Abstract
Renal ischemia/reperfusion-injury (IRI) is a common disease in clinic, which is also the most common cause of acute kidney failure. Previous investigations has illustrated that catalpol has neuroprotective, anti-inflammatory, and anti-hepatitis virus effects. This study was designed to investigate the protective effect of catalpol on renal IRI mice through suppressing phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt)-endothelial nitric oxide synthase (eNOS) and against inflammation, and the possible underlying mechanism. Firstly, we used renal IRI model to analyze blood urea nitrogen and serum creatinine levels in renal IRI mice. Next, real-time PCR and western blotting were used to detect the expression of KIM-1 and the expression of PI3K, Akt and eNOS levels in renal IRI, respectively. In addition, activities of tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-10 (IL-10) in renal IRI mice were measured with respective TNF-α, IL-1β, IL-6 and IL-10 ELISA kits. Our results showed that catalpol clearly reduced blood urea nitrogen, serum creatinine levels and the expression of KIM-1 in renal IRI mice. Meanwhile, we found that catalpol markedly reduced the expression of PI3K, Akt and eNOS levels in renal IRI group. Suppressing of the PI3K/Akt-eNOS and the TNF-α, IL-1β, IL-6 and IL-10 activities was involved in the protective effect of catalpol on renal IRI. Collectively, catalpol protected renal IRI via inhibiting PI3K/Akt-eNOS signaling and inflammatory responses.
Keywords: Catalpol, renal ischemia/reperfusion-injury, PI3K/Akt, eNOS, inflammation
Introduction
Kidney damage caused by renal ischemia/reperfusion-injury (IRI) is a common disease clinically [1]. In shock and kidney transplant patients, it is the most common cause of acute kidney failure. Over the past 40 years, acute renal necrosis rate of shock patients was always close to 50%, without significant change [2,3]. For the treatment of renal ischemia-reperfusion injury, supportive therapy remains, therefore, improved treatment strategies to prevent renal IR-injury, is drawing attention from scholars [4].
Endothelial progenitor cells are vascular endothelial cells that can be differentiated into precursor cells. Studies show that endothelial progenitor cells are not only involved in human embryonic angiogenesis, but also participate in postnatal angiogenesis and endothelial repair process after injury [5], the number and the dysfunction of endothelial progenitor cells play a key role in the development of renal vascular complications. Function disorders of renal endothelial progenitor cells include nitric oxide synthase (NOS)/nitric oxide (NO), oxidative stress and certain cytokines. Endothelial nitric oxide synthase (eNOS) is a key enzyme in the regulation of endothelial-derived NO production [6]; Phosphatidylinositol3-kinase (PI3K)/Akt signaling pathway is an important signaling pathway to regulate a variety of cellular metabolism, growth, migration and proliferation, which is an important measure in the treatment of renal IR-injury [7].
Inflammatory response can significantly promote renal IR-injury [8-10]. In IR-injury, polymorphonuclear cell or proximal convoluted tubule and mesangial cell in kidney generate bradykinin, histamine, leukotrienes, PAF and other activating factors, tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and other pro-inflammatory cytokines, and monocyte chemoattractant protein-1 (MCP-1), interferon induced protein-10 (IP-10) and other chemokines, leading to leukocyte and activation of chemotaxis, thus migrating to the injury site [11]. Then, leukocyte becomes adhered tightly to the skin from rolling within the vessel by the interaction with vascular endothelial cells, further migrating to endoderm, and eventually crosses endoderm and reaches the extravascular tissue to play a role [12]. On one hand, inflammatory cells directly damage the reperfusion tissue cells by the release of and oxidase enzyme and hydrolyticenzyme, on the other hand, the adhered neutrophil hand blocks the microvascular bed, further increasing the circulation disorders [13].
Catalpol has anti-cancer, neuroprotective, anti-inflammatory, diuretic, hypoglycemic and anti-hepatitis virus effects, etc., which is an iridoid glucoside compound, mainly present in the rehmannia and other plants [14]. Zhang et al. suggested that the effects of catalpol on mitochondrial function could occur through inhibiting NOS activity [15]. Taken together, Zhang et al. indicated that catalpol could possess therapeutic potential against lipopolysaccharide -induced acute systemic inflammation by attenuating NF-kappaB activation [16]. However, the role of catalpol on renal IRI disease is not clear. For this purpose, we investigated whether catalpol possesses the essential activity on renal ischemia/reperfusion injury as an anti-inflammatory medicine has not been investigated. We further elucidated that the anti-inflammatory activity of catalpol and the underlying mechanism involved.
Materials and methods
Drug and reagents
Catalpol (with a purity > 96%) was purchased from Sigma (Shanghai, China). BCA Protein Assay Kit was purchased from Sangon Biotech (Shanghai, China). Trizol reagent was purchased from Invitrogen (Chicago, USA). PrimeScript RT Master Mix and ABI 7500 system were purchased from Takara (Japan). Urea Nitrogen Diacetylmonoxime Test Kit and Creatinine LiquiColor Test (Kinetic) were purchase from Tiangen (Beijing, China). TNF-α, IL-1β, IL-6 and IL-10 ELISA kits were obtained from Tiangen (Beijing, China).
Ethics statement and animal preparation
This study was approved by the Animal Experiment Committee of Wuhan University. C57BL/6 mice were originally from Animal Lab of Wuhan University, Renmin Hospital. All C57BL/6 mice were carried out according to the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals. The mice (9 weeks old) were bred in the animal facility of Wuhan University with specific pathogen free conditions, and housed under 12 hours/12 hours dark and light cycle with free access to water and food. After 1 week of acclimation, the mice were used for renal ischemia/reperfusion surgery as described previously [15.1-17.1]. The mice were randomly allocated into five groups with each containing 8 mice: (1) control group (Con), which was normal rats that received physiological saline (i.p.); (2) ischemia/reperfusion-injury (IRI) group, in which the mice were subjected to renal ischemia for 1 h; (3-5) catalpol (Cat) group, in which the mice were administered (25, 50 and 100 mg/kg, i.p.) [17] catalpol 1 h prior to I/R induction.
Sample collection
When the mice were sacrificed, the whole blood was collected and kidney tissue was excised. Then, the blood was kept on ice and added with an equal volume of 9 mM EDTA in phosphate buffered saline. Meanwhile, kidney tissue was dissected on an ice plate to separate the cortical and medullar part. All samples were freshly frozen in liquid nitrogen for storing.
Assessment of renal function
The blood samples were collected and clotted in the room temperature. The samples were centrifuged at 12000 g for 5 min and then the supernatants were collected as serum. The blood urea nitrogen (BUN) level was examined with Urea Nitrogen Diacetylmonoxime Test Kit (Tiangen, Beijing, China) following the manual. The blood urea nitrogen (BUN) level was examined with Urea Nitrogen (BUN) Diacetylmonoxime Test Kit from Stanbio Laboratory (Tiangen, Beijing, China) following the manual.
Real-time PCR analysis of KIM-1 expression
Total RNA was isolated from renal tissues using Trizol according to the manufacturer’s instructions (Invitrogen, Chicago, USA). cDNA was reverse transcription reaction using PrimeScript RT Master Mix (Takara, Japan). Real-time PCR amplifications were carried out using the ABI 7500 system (Takara, Japan). All PCR primers were compound and purchased from biosune Biotech (Shanghai, China). KIM-1-F: 5’-TGGAGATTCCTGGATGGT-3’; KIM-1-R: 5’-GAGGTGGAGACTCTGGTTGA-3’; U6-F: 5’-CGCTTCGGCAG CACATATACTA-3’; and U6-R: 5’-CGCTTCACGAATTTGCGTGTCA.
Western blotting
Renal tissue samples were ground into a powder with liquid nitrogen and lysed in RIPA buffer. The amount of whole cell proteins was determined by BCA Protein Assay Kit (Sangon Biotech, Shanghai, China). Next, the proteins were electrophoresed with 10% polyacrylamide gel containing SDS and transferred into nitrocellulose membranes (Millipore Corporation, Bedford, MA, USA) at 4°C for 2-4 hours. The membrane was then blocked with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1-2 hour. The membrane was incubated with anti-PI3K (1:1000, BeastBio, Shanghai, China), anti-Akt (1:1500, BeastBio, Shanghai, China), anti-eNOS (1:500, BeastBio, Shanghai, China) and β-actin (1:500, BeastBio, Shanghai, China) overnight at 4°C with shaking. The membrane was washed with TBST and then incubated with IgG conjugates for 2 hours. Finally, membranes were washed with TBST and bands were observed by an enhanced ECL chemiluminescence system (Pierce, Rockford, USA).
Activities of TNF-α, IL-1β, IL-6 and IL-10
Activities of TNF-α, IL-1β, IL-6 and IL-10 were determined in samples using the commercially TNF-α, IL-1β, IL-6 and IL-10 ELISA kits. The measurement was performed step by step according to manufacturer’s protovarian cancerol (Tiangen, Beijing, China). Samples were analyzed spectrophotometrically at 450 nm of absorbance.
Statistical analysis
All results are analyzed with SPSS 17.0 software and expressed as mean ± SD. Statistical analysis was performed by using a one way Analysis of Variance. In all cases, P value < 0.05 was considered with statistical significance.
Results
Assessment of renal function
The chemical structure of catalpol was shown in Figure 1. We evaluated renal function by blood urea nitrogen levels and serum creatinine levels in serum samples collected. When compared with control mice, IRI caused a significant increase in the plasma levels of urea nitrogen and creatinine levels (Figure 2A, 2B), suggesting a significant degree of renal dysfunction. When compared with IRI mice, plasma levels of urea and creatinine were significantly reduced because of the treatment of catalpol (25, 50 and 100 mg/kg) for 12 hours, therefore, renal dysfunction was significantly cured in mice that were subjected to IRI (Figure 2A, 2B).
Figure 1.
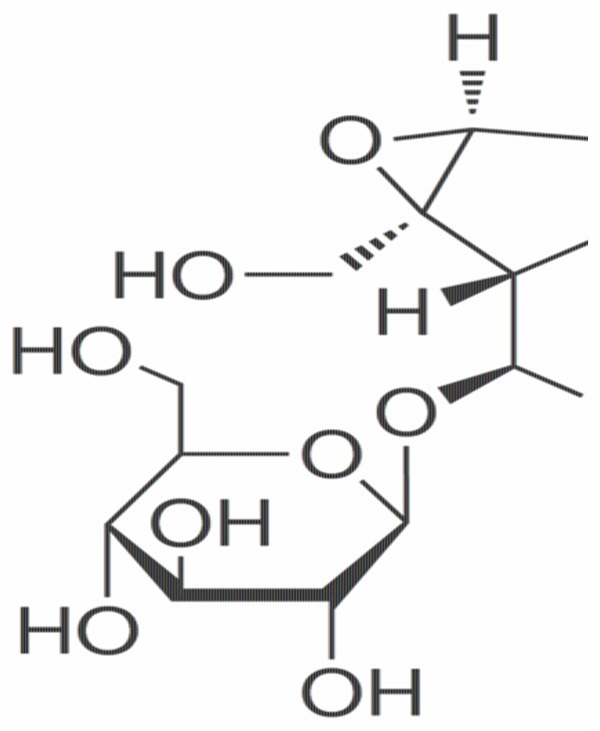
The chemical structure of catalpol.
Figure 2.
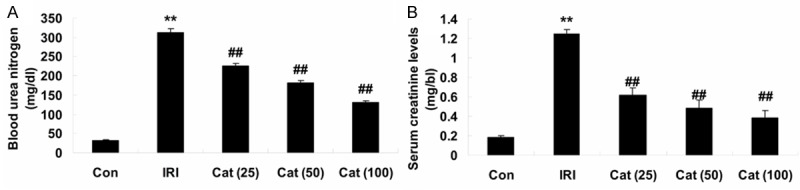
Assessment of renal function. Effect of catalpol on the Blood urea nitrogen (A) and serum creatinine (B). **P < 0.01 compared with Con group; ##P < 0.01 compared with IRI group. Con, control group; IRI, ischemia/reperfusion-injury group; Cat (25), catalpol (25 mg/kg)-treated group; Cat (50), catalpol (50 mg/kg)-treated group; Cat (100), catalpol (100 mg/kg)-treated group.
Real-time PCR analysis of KIM-1 expression
To expand these findings, we examined KIM-1 expression in all experiment mice. When compared with control mice, the significant elevation of KIM-1 expression was found in IRI mice (Figure 3). As expected, the expression level of KIM-1 was significantly lower than that of IRI mice, after the treatment of catalpol (25, 50 and 100 mg/kg) for 12 hours (Figure 3).
Figure 3.
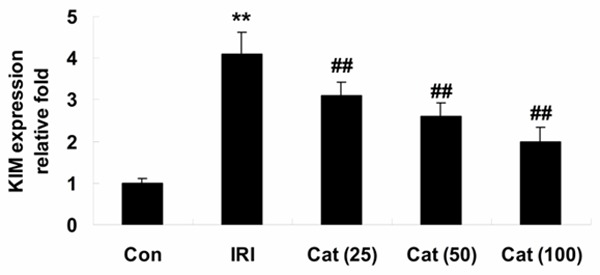
Real-time PCR analysis of KIM-1 expression. **P < 0.01 compared with Con group; ##P < 0.01 compared with IRI group. Con, control group; IRI, ischemia/reperfusion-injury group; Cat (25), catalpol (25 mg/kg)-treated group; Cat (50), catalpol (50 mg/kg)-treated group; Cat (100), catalpol (100 mg/kg)-treated group.
Western blot assays for PI3K, Akt and eNOS
To further investigate the above findings, we detected the expressions of PI3K, Akt and eNOS protein in all experiment mice. When compared with control mice, the expression of PI3K and Akt of kidneys that were significantly reduced in renal IRI mice (Figure 4A-C). In contrast, the expressions of PI3K and Akt were demonstrated a marked augment in Cat-treatment group, compared to that of renal IRI group (Figure 4A-C). Meanwhile, when compared with control mice, the expression of eNOS was significantly decreased in renal IRI mice (Figure 4A, 4D). On the contrary, the expression of eNOS was demonstrated a marked reduction in Cat-treatment group, compared to that of renal IRI group (Figure 4A, 4D).
Figure 4.
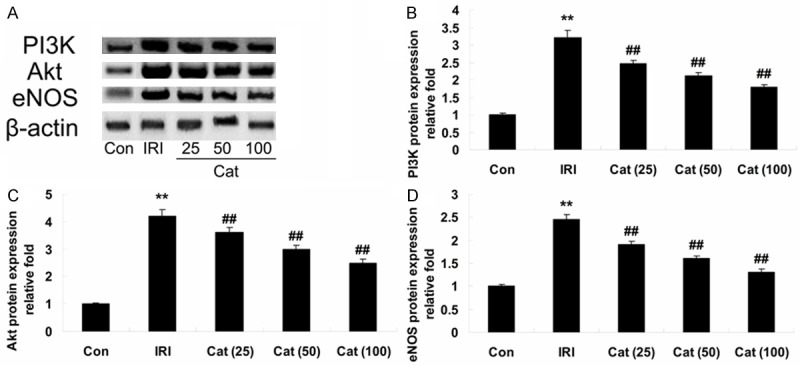
Western blot assays for PI3K, Akt and eNOS. Indicated representative western blotting analysis of PI3K, Akt and eNOS protein levels (A), statistical analysis of PI3K, Akt and eNOS protein levels (B-D), respectively, in different groups. **P < 0.01 compared with Con group; ##P < 0.01 compared with IRI group. Con, control group; IRI, ischemia/reperfusion-injury group; Cat (25), catalpol (25 mg/kg)-treated group; Cat (50), catalpol (50 mg/kg)-treated group; Cat (100), catalpol (100 mg/kg)-treated group.
Activities of inflammatory cytokines
To better understand the impact of inflammatory cytokines during renal IRI, we examined the activities of TNF-α, IL-1β, IL-6 and IL-10 in the renal tissues. When compared with control mice, the activities of TNF-α, IL-1β, IL-6 and IL-10 were significantly augmented in renal IRI mice (Figure 5A-D). In some areas, we found the activities of TNF-α, IL-1β, IL-6 and IL-10 were significantly reduce on account of the treatment of catalpol (25, 50 and 100 mg/kg) for 12 hours (Figure 5A-D).
Figure 5.
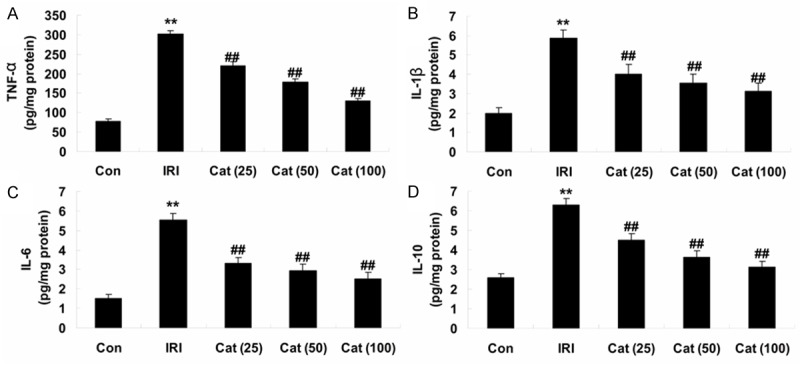
Alterations in the activities of inflammatory cytokines. Indicated the activities of TNF-α, IL-1β, IL-6 and IL-10 (A-D) respectively, in different groups. **P < 0.01 compared with Con group; ##P < 0.01 compared with IRI group. Con, control group; IRI, ischemia/reperfusion-injury group; Cat (25), catalpol (25 mg/kg)-treated group; Cat (50), catalpol (50 mg/kg)-treated group; Cat (100), catalpol (100 mg/kg)-treated group.
Discussion
Renal IRI refers to the tissue or organ damage that the tissue or organs regain the blood perfusion after ischemia. Kidney belongs to the high blood perfusion organs, very sensitive to ischemia and reperfusion, which is prone to be damaged. Our results provided the treatment of catalpol could significantly reduce the serum of urea and creatinine levels in serum IRI mice. Dong et al. reported that catalpol could significantly reduce the levels of 24 h urinary protein excretion, serum creatinine, blood urea nitrogen on diabetic nephropathy in rats [18]. Meanwhile, Sohotnik et al explained that urinary excretion of NGAL and KIM-1 substantially increased following I/R insult [19]. In this study, we were the first to show that catalpol could significantly reduce the expression of KIM-1 levels in renal IRI mice.
PI3K/Akt signal pathway participates in the process that endothelial progenitor cells are differentiated into endothelial cells. High-density lipoprotein can promote progenitor cell differentiation into endothelial-like cells by activating PI3K/Akt signaling pathway; HMG-CoA reductase inhibitor and vascular endothelial growth factor can promote the differentiation of progenitor cells by activating NOS by PI3K/Akt signaling pathway [20-22]. These studies indicate that PI3K/Akt is an important way to promote the proliferation and differentiation of progenitor cells.
NO promotes proliferation, migration and tube formation of endothelial cells, which is a key indicator that impacts endothelial progenitor cell function. NOS/NO is an important way to promote proliferation, adhesion, migration and in vitro angiogenesis of progenitor cells. NOS has three types: type I nNOS, type II iNOS and type III eNOS, which is widely present in endothelial cells. Due to the close relationship between endothelial progenitor cells and endothelial cells, it can be speculated that eNOS/NO may be an important way to promote proliferation, adhesion, migration and in vitro angiogenesis of progenitor cells. eNOS is a key enzyme in the regulation of endothelial-derived NO production, regulated by PI3K/Akt signaling pathway, thus affecting progenitor cells by PI3K/Akt/eNOS signaling pathways, and thereby treating renal IR-injury. Additionally, we found that catalpol markedly reduced the expression of PI3K, Akt and eNOS levels in renal IRI group. Hu et al. reported that catalpol inhibits apoptosis of endothelium through the PI3K/Akt signaling pathway [23]. Zhang et al. suggest that catalpol significantly decreased reactive oxygen species production and NOS activities in mice [15].
Some reports have evaluated propofol could significantly reduce IL-6, IL-8, TNF-α levels in renal IRI [24]. Nitrosothiols protected on the early inflammatory (IL-6 and IL-10) response to kidney IRI rats [25]. In this study, we found that catalpol significantly reduced the activities of TNF-α, IL-1β, IL-6 and IL-10 in renal IRI mice. Catalpol protected lipopolysaccharide -induced acute lung injury through down-regulated of TNF-α, IL-6, IL-4 and IL-1β [26]. Xiao et al. reported that catalpol ameliorates sodium taurocholate-induced acute pancreatitis of reduction of interleukin (IL)-1β, IL-6 and TNF-α levels in rats [27].
In summary, the results of the present study provide strong evidence that the treatment of catalpol attenuated renal IRI in mice and the protective mechanism was associated, at least in part, with suppressing PI3K/Akt-eNOS signaling and inflammation. The results also suggest the possibility that the treatment of catalpol is a potential therapeutic strategy for the prevention and therapy of renal IRI and the associated with PI3K/Akt-eNOS and inflammation.
Acknowledgements
These works were supported by the Grants from the National Science Foundation of China (81100519 to J.Z).
Disclosure of conflict of interest
None.
References
- 1.Gill N, Nally JV Jr, Fatica RA. Renal failure secondary to acute tubular necrosis: epidemiology, diagnosis, and management. Chest. 2005;128:2847–2863. doi: 10.1378/chest.128.4.2847. [DOI] [PubMed] [Google Scholar]
- 2.Rouschop KM, Roelofs JJ, Claessen N, da Costa Martins P, Zwaginga JJ, Pals ST, Weening JJ, Florquin S. Protection against renal ischemia reperfusion injury by CD44 disruption. J Am Soc Nephrol. 2005;16:2034–2043. doi: 10.1681/ASN.2005010054. [DOI] [PubMed] [Google Scholar]
- 3.Cai Y, Xu H, Yan J, Zhang L, Lu Y. Molecular targets and mechanism of action of dexmedetomidine in treatment of ischemia/reperfusion injury. Mol Med Rep. 2014;9:1542–1550. doi: 10.3892/mmr.2014.2034. [DOI] [PubMed] [Google Scholar]
- 4.Mejia-Vilet JM, Ramirez V, Cruz C, Uribe N, Gamba G, Bobadilla NA. Renal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactone. Am J Physiol Renal Physiol. 2007;293:F78–86. doi: 10.1152/ajprenal.00077.2007. [DOI] [PubMed] [Google Scholar]
- 5.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 6.Kinaci MK, Erkasap N, Kucuk A, Koken T, Tosun M. Effects of quercetin on apoptosis, NF-kappaB and NOS gene expression in renal ischemia/reperfusion injury. Exp Ther Med. 2012;3:249–254. doi: 10.3892/etm.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Yao Y, Xiao F, Lan X, Yu C, Zhang Y, Jiang C, Yang J, Pei G, Li Y, Rong S, Hu S, Li J, Xu G. Administration of dexamethasone protects mice against ischemia/reperfusion induced renal injury by suppressing PI3K/AKT signaling. Int J Clin Exp Pathol. 2013;6:2366–2375. [PMC free article] [PubMed] [Google Scholar]
- 8.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 9.Kelly KJ. Acute renal failure: much more than a kidney disease. Semin Nephrol. 2006;26:105–113. doi: 10.1016/j.semnephrol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Lu CY, Hartono J, Senitko M, Chen J. The inflammatory response to ischemic acute kidney injury: a result of the ‘right stuff’ in the ‘wrong place’? Curr Opin Nephrol Hypertens. 2007;16:83–89. doi: 10.1097/MNH.0b013e3280403c4e. [DOI] [PubMed] [Google Scholar]
- 11.Li YW, Zhang Y, Zhang L, Li X, Yu JB, Zhang HT, Tan BB, Jiang LH, Wang YX, Liang Y, Zhang XS, Wang WS, Liu HG. Protective effect of tea polyphenols on renal ischemia/reperfusion injury via suppressing the activation of TLR4/NF-kappaB p65 signal pathway. Gene. 2014;542:46–51. doi: 10.1016/j.gene.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Qin Z, Lv E, Zhan L, Xing X, Jiang J, Zhang M. Intravenous pretreatment with emulsified isoflurane preconditioning protects kidneys against ischemia/reperfusion injury in rats. BMC Anesthesiol. 2014;14:28. doi: 10.1186/1471-2253-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cong G, Cui L, Zang M, Hao L. Attenuation of renal ischemia/reperfusion injury by a polysaccharide from the roots of Dipsacus asperoides. Int J Biol Macromol. 2013;56:14–19. doi: 10.1016/j.ijbiomac.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Liu YR, Lei RY, Wang CE, Zhang BA, Lu H, Zhu HC, Zhang GB. Effects of catalpol on ATPase and amino acids in gerbils with cerebral ischemia/reperfusion injury. Neurol Sci. 2014;35:1229–1233. doi: 10.1007/s10072-014-1687-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Liu W, Niu X, An L. Systemic administration of catalpol prevents D-galactose induced mitochondrial dysfunction in mice. Neurosci Lett. 2010;473:224–228. doi: 10.1016/j.neulet.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 16.Zhang A, Hao S, Bi J, Bao Y, Zhang X, An L, Jiang B. Effects of catalpol on mitochondrial function and working memory in mice after lipopolysaccharide-induced acute systemic inflammation. Exp Toxicol Pathol. 2009;61:461–469. doi: 10.1016/j.etp.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Chen Z, Xu F, Zhu C, Fang F, Shu S, Li M, Ling C. Radio-protective effect of catalpol in cultured cells and mice. J Radiat Res. 2013;54:76–82. doi: 10.1093/jrr/rrs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Z, Chen CX. Effect of catalpol on diabetic nephropathy in rats. Phytomedicine. 2013;20:1023–1029. doi: 10.1016/j.phymed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Sohotnik R, Nativ O, Abbasi A, Awad H, Frajewicki V, Bishara B, Sukhotnik I, Armaly Z, Aronson D, Heyman SN, Nativ O, Abassi Z. Phosphodiesterase-5 inhibition attenuates early renal ischemia-reperfusion-induced acute kidney injury: assessment by quantitative measurement of urinary NGAL and KIM-1. Am J Physiol Renal Physiol. 2013;304:F1099–1104. doi: 10.1152/ajprenal.00649.2012. [DOI] [PubMed] [Google Scholar]
- 20.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int. 2005;67:1672–1676. doi: 10.1111/j.1523-1755.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 22.Besler C, Doerries C, Giannotti G, Luscher TF, Landmesser U. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther. 2008;6:1071–1082. doi: 10.1586/14779072.6.8.1071. [DOI] [PubMed] [Google Scholar]
- 23.Hu L, Sun Y, Hu J. Catalpol inhibits apoptosis in hydrogen peroxide-induced endothelium by activating the PI3K/Akt signaling pathway and modulating expression of Bcl-2 and Bax. Eur J Pharmacol. 2010;628:155–163. doi: 10.1016/j.ejphar.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Chou WP, Pei L. Effects of propofol on renal ischemia/reperfusion injury in rats. Exp Ther Med. 2013;6:1177–1183. doi: 10.3892/etm.2013.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Criado FJ, Rodriguez-Barca P, Garcia-Cenador MB, Rivas-Elena JV, Grande MT, Lopez-Marcos JF, Mourelle M, Lopez-Novoa JM. Protective effect of new nitrosothiols on the early inflammatory response to kidney ischemia/reperfusion and transplantation in rats. J Interferon Cytokine Res. 2009;29:441–450. doi: 10.1089/jir.2008.0100. [DOI] [PubMed] [Google Scholar]
- 26.Fu K, Piao T, Wang M, Zhang J, Jiang J, Wang X, Liu H. Protective effect of catalpol on lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2014;23:400–6. doi: 10.1016/j.intimp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Xiao WQ, Yin GJ, Fan YT, Qiu L, Cang XF, Yu G, Hu YL, Xing M, Wu de Q, Wang XP, Hu GY, Wan R. Catalpol ameliorates sodium taurocholate-induced acute pancreatitis in rats via inhibiting activation of nuclear factor kappa B. Int J Mol Sci. 2014;15:11957–11972. doi: 10.3390/ijms150711957. [DOI] [PMC free article] [PubMed] [Google Scholar]


