Abstract
Tantalum rod implant following core decompression is reported to be effective in early stage of osteonecrosis of the femoral head (ONFH). The purpose of this study was to assess the survivorship and prognostic factors for radiographic progression and conversion to total hip arthroplasty (THA) after treatment with a modified tantalum implant technology. 59 consecutive hips (45 patients) in whom ONFH was treated with core decompression, impaction bone grafting of 2 mm-composite bone filling material, and insertion of a porous tantalum implant. 57 hips (44 patients, mean age 43 years, range 21 to 70 years) with Steinberg Stage I-IVA ONFH were available for follow-up at a mean of 44.8 months (rang, 11 to 62 months). Outcome measures included HHS (Harris Hip Score), radiographic outcome, and survivorship analysis with reversion to THA. Radiographic progression occurred in 17 hips (17/57, 29.82%). 11 hips (11/57, 19.30%) were converted to THA. The overall survival rate was 72.49% at 60 months post-operatively. After logistic regression analysis, corticosteroid use and bone marrow edema were found to be predictors of radiographic progression. The Cox proportional-hazard model revealed that bone marrow edema was an independent prognostic factor for conversion to THA. This modified technology may make patients avoid the use of corticosteroid, especially those without bone marrow edema, and obtains encouraging survival rates and a delay in or prevention of THA.
Keywords: Osteonecrosis of the femoral head, porous tantalum implant, prognostic factors, bone marrow edema, survivorship analysis, radiographic progression
Introduction
ONFH is a pathological state with multiple possible etiologies that causes decreased vascular supply to the subchondral bone of the femoral head, resulting in osteocyte death and collapse of the articular surface [1,2]. It remains a difficult disease to treat because it typically affects young patients in their one third to fifth decades of life and a considerable proportion of patients suffer from a bilateral hip involvement.
Operative management alternatives for ONFH vary from joint salvaging procedures including proximal femur rotational osteotomy, core decompression sequestrectomy and replacement with bone grafting, non-vascularized cancellous or cortical bone grafting of the lesion, muscle-pedicle bone grafting, free vascularized fibular grafting and multiple small tantalum pegs [3-12]. In which, the two most commonly used procedures are core decompression and free vascularized fibular grafting.
Alternatively, a method combining core decompression and insertion of an osteonecrosis intervention implant has been developed. This method was first proposed by Pedersen et al. in 1997 [13]; they indicated that a porous tantalum rod was a reasonable mechanical substitute for a fibular graft. Since 2005, a number of investigations using the porous tantalum rod following core decompression for ONFH treatment have been reported [14-21]. However, the clinical outcomes, postoperative weight-bearing time and the role of porous tantalum implant are still controversial [18,21].
In the present study, we reported a modified porous tantalum implant technology and performed a survivorship analysis for patients with ONFH undergoing this modified technology in our institution. Additionally, some independent prognostic factors for radiographic progression and conversion to THA were identified.
Patients and methods
Patients
Inclusion and exclusion criteria
This prospective study was conducted during June 2006 to January 2009. Patients who all signed informed consent forms were offered the tantalum implant (Trabecular Metal, Zimmer, Co. USA) procedure when they were unwilling to have treatment with free vascularized fibular graft or THA. After approval from the institutional review board, the study was approved by the local ethical committee of our institution. The diagnosis of “ONFH” was set by radiological and clinical evidence. The inclusion criteria were as follows: Patients between 18 and 70 years, with a body mass index of less than 40 and Steinberg stage I, II, III, or IVA ONFH were enrolled. Exclusion criteria were: ONFH with serious subchondral collapse (Steinberg IVB, and IVC) or the complete destruction of the hip joint (Steinberg V and Steinberg VI); Patients with a history of previous core decompression, bone grafting, and proximal femoral osteotomy in the affected hip; Patients who had undergone a previous treatment for avascular necrosis in the affected hip, such as electromagnetic and ultrasound stimulation, or taken medications intended to intervene in or treat the disease.
Study population
45 patients with 59 osteonecrotic hips treated with tantalum implants were initially entered in the study and signed an informed consent form prior to enrollment. 1 patient (1 hip) was lost to follow-up six weeks after surgery, while another patient with bilateral involvement had an early failure in left hip 5 months after surgery due to deep infection and therefore received tantalum rod implant removal. 57 hips (44 patients, 5 females and 39 males; mean 43 years, rang 21 to 70 years) were available at a mean follow-up of 44.8 months (rang, 11 to 62 months).
In all, 25 patients (25/44, 56.81%) had bilateral involvement (13 patients with bilateral tantalum implants; 1 patient with a unilateral tantalum implant and with a previous contralateral THA; 3 patients with a unilateral tantalum implant and simultaneously with a contralateral THA; 3 patients with a unilateral tantalum implant and simultaneously with a contralateral porous hydroxylapatite composite bone grafting, and another 5 patients with a unilateral tantalum implant and simultaneously had a contralateral percutaneous multiple small-diameter drilling).
Modified tantalum implant surgical technique
With use of fluoroscopic guidance, the guidewire was then placed into the center of the osteonecrotic lesion, typically in the anterolateral portion of the femoral head. After a minimally invasive lateral approach (2 to 3 cm skin incision), core decompression, with use of three cannulated drills (8, 9, and 10 mm), was then used to remove bone up to the subchondral level. The cannulated drill bit and guidewire were then removed, and expanding scraper of various diameters was used to progressively decompress the area of osteonecrosis. The sequestrum or bone marrow fat in the necrotic area was removed using a long-handled curette. Then granular porous medical nano-hydroxyapatite/polyamide 66 composite bone filling material (nano-apatite composite, Sichuan National Nano Technology Co., Ltd, Chengdu, China) were implanted in the proximal bone tunnel to the length of 2 mm. Repeated filling and compaction of the particles was performed using a pushing bar to ensure close suppress [22]. After measuring and tapping, the implant was threaded into the final position until the implant abutted the end of suppressed bone particles. Wound closure was performed after placing a suction drainage tube at the outer edge of the incision. Bilateral procedures were performed in the same operative period, whether they were both tantalum implants or one tantalum implant and the other kind of hip joint operation. All surgeries were performed by the same team of orthopedic surgeons (Liu Y and Liu S).
Postoperative management and rehabilitation
Postoperative care consisted of removal the drainage tube 24 to 48 h after surgery, prophylactic intravenous antibiotic (Cefazolin 1 to 2 g IV 8 h) for the first 48 hours after surgery to prevent wound infection and anticoagulation therapy (Low Molecular Weight Heparin 5000 IU SC qd) for at least three days.
Weight-bearing was not allowed within the first 3 months after surgery. Partial weight-bearing crutch walking was allowed thereafter and full weight-bearing was allowed 6 months after surgery. Patients who had persistent pain and limitation in function and who were not satisfied with the outcome following tantalum rod insertion were assessed by a single surgeon (Liu S) for conversion to THA.
Follow-up and outcome assessment
Follow-up examinations were scheduled at 1, 3, 6 and 12 months, and then once a year. The evaluation parameters included Harris hip score, radiographic examination and MR images of the affected hip. Radiographs of the affected hip in anteroposterior and lateral views were used to assess the size of the lesion, congruency of the femoral head, the presence of a crescent sign and degenerative changes of the hip joint. MR images were used to evaluate the change in the size of the lesion, bone marrow edema and joint effusion. The initial stage and the extent of involvement of the femoral head were assessed radiographically according to the classification system of Steinberg [23]. Necrosis area of more than 30% was defined as large osteonecrotic lesion. Bone marrow edema was defined as an ill-defined area of low signal intensity on T1-weighted images, with corresponding high signal intensity on T2-weighted or inversion recovery images localizing to the femoral head, neck, and intertrochanteric region [24-27]. The joint fluid was graded on the basis of the coronal images as follows: 0, no fluid; 1, minimal fluid; 2, enough fluid to surround the femoral neck (Figure 4); and 3, distention of capsule recesses [27]. Joint effusion was defined as grade ≥ 2 joint fluids. Throughout the study, clinical evaluation was done by a single observer (not a surgeon). Two radiologists (Zhang H & Liu W) blinded to the nature of study read and reported the results of radiograph and MR studies. To circumvent the problem of intra-observer and interobserver variability in radiographic assessing, they independently evaluated the radiographs. If there was a disagreement, a third person interpreted the films until a unanimous decision could be made.
Figure 4.
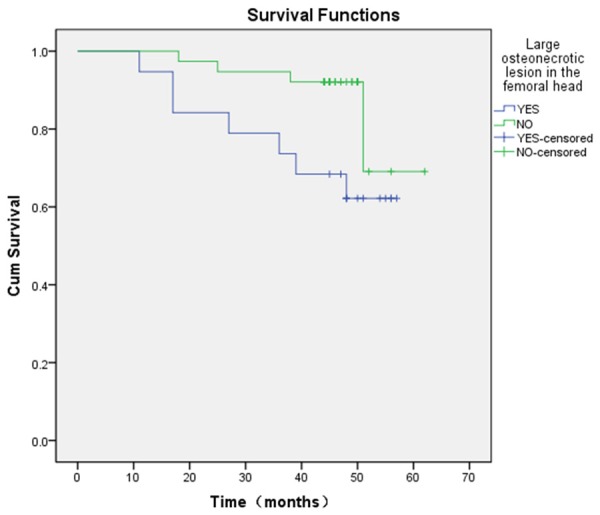
Kaplan-Meier survival curve, stratified according to osteonecrotic lesion in the femoral head. The end point is revision to THA shows the estimated survival rates were 69.08% at 60 months for hips without large osteonecrotic lesion in the femoral head (95% CI, 17.50% to 92.45%) and 62.20% at 60 months for hips with large osteonecrotic lesion in the femoral head (95% CI, 36.44% to 79.99%) (= 4.24, P = 0.040).
Statistical analysis
Statistical analysis of the data was performed with use of Statistical Package for the Social Sciences (SPSS) software (version 13.0 for Windows; SPSS, Chicago, Illinois). The Student t test was used for the comparison of means between preoperative Harris hip scores and postoperative Harris hip scores. Nominal variables were tested with use of the chi-square test or the Fisher’s exact test. A Kaplan-Meier survival analysis, with revision to THA as the end point, was performed. A comparison of Kaplan-Meier curves for stratification factors was performed with the log-rank test (Mantel-Cox). Multi-factor analysis was performed with use of the logistic regression analysis to identify the independent prognostic factors related to radiographic progression, and with use of the Cox proportional-hazards model to determine the independent prognostic factors associated with conversion to THA. All tests were two-sided. The results were considered to be significant at P < 0.05.
Results
Hip scores
The average postoperative Harris hip score for the 57 hips available for clinical evaluation at last follow-up was 78 ± 2.95 points (range, 56 to 100 points [20 excellent, 18 good, 4 fair, 15 poor]), whereas the average preoperative Harris hip score was 59 points ± 2.80 (range, 38 to 80 points [5 excellent, 1 good, 3 fair, 48 poor]) (t = 6.29, P < 0.001).
Radiographic progression
According to preoperative Steinberg stage of the disease, radiographic progression occurred in 17 hips (29.8%) after insertion of the porous tantalum implants in follow-up examinations. 1 stage-I hip showed flattening of the femoral head with depression (stage-IV). 9 stage-II hips showed radiographic progression: 7 hips progressed to stage-III, 2 hips showed progression to stage-IV. 3 stage-III hips showed joint space narrowing (stage-V). 1 stage-IV hip showed progression to stage-V. 1 stage-III and 2 stage-IV hips showed progression in the same stage.
By logistic regression analysis, corticosteroid use (hazard ratio, 41.32; 95% confidence interval (CI), 0.06 to 0.97; Wald = 13.59; P < 0.001), bone marrow edema (hazard ratio, 0.22; 95% CI, 0.05 to 0.99; Wald = 3.91, P = 0.048) were found to be two predictors of radiographic progression. With regard to underlying risk factors, 11 of 23 hips with corticosteroid-induced osteonecrosis occurred radiographic progression according to preoperative Steinberg stage of the disease, whereas only 6 of 34 hips with non corticosteroid-induced osteonecrosis occurred radiographic progression (relative risk = 2.71; 95% CI, 1.17 to 6.29; x2 = 5.970, P = 0.015). Meanwhile, concerning bone marrow edema, 13 of 32 hips with bone marrow edema occurred radiographic progression according to preoperative Steinberg stage of the disease, whereas only 4 of 25 hips without bone marrow edema occurred radiographic progression (relative risk = 2.54; 95% CI, 0.99 to 12.93; x2 = 4.066, P = 0.044).
Conversion to THA
11 hips (19.30%) were converted into THA at an average time of 44.8 months (range, 11 to 62 months) after insertion of the porous tantalum implant. The patients who had a revision included 8 men and 3 women (average age, 50 years; range, 34 to 68 years). The Kaplan-Meier survival analysis for all hips (Figure 1) showed that the probability for not requiring revision to THA after insertion of a porous tantalum implant was 98.25% (95% CI, 88.19% to 99.75%) at 12 months, 92.98% (95% CI, 82.37% to 97.31%) at 24 months, 89.47% (95% CI, 78.06% to 95.13%) at 36 months, 84.21% (95% CI, 71.85% to 91.45%) at 48 months, and 72.49% (95% CI, 49.58% to 86.29%) at 60 months.
Figure 1.
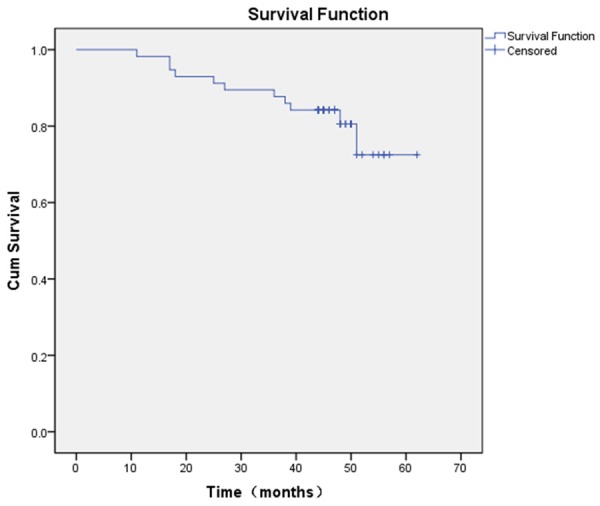
Kaplan-Meier survival curve shows the survival rates were 98.25% (95% CI, 88.19% to 99.75%) at 12 months, 92.98% (95% CI, 82.37% to 97.31%) at 24 months, 89.47% (95% CI, 78.06% to 95.13%) at 36 months, 84.21% (95% CI, 71.85% to 91.45%) at forty-eight months, and 72.49% (95% CI, 49.58% to 86.29%) at 60 months.
10 of 32 hips (31.25%) accompanied with bone marrow edema required conversion into THA, whereas only 1 of 25 hips (4.00%) not accompanied with bone marrow edema required conversion into THA (relative risk = 7.81; 95% CI, 1.07 to 57.03; x2 = 5.057, P = 0.025). A comparison of Kaplan-Meier curves showed significantly lower survival rates (x2 = 7.429, P = 0.006) for hips accompanied with bone marrow edema (65.34% at 60 months; 95% CI, 43.44% to 80.48%) than for those not accompanied with bone marrow edema (85.71% at 60 months; 95% CI, 33.41% to 97.86%) (Figure 2).
Figure 2.
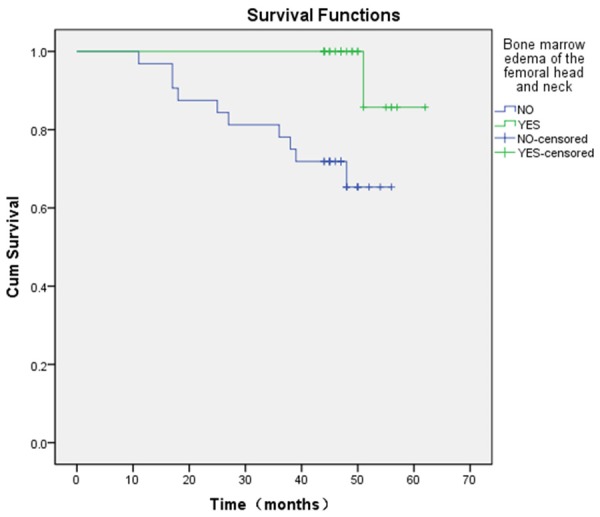
Kaplan-Meier survival curve, stratified according to whether accompanied with bone marrow edema shows the estimated survival rates were 85.71% at 60 months for hips not accompanied with bone marrow edema (95% CI, 43.44% to 80.48%) and 67.37% at 60 months for hips accompanied with bone marrow edema (95% CI, 33.41% to 97.86%) (= 7.429, P = 0.006).
10 of 34 hips (29.41%) accompanied with joint effusion on preoperative MRI imaging required conversion into THA, whereas only 1 of 23 (4.34%) hips in patients not accompanied with joint effusion on preoperative MRI imaging required conversion into THA (relative risk = 6.76; 95% CI, 0.93 to 49.31; x2 =4.041, P = 0.044). A comparison of Kaplan-Meier curves showed significantly lower survival rates (x2 = 5.910, P = 0.015) for hips accompanied with joint effusion on preoperative MRI imaging (62.20% at 60 months; 95% CI, 46.27% to 81.71%) than for those not accompanied with joint effusion on preoperative MRI imaging (83.33% at 60 months; 95% CI, 27.31% to 97.47%) (Figure 3).
Figure 3.
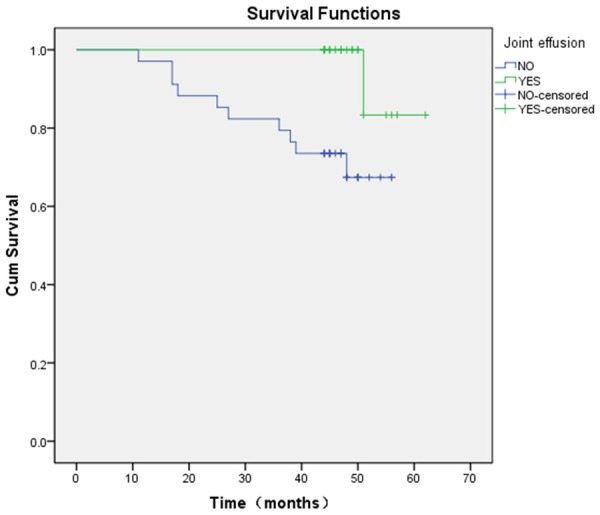
Kaplan-Meier survival curve, stratified according to whether accompanied with joint effusion shows the estimated survival rates were 83.33% at 60 months for hips not accompanied with joint effusion (95% CI, 27.31% to 97.47%) and 67.40% at 60 months for hips accompanied with joint effusion (95% CI, 46.27% to 81.71%) (= 5.910, P = 0.015).
7 of 19 hips (63.64%) with large osteonecrotic lesion in the femoral head required conversion into THA, whereas only 4 of 38 hips (10.52%) without large osteonecrotic lesion in the femoral head required conversion into THA (relative risk = 3.69; 95% CI, 1.32 to 22.02; x2 = 4.069, P = 0.044). A comparison of Kaplan-Meier curves showed significantly lower survival rates (x2 = 4.24, P = 0.040) for hips with large osteonecrotic lesion in the femoral head (62.20% at 60 months; 95% CI, 36.44% to 79.99%) than hips without large osteonecrotic lesion in the femoral head (69.08% at 60 months; 95% CI, 17.50% to 92.45%) (Figure 4).
5 of 13 hips (39.46%) in patients with chronic systemic disease required conversion into THA, whereas only 6 of 44 hips (13.64%) in patients without chronic systemic disease required conversion into THA (relative risk = 2.82; 95% CI, 1.02 to 7.77; x2 = 2.2537, P = 0.111). There was a trend towards lower survival rates for hips with chronic systemic disease than for hips without chronic systemic disease (x2 = 3.072, P = 0.080).
Meanwhile, with regard to preoperative Steinberg stage, there existed a trend towards lower survival rates for the higher stage hips than for the lower stage hips (x2 = 6.488, P = 0.090).
However, with the numbers studied, no significant difference were found among the survival curves when stratified by gender (x2 = 2.035, P = 0.154), age (x2 = 1.740, P = 0.187), etiology (x2 = 5.223, P = 0.156), corticosteroid intake(x2 = 2.517, P = 0.113), bilateral disease treated with tantalum implant (x2 = 0.008, P = 0.928), preoperative collapse of the femoral head (x2 = 0.139, P = 0.709), more than 80 points according to preoperative Harris hip score (x2 = 2.068, P = 0.150). The survival probability at 36 months and 60 months by variable demographic and radiographic parameters are summarized in Table 1.
Table 1.
Analysis of survival probability at 36 months and 60 months by variable demographic and radiographic parameters
| Parameter | Number of Hips | Conversion to THA | Survival Probability at 36 Months (SE) | Survival Probability at 60 Months (SE) | Log-rank test |
|---|---|---|---|---|---|
| Overall Study | 57 | 11 | 89.47% (4.06%) | 72.49% (9.28%) | |
| Gender# | P = 0.141 | ||||
| Male | 50 | 8 | 90.00% (0.42%) | 74.44% (10.97%) | |
| Female | 7 | 3 | 85.71% (13.23%) | 57.14% (18.70%) | |
| Age | P = 0.161 | ||||
| ≤ 50 years | 40 | 6 | 95.00% (3.45%) | 74.06% (12.45%) | |
| > 50 years | 15 | 5 | 76.47% (10.29%) | 70.59% (11.05%) | |
| Etiology | P = 0.982 | ||||
| Corticosteroid-related | 23 | 7 | 86.95% (70.20%) | 67.19% (10.50%) | |
| Idiopathic | 12 | 3 | 75.00% (0.2150%) | 75.00% (21.50%) | |
| Alcoholic | 17 | 1 | 1.0000% (0.0000%) | 75.00% (21.65%) | |
| Posttraumatic | 5 | 0 | 100.00% (0.00%) | 100.00% (0.00%) | |
| Chronic systemic disease | P = 0.081 | ||||
| Yes | 13 | 5 | 76.93% (11.69%) | 46.16% (20.69%) | |
| No | 44 | 6 | 93.18% (3.80%) | 83.10% (6.99%) | |
| Corticosteroid use | P = 0.099 | ||||
| Yes | 23 | 7 | 86.95% (7.02%) | 67.19% (10.50%) | |
| No | 34 | 4 | 91.17% (4.86%) | 75.97% (14.45%) | |
| Bilateral disease treated with Tantalum implant | P = 0.982 | ||||
| Unilateral | 32 | 6 | 93.74% (4.28%) | 79.93% (7.46%) | |
| Bilateral | 25 | 5 | 83.99% (7.33%) | 71.99% (12.76%) | |
| Preoperative Steinberg Stage | P = 0.094 | ||||
| I | 4 | 0 | 100.00% (0.00%) | 100.00% (0.00%) | |
| II | 22 | 5 | 90.91% (6.13%) | 75.97% (9.49%) | |
| III | 6 | 3 | 66.66% (19.24%) | 50.00% (20.41%) | |
| IVA | 25 | 3 | 92.00% (5.43%) | 46.00% (32.64%) | |
| Preoperative Harris hip score more than 80 points | P = 0.150 | ||||
| Yes | 8 | 0 | 100.00% (0.00%) | 100.00% (0.00%) | |
| No | 49 | 11 | 87.76% (4.68%) | 67.67% (10.78%) | |
| Bone marrow edema | P = 0.006 * | ||||
| Yes | 32 | 10 | 81.35% (6.99%) | 65.34% (9.54%) | |
| No | 25 | 1 | 85.71% (13.22%) | 85.71% (13.22%) | |
| Joint effusion | P = 0.015 * | ||||
| Yes | 34 | 10 | 82.35% (6.54%) | 67.40% (9.08%) | |
| No | 23 | 1 | 100.00% (0.00%) | 83.33% (15.21%) | |
| Preoperative collapse of the femoral head | P = 0.709 | ||||
| Yes | 26 | 5 | 92.31% (5.22%) | 79.32% (8.38%) | |
| No | 31 | 6 | 87.09% (6.61%) | 41.93% (29.83%) | |
| Extent of osteonecrotic lesion | P = 0.040 * | ||||
| Small and Medium (< 30%) | 19 | 7 | 94.74% (3.62%) | 69.08% (20.21%) | |
| Large (> 30%) | 38 | 4 | 78.95% (9.36%) | 62.20% (11.36%) | |
| Post-operative radiographic progression | P < 0.001 * | ||||
| Yes | 17 | 9 | 70.59% (11.05%) | 41.18% (13.60%) | |
| No | 40 | 2 | 97.50% (2.47%) | 95.00% (3.45%) |
THA: Total Hip Arthroplasty, SE: Standard Error.
P < 0.05 was considered significant.
Bold value indicate the significant P value.
gender reported as number of hips.
The Cox proportional-hazard model (selection = stepwise) revealed that bone marrow edema (hazard ratio = 10.326; 95% CL, 1.31 to 81.54; x2 = 8.617, P = 0.003), was the independent prognostic factor related to conversion into THA. Meanwhile, the Cox proportional-hazard model showed that, with the numbers available for study, there was no statistically significant association was found between conversion to THA and such factors as gender, age over fifty years, etiology, associated chronic systemic disease, corticosteroid use, bilateral disease treated with tantalum implant, preoperative Steinberg Stage, Harris hip score more than 80 points, joint effusion on preoperative MRI imaging, collapse of the femoral head, osteonecrotic lesion size.
Radiographs from a representative case of a 53-year-old male patient with bilateral necrosis in stage I C (right, R) and stage I B (left, L) treated with modified porous tantalum implant technology. Collapse of the right hip (arrow) was noticed at 24 months after surgery. Tantalum rod of the right hip was replaced by total hip arthroplasty in the revision operation at 25 months after surgery. X-rays are shown as taken before surgery (a), 8 months after surgery (b), 24 months after surgery (c) and 25 months after surgery (d) (Figure 5).
Figure 5.
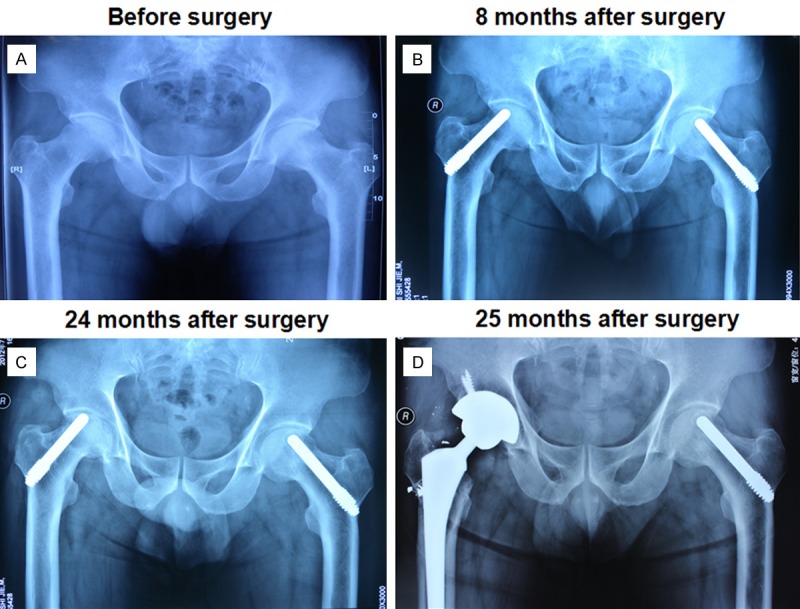
Radiographs from a representative case of a 53-year-old male patient with bilateral necrosis in stage I C (right, R) and stage I B (left, L) treated with modified porous tantalum implant technology. Collapse of the right hip (arrow) was noticed at 24 months after surgery. Tantalum rod of the right hip was replaced by total hip arthroplasty in the revision operation at 25 months after surgery. X-rays are shown as taken before surgery (A), 3 months after surgery (B), 18 months after surgery (C) and 25 months after surgery (D).
Discussion
In order to improve the treatment of ONFH, emerging technologies have been described [1,2,12]. The development of an osteonecrosis intervention implant was supposed to improve the treatment of early and intermediate stages ONFH [14-18]. Possible benefits were thought to include the advantages of the core decompression: reduction of the intraosseous pressure and reperfusion with the possibility of regeneration [5,6]. Additional advantages were supposed to be the structural support, early postoperative load-bearing, the low donor-site morbidity and the minimally invasive procedure [14-18].
Veilette et al. [14] reported the outcome of treatment of ONFH (the majority of patients had osteonecrosis of stage II according to Steinberg) with the tantalum implant and postoperative non-weight bearing for six weeks. The survival rates of 60 hips with ONFH were 92% after 12 months, 82% after 24 months and 68% after 48 months. With a minimum of 2 years of follow-up and an average follow-up of 39 months (range, 27 to 59 months), Shuler and colleagues [15] presented a survival rate of 86% after insertion of the tantalum implant in patients with Upenn stage I and II ONFH and postoperative six-weeks protected weight bearing. Tsao et al. [16] described a survival rate of 85% at 12 months, 79% at 24 months and 73% at 48 months for all Steinberg stage II hips. However, in the multicenter study of Tsao, information about the postoperative procedure is not included. Nadeau et al. [17] reported a survival rate of 42.5% after 48 months and 10 out of 18 (55.6%) tantalum implant procedures failed for the treatment of Steinberg stage III and IV ONFH with an average follow-up of 23.2 months (range, 12 to 48 months). The patients were instructed to be non-weight bearing for three weeks, to partial weight bear for an additional three weeks and then to bear weight as tolerated postoperatively. Varitimidis et al. [19] studied retrospectively 26 hips after tantalum rod implantation for treatment of Steinberg stage II-IV ONFH and postoperative partial weight bearing for 6 weeks. Survivorship was 70% at a mean 38 months (range, 15-71 months) follow-up. In contrast, Floerkemeier et al. [21] reported a survival rate was 44% after implantation of an osteonecrosis intervention rod after a mean follow-up of 1.45 years. From 19 patients with 23 ARCO stage I and II ONFH, there were 13 cases in which a THA was necessary. Patients were allowed to increase weight-bearing gradually as tolerated after surgery. Although backscattered scanning electron microscopy confirmed the presence of bone ingrowth in thirteen (87%) of the fifteen retrieved porous tantalum implants, the mean extent of bone ingrowth was only 1.9% (range, 0% to 4.4%) [18]. Tanzer M et al. [18] concluded that the retrieved implants were associated with little bone ingrowth and insufficient mechanical support of subchondral bone. The implant design, the surgical technique, its application, and the clinical characteristics of candidates for this procedure should continue to be monitored closely.
In the current study, with weight-bearing being not allowed within the first 3 months after surgery, 11 hips (19.3%) were converted to a THA at an average of 29.7 months (range, 11-51 months) after insertion of the porous tantalum implant. The survival rate of 57 hips with ONFH was 98.20% at 12 months, 92.98% at 24 months, 89.47% at 36 months, 84.21% at 48 months and 72.49% at 60 months. Survival rates for hips without bone marrow edema were 85.71% at 60 months. The majority of the survivors revealed an almost unchanged or improved radiological appearance (70.2%). Patients who did not require THA had increased Harris hip scores by 22 points, and patients who eventually required arthroplasty decreased by 14 points.
The early result of the present study supports and even exceeds the mainly encouraging result described by several teams [14-17] and Varitimidis et al. [19]. A possible reason may be the wide excision of the necrotic bone of femoral head, addition of porous medical composite bone filling material, and variation of postoperative load bearing. The suggested advantage of this treatment of an earlier load-bearing may have a negative influence on the outcome of the treatment (Table 2). Further studies analysing the outcome of implanted osteonecrosis intervention rod would be helpful.
Table 2.
Literature review of result of the porous tantalum implant
| Study/Year/design | No. of Hips | Specific selection | Time of weight bearing postoperatively | Mean duration of follow-up | Survivorship |
|---|---|---|---|---|---|
| Veilette et al. [14] (2006) (R) | 58 | Steinberg stage I (1 hips), II (49 hips) and III (8 hips) | Non-weight bearing for six weeks | 24 months | 68.1% at 48 months |
| Shuler et al. [15] (2007) (P) | 24 | Upenn stage I (2 hips) and II (22 hips) | Six-weeks protected weight bearing | 39 months (range, 27-59 months) | 86% |
| Tsao et al. [16] (2005) (P) | 94 | Steinberg stage I (1 hips) and II (93 hips) | 72.5% at 48 months | ||
| Nadeau et al. [17] (2007) (P) | 18 | Steinberg stage III (3 hips) and IV (15 hips) | Non-weight bearing for three weeks, to partial weight bear for an additional three weeks and then to bear weight as tolerated | 23.2 months (range 12 to 48) | ≈42.5% at 48 months |
| Varitimidis et al. [19] (2009) (P) | 26 | Steinberg stage I (9 hips), II (7 hips) and III (10 hips) | Partial weight bearing for 6 weeks | 38 months (range, 15-71 months) | 70% at 6 years |
| Floerkemeier et al. [21] (2011) (R) | 23 | ARCO stage I and II | Allowed to increase weight-bearing gradually as tolerated after surgery | 529 days (1.45 years) (range 120-1,348 days)* | 44%# |
| Liu Y et al. (current study) (P) | 57 | Steinberg stages I (4 hips), II (22 hips), III (6 hips) and IVA (25 hips) | Weight-bearing was not allowed within the first 3 months. Partial weight-bearing crutch walking was allowed thereafter and full weight-bearing was allowed 6 months. | 44.8 months (rang, 11-62 months) | 84.21% at 48 months 72.49% at 60 months |
The time until conversion to THR;
This data may be wrong.
The results of previous studies have suggested that bone marrow edema is a poor prognostic sign since it develops after the onset or worsening of hip pain and correlates with the subsequent collapse of the femoral head suggesting progression to advanced ONFH [24-27]. Iida et al. [26] reported bone marrow edema was not present on initial MR imaging of osteonecrosis. They concluded that bone marrow edema should be considered a marker for potential progression to advanced osteonecrosis. Ito et al. [25] reported that the final radiographic stage of the 28 hips that showed bone marrow edema was significantly advanced compared with those without bone marrow edema. Bone marrow edema strongly correlated with necrotic volume and was the most significant risk factor for worsening of hip pain.
In agreement with these studies, the present study identified bone marrow edema was not only the independent prognostic factor related to conversion to radiographic progression, but the independent predictor of THA, regardless of the stage of the disease. Meanwhile, although we had a limited number of hips accompanied by bone marrow edema in this study, our results suggested significantly lower survival rates for hips accompanied by bone marrow edema (65.34% at 60 months) than in patients without bone marrow edema (85.71% at 60 months). The relative risk of requiring conversion to THA were 7.81 times higher in hips accompanied by bone marrow edema than without accompanied by bone marrow edema.
Although bone marrow edema seems to have a stronger association with pain than does joint effusion in ONFH, joint effusions are frequently correlated with pain and are commonly found together with bone marrow edema [23]. Huang et al. [27] discovered both bone marrow edema and joint effusions existed with a peak occurrence in stage III disease. Chan et al. [28] concluded that the amount of joint fluid correlates well with the stage of ONFH.
Similar to previous studies, although we were unable to identify joint effusion on preoperative MRI imaging as an independent prognostic factor for radiographic progression and conversion to THA there is a statistically significant difference between the overall survival rates for hips stratified according to joint effusion, the relative risk of requiring conversion to THA were 6.76 times higher in patients accompanied by joint effusion on preoperative MRI imaging than in patients without joint effusion in our present study.
Many reports in the literature regarding the natural history of osteonecrosis and the result of core decompression with or without bone grafting [7,29,30] have documented marked differences in prognosis between hips that have had collapse and those that have not. Meanwhile, the stage of the disease is found to be an important prognostic factor in the outcome of free vascularized fibular grafting [10,11,31]. The meta-analysis that evaluated studies with core decompression technique or conservative treatment for femoral head osteonecrosis, demonstrated that further surgical intervention was necessary in 16%, 37%, and 71% after core decompression of osteonecrosis stages I, II and III, respectively, according to Steinberg’s classification [5].
In the current study, we discovered that there was a greater trend for the higher stage hips to require conversion into THA, however we were unable to identify the preoperative Steinberg stage and preoperative collapse of the femoral head as the independent prognostic factor for radiographic progression or conversion to THA.
Another important factor in patient selection has been the underlying associated risk factors for the osteonecrosis. The results of several studies have suggested that outcomes are worse for patients who have corticosteroid-associated osteonecrosis [32,33]. Veillette et al. [16] identified the use of corticosteroids as an independent prognostic factor for radiographic progression, regardless of the stage of the disease. Bozic et al. [34] demonstrated an independent relationship between the use of corticosteroids and survival of the hip in their survival analysis of hips that were treated with core decompression for osteonecrosis. However, the present study merely discovered the use of corticosteroids to be an independent prognostic factor for radiographic progression.
Among various related factors, size of a necrotic lesion is considered an important factor predicting collapse of the femoral head in the early stages of osteonecrosis. Bassounas et al. [35] discovered the mean lesion size was 28% of the sphere equivalent of the femoral head, 24 ± 12% for the successful hips and 37 ± 9% for the failed (P < 0.001). Using three-dimensional quantitative analysis of lesion morphology, Nishii et al. [36] demonstrated that lesion volume is strongly correlated with risk of collapse. Motomura et al. [37] found that collapse began at the lateral boundary of the necrotic lesion and that the size of the necrotic lesion seemed to contribute to its distribution. Ito et al. [26] suggested that the necrotic volume was one possible risk factor to predict the outcome.
In agreement with these studies, the present study determined a statistically significant difference between the survival rates for hips stratified according to size of a necrotic lesion in the femoral head, the relative risk of requiring conversion to THA was 3.69 times higher in patients with large osteonecrotic lesion than in patients without large osteonecrotic lesion. However we were unable to identify size of a necrotic lesion as an independent prognostic factor for radiographic progression and conversion to THA.
It is believed chronic systemic disease often have bone-mineralization defects and osteoporosis with poor bone quality [38]. Veillette et al. [16] identified chronic systemic disease as an independent prognostic factor for failure and conversion to THA using Cox proportional-hazard model. In contrast to previous studies, although in our study the relative risk of requiring conversion to THA were 2.82 times higher in patients with chronic systemic disease than in patients without that disease, we were unable to show a statistically significant difference between the overall survival rates for hips stratified according to the chronic systemic disease. Furthermore, we were neither able to identify chronic systemic disease as an independent predictor of radiographic progression, nor an independent prognostic factor for conversion to THA.
Limitations of the study include the small number of hips studied with no control patients treated with core decompression combined with other methods or patients treated non-operatively. Another limitation relies on the fact that the follow-up time is relatively short. Further studies are also needed to more comprehensively determine the longer term efficacy of the treatment approach described in this study and to determine which patients might be more likely to benefit from this treatment approach.
In summary, the treatment of early and intermediate stage osteonecrosis of the femoral head with a modified porous tantalum implant technology can be accomplished with a minimally invasive technique, no donor-site morbidity, and few major device-related complications. For patients without use of corticosteroids and without large osteonecrotic lesion, and especially for those who do not have bone marrow edema nor joint effusion on preoperative MRI imaging, the clinical results from our study show highly encouraging survival rates and a delay in or prevention of progressive articular collapse and THA. However, we have to indicate that complications of our study include one case of deep infection successfully managed with a one-stage tantalum implant extraction and lavage-drainage operation and three case of trochanteric bursitis. What a pity, the supposed advantage of porous tantalum implant of early postoperative load-bearing was not proved in our present study.
Acknowledgements
The work is supported by Application Study of Capital Clinical Characteristics of China (No Z121107001012093).
Disclosure of conflict of interest
None.
References
- 1.Kaushik AP, Das A, Cui Q. Osteonecrosis of the femoral head: An update in year 2012. World J Orthop. 2012;3:49–57. doi: 10.5312/wjo.v3.i5.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63:16–28. doi: 10.1016/j.ejrad.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Zhao G, Yamamoto T, Ikemura S, Motomura G, Mawatari T, Nakashima Y, Iwamoto Y. Radiological outcome analysis of transtrochanteric curved varus osteotomy for osteonecrosis of the femoral head at a mean follow-up of 12.4 years. J Bone Joint Surg Br. 2010;92:781–786. doi: 10.1302/0301-620X.92B6.23621. [DOI] [PubMed] [Google Scholar]
- 4.Ito H, Tanino H, Yamanaka Y, Nakamura T, Takahashi D, Minami A, Matsuno T. Long-term results of conventional varus half-wedge proximal femoral osteotomy for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Br. 2012;94:308–314. doi: 10.1302/0301-620X.94B3.27814. [DOI] [PubMed] [Google Scholar]
- 5.Castro FP Jr, Barrack RL. Core decompression and conservative treatment for avascular necrosis of the femoral head: a meta-analysis. Am J Orthop (Belle Mead NJ) 2000;29:187–194. [PubMed] [Google Scholar]
- 6.Marker DR, Seyler TM, Ulrich SD, Srivastava S, Mont MA. Do modern techniques improve core decompression outcomes for hip osteonecrosis? Clin Orthop Relat Res. 2008;466:1093–1103. doi: 10.1007/s11999-008-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei BF, Ge XH. Treatment of osteonecrosis of the femoral head with core decompression and bone grafting. Hip Int. 2011;21:206–210. doi: 10.5301/HIP.2011.6525. [DOI] [PubMed] [Google Scholar]
- 8.Baksi DP, Pal AK, Baksi DD. Long-term results of decompression and muscle-pedicle bone grafting for osteonecrosis of the femoral head. Int Orthop. 2009;33:41–47. doi: 10.1007/s00264-007-0455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watters TS, Browne JA, Orlando LA, Wellman SS, Urbaniak JR, Bolognesi MP. Cost-effectiveness analysis of free vascularized fibular grafting for osteonecrosis of the femoral head. J Surg Orthop Adv. 2011;20:158–167. [PubMed] [Google Scholar]
- 10.Korompilias AV, Beris AE, Lykissas MG, Kostas-Agnantis IP, Soucacos PN. Femoral head osteonecrosis: why choose free vascularized fibula grafting. Microsurgery. 2011;31:223–228. doi: 10.1002/micr.20837. [DOI] [PubMed] [Google Scholar]
- 11.Eward WC, Rineer CA, Urbaniak JR, Richard MJ, Ruch DS. The vascularized fibular graft in precollapse osteonecrosis: is long-term hip preservation possible? Clin Orthop Relat Res. 2012;470:2819–2826. doi: 10.1007/s11999-012-2429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malizos KN, Papasoulis E, Dailiana ZH, Papatheodorou LK, Varitimidis SE. Early results of a novel technique using multiple small tantalum pegs for the treatment of osteonecrosis of the femoral head: a case series involving 26 hips. J Bone Joint Surg Br. 2012;94:173–178. doi: 10.1302/0301-620X.94B2.27287. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen DR, Brown TD, Poggie RA. Finite element characterization of a porous tantalum material for treatment of avascular necrosis. Trans Orthop Res Soc. 1997;22:598. [Google Scholar]
- 14.Veillette CJ, Mehdian H, Schemitsch EH, McKee MD. Survivorship analysis and radiographic outcome following tantalum rod insertion for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):48–55. doi: 10.2106/JBJS.F.00538. [DOI] [PubMed] [Google Scholar]
- 15.Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplasty. 2007;22:26–31. doi: 10.1016/j.arth.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Tsao AK, Roberson JR, Christie MJ, Dore DD, Heck DA, Robertson DD, Poggie RA. Biomechanical and clinical evaluations of a porous tantalum implant for the treatment of early-stage osteonecrosis. J Bone Joint Surg Am. 2005;87(Suppl 2):22–27. doi: 10.2106/JBJS.E.00490. [DOI] [PubMed] [Google Scholar]
- 17.Nadeau M, Séguin C, Theodoropoulos JS, Harvey EJ. Short term clinical outcome of a porous tantalum implant for the treatment of advanced osteonecrosis of the femoral head. Mcgill J Med. 2007;10:4–10. [PMC free article] [PubMed] [Google Scholar]
- 18.Tanzer M, Bobyn JD, Krygier JJ, Karabasz D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J Bone Joint Surg Am. 2008;90:1282–1289. doi: 10.2106/JBJS.F.00847. [DOI] [PubMed] [Google Scholar]
- 19.Varitimidis SE, Dimitroulias AP, Karachalios TS, Dailiana ZH, Malizos KN. Outcome after tantalum rod implantation for treatment of femoral head osteonecrosis: 26 hips followed for an average of 3 years. Acta Orthop. 2009;80:20–25. doi: 10.1080/17453670902804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floerkemeier T, Lutz A, Nackenhorst U, Thorey F, Waizy H, Windhagen H, von Lewinski G. Core decompression and osteonecrosis intervention rod in osteonecrosis of the femoral head: clinical outcome and finite element analysis. Int Orthop. 2011;35:1461–1466. doi: 10.1007/s00264-010-1138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floerkemeier T, Thorey F, Daentzer D, Lerch M, Klages P, Windhagen H, von Lewinski G. Clinical and radiological outcome of the treatment of osteonecrosis of the femoral head using the osteonecrosis intervention implant. Int Orthop. 2011;35:489–495. doi: 10.1007/s00264-009-0940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Liu S, Su X. Core decompression and implantation of bone marrow mononuclear cells with porous hydroxylapatite composite filler for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2013;133:125–133. doi: 10.1007/s00402-012-1623-3. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 24.Koo KH, Ahn IO, Kim R, Song HR, Jeong ST, Na JB, Kim YS, Cho SH. Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: prospective study with serial MR images. Radiology. 1999;213:715–722. doi: 10.1148/radiology.213.3.r99dc06715. [DOI] [PubMed] [Google Scholar]
- 25.Ito H, Matsuno T, Minami A. Relationship between bone marrow edema and development of symptoms in patients with osteonecrosis of the femoral head. AJR Am J Roentgenol. 2006;186:1761–1770. doi: 10.2214/AJR.05.0086. [DOI] [PubMed] [Google Scholar]
- 26.Iida S, Harada Y, Shimizu K, Sakamoto M, Ikenoue S, Akita T, Kitahara H, Moriya H. Correlation between bone marrow edema and collapse of the femoral head in steroid-induced osteonecrosis. AJR Am J Roentgenol. 2000;174:735–743. doi: 10.2214/ajr.174.3.1740735. [DOI] [PubMed] [Google Scholar]
- 27.Huang GS, Chan WP, Chang YC, Chang CY, Chen CY, Yu JS. MR imaging of bone marrow edema and joint effusion in patients with osteonecrosis of the femoral head: relationship to pain. AJR Am J Roentgenol. 2003;181:545–549. doi: 10.2214/ajr.181.2.1810545. [DOI] [PubMed] [Google Scholar]
- 28.Chan WP, Liu YJ, Huang GS, Jiang CC, Huang S, Chang YC. MRI of joint fluid in femoral head osteonecrosis. Skeletal Radiol. 2002;31:624–630. doi: 10.1007/s00256-002-0531-y. [DOI] [PubMed] [Google Scholar]
- 29.Bednarek A, Atras A, Gągała J, Kozak Ł. Operative technique and results of core decompression and filling with bone grafts in the treatment of osteonecrosis of femoral head. Ortop Traumatol Rehabil. 2010;12:511–518. [PubMed] [Google Scholar]
- 30.Mont MA, Marulanda GA, Seyler TM, Plate JF, Delanois RE. Core decompression and nonvascularized bone grafting for the treatment of early stage osteonecrosis of the femoral head. Instr Course Lect. 2007;56:213–220. [PubMed] [Google Scholar]
- 31.Korompilias AV, Lykissas MG, Beris AE, Urbaniak JR, Soucacos PN. Vascularised fibular graft in the management of femoral head osteonecrosis: twenty years later. J Bone Joint Surg Br. 2009;91:287–293. doi: 10.1302/0301-620X.91B3.21846. [DOI] [PubMed] [Google Scholar]
- 32.Mont MA, Jones LC. Management of osteonecrosis in systemic lupus erythematosus. Rheum Dis Clin North Am. 2000;26:279–309. doi: 10.1016/s0889-857x(05)70139-3. [DOI] [PubMed] [Google Scholar]
- 33.Colwell CW Jr, Robinson CA, Stevenson DD, Vint VC, Morris BA. Osteonecrosis of the femoral head in patients with inflammatory arthritis or asthma receiving corticosteroid therapy. Orthopedics. 1996;19:941–946. doi: 10.3928/0147-7447-19961101-07. [DOI] [PubMed] [Google Scholar]
- 34.Bozic KJ, Zurakowski D, Thornhill TS. Survivorship analysis of hips treated with core decompression for nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1999;81:200–209. doi: 10.2106/00004623-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Bassounas AE, Karantanas AH, Fotiadis DI, Malizos KN. Femoral head osteonecrosis: volumetric MRI assessment and outcome. Eur J Radiol. 2007;63:10–15. doi: 10.1016/j.ejrad.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Nishii T, Sugano N, Ohzono K, Sakai T, Sato Y, Yoshikawa H. Significance of lesion size and location in the prediction of collapse of osteonecrosis of the femoral head: a new three-dimensional quantification using magnetic resonance imaging. J Orthop Res. 2002;20:130–136. doi: 10.1016/S0736-0266(01)00063-8. [DOI] [PubMed] [Google Scholar]
- 37.Motomura G, Yamamoto T, Yamaguchi R, Ikemura S, Nakashima Y, Mawatari T, Iwamoto Y. Morphological analysis of collapsed regions in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2011;93:184–187. doi: 10.1302/0301-620X.93B225476. [DOI] [PubMed] [Google Scholar]
- 38.Moe SM, Drüeke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]


