Abstract
Aim: The present study aimed to investigate the role of Interleukin 15 (IL-15) in protein degradation in skeletal muscle mediated by the ubiquitin-proteasome pathway (UPP) in a rat model of chronic obstructive pulmonary disease (COPD). Methods: The COPD model was established in 30 Sprague Dawley (SD) rats through the combination of passive smoking and intratracheal injection of lipopolysaccharide (LPS). The pathological changes in lungs and bronchi of COPD rats (n=30) were compared with that of control rats (n=15). The levels of IL-15 and tumor necrosis factor-α (TNF- α) in the serum, diaphragm, gastrocnemius, and the intercostal muscles were measured using enzyme-linked immunosorbent assay (ELISA), and compared between the COPD and control rats. In addition, the expression of E2-14K, MAFbx, and ubiquitin (Ub) was evaluated using quantitative real-time PCR and western blot and compared between the COPD and control rats. Results: The levels of IL-15 and TNF-α in the serum, diaphragm, gastrocnemius, and the intercostal muscles in COPD rats were significantly higher than that in control rats. The expression of E2-14K, MAFbx, and ubiquitin (Ub) in the diaphragm, gastrocnemius, and the intercostal muscles in COPD rats was also significantly higher than that in the control rats. In addition, we identified positive correlation between the levels of IL-15 and TNF-α. Positive correlation was also identified between the levels of IL-15 and E2-14K, MAFbx, and Ub. Conclusion: Our results suggest that IL-15 inhibited the protein degradation in skeletal muscle in COPD rats, which may be mediated by the TNF-α and UPP pathway.
Keywords: Chronic obstructive pulmonary disease, ubiquitin-proteasome pathway, IL-15, tumor necrosis factor-α
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most common respiratory diseases. It has been reported that the incidence of COPD in China was 8.2% [1]. COPD is characterized by persistent airflow obstruction, accompanied by increased chronic inflammatory reaction of airways and lungs to noxious particles or gas. With the disease progresses, respiratory muscle fatigue and respiratory insufficiency may occur due to protein degradation in respiratory muscles, and approximately 24%-71% of COPD patients have various degrees of malnutrition [2]. The ubiquitin-proteasome pathway (UPP), which plays an essential role in the degradation of most short-lived proteins, is the principal mechanism underlying the catabolism of proteins in eukaryotic cells. The ATP-dependent UPP plays a critical role in protein degradation in skeletal muscle. It has been shown that UPP is the major mechanism underlying the high level of protein degradation in diaphragm in COPD [3,4]. Interleukin 15 (IL-15) is a cytokine secreted by multiple types of cells. It has been reported that IL-15 inhibited protein degradation in skeletal muscle in tumor patients and aging mice through down-regulating UPP [5]. In addition, it has been reported that TNF-α promoted protein degradation in the skeletal muscle through improving the expression and activity of ubiquitin and its regulators [6]. Therefore, IL-15 and TNF-α may interact with the UPP to regulate the metabolism of proteins in skeletal muscle, and be involved in the development of COPD. Currently, studies on the relationship among IL-15, TNF-α, and UPP components are limited. In the present study, we established a COPD model in rats through the combination passive smoking and intratracheal injection of lipopolysaccharide (LPS), and investigated the relationship and role of IL-15, TNF-α, and UPP components in the development of COPD.
Materials and methods
Ethical standards
All animal studies have been approved by The Institute Research Medical Ethics Committee of Guangzhou Medical University, and conducted the National Institutes of Health (NIH) Guide and Declaration of Helsinki.
Establishment of the COPD model in rats
Forty-five male Sprague Dawley (SD) rats (8 weeks of age, 222.75±6.37 g) of specific pathogen free (SPF) grade were purchased from the Medical Animal Experimental Center of Guangdong Province. Animals were randomly divided into two groups: the COPD group (n=30) and the control group (n=15). The SD rats were housed in the animal experimental laboratory in the Institute of Respiratory Diseases, First Affiliated Hospital of Guangzhou Medical College under condition of 22°C~26°C, 40-70% humidity, 10-20 times/h of ventilation, and positive pressure of 20~50 pa.
The COPD model was established according to our previous study [4]. Briefly, the SD rat were anesthetized through intraperitoneal injection of 10% chloral hydrate (4 ml/kg), and tracheotomy was conducted for slow intratracheal administration of 0.9% saline (0.2 ml) for the control group or 200 μg LPS (0.2 ml, Sigma, USA) for the COPD group. Intratracheal injection of saline or LPS was conducted for a total of two times on the first and fifteenth days. Except for the two days when intratracheal injection of saline or LPS was conducted, the SD rats received passive smoking two times (9:00-10:10 am and 15:00-16:10 pm) per day for a total of four weeks. Five cigarettes (Golden Xuchang, Henan, China. tar 13 mg, nicotine 13 mg) were used in each time of passive smoking and lasted for 30 mins. The interval between each cigarette was 10 minutes.
After the four weeks of passive smoking, the SD rat were anesthetized through intraperitoneal injection of 10% chloral hydrate (4 ml/kg), and tracheotomy and endotracheal intubation were conducted to test the airway resistance and lung compliance using the Buxco animal lung function RC system (Buxco, USA).
After pulmonary function testing mentioned above, the abdominal cavity of SD rats was surgically opened to collect 3 ml blood from the exposed inferior vena cava using disposable lancets. The collected blood was centrifuged in Eppendorf tubes at 4°C and 3000 rpm/min for 10 mins. The serum was then transferred into new Eppendorf tubes and stored at -80°C for follow-up experiment. Then, the diaphragm, gastrocnemius, and intercostal muscles were surgically dissected from rats and immediately stored in liquid nitrogen.
Histopathology
Histopathological examination of the lungs and bronchi of COPD rats and control animals was conducted according to our previous study [5].
Evaluation of the levels of IL-15 and TNF-α in serum and skeletal muscle using enzyme-linked immunosorbent assay (ELISA)
Skeletal muscle (100 mg) was completely homogenized in 1 ml PBS and centrifuged at 4°C and 3500 r/min for 10 mins. The levels of IL-15 and TNF-α in the supernatant of homogenized skeletal muscle and serum were evaluated using the Rat TNF-α ELISA kit according to the manufacture’s instruction (Thermo Fisher Scientific Inc., Rockford, IL, USA).
Evaluation of the mRNA levels of E2-14k, MAFbx, and Ub in skeletal muscle using quantitative real-time PCR
Skeletal muscle (100 mg) was homogenized in 1 ml PBS on ice and total RNA was isolated from the skeletal muscle using the Trizol reagent according to the manufacture’s instruction (Gibco, Grand Island, NY, United States)). The purity of isolated RNA was measured on a UV spectrophotometer.
The mRNA in isolated total RNA was reversely transcribed into cDNA at 37°C for 15 mins in a 10 ul reaction system. Quantitative real-time PCR was conducted using the SYBR® Premix Ex Taq™ (TaKaRa Biotechnology Ltd, Dalian, China) in quantitative real-time PCR thermocycler (Life Technology, Carlsbad, CA, USA). The primers used in quantitative PCR assay are: E2-14k: forward: 5’-AGGC TCATGCGGGATTTCA-3’, reverse: 5’-AACCTACGGTTGGTGGTTTAT TTG-3’; MAFbx: forward: 5’-CAACATGTGGGT GTATCGAATGGT-3’, reverse: 5’-TGATGTTCAGTTGTAAGCACACAGG-3’; Ub forward: 5’-CCAATGGCGGTTAAT GACCTT-3’, 5’-TTTCGATGGGGCTTGAGGATT-3’; GAPDH: forward: 5’-GGCACAGTCAAGGCTGAGA ATG-3’, reverse: 5’-ATGGTGGTGAAGACGCCA GTA-3’.
The PCR primers were synthesized by TaKaRa Biotechnology Ltd (Dalian, China). The cDNA template used in quantitative real-time PCR was diluted for 100, 101, 102, 103, and 104 times and 2 μl cDNA was used to establish the standard curve of each gene. Quantitative real-time PCR was conducted in a 20 μl PCR reaction system including 10 μl SYBR Premix Ex Taq, 2 μl forward and reverse primers (10 μM), 2 μl cDNA temple, and 6.4 μl ddH2O. GAPDH gene was used as an internal control. The PCR amplification program was as follows: initial denaturation at 95°C for 30 s, 40 cycles of 95°C for 5 s, amplification at 60°C for 30 s, and a melting curve analysis at 95°C for 15 s, 60°C for 60 s, and 95°C for 15 s. Quantitative real-time PCR assay for each gene was conducted in triplicate. The relative mRNA level of each gene was calculated according to the delta Ct method.
Evaluation of the protein levels of E2-14k, MAFbx, and Ub in skeletal muscle using Western blot analysis
Skeletal muscle (100 mg) was completely homogenized in 1 ml protein isolation buffer and centrifugated at 4°C and 1000 rpm/min for 10 mins. The supernatant containing total proteins was transferred to a new Eppendorf tube and the protein was quantified using the Bradford method. The isolated proteins were stored at -80°C for Western blot analysis.
Western blot analysis was performed as described in our previous report [4]. Briefly, protein samples were mixed with 5 x loading buffer at a 1: 4 ratio and boiled for 10 mins in water bath for protein denaturation. Equal amounts of protein (30 μg in 20 μl) from both the COPD model and control rats were separated by 10% SDS-PAGE. The proteins were separated at 80 V for 30 mins and followed by 100 V for 60 mins after the bromophenol blue run into the separate gel from the laminated gel. Proteins were then transferred to a PVDF membrane at 250 mA for 60 mins. The membranes were blocked with 5% non-fat milk for 60-120 mins and incubated with primary antibodies at 4°C overnight followed by incubation at room temperature for 30 mins. The primary antibodies were 1:500 E2-14k, (CST), 1:500 MAFbx (Santa Cruz Biotechnology), 1:500 Ub (Santa Cruz Biotechnology), and 1:1000 GAPDH (Santa Cruz Biotechnology). The PVDF membrane was incubated with 1:1000 secondary antibody (Bioworld Technology Inc., MN, USA). The target proteins were detected in the VILBER LOURMAT system using an enhanced chemiluminescence (ECL) kit (Pierce, Thermo Fisher Scientific Inc., Rockford, IL, USA). Each experiment was conducted in triplicate and the mean value of protein level was normalized according to the expression of GAPDH to evaluate the expression of proteins E2-14k, MAFbx, and Ub.
Statistical analyses
The data were presented as mean ± SD. Statistical analyses were conducted using the SPSS 17.0 software. Measurement data were analyzed using the two independent samples t-test. Pearson linear correlation analysis was used to analyze the correlation between two variables. P values less than 0.05 were considered statistically significant.
Results
The weight of COPD rats was significantly lower than that of the control rats
During the 2nd and 4th weeks of establishing the COPD model, the weight of rats of the COPD group (90.430±13.941) was significantly lower than that of the control rats (117.746±10.325) (P<0.05) (Figure 1).
Figure 1.
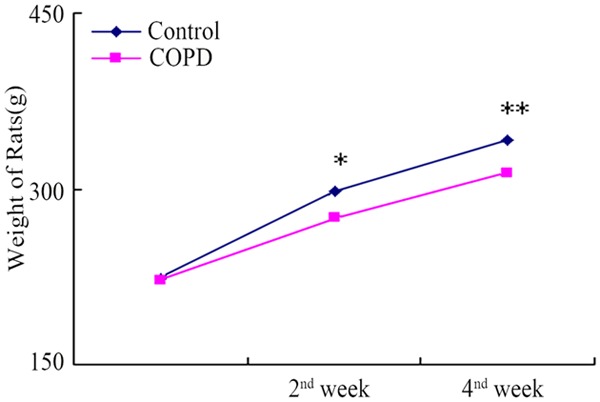
Comparison of the weight changes between the COPD and control rats. During the 2nd and 4th weeks of establishing the COPD model, the weight of COPD rats was significantly lower than that of control rats. *, **P<0.05.
Histopathological changes of the lung tissues in COPD rats
The lungs of COPD rats were significantly larger than that of control rats. Compared to the lungs of control rats, the lungs of COPD mice was pale with poor elasticity, and scattered bleeding under lung capsular was observed.
Under light microscopy, the alveolar space was larger and the alveolar wall was thinner in COPD lungs than that in normal lungs. Some alveoli broke and fused into bullae. In addition, infiltration of inflammatory cells in mucosa, detachment of epithelial cells, increased goblet cells, trachea cilia disorders and detachment, and proliferation of fibrous connective tissue and smooth muscle were also observed in COPD bronchi (Figure 2).
Figure 2.
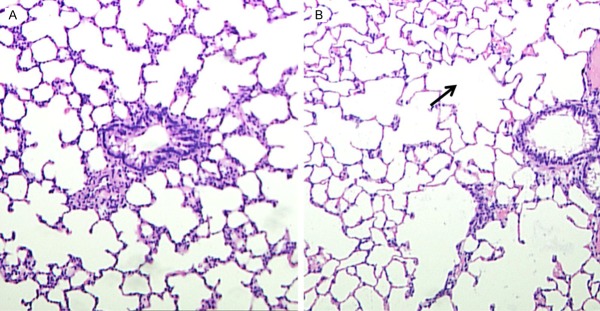
Histopathological changes in the lung of COPD rats. In COPD rats (left), the alveolar space was larger and the alveolar wall was thinner than that in normal lungs (right). In addition, some alveoli fused into bullae in COPD lungs (black arrow). (HE staining, ×100).
The pulmonary function of COPD rats was impaired
Compared to the control rats, the COPD rats exhibited significantly increased airway resistance (P<0.05) and significantly reduced lung compliance (P<0.05) (Table 1).
Table 1.
The results of pulmonary functional tests (x̅ ± s)
| Group | Airway resistance (kpa*mL-1*s-1) | Lung compliance (mL/cmH2O) |
|---|---|---|
| Control (n=15) | 0.295±0.479 | 0.527±0.059 |
| COPD (n=45) | 0.489±0.072* | 0.362±0.061* |
P<0.05 compared to the normal controls.
1 cm H2O=0.098 kpa.
The levels of IL-15 and TNF-α and the expression of E2-14k, MAFbx, and Ub in COPD rats were significantly higher than that in the control rats
Based on ELISA, the levels of IL-15 and TNF-α in the serum, diaphragm, gastrocnemius, and intercostal muscles of COPD rats were significantly higher than that in the control rats (P<0.05) (Figure 3). Based on quantitative real-time PCR and Western blot analyses, the levels of E2-14k, MAFbx, and Ub mRNA (Figure 4) and proteins (Figure 5) were significantly higher than that in control rats.
Figure 3.
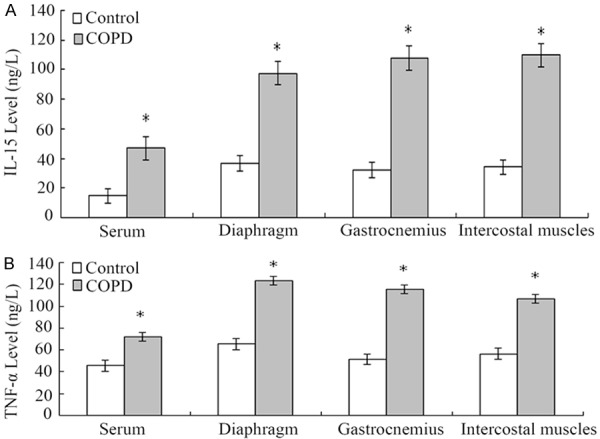
Comparison of the levels of IL-15 and TNF-α in the serum, diaphragm, gastrocnemius, and intercostal muscles between COPD and control rats. A. The levels of IL-15 in the serum, diaphragm, gastrocnemius, and intercostal muscles in COPD rats were significantly higher than that in the control rats, respectively (*P<0.05). B. The levels of TNF-α in the serum, diaphragm, gastrocnemius, and intercostal muscles in COPD rats were significantly higher than that in the control rats, respectively (*P<0.05).
Figure 4.
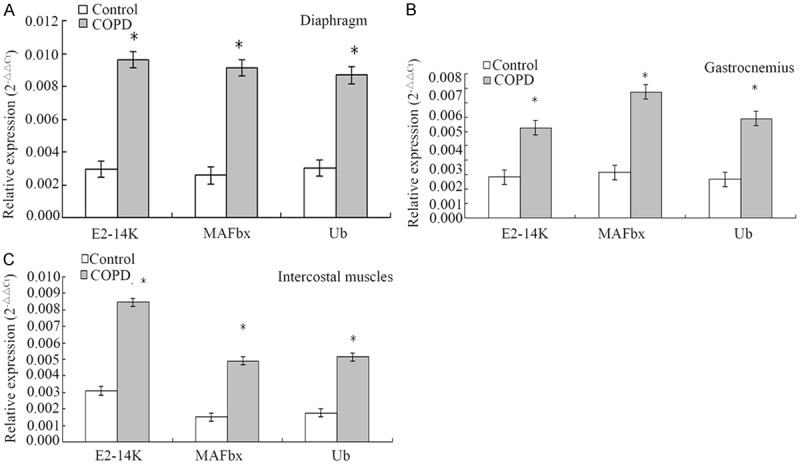
Comparison of the mRNA levels of E2-14K, MAFbx, and Ub in the diaphragm, gastrocnemius, and intercostal muscles between the COPD and control rats based on quantitative real-time PCR assay. A. The mRNA levels of E2-14K, MAFbx, and Ub in the diaphragm in the COPD rats were significantly higher than that in the control rats (*P<0.05). B. The mRNA levels of E2-14K, MAFbx, and Ub in the gastrocnemius in the COPD rats were significantly higher than that in the control rats (*P<0.05). C. The mRNA levels of E2-14K, MAFbx, and Ub in the intercostal muscles in the COPD rats were significantly higher than that in the control rats (*P<0.05).
Figure 5.
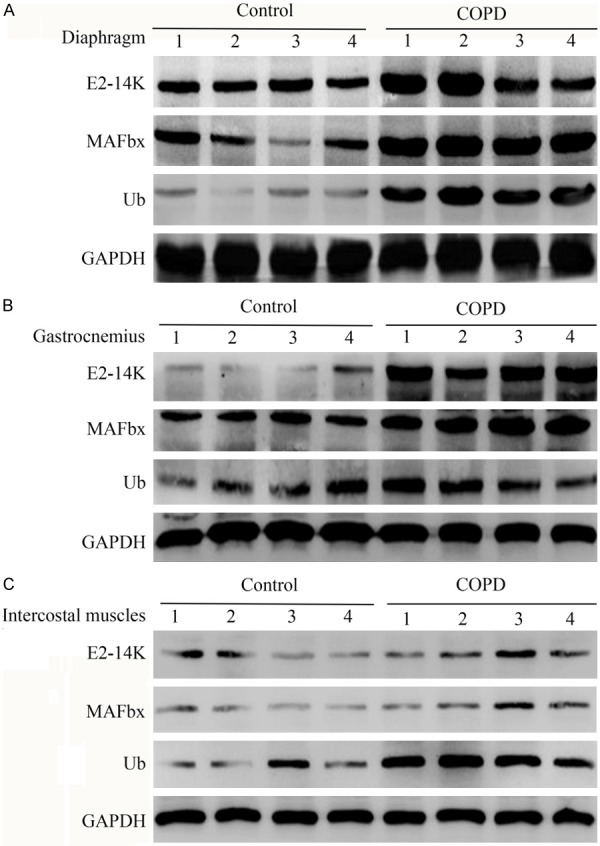
Comparison of the protein levels of E2-14K, MAFbx, and Ub in the diaphragm, gastrocnemius, and intercostal muscles between the COPD and control rats based on Western blot analysis. A. The protein levels of E2-14K, MAFbx, and Ub in the diaphragm in the COPD rats were significantly higher than that in the control rats (*P<0.05). B. The protein levels of E2-14K, MAFbx, and Ub in the gastrocnemius in the COPD rats were significantly higher than that in the control rats (*P<0.05). C. The protein levels of E2-14K, MAFbx, and Ub in the intercostal muscles in the COPD rats were significantly higher than that in the control rats (*P<0.05).
Correlation analyses
Positive correlation was found between the levels of IL-15 and TNF-α in the serum (r=0.75), diaphragm (r=0.81), gastrocnemius (r=0.82), and intercostal muscles (r=0.78) of COPD mice (P<0.05). In addition, the levels of IL-15 positively correlated with the expression of E2-14K, MAFbx, and Ubin in the diaphragm (r=0.88, r= 0.86, and r=0.87, respectively), gastrocnemius (r=0.85, r=0.87, and r=0.76, respectively), and intercostal muscles (r=0.85, r=0.80, and r=0.84, respectively) (P<0.05). However, the weight changes of COPD rats negatively correlated with the levels of IL-15 in the serum (r=0.90), diaphragm (r=0.85), gastrocnemius (r=0.82), and intercostal muscles (r=0.82) of COPD rats (P<0.05).
Discussion
In the present study, we established a COPD model in rats and compared the levels of IL-15, TNF-α, and a number of UPP components such as E2-14K, MAFbx, and Ub in the serum, diaphragm, gastrocnemius, and intercostal muscles between COPD and control rats. The histopathological changes of lungs in COPD rats such as nonspecific airway inflammation and emphysema were highly similar with that in human COPD. Therefore, intratracheal injection of LPS combined with passive smoking is an efficient way to induce COPD in rats.
Respiratory muscles are classified into skeletal muscle based on embryological, morphological and functional features. As in other cell types, the lysosomal pathway, the calcium-calpain pathway, and the UPP [4] were the three major protein degradation pathways in skeletal muscle cells. The UPP is a highly selected and ATP-dependent pathway, and plays an important role in protein degradation in skeletal muscles. Ubiquitin (Ub), ubiquitin activating enzyme E1, ubiquitin-conjugating enzyme E2, ubiquitin ligase E3, 26S proteasome, and de-ubiquitination enzymes are the major components of the UPP that is widely distributed in eukaryotic cells. Han et al. have reported that the levels of Ub mRNA and E2-14k protein in COPD rats were significantly higher than that in control rats [7]. E2-14k is a member of the ubiquitin carrier protein E2 family, which plays an essential role in the binding between ubiquitin and substrate proteins. In the present study, we found that the mRNA levels of E2-14k and Ub in the diaphragm, gastrocnemius, and intercostal muscles in COPD rats were significantly higher than that in control rats. In addition, E3 plays an important role in the process of ubiquitination through the determination of the time and specificity of the substrate protein ubiquitination. Lecker et al. have shown that muscle-specific ligase MAFbx and MuRF1 were important proteins involved in the regulation of muscle atrophy [8]. Ottenheijm et al. also reported that the mRNA level of MAFbx was increased in COPD patients [9]. Similar results have also been reported by Doucet et al. [10]. and Plant et al [11]. In the present study, we evaluated the expression of E2-14k, MAFb, and Ub, which are the major components of the UPP, in skeletal muscle in COPD rats and compared their levels with that in control rats. We found that the mRNA and protein levels of E2-14k, MAFb, and Ub in the skeletal muscles in COPD rats were higher than that in control rats. The changes in the expression of E2-14k, MAFb, and Ub were not only found in respiratory muscles but also in peripheral muscle gastrocnemius. Therefore, activation of the UPP in COPD is a systematic event, which affecting both respiratory and peripheral muscles.
With the progress in studies on the pathogenesis of COPD, it has been widely accepted that COPD is a systematic diseases related to inflammation rather than an inflammatory disease limited in the lung [12]. Ottenheijm et al. reported that the content of myosin in the diaphragmatic muscle of mild and moderate COPD patients (GOLD grade I and II) decreased 30% [13]. In addition, nutrition support therapy alone did not significantly prevent the degradation of skeletal muscle proteins in COPD patients with malnutrition [14]. Therefore, COPD has been considered as a skeletal muscle disease [15]. In the present study, we found that the weight of COPD rats was significantly lower than that of the normal control rats. In addition, the levels of E2-14k, MAFbx, and Ub in COPD rats in the diaphragm, gastrocnemius, and intercostal muscleswere significantly higher than that in the control rats, which may suggest that the activated UPP pathway lead to the increased degradation of proteins in respiratory muscles or all skeletal muscles in the body and the decrease of the weight of COPD rats.
Interleukin-15 (IL-15) is a cytokine first isolated and cloned from a monkey kidney cell line in 1994 [16]. It has been shown that IL-15 is widely expressed in mammalian tissues, especially the skeletal muscle in which the mRNA level of IL-15 is extremely high [17]. Carbo et al. has reported that IL-15 treatment inhibited protein degradation in rats of malignant tumors [18]. In addition, overexpression of IL-15 in mouse myoblast C2C12 led to skeletal muscle hypertrophy. [19]. Carbo et al. also reported that the role of IL-15 in the prevention of protein degradation is mediated by the ATP-dependent UPP pathway. Therefore, IL-15 may prevent the degradation of proteins in some diseases such as cancers. However, the role of IL-15 in the pathogenesis of COPS is largely unknown. In the present study, we found that the levels of IL-15 in the serum, diaphragm, gastrocnemius, and intercostal muscles in COPD rats were significantly higher than that in the normal control rats. In addition, the IL-15 level in skeletal muscles was significantly higher than that in serum in COPD rats, which is consistent with previous studies. We also identified positive association between the IL-15 level and the expression of E2-14K, MAFbx, and Ub in diaphragm, gastrocnemius, and intercostal muscles in COPD rats. Furthermore, negative association between the animal weight and the expression of E2-14K, MAFbx, Ub, and IL-15 in diaphragm, gastrocnemius, and intercostal muscles was observed in COPD rats. Therefore, we speculate that UPP, the major protein degradation pathway, was activated in COPD rats to promote the degradation of proteins in respiratory muscles. Increased expression IL-15 may be a natural response of the body to the degradation of proteins in respiratory muscles. It has been reported that exogenous IL-15 reduced the degradation of skeletal muscle protein in rats by ten times [20]. While IL-15 is a protective mechanism preventing the degradation of skeletal muscle proteins, multiple factors such inflammatory factors, immunoregulatory factors, TNF-α, the NF-kB (p65) pathway, and limited expression of endogenous IL-15 may be involved in the degradation of skeletal muscle proteins in COPD and IL-15 may not be sufficient to effectively inhibit the degradation of respiratory muscles in COPD. Therefore, increased expression IL-15 was not able to reverse the development of COPD and increased expression of IL-15 was observed with the upregulation of UPP components and TNF-α in COPD rats.
TNF-α is a polypeptide cytokine secreted by monocytes-macrophages, T lymphocytes, and endothelial cells. TNF-α is one of the major mediator involved in the systemic inflammatory response syndrome. The previous study has shown that TNF-α increased the degradation of muscle proteins in mice [21]. In addition, Hober et al. have reported that TNF-α treatment increased the expression of ubiquitin and ubiquitin-binding proteins as well as the degradation of skeletal muscle proteins in vitro and in vivo [22]. Taken together, TNF-α is involved in the degradation of muscle proteins in inflammation-related diseases.
In the mouse shock model caused by E. coli infection, IL-15 inhibited the apoptosis induced by TNF-α [23]. The activated IL-15 receptor competitively bound to the specific adapter proteins of type I TNF-α receptor to inhibit the biological activities of TNF-α. In the present study, we found positive correlation between the levels of IL-15 and TNF-α in the serum, diaphragm, gastrocnemius, and intercostal muscles in COPD rats. In addition, the expression of both IL-15 and TNF-α in the serum, diaphragm, gastrocnemius, and intercostal muscles in COPD rats was positively associated with the expression of the components of the UPP pathway, such as E2-14K, MAFbx, and Ub. In COPD rats, the expression of TNF-α in respiratory muscles was increased, resulting in the activation of the UPP pathway and the degradation of skeletal muscle proteins. It has been reported that TNF-α antagonist could not effectively increase the quality of skeletal muscle [24]. Therefore, we speculate that IL-15 prevented the degradation of skeletal muscle proteins in COPD through TNF-α and the UPP pathway, however, the UPP may not be the only pathway involved in the development of COPD.
Degradation of skeletal muscle proteins is one of the characteristic features of COPD. In summary, we identified the correlation among IL-15, TNF-α, and a number of components of the UPP pathway based on a rat model of COPD. Our results suggest the interaction among IL-15, TNF-α, and the UPP pathway contributed to the pathogenesis of COPD. However, the underlying mechanisms are still not fully understood and need to be further investigated. IL-15 may lead to the development of novel drugs preventing the degradation of skeletal muscle proteins for the treatment of COPD.
Acknowledgements
This study was supported by Project Funding for the Construction of National Key Clinical Specialty.
Disclosure of conflict of interest
None.
References
- 1.Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, Chen B, Wang C, Ni D, Zhou Y, Liu S, Wang X, Wang D, Lu J, Zheng J, Ran P. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176:753–760. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 2.Laaban JP. [Nutrition and chronic respiratory failure] . Ann Med Interne (Paris) 2000;151:542–548. [PubMed] [Google Scholar]
- 3.Ottenheijm CA, Heunks LM, Hafmans T, van der Ven PF, Benoist C, Zhou H, Labeit S, Granzier HL, Dekhuijzen PN. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:527–534. doi: 10.1164/rccm.200507-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng FX, Liu Z, Liang Z, Fan W. Skeletal muscle protein degradation mechanism in chronic obstructive pulmonary disease rats. Int J Respir. 2011;31:1770–1776. [Google Scholar]
- 5.Figueras M, Busquets S, Carbo N, Barreiro E, Almendro V, Argiles JM, Lopez-Soriano FJ. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2004;569:201–206. doi: 10.1016/j.febslet.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 6.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han F, Xu W, Luo Y, Yang T, Chen Y, Zhang Y. The Ubiquitin-Proteasome Pathway is Activated in the Diaphragm of COPD Rat. Chin J Respir Crit Care Med. 2010;9:141–144. [Google Scholar]
- 8.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 9.Ottenheijm CA, Heunks LM, Li YP, Jin B, Minnaard R, van Hees HW, Dekhuijzen PN. Activation of the ubiquitin-proteasome pathway in the diaphragm in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:997–1002. doi: 10.1164/rccm.200605-721OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doucet M, Russell AP, Leger B, Debigare R, Joanisse DR, Caron MA, LeBlanc P, Maltais F. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:261–269. doi: 10.1164/rccm.200605-704OC. [DOI] [PubMed] [Google Scholar]
- 11.Plant PJ, Brooks D, Faughnan M, Bayley T, Bain J, Singer L, Correa J, Pearce D, Binnie M, Batt J. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2010;42:461–471. doi: 10.1165/rcmb.2008-0382OC. [DOI] [PubMed] [Google Scholar]
- 12.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 13.Ottenheijm CA, Heunks LM, Hafmans T, van der Ven PF, Benoist C, Zhou H, Labeit S, Granzier HL, Dekhuijzen PN. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:527–534. doi: 10.1164/rccm.200507-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schols AM. Nutritional and metabolic modulation in chronic obstructive pulmonary disease management. Eur Respir J Suppl. 2003;46:81s–86s. doi: 10.1183/09031936.03.00004611. [DOI] [PubMed] [Google Scholar]
- 15.Rutten EP, Franssen FM, Engelen MP, Wouters EF, Deutz NE, Schols AM. Greater whole-body myofibrillar protein breakdown in cachectic patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2006;83:829–834. doi: 10.1093/ajcn/83.4.829. [DOI] [PubMed] [Google Scholar]
- 16.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, Et A. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 17.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 18.Carbo N, Lopez-Soriano J, Costelli P, Busquets S, Alvarez B, Baccino FM, Quinn LS, Lopez-Soriano FJ, Argiles JM. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br J Cancer. 2000;83:526–531. doi: 10.1054/bjoc.2000.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res. 2002;280:55–63. doi: 10.1006/excr.2002.5624. [DOI] [PubMed] [Google Scholar]
- 20.Quinn LS, Haugk KL, Damon SE. Interleukin-15 stimulates C2 skeletal myoblast differentiation. Biochem Biophys Res Commun. 1997;239:6–10. doi: 10.1006/bbrc.1997.7414. [DOI] [PubMed] [Google Scholar]
- 21.García-Martínez C, Llovera M, Agell N, López-Soriano FJ, Argilés JM. Ubiquitin Gene Expression in Skeletal Muscle Is Increased by Tumor Necrosis Factor-α. Biochem Biophys Res Commun. 1994;201:682–686. doi: 10.1006/bbrc.1994.1754. [DOI] [PubMed] [Google Scholar]
- 22.Hobler SC, Wang JJ, Williams AB, Melandri F, Sun X, Fischer JE, Hasselgren PO. Sepsis is associated with increased ubiquitinconjugating enzyme E214k mRNA in skeletal muscle. Am J Physiol. 1999;276:R468–R473. doi: 10.1152/ajpregu.1999.276.2.R468. [DOI] [PubMed] [Google Scholar]
- 23.Hiromatsu T, Yajima T, Matsuguchi T, Nishimura H, Wajjwalku W, Arai T, Nimura Y, Yoshikai Y. Overexpression of interleukin-15 protects against Escherichia coli-induced shock accompanied by inhibition of tumor necrosis factor-alpha-induced apoptosis. J Infect Dis. 2003;187:1442–1451. doi: 10.1086/374643. [DOI] [PubMed] [Google Scholar]
- 24.Bulfone-Paus S, Bulanova E, Budagian V, Paus R. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. Bioessays. 2006;28:362–377. doi: 10.1002/bies.20380. [DOI] [PubMed] [Google Scholar]


