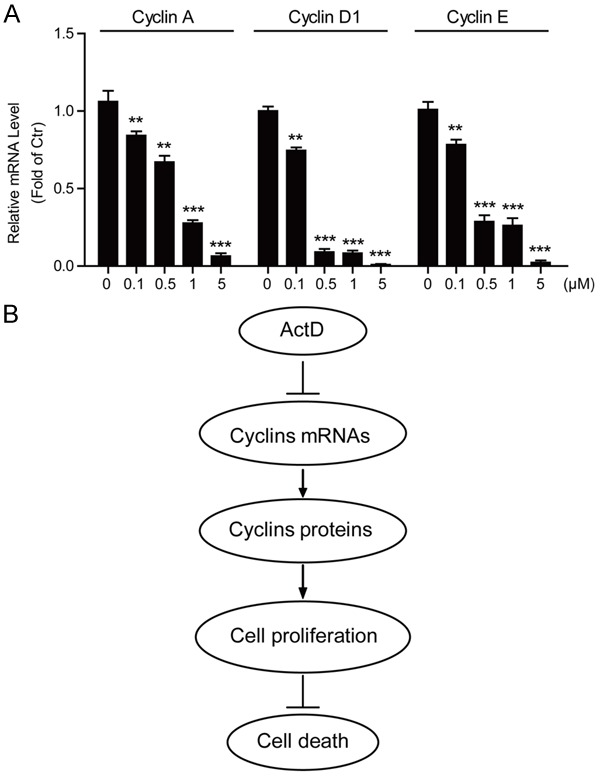
Actinomycin D decreases cyclin gene transcriptions in MG63 cells. A. Histograms showing that the mRNA levels of Cyclin A, Cyclin D1 and Cyclin E are decreased in MG63 cells by Actinomycin D treatment (0, 0.1, 0.5, 1 and 5 μM for 24 hours). Results are averages of three independent experiments. Data represent mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001. B. Schematic representation highlighting the molecular link between Actinomycin D, cyclin gene expression and cell death in MG63 human osteosarcoma cells. Actinomycin D inhibits gene transcription and then protein expression of cyclin proteins, leading to the arrest of cell cycles and proliferations of MG63 cells, which finally promoting cell apoptosis.
Discussion
Uncontrolled cellular proliferation is the hallmark of cancer, and proliferation rate, that is the rate of tumor cell division, is linked to prognosis for several types of cancer, such as osteosarcoma [24,25]. Therefore, targeted inhibition of cell proliferation may afford clues for cancer therapy. In the present study, we demonstrated that Actinomycin D (ActD) may impair osteosarcomatous proliferations and lead to significant apoptosis in MG63 human osteosarcoma cells. Moreover, the proliferative inhibitory effects of ActD on osteosarcoma cells may be at least partly dependent on cyclin protein expressions, because we noted that ActD treatment would inhibit either cyclin gene transcriptions or protein translations. Thus, the reduced cyclin protein expressions may be responsible for the cell cycle arrest and consequently apoptosis (Figure 6B). Taken together, our work have revealed a novel mechanism by which ActD inhibits osteosarcoma cell proliferations and induces apoptosis, and will provide an useful clue to chemotherapy in future treatment of osteosarcoma.
Osteosarcoma is the most common form of malignant bone cancer in humans. Multidrug chemotherapy and aggressive surgical techniques have improved survival; however, the prognosis for human patients with metastatic disease remains a serious problem [26]. Early metastasis, chemotherapy resistance, and altered expression of tumorous proteins together contribute to the development of osteosarcoma [27]. Therefore, the search for more effective anti-osteosarcoma agents is necessary and urgent. Here, our work demonstrates that ActD, a transcription blocker, could inhibit osteosarcoma cell proliferations and thus induce apoptosis to these cells. It’s noted that ActD exhibits superb effects on osteosarcomatous apoptosis at high concentration (1 and 5 μM). This result is consistent with previous reports, showing that high doses (1 mg/mL) of ActD block transcription of all RNA species, whereas low doses (100 ng/mL) cause preferential inhibition of ribosomal RNA synthesis [16]. Nevertheless, our work reveals that ActD is of potential to induce apoptosis in osteosarcoma cells by cell cycle arrest.
Apoptosis has been identified as a central mechanism for the induction of cell death by cytostatic drugs [16,28]. Cytostatic drugs seem to trigger diverse pathways, leading to apoptosis including the activation of death receptor/ligand systems, mitochondrial changes, DNA damage or inhibition of protein synthesis. And ActD is a potent inducer of apoptosis in a variety of cells in vitro and in vivo, by binding to DNA and inhibiting RNA and protein synthesis. In present study, we further revealed that ActD was able to induce cell cycle arrest by blocking cyclin protein expressions. Actually, there are evidences showing that cyclins are potential oncogenes of human cancer. For example, both cyclin D1 and cyclin E are deregulated in a substantial fraction of human cancers, including cancers of the breast, prostate, lung, gut, pancreas and head and neck, and in many cases this is associated with effects on prognosis [9]. Moreover, overexpression of cyclin D1 in the mammary gland leads to hyperplasia and eventually to carcinoma [12]. Thus, inhibitory effects of ActD on cyclins support the notion that ActD could work as a potent inducer of apoptosis in osteosarcoma cells.
Conclusion
The present results showed that ActD may impair osteosarcomatous proliferations and lead to significant apoptosis in MG63 human osteosarcoma cells, by inhibiting cyclins expressions and arresting cell-cyle progression. Our work have revealed a novel mechanism by which ActD inhibits osteosarcoma cell proliferations and induces apoptosis, and will provide an useful clue to chemotherapy in future treatment of osteosarcoma.
Disclosure of conflict of interest
None.
References
- 1.Zhang Y, Hu H, Song L, Cai L, Wei R, Jin W. Epirubicin-mediated expression of miR-302b is involved in osteosarcoma apoptosis and cell cycle regulation. Toxicol Lett. 2013;222:1–9. doi: 10.1016/j.toxlet.2013.06.242. [DOI] [PubMed] [Google Scholar]
- 2.Gong C, Liao H, Wang J, Lin Y, Qi J, Qin L, Tian LQ, Guo FJ. LY294002 induces G0/G1 cell cycle arrest and apoptosis of cancer stem-like cells from human osteosarcoma via down-regulation of PI3K activity. Asian Pac J cancer Prev. 2012;13:3103–3107. doi: 10.7314/apjcp.2012.13.7.3103. [DOI] [PubMed] [Google Scholar]
- 3.Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AH, Hogendoorn PC, Egeler RM. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer. 2011;47:2431–2445. doi: 10.1016/j.ejca.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 5.D’Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin oncol. 2011;38:S19–29. doi: 10.1053/j.seminoncol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C, Trepel J, Meltzer P, Helman L. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res. 2001;61:3750–3759. [PubMed] [Google Scholar]
- 7.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Ann Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 9.Musgrove EA. Cyclins: roles in mitogenic signaling and oncogenic transformation. Growth factors. 2006;24:13–19. doi: 10.1080/08977190500361812. [DOI] [PubMed] [Google Scholar]
- 10.Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 11.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nature Rev Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 12.Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 13.Inomata M, Uchino S, Tanimura H, Shiraishi N, Adachi Y, Kitano S. Amplification and overexpression of cyclin D1 in aggressive human esophageal cancer. Oncol Rep. 1998;5:171–176. doi: 10.3892/or.5.1.171. [DOI] [PubMed] [Google Scholar]
- 14.Akervall JA, Michalides RJ, Mineta H, Balm A, Borg A, Dictor MR, Jin Y, Loftus B, Mertens F, Wennerberg JP. Amplification of cyclin D1 in squamous cell carcinoma of the head and neck and the prognostic value of chromosomal abnormalities and cyclin D1 overexpression. Cancer. 1997;79:380–389. [PubMed] [Google Scholar]
- 15.Szeberenyi J. The effect of actinomycin D on RNA metabolism in human cells. Biochem Mol Biol Edu. 2006;34:50–51. doi: 10.1002/bmb.2006.49403401050. [DOI] [PubMed] [Google Scholar]
- 16.Kleeff J, Kornmann M, Sawhney H, Korc M. Actinomycin D induces apoptosis and inhibits growth of pancreatic cancer cells. Int J Cancer. 2000;86:399–407. doi: 10.1002/(sici)1097-0215(20000501)86:3<399::aid-ijc15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Ginell S, Lessinger L, Berman HM. The crystal and molecular structure of the anticancer drug actinomycin D--some explanations for its unusual properties. Biopolymers. 1988;27:843–864. doi: 10.1002/bip.360270511. [DOI] [PubMed] [Google Scholar]
- 18.Hou MH, Robinson H, Gao YG, Wang AH. Crystal structure of actinomycin D bound to the CTG triplet repeat sequences linked to neurological diseases. Nucl Acids Res. 2002;30:4910–4917. doi: 10.1093/nar/gkf619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green DM, Beckwith JB, Breslow NE, Faria P, Moksness J, Finklestein JZ, Grundy P, Thomas PR, Kim T, Shochat S. Treatment of children with stages II to IV anaplastic Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J. Clin. Oncol. 1994;12:2126–2131. doi: 10.1200/JCO.1994.12.10.2126. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Cai S, Cai L, Li X. Anti-apoptotic effect of hepatocyte growth factor from actinomycin D in hepatocyte-derived HL7702 cells is associated with activation of PI3K/Akt signaling. Toxicol Lett. 2006;165:142–148. doi: 10.1016/j.toxlet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Ma W, Teng Y, Hua H, Hou J, Luo T, Jiang Y. Upregulation of heat shock protein 27 confers resistance to actinomycin D-induced apoptosis in cancer cells. FEBS J. 2013;280:4612–4624. doi: 10.1111/febs.12432. [DOI] [PubMed] [Google Scholar]
- 22.Wang MJ, Liu S, Liu Y, Zheng D. Actinomycin D enhances TRAIL-induced caspase-dependent and -independent apoptosis in SH-SY5Y neuroblastoma cells. Neurosci Res. 2007;59:40–46. doi: 10.1016/j.neures.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Merkel O, Wacht N, Sifft E, Melchardt T, Hamacher F, Kocher T, Denk U, Hofbauer JP, Egle A, Scheideler M, Schlederer M, Steurer M, Kenner L, Greil R. Actinomycin D induces p53-independent cell death and prolongs survival in high-risk chronic lymphocytic leukemia. Leukemia. 2012;26:2508–2516. doi: 10.1038/leu.2012.147. [DOI] [PubMed] [Google Scholar]
- 24.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama T, Dass CR, Choong PF. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol Cancer Ther. 2008;7:3461–3469. doi: 10.1158/1535-7163.MCT-08-0530. [DOI] [PubMed] [Google Scholar]
- 27.Osaki M, Takeshita F, Sugimoto Y, Kosaka N, Yamamoto Y, Yoshioka Y, Kobayashi E, Yamada T, Kawai A, Inoue T, Ito H, Oshimura M, Ochiya T. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther. 2011;19:1123–1130. doi: 10.1038/mt.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bock J, Szabo I, Jekle A, Gulbins E. Actinomycin D-induced apoptosis involves the potassium channel Kv1.3. Biochem Biophys Res Commun. 2002;295:526–531. doi: 10.1016/s0006-291x(02)00695-2. [DOI] [PubMed] [Google Scholar]


