Abstract
Cigarette smoke-induced airway inflammation is one of the most important features of chronic airway diseases. Studies suggest that chrysin possesses strong anti-inflammatory properties and this study aimed to investigate the effect of chrysin on cigarette smoke-induced airway inflammation in mice. Mice with exposure to cigarette smoke were intraperitonealy injected with chrysin (10, 20 mg/kg·d). TNF-α, IL-1β and IL-8 levels in bronchoalveolar lavage fluid were determined by ELISA. MPO level in lung homogenates was tested by a MPO kit. The expression of signaling proteins in lung tissue, phosphorylation ERK and p38 was detected using Western Blot. Cigarette smoke exposure increased the release of inflammatory cytokines TNF-α, IL-1β, IL-8 in bronchoalveolar lavage fluid and the expression of MPO in lung tissue. Chrysin pretreatment inhibited cigarette smoke-induced airway inflammation, inflammatory cytokines release, and MPO expression. Cigarette smoke exposure also increased the expression of phosphorylation ERK and p38, meanwhile, chrysin intervention can inhibit such changes. In summary, chrysin inhibits cigarette smoke exposure-induced airway inflammation in mice, which may partly act through inhibition of ERK and p38 phosphorylation.
Keywords: Cigarette smoke, airway inflammation, chrysin
Introduction
Consistent airway inflammation is one of the most important pathogenic characteristics of chronic inflammatory airway diseases such as chronic obstructive pulmonary disease (COPD) [1]. Cigarette smoke (CS), which contains thousands of toxic substances, is a well-known etiological factor in the development of COPD [2,3]. CS exposure damages the airway epithelium, induces airway inflammation and high oxidative stress status, which leading to airway mucus hypersecretion, airway obstruction, and finally with an irreversible airflow limitation and progressive decline in lung function [4,5]. To protect airway form CS-induced airway inflammation will be of great importance in the prevention and management of COPD.
Flavonoids are plant polyphenolic compounds that comprise several classes and are effective in suppressing inflammation and oxidative stress [6], which may provide potential treatment for inflammatory airway disease. Chrysin is a naturally-occurring flavone that is commonly found in flowers, and it has also been reported to possess anti-oxidant, anti-inflammation, anti-diabetogenic, and anti-cancerous properties [7,8]. Growing studies pay attention to the effects of chrysin on respiratory diseases, such as Staphylococcus aureus pneumonia, asthma and lung fibrosis [9-11], however, there is no study examined the effect of chrysin on CS-induced airway inflammation. This study aimed to investigate the potential effect of chrysin on CS-induced airway inflammation in an experimental mouse model.
Materials and methods
Animals
Animal preparation was handled-according to the Laboratory Animal Care Guidelines of West China School of Medicine, Sichuan University. Specific pathogen-free, male BALB/c mice (6-8 weeks) were purchased from Dashuo Biological Technology Co, Ltd (Chengdu, China). Mice were housed in a temperature- and humidity controlled facility and kept on a 12-h light/dark cycle, with free access to water and laboratory feed.
Mice were randomly divided into the following four groups (n=4 per group): control group (Con group), which was not exposed to CS; Cigarette smoke-exposed group (CS group), which received placebo and was exposed to CS; Cigarette smoke -exposed low-dose chrysin group (CS+Chrysin(L)), which received 10 mg/kg chrysin (q.o.d.) and was subsequently exposed to CS; and the Cigarette smoke-exposed high-dose chrysin group (CS+Chrysin(H)), which received 20 mg/kg chrysin (q.o.d.) and was subsequently exposed to CS.
CS+Chrysin(L) and CS+Chrysin(L) mice received chrysin by intraperitoneal injection at the above mentioned doses 30 min before CS exposure, and then exposed to the smoke of five commercially available cigarettes (Jiaozi, China Tobacco Chuanyu Industrial Co., Ltd.; 1.1 mg nicotine and 11 mg tar per cigarette) for 30 min twice daily, 6 days per week for 4 weeks, following the methods of Yang et al [4]. After 4 weeks of CS exposure, all the mice were sacrificed by intraperitoneal 3% sodium pentobarbital, followed by exsanguination from the right ventricle to allow tissue sample collection.
Bronchoalveolar lavage fluid (BALF) analysis
Inflammatory cell counting
The right lung was lavaged three times with 0.5 ml of saline, with a recovery rate of 90%. The BALF samples were centrifuged at 1,000 g for 5 minutes, and the supernatants were removed and stored at -80°C for cytokines measurement. The pelleted cells were re-suspended in 0.2 ml phosphate-buffered saline, the total cell number was determined by a hemocytometer. Differential cell count was performed by cytocentrifugation (Cytopro7620, Wescor, Utah, USA) at 700 rpm for 10 min and stained with Wright’s stain (200 cells were counted for each mouse).
Inflammatory cytokines detection
Levels of IL-1β, IL-8, and TNF-α were measured in BALF using commercially available enzyme-linked immunosorbent assay (ELISA) kits for mouse cytokines (Xitang Bio-Technology Co., Ltd., Shanghai, China). The manufacturer’s instructions were strictly followed during the ELISA experiments.
Lung histopathology examination
The left mouse lung was not lavaged and was immersed in 4% phosphate-buffered paraformaldehyde to allow complete fixation, after which it was embedded in paraffin, sectioned (4 μm), and stained with hematoxylin and eosin (H&E) to evaluate morphological changes in lungs.
Myeloperoxidase (MPO) measurement
Snap-frozen lung tissue was homogenated in phosphate-buffered saline and the MPO was determined using a MPO assay kit (Nnajing Jiancheng Bioengineering Institute, Nanjing, China) with the reagents provided according to the manufacturer’s instructions.
Western blot analysis
Lung tissues were lysed in RIPA buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 2 mM sodium fluoride, 2 mM EDTA, 0.1% SDS, and PMSF. Protein concentrations from whole lung extracts were determined by a BCA protein assay kit (Thermo Fisher Scientific Inc., MA, USA). To analyze P-ERK, P-P38MAPK, ERK, P38MAPK, total protein (20 μg) was fractionated by 10% SDS polyacrylamide gel electrophoresis and transferred to PVDF membranes. Membranes were blocked for 1 h at room temperature with 5% BSA in TBS-Tween and incubated overnight at 4°C with the appropriate primary antibodies. Primary antibodies were anti-ERK mAb, anti-phospho-ERK mAb, anti-p38MAPK, and anti-phospho-p38 mAb (Cell Signaling Technology, Beverly, MA, USA). After incubation with horseradish peroxidase-conjugated second antibodies (Cell Signalling Technology), the immune complexes were detected with enhanced chemiluminescence reagents (Millipore, Billerica, USA). Band intensities were quantified using computerized image analysis (QuantityOne-software, Bio-Rad, Hercules, CA).
Statistical analysis
All values are expressed as Mean ± SD. Statistical analysis was carried out using one-way ANOVA, followed by the LSD significant difference test (SPSS for Windows version 18.0, Chicago, IL, USA). A significant difference was defined at P>0.05.
Results
Chrysin prevented CS-induced mouse airway histopathological changes
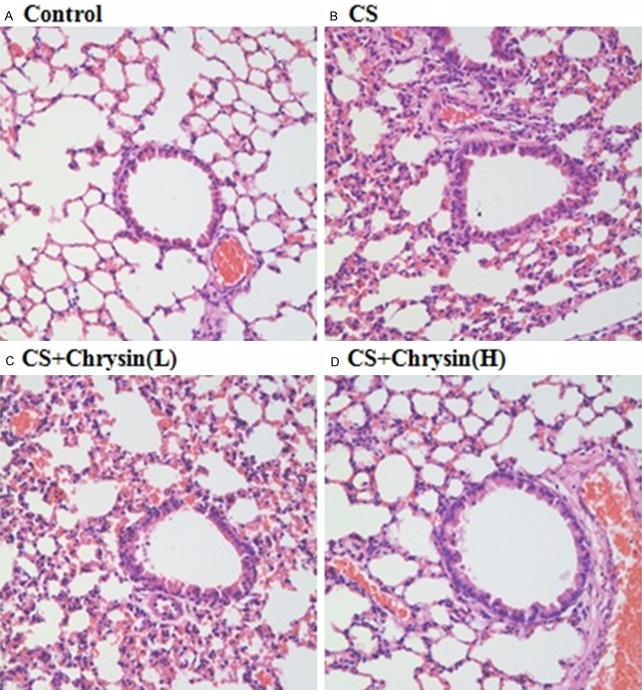
In this study, H&E staining was used to evaluate histopathological changes in mouse airways. Four-week’s CS exposure induced significant airway inflammatory response manifested as thickening of the airway epithelium, lumen obstruction by mucus and cell debris, and inflammatory cell infiltration (Figure 1A-D). Such changes were significantly attenuated by chrysin pretreatment, especially with high dose chrysin.
Figure 1.
The effect of chrysin on CS-induced lung histological changes. Lung tissues were analyzed by hematoxylin and eosin staining, A. Control, no chrysin pretreatment and no exposure to cigarette smoke; B. CS, no chrysin pretreatment and exposure to cigarette smoke; C. CS+Chrysin(L), mice pretreated with 10 mg/kg chrysin and exposed to cigarette smoke; D. CS+Chrysin (H), mice pretreated with 20 mg/kg chrysin and exposed to cigarette smoke. Scale bars =100 μm.
Chrysin attenuated CS-induced inflammatory cell influx and inflammatory cytokines release in BALF
Total and differential cell counts were performed in BALF to investigate the effect of chrysin on CS-induced inflammation in mouse airways. Total BALF cell counts and differential cells counts were significantly higher in CS-exposed mice than in the control group. High dose of chrysin pretreatment significantly reduced the CS-induced recruitment of total cells and differential cells to BALF (Figure 2), and suppressed the CS-induced influx of inflammatory cells into BALF. BALF levels of IL-1β, IL-8, and TNF-α were significantly higher in the samples from CS-exposed mice than in the samples from control animals. Pretreatment with high dose chrysin significantly reduced the CS-induced increases in IL-1β, IL-8, and TNF-α in BALF (Figure 3).
Figure 2.
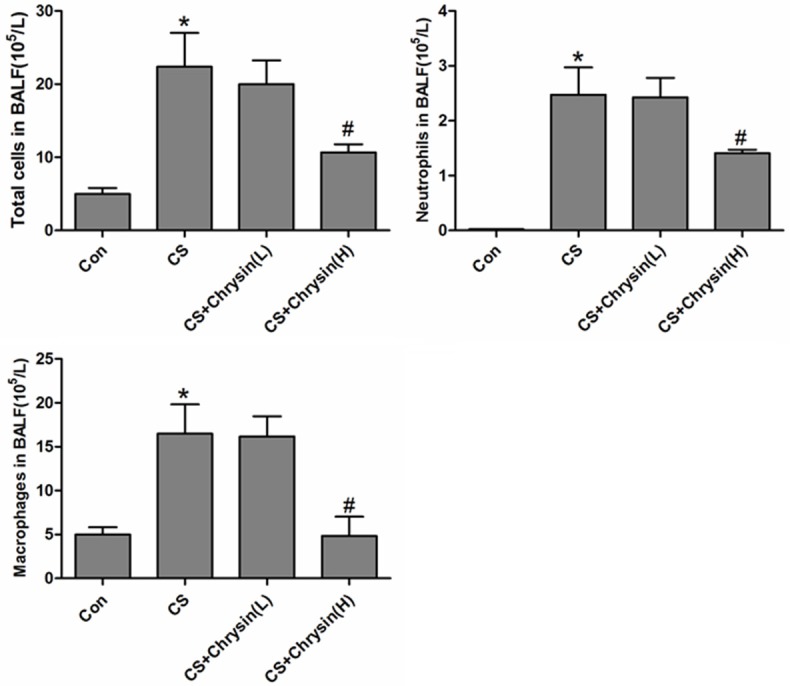
The effects of chrysin on BALF cellularity in CS-exposed mice. Differential cell count in BALF was determined by cytocentrifugation and Wright’s stain. A. Control, no chrysin pretreatment and no exposure to cigarette smoke; B. CS, no chrysin pretreatment and exposure to cigarette smoke; C. CS+Chrysin(L), mice pretreated with 10 mg/kg chrysin and exposed to cigarette smoke; D. CS+Chrysin(H), mice pretreated with 20 mg/kg chrysin and exposed to cigarette smoke. *P<0.05 with respect to the control group, #P<0.05 with respect to the cigarette smoke-exposed group.
Figure 3.
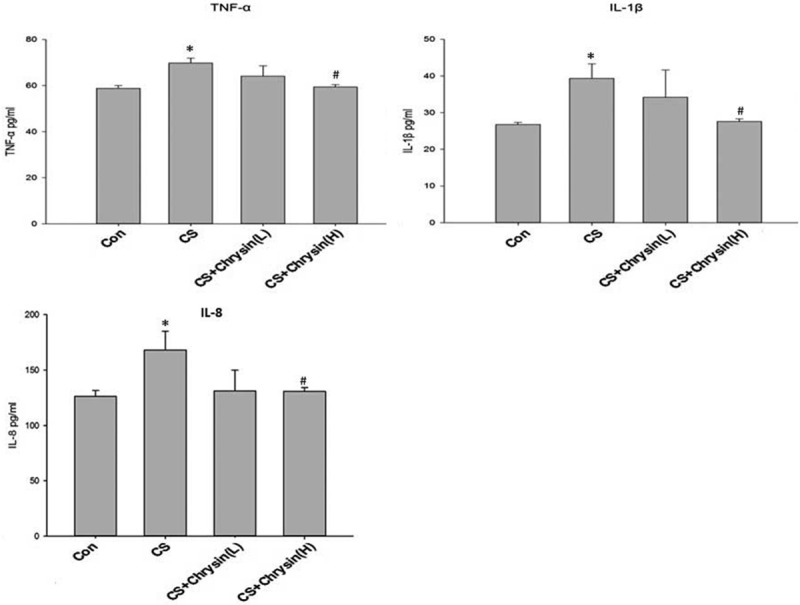
The effects of chrysin on the production of BALF cytokines. BALF was collected from different groups of mouse lung. The concentrations of TNF-α (left), IL-1β (middle), and IL-8 (right) in BALF were analyzed by ELISA. Values are expressed as mean ± SD. A. Control, no chrysin pretreatment and no exposure to cigarette smoke; B. CS, no chrysin pretreatment and exposure to cigarette smoke; C. CS+Chrysin(L), mice pretreated with 10 mg/kg chrysin and exposed to cigarette smoke; D. CS+Chrysin(H), mice pretreated with 20 mg/kg chrysin and exposed to cigarette smoke. *P<0.05 with respect to the control group, #P<0.05 with respect to the cigarette smoke-exposed group.
Chrysin suppressed CS-induced MPO expression in lung tissue
Four weeks of CS-exposure significantly increased the MPO levels in lung tissue of mice, and high dose of chrysin treatment significantly inhibited CS-induced MPO expression (Figure 4).
Figure 4.
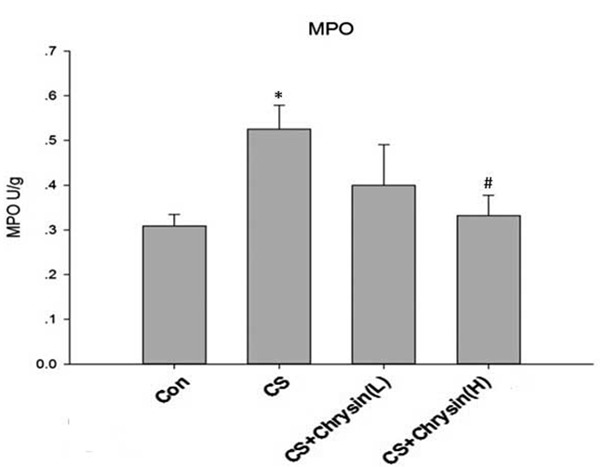
The effects of chrysin on lung MPO activity in CS-treated mice. Lung sections from different groups were homogenized, and the MPO activity was determined spectrophotometrically. A. Control, no chrysin pretreatment and no exposure to cigarette smoke; B. CS, no chrysin pretreatment and exposure to cigarette smoke; C. CS+Chrysin(L), mice pretreated with 10 mg/kg chrysin and exposed to cigarette smoke; D. CS+Chrysin(H), mice pretreated with 20 mg/kg chrysin and exposed to cigarette smoke. *P<0.05 with respect to the control group, #P<0.05 with respect to the cigarette smoke-exposed group.
Chrysin inhibited CS-induced ERK and P38 phosphorylation
To explore the possible mechanisms involved in CS-induced airway inflammation, the expression levels of p-ERK and p-p38 were examined. Lung tissues of CS-exposed mice showed higher levels of phosphorylated ERK and P38 than did lung tissues of control mice, and chrysin pretreatment attenuated these effects of CS (Figure 5).
Figure 5.
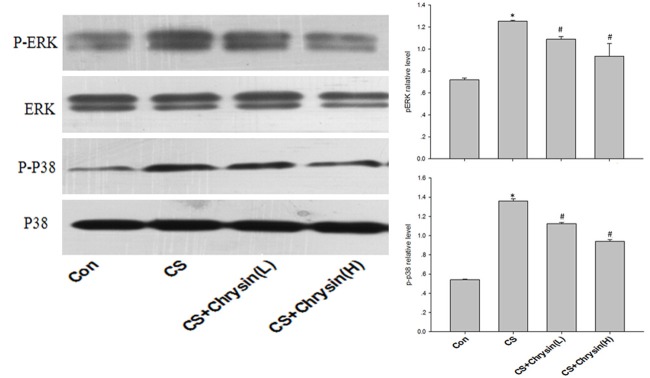
Expression of p-p38 and p-ERK in mice lungs. p-p38 and p-ERK levels were measured by Western blotting in mouse lungs (left) and quantified by densitometry (right). A. Control, no chrysin pretreatment and no exposure to cigarette smoke; B. CS, no chrysin pretreatment and exposure to cigarette smoke; C. CS+Chrysin(L), mice pretreated with 10 mg/kg chrysin and exposed to cigarette smoke; D. CS+Chrysin(H), mice pretreated with 20 mg/kg chrysin and exposed to cigarette smoke. *P<0.05 with respect to the control group, #P<0.05 with respect to the cigarette smoke-exposed group.
Discussion
In this study, CS was used to establish airway inflammation model in mouse, which causes significant release of inflammatory cytokines in BALF, MPO expression, and ERK, P38 phosphorylation in mouse lung, and pretreatment with chrysin attenuated these effects in vivo.
In our study, chrysin protects mouse airway from CS-induced inflammatory response through inhibiting the release of inflammatory cytokines, including IL-1β, IL-8, and TNF-α, which play a role in the pathogenesis of COPD. Recent studies suggest that IL-1β were significantly increased in total lung tissue and induced sputum of patients with COPD, respectively, compared with never-smokers, and IL-1β should be considered as an important mediator in CS-induced inflammation and COPD [12]. Our previous study found that CS increases IL-8 expression in the lung tissues of COPD patients, and IL-8 is associated with stages of COPD, which may serve as an indicator for clinical progress [13]. TNF-α is a cytokine that regulating inflammatory activities, it is an important chemotactic protein for neutrophils, studies suggest that TNFα levels are increased in the blood and sputum of COPD patients, play a role in the pathogenesis and clinical course of COPD [14,15], Many clinical trials have investigated the therapeutic potential of specific cytokines inhibition for COPD, but with unsatisfactory results, anyway, further studies are needed to determine the detailed role of such inflammatory cytokines in COPD, a specific group of COPD patients should be targeted with specific anti-cytokine therapy if there is evidence of high expression of that cytokine and there are features of the clinical expression of COPD that will respond to therapy [16].
MPO is a heme-containing peroxidase expressed abundantly in neutrophils, it is one of the principal enzymes released from secondary granules following neutrophil activation, and plays an important role in driving inflammatory reactions and tissue oxidation. Many studies suggest that sputum MPO levels were increased in stable COPD patients when compared with normal controls, and such changes was especially pronounced during COPD exacerbations, sputum MPO might be a promising biomarker for guiding COPD management [17]. Recent study found that MPO inhibitor intervention can stop progression of emphysema and small airway remodeling even when treatment starts relatively late in the course of long-term smoke exposure, suggesting inhibition of MPO may be a novel and useful therapeutic treatment for COPD [18]. In present study, we confirmed CS-induced MPO high expression in lung tissue, and chrysin intervention decreased MPO expression, suggesting its potential mechanism in suppressing CS-induced airway inflammation.
MAPK signaling pathways containing ERK and P38 play an important role in the pathogenesis of COPD. Studies showed a significantly increased expression of phospho-p38 MAPK and IL-8 in the sputum samples of the COPD patients, and the p38 MAPK activity was remarkably correlated with the CXCL8 level and neutrophils infiltration in the airway, and the decline of lung function in the COPD patients [19]. Gaffey et al reported that phospho-p38 was increased in lung tissues of COPD patients, and inhibition of P38 MAPK may supply a novel therapy choice for COPD [20]. A clinical trial has investigated the efficacy of the oral p38 inhibitor PH-797804 on COPD patients, and PH-797804 demonstrated improvements over placebo in lung function parameters and dyspnea in patients with moderate to severe COPD [21]. Liu et al reported that increased expression of ERK might be involved in the pathogenesis of COPD patients, and phosphorylated ERK is involved in pro-inflammatory cytokines production and airway mucus hyepersecretion [22-23]. Our studies confirmed that P38 and ERK phosphorylation involved in CS-induced airway inflammation, therapy or drugs targeting on these signaling pathways may help to attenuate CS-induced airway inflammation. What should be pointed out is that the present study included a limited number of mice and further studies are needed to confirm the protective role of chrysin on CS-induced airway inflammation and investigate its possible mechanism, and how to translate the preclinical study findings into clinical studies.
Conclusion
Taken together, our study confirmed that CS exposure can induce significant airway inflammation, increase lung tissue MPO expression, phospho-p38 MAPK and ERK, pretreatment with chrysin may attenuate these changes, thus, chrysin shows the potential to treat CS-induced inflammatory airway disorders.
Acknowledgements
This work was supported by grants 81230001 and 81300032 from the National Natural Science Foundation of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo L, He F, Sergakis GG, Koozehchian MS, Stimpfl JN, Rong Y, Diaz PT, Best TM. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am J Physiol Lung Cell Mol Physiol. 2014;307:L205–L218. doi: 10.1152/ajplung.00330.2013. [DOI] [PubMed] [Google Scholar]
- 3.Campesi I, Carru C, Zinellu A, Occhioni S, Sanna M, Palermo M, Tonolo G, Mercuro G, Franconi F. Regular cigarette smoking influences the transsulfuration pathway, endothelial function, and inflammation biomarkers in a sex-gender specific manner in healthy young humans. Am J Transl Res. 2013;5:497–509. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang T, Luo F, Shen Y, An J, Li X, Liu X, Ying B, Liao Z, Dong J, Guo L, Wang T, Xu D, Chen L, Wen F. Quercetin attenuates airway inflammation and mucus production induced by cigarette smoke in rats. Int Immunopharmacol. 2012;13:73–81. doi: 10.1016/j.intimp.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 5.John G, Kohse K, Orasche J, Reda A, Schnelle-Kreis J, Zimmermann R, Schmid O, Eickelberg O, Yildirim AÖ. The composition of cigarette smoke determines inflammatory cell recruitment to the lung in COPD mouse models. Clin Sci (Lond) 2014;126:207–221. doi: 10.1042/CS20130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rehman MU, Tahir M, Khan AQ, Khan R, Lateef A, Oday-O-Hamiza , Qamar W, Ali F. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett. 2013;216:146–158. doi: 10.1016/j.toxlet.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Sirovina D, Orsolić N, Koncić MZ, Kovacević G, Benković V, Gregorović G. Quercetin vs chrysin: effect on liver histopathology in diabetic mice. Hum Exp Toxicol. 2013;32:1058–1066. doi: 10.1177/0960327112472993. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Qiu J, Dong J, Li H, Luo M, Dai X, Zhang Y, Leng B, Niu X, Zhao S, Deng X. Chrysin protects mice from Staphylococcus aureus pneumonia. J Appl Microbiol. 2011;111:1551–1558. doi: 10.1111/j.1365-2672.2011.05170.x. [DOI] [PubMed] [Google Scholar]
- 10.Wadibhasme PG, Ghaisas MM, Thakurdesai PA. Anti-asthmatic potential of chrysin on ovalbumin-induced bronchoalveolar hyperresponsiveness in rats. Pharm Biol. 2011;49:508–515. doi: 10.3109/13880209.2010.521754. [DOI] [PubMed] [Google Scholar]
- 11.Kilic T, Ciftci O, Cetin A, Kahraman H. Preventive Effect of Chrysin on Bleomycin-Induced Lung Fibrosis in Rats. Inflammation. 2014;37:2116–2124. doi: 10.1007/s10753-014-9946-6. [DOI] [PubMed] [Google Scholar]
- 12.Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Vanden Berghe T, Vandenabeele P, Lambrecht BN, Joos GF, Brusselle GG. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J. 2011;38:1019–1028. doi: 10.1183/09031936.00158110. [DOI] [PubMed] [Google Scholar]
- 13.Hu XR, Han SX, Wang T, Zhang MK, Chen L, Wen FQ. Association between interleukin-8 in lung tissues and stages of chronic obstructive pulmonary diseases. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:885–888. [PubMed] [Google Scholar]
- 14.Chiang CH, Chuang CH, Liu SL. Transforming growth factor-β1 and tumor necrosis factor-α are associated with clinical severity and airflow limitation of COPD in an additive manner. Lung. 2014;192:95–102. doi: 10.1007/s00408-013-9520-2. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFalpha in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:397–412. doi: 10.2147/COPD.S42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu A, Ge D, Zhang J, Teng Y, Yuan C, Huang M, Adcock IM, Barnes PJ, Yao X. Sputum myeloperoxidase in chronic obstructive pulmonary disease. Eur J Med Res. 2014;19:12. doi: 10.1186/2047-783X-19-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Churg A, Marshall CV, Sin DD, Bolton S, Zhou S, Thain K, Cadogan EB, Maltby J, Soars MG, Mallinder PR, Wright JL. Late intervention with a myeloperoxidase inhibitor stops progression of experimental chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:34–43. doi: 10.1164/rccm.201103-0468OC. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Xie M, He X, Gao H. Activity of sputum p38 MAPK is correlated with airway inflammation and reduced FEV1 in COPD patients. Med Sci Monit. 2013;19:1229–1235. doi: 10.12659/MSM.889880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaffey K, Reynolds S, Plumb J, Kaur M, Singh D. Increased phosphorylated p38 mitogen-activated protein kinase in COPD lungs. Eur Respir J. 2013;42:28–41. doi: 10.1183/09031936.00170711. [DOI] [PubMed] [Google Scholar]
- 21.MacNee W, Allan RJ, Jones I, De Salvo MC, Tan LF. Efficacy and safety of the oral p38 inhibitor PH-797804 in chronic obstructive pulmonary disease: a randomised clinical trial. Thorax. 2013;68:738–745. doi: 10.1136/thoraxjnl-2012-202744. [DOI] [PubMed] [Google Scholar]
- 22.Liu K, Liu XS, Yu MQ, Xu YJ. Change of extracellular signal-regulated kinase expression in pulmonary arteries from smokers with and without chronic obstructive pulmonary disease. Exp Lung Res. 2013;39:162–172. doi: 10.3109/01902148.2013.788234. [DOI] [PubMed] [Google Scholar]
- 23.Xiao J, Wang K, Feng YL, Chen XR, Xu D, Zhang MK. Role of extracellular signal-regulated kinase 1/2 in cigarette smoke-induced mucus hypersecretion in a rat model. Chin Med J (Engl) 2011;124:3327–3333. [PubMed] [Google Scholar]