Abstract
Background: Primary open-angle glaucoma (POAG) is the most common form of glaucoma with a genetic predisposition. The relationship between polymorphisms in MYOC or APOE promoter region and POAG has been addressed in many case-control studies, but the published results were not consistent. Methods: A meta-analysis assessing the association between five single nucleotide polymorphisms (SNPs) (in MYOC promoter: rs12035719 and rs2075648; in APOE promoter: rs405509, rs769446 and rs449647) and the risk of POAG was performed based on included studies from literature research. In fixed effect model or random effect model, the Mantel-Haenszel (M-H) pooled odds ratios (ORs) and 95% confidence intervals (95% CIs) were used to evaluate the genetic association. Stratification analysis was also conducted to test the association within Asian or Caucasian populations. Results: Twenty five case-control studies within multiple populations were identified and no publish bias was observed. Significant association was detected between POAG risk and MYOC rs2075648 in Caucasian (GA+AA vs. GG, OR=0.587, 95% CI=0.437-0.788, P < 0.001). For other SNPs and in other ethnic populations, no statistic evidence was detected for significant association between them and the development of POAG. Conclusions: This meta-analysis suggested a genetic association between one of MYOC polymorphism (rs2075648) and the risk of POAG only in Caucasian population. The significant heterogeneity for this locus might imply the different POAG genetic basis among different populations.
Keywords: APOE, glaucoma, meta-analysis, MYOC, polymorphism
Introduction
Glaucoma is a group of optic diseases characterized by optic disc cupping and loss of visual caused by elevated intra-ocular pressure (IOP) and is predicted to involve 79.6 million patients by 2020 [1]. Primary open-angle glaucoma (POAG, OMIM137760) is a common form of glaucoma, which could be subdivided into chronic open-angle glaucoma (COAG) and juvenile open-angle glaucoma (JOAG). Family aggregation and high concordance in monozygotic twin pairs (98.0% in monozygotic twins pairs and 72.0% in twin/spouse pairs) implied strong genetic basis for POAG [2,3]. The first predisposing gene for POAG, MYOC (myocilin, also called trabecular meshwork-induced glucocorticoid response protein, TIGR) was identified in 1997 at the GLC1A locus [4], and latter identified POAG genes include OPTN [5] and WDR36 [6]. However currently only about 5% of POAG could be attributed to Mendelian form mutation, other POAG cases may have a complex genetic basis involving multiple minor-effect genes [7].
As the first recognized POAG gene, MYOC mutations cause most cases of autosomal dominant JOAG and lead to 4.6% of adult-onset POAG [8]. While some mutations in MYOC (such as GLN368STOP) have been detected in many association studies, the normal function of its encoding protein remains unclear [7,9]. In 2001, Colomb, E. et al. [10] found that a single nucleotide polymorphism (SNP) in MYOC promoter, -1000C/G (also designated as MYOC.mt1 or rs12035719), was associated with increased IOP and severity of damaged visual field in POAG. Subsequent studies (Table 1) further explored the association between this SNP and the development or clinical features of POAG, but their findings were inconsistent. In 2008, a systematic review and meta-analysis [11] evaluating the association between rs12035719 and the risk of POAG was published, with only four studies included. Another SNP in the promoter region of MYOC, -83G>A (rs2075648), was also analyzed in many POAG genetic association studies (Table 1). These case-control studies were limited in their sample size and there haven’t been meta-analysis performed to examine the relationship between POAG and MYOC rs2075648 to date.
Table 1.
Characteristics of the included studies
| Study | Population | Ethnicity | SNPa | POAGs:Controls | Genotyping method |
|---|---|---|---|---|---|
| Fingert, J. H. 1999b [24] | USA Australian and Canadian | Caucasian | rs2075648 | 1284:91 | SSCP |
| African American | Negroid | rs2075648 | 312:40 | SSCP | |
| Suzuki, R. 2000 [57] | Japan | Asian | rs2075648 | 30:36 | unspecified |
| Colomb, E. 2001 [10] | French | Caucasian | rs12035719 | 142:94 | ASO |
| Mabuchi, F. 2001 [58] | Japanese | Asian | rs2075648 | 119:100 | SSCP |
| Alward, W. L. 2002 [25] | USA | Caucasian | rs12035719 | 393:92 | SSCP and RFLP |
| Copin, B. 2002 [18] | French | Caucasian | rs405509, rs769446, rs449647 | 191:102 | unspecified |
| Hulsman, C. A. 2002 [59] | Dutch | Caucasian | rs2075648 | 40:94 | SSCP |
| Mukhopadhyay, A. 2002 [60] | Indian | Asian | rs2075648 | 56:51 | PCR-RFLP |
| Melki, R. 2003 [61] | French | Caucasian | rs2075648 | 237:108 | DHPLC |
| Fan, B. J. 2004 [36] | Chinese | Asian | rs2075648 | 88:94 | HTCSGE |
| rs12035719 | 212:221 | HTCSGE and RFLP | |||
| Fan, B. J.2005 [28] | Chinese | Asian | rs405509, rs769446, rs449647 | 400:281 | PCR-RFLP |
| rs2075648 | HTCSGE | ||||
| Ozgul, R. K. 2005 [62] | Turkish | Caucasian | rs12035719 | 88:123 | PCR-RFLP |
| Saura, M. 2005 [63] | Galician | Caucasian | rs2075648 | 79:109 | SSCP and RFLP |
| Bhattacharjee, A. 2007 [64] | Indian | Asian | rs2075648 | 315:100 | PCR-sequencing |
| Kumar, A. 2007 [65] | Indian | Asian | rs2075648 | 116:98 | PCR-SSCP |
| Lopez-Martinez, F. 2007 [66] | Spanish | Caucasian | rs2075648, rs12035719 | 110:98 | PCR-sequencing |
| Yen, Y. C. 2007 [67] | Taiwanese | Asian | rs12035719 | 48:100 | PCR-sequencing |
| Jia, L. Y. 2009 [38] | Chinese | Asian | rs2075648 | 175:200 | PCR-sequencing |
| rs405509, rs769446, rs449647 | TaqMan | ||||
| Sohn, S. 2010 [68] | Korean | Asian | rs2075648 | 60:74 | PCR-sequencing |
| Kasahara, N. 2011 [69] | Brazilian | Caucasian | rs12035719 | 167:130 | TaqMan |
| Whigham, B. T. 2011 [70] | Southern African | Negroid | rs2075648 | 113:131 | PCR-sequencing |
| Banerjee, D. 2012 [71] | Indian | Asian | rs2075648 | 250:100 | PCR-sequencing |
| Buentello-Volante, B. 2013 [72] | Mexican | Caucasian | rs12035719 | 118:100 | PCR-sequencing |
| Nowak, A. 2013 [72] | Polish | Caucasian | rs449647 | 183:209 | PCR-RFLP |
| Saglar, E. 2014 [73] | Turkish | Caucasian | rs405509 | 75:122 | PCR-RFLP |
PCR: polymerase chain reaction; RFLP: restricted fragment length polymorphism; SSCP: single strand conformation polymorphism; ASO: allele-specific oligonucleotide; DHPLC: denaturing high performance liquid chromatography; HTCSGE: high throughput conformation sensitive gel electrophoresis; POAG: primary open-angle glaucoma.
Here we only listed our concerned SNPs in the included studies, which might discussed SNPs other than the five SNPs we focused.
The association study in this literature was subdivided into two dependent studies (in Caucasian and in Negroid) given the ethnic heterogeneity of its samples.
APOE encodes the major apolipoprotein in central nerve system and its polymorphism plays a role in Alzheimer’s disease (AD) and coronary heart disease [12,13]. The optic nerve injury in POAG has some similarity with the involved neuron in AD [14] and the association studies between APOE polymorphisms and AD has proved evidence for genetic predisposition [15], suggesting a potential role of APOE in the development of POAG. The association between APOE polymorphisms and POAG has been discussed in a number of case-control studies, but the results remained controversial [16,17]. In 2002, Copin, B. et al. found that the APOE promoter SNPs, which were valued in AD, also modified the POAG phenotype and might have an interaction with a SNP (rs12035719) in the MYOC promoter [18]. The subsequent studies (listed in Table 1) focused on the relation between three APOE promoter SNPs, namely -219T>G (rs405509), -427T/C (rs769446) and -491A>T (rs449647), and POAG, but their conclusions were not in agreement.
To give a relatively generalized and precise estimation of the association between the genetic polymorphisms in MYOC promoter and APOE promoter with the risk of POAG, we performed this literature based meta-analysis, which include five SNPs: rs2075648, rs12035719, rs405509, rs769446 and rs449647.
Methods
Search strategy
We conducted literature searches in PubMed, Web of Science, and China Biological Medicine Database (CBMD) to indentify related published articles up to September 2014. The following terms and their possible combinations were used: MYOC (or myocilin, trabecular meshwork-induced glucocorticoid response protein, TIGR, GLC1A), APOE (or apolipoprotein E), promoter, SNP (or respective name of the five included SNPs: rs2075648, rs12035719, rs405509, rs769446, rs449647 or their traditional names) and primary open angle glaucoma (or POAG).
Inclusion and exclusion criteria
The included studies must meet the following criteria: (1), evaluation the associations between the promoter polymorphisms of MYOC or APOE and POAG; (2), original and independent case-control study; (3), containing sufficient data for extraction for meta-analysis; (4), if genotype data were provided, there should be no significant deviation from Hardy-Weinberg equilibrium (HWE) in χ2 test. Studies were excluded if they were only in vitro or in vivo mechanism studies or family linkage studies without population studies. Reviews or other studies based on earlier published data were also excluded. The literature language is restricted to English.
Data extraction
Data extraction was performed independently by 2 coauthors. Disagreements were resolved by discussion. The following information, in addition to published statistical data, was extracted: first author, publication year, population ethnicity, number of cases and controls, genotyping methods.
Statistical analysis
Heterogeneity among studies was assessed by Cochran’s chi-square test and by the I2 statistic: I2 < 25% implies slight heterogeneity while I2 > 50% implies notable heterogeneity [19]. In the test of heterogeneity, P < 0.10 indicating significant heterogeneity; otherwise, P < 0.05 was considered statistically significant. If no significant heterogeneity detected among studies, the fixed-effects model was used, which assumes that the variability is due to random variation; otherwise, the random-effects model is used [20]. Random-effect model could accommodate the possible different environment effect among studies. The pooled odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated by the Mantel-Haenszel (M-H) method [21]. Subgroup analyses were performed by ethnicity and other environmental effects were ignored considering the relatively great heterogeneity. Begg’s funnel plots and Egger’s tests were used to detect publication bias: the more symmetrical the funnel plot, the less probability of publication bias. Egger’s linear regression method was used to measure the asymmetry of Begg’s funnel plots: the larger the deviation of the intercept from zero, the more asymmetrical the funnel plot [22]. Statistical analyses involved were performed using the statistic software STATA version 12.0 (STATA Corporation, College Station, TX, USA).
Results
Characteristics of included studies
Among the 252 electronically or manually identified articles, 25 articles were eventually included in our meta-analysis. The POAG patients in the study by Alward, W. L. et al. [23] were included in the study by Fingert, J. H. et al. [24] and only the latter were included in this study; the study by Alward, W. L. et al. published in 2002 [25] also included some patients in previous studies [23,24], but it was included here for the evaluation of another SNP (rs12035719, Table 1). The 91 patients in the study by Lam, D. S. C. et al. [26] were included in the study by Pang, C. P. et al. [27], and the 187 POAG patients in the latter study were again included in the study by Fan, B. J. et al. [28]; another study by Lam, C. Y. and Fan, B. J. et al. published next year [29] also provided the same genotype data of 400 POAG patients in rs12035719. Among the four researches of overlapping populations, only Fan’s study published in 2005 [28] were included in this meta-analysis. The detailed process was displayed in Figure 1 and the characteristics of finally included studies were listed in Table 1.
Figure 1.
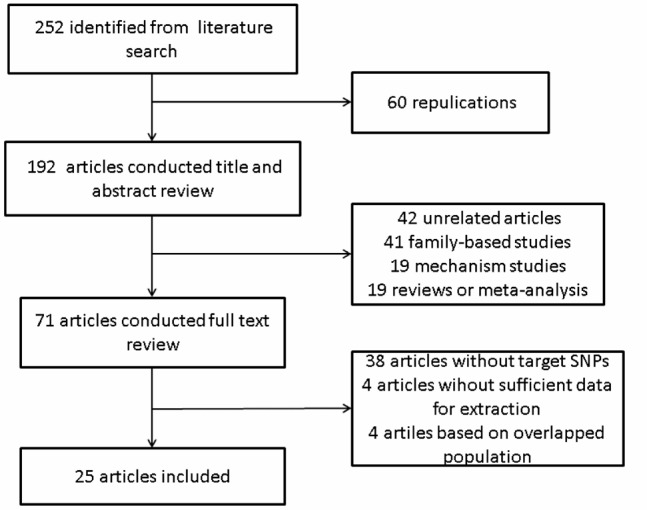
Flow chart of literature search and study selection.
Estimation of association
The main calculation results were shown in Table 2. We first assessed the publication bias of included studies for each SNP. The Begg’s funnel plots for rs12035719 and rs2075648 were quite symmetrical (Figure 4) and the p values of Begg’s test and Egger’s test were all larger than 0.1 (Table 2), which suggested no publication bias in any discussed SNP.
Table 2.
Results of meta-analysis for MYOC and APOE promoter polymorphisms and risk of POAG
| Polymorphism (comparison) | Population | No. of studies | Test of publish bias (p-value) | Test of association | Test of heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Egger’s test | Begg’s testa | OR (95% CI) | P-valueb | Effect Model | P-value | I 2 | |||
| rs2075648 (GA+AA vs. GG) | Overall | 17 | 0.554 | 0.592 | 0.849 (0.662-1.088) | 0.196 | random | 0.019 | 46.4% |
| Asian | 10 | 0.328 | 0.371 | 0.996 (0.744-1.333) | 0.978 | random | 0.059 | 45.1% | |
| Negroid | 2 | - | 1.000 | 2.118 (0.080-56.354) | 0.654 | random | 0.124 | 57.8% | |
| Caucasian | 5 | 0.309 | 0.806 | 0.587 (0.437-0.788) | 0.000 | fixed | 0.419 | 0.0% | |
| rs12035719 (C vs. G) | Overall | 8 | 0.777 | 0.536 | 1.017 (0.840-1.231) | 0.184 | fixed | 0.184 | 30.5% |
| Caucasian | 6 | 0.516 | 1.000 | 0.991 (0.775-1.269) | 0.946 | fixed | 0.140 | 39.8% | |
| Asian | 2 | - | 1.000 | 1.056 (0.781-1.429) | 0.772 | fixed | 0.189 | 42.0% | |
| rs405509 (G vs. T) | Overall | 4 | 0.384 | 1.000 | 1.022 (0.878-1.188) | 0.782 | fixed | 0.503 | 0.0% |
| Caucasian | 2 | - | 1.000 | 0.976 (0.750-1.270) | 0.856 | fixed | 0.614 | 0.0% | |
| Asian | 2 | - | 1.000 | 1.045 (0.869-1.257) | 0.642 | fixed | 0.166 | 48.0% | |
| rs769446 (C vs. T) | overall | 3 | 0.985 | 1.000 | 0.962 (0.658-1.408) | 0.842 | fixed | 0.392 | 0.0% |
| rs449647 (T vs. A) | overall | 4 | 0.442 | 0.308 | 1.257 (0.991-1.594) | 0.059 | fixed | 0.225 | 31.2% |
| Caucasian | 2 | - | 1.000 | 1.272 (0.970-1.668) | 0.081 | fixed | 0.143 | 53.5% | |
| Asian | 2 | - | 1.000 | 1.207 (0.735-1.982) | 0.458 | fixed | 0.140 | 54.1% | |
POAG: primary open-angle glaucoma.
Continuity-corrected P-value.
Significance test of OR=1.
Figure 4.
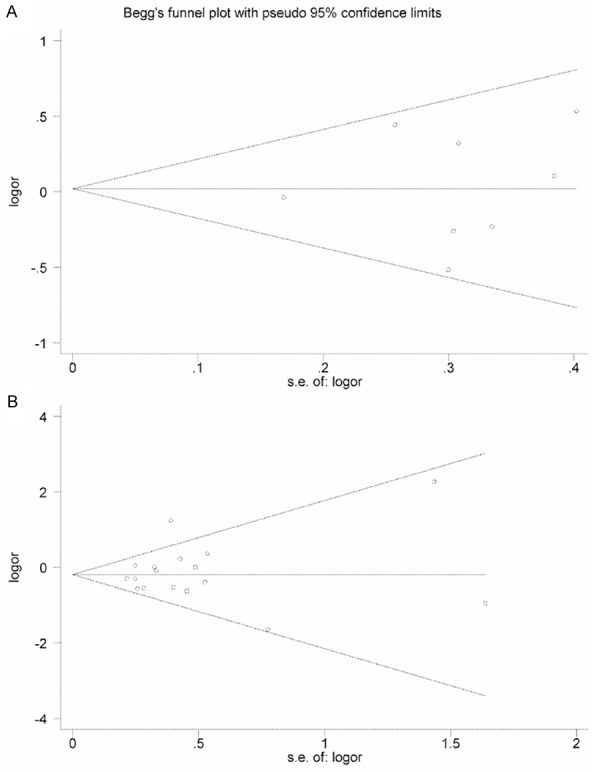
Begg’s funnel plots with pseudo 95% confidence limits. A: rs12035719, overall; B: rs2075648, overall. The circles represent separate studies and its size represents the sample size of corresponding study.
For rs12035719, 8 studies (1278 POAG patients and 958 controls in total) were involved without publication bias (Figure 4A). The association was not statistically significant in addictive genetic model (C vs. G pooled OR=1.017, P=0.184; Table 1 and Figure 2). After stratified by ethnicity, the association was still not statistically proved in either Caucasian (P=0.946) and Asian (P=0.772). The association was also not observed in dominant or recessive model (data not shown).
Figure 2.
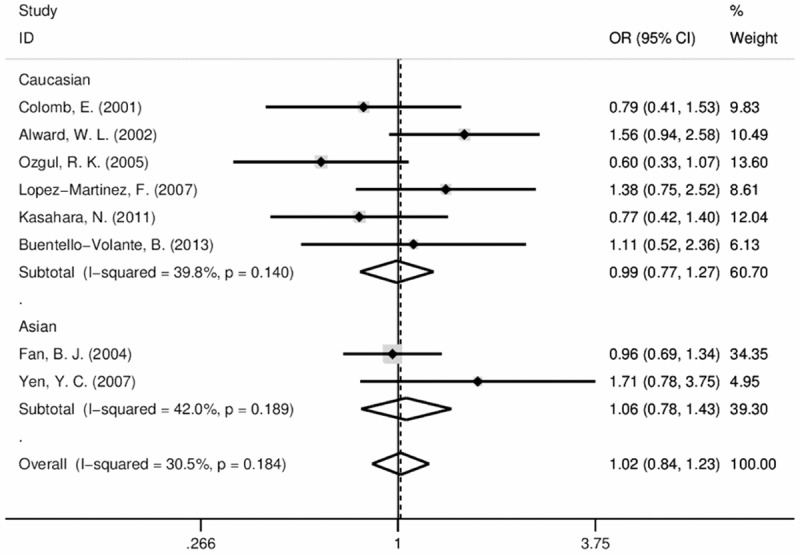
Forest plots of odds ratios (ORs) and 95% confidence intervals (95% CIs) of rs12035719 in MYOC promoter in the case-control studies. The size of the gray square represents the relative weight of each study.
For rs2075648, 17 studies (3719 patients and 1806 controls) were included for calculation. Since many of published literatures only provide the number of mutation without exact genotype data, the comparison were conducted only in dominant genetic model, namely GA+AA vs. GG in patients and controls. No publication bias was detected overall or in each ethnic subgroup (Figure 4B) but notable or moderate heterogeneity within studies exists except in Caucasian. The significant association was only found in Caucasian (OR=0.587, 95% CI: 0.437-0.788, P < 0.001; Table 1 and Figure 3). No association was found in Asian subgroups (OR=0.996, P=0.978) although data from ten studies were pooled.
Figure 3.
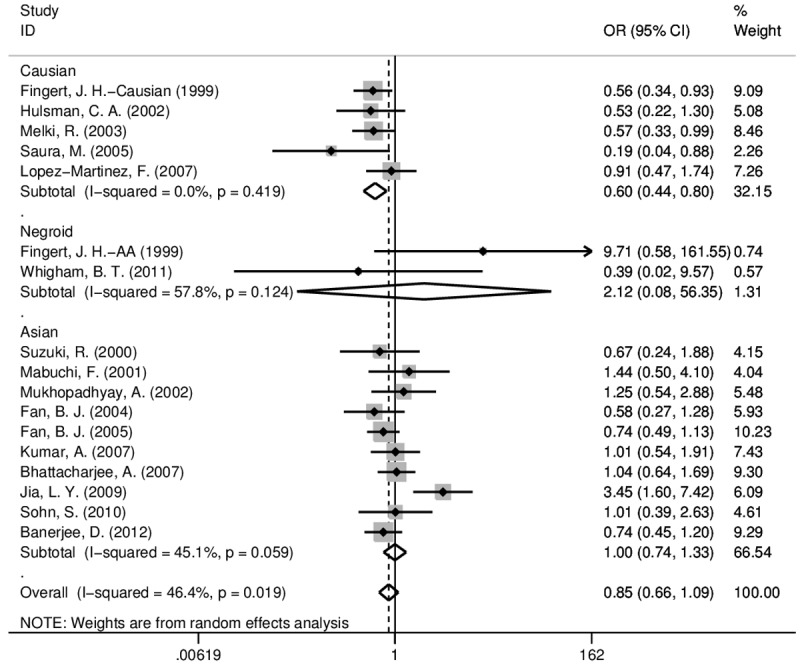
Forest plots of odds ratios (ORs) and 95% confidence intervals (95% CIs) of rs2075648 in MYOC promoter in the case-control studies. The size of the gray square represents the relative weight of each study. AA: African American.
For the three SNPs in the promoter region of APOE, rs405509 (four included studies, 842 patients and 705 controls), rs769446 (3 included studies, 767 patients and 583 controls) and rs449647 (four included studies, 950 patients and 792 controls), the meta-analysis results showed no evidence for significant association between any of them and the risk of POAG (rs405509 G vs. T OR=1.022 P=0.782; rs769446 C vs. T OR=0.962 P=0.842; rs449647 T vs. A OR=1.257 P=0.059; Table 2). The results calculated in dominant or recessive genetic model were similar with addictive genetics model (data not shown). The ethnic stratification meta-analysis still statistically suggested no significant association (Table 2).
Discussion
As the most common type of glaucoma, POAG is contributed by complex genetic factors [30]. In this meta-analysis, to assess the association between MYOC or APOE promoter variants and the risk of POAG in Asian and Caucasian populations, twenty five studies addressing one or some of two SNPs in MYOC promoter (rs2075648 and rs12035719) and three SNPs in APOE promoter (rs405509, rs769446 and rs449647) were included. While significant association was detected for rs2075648 in the Caucasian population, the results showed no evidence for other SNPs or in other populations.
MYOC is the first identified POAG gene and it encodes a functional unclear protein, myocilin, which is expressed in most tissues of the body and in almost every ocular tissue [31,32]. The mutations in MYOC coding region have already been showed strong relationship with the development of both JOAG and COAG. In 2012, a meta-analysis evaluated the associations between POAG risk and myocilin polymorphisms, which conducted calculations on five mutations (R46X, R76K, Y347Y, T353I, and Q368X) and found two of them (Q368X and T353I) significantly associated with POAG [33]. The expression of myocilin in trabecular meshwork was valued because it might be the cause for elevation of IOP in POAG by resisting aqueous humor outflow [9,34]. Immunohistochemical study showed an increased expression of myocilin in the trabecular meshwork of patients with COAG [34]. However, the expression is not altered in the blood of POAG patients, suggesting that the specific altered expression might contribute to POAG pathogenesis in related tissues [35].
The importance of MYOC transcription regulation in POAG was revealed by some studies [10,36] which evaluated the role of rs12035719 (MYOC.mt1) in MYOC promoter and obtained conflicting results. The minor allele (C) is more common in Asian than in European (20% vs. 10%, 1000 genomes project). In the former meta-analysis for rs12035719 including researches up to 2005 [11], the results suggested no significant association and the publication bias was found using Egger’s test. In this updated meta-analysis for rs12035719, the result were still not significant but no publication bias detected (C vs. G, in Egger’s test, intercept=1.615, 95%=-3.74-4.43, P=0.777; the results in other genetic models were similar).
Another MYOC promoter SNP rs2075648 (-83G>A) is located nearer to the transcription start site and involved in MYOC basal transcription. According to 1000 genomes project, the minor allele (A) frequency is 5% in Asian and 15% in European. Promoter study has proved that the SNP is within a canonical E-box sequence, which is responsible for the binding of transcription factors [37]. The rs2075648 is also in a linkage disequilibrium (LD) block and showed strong LD with an exon polymorphism c.227G>A [38]. Our meta-analysis showed significant heterogeneity within the effect size of overall 17 studies on this locus, but the heterogeneity was eliminated in Caucasian subgroup, suggesting the genetic heterogeneity in POAG between different ethnic populations. The M-H pooled OR in Caucasian is 0.587, which statistically suggests that rs2075648 mutation carriers Caucasian (GA or AA) have lower chance to develop POAG. Nevertheless, the heterogeneity remains significant and the pooled OR is not significantly different from 1 in Asian subgroup, which might be contributed to the complicated genetic backgrounds within the Asian cohorts. The heterogeneous genetic basis of POAG among geographic or ethnic populations has been suggested in many studies, for examples, many MYOC mutations were only observed in specific populations and founder effects might contribute to the heterogeneity [39].
The APOE encoding protein, apolipoprotein E, is mainly expressed in central nervous system and plays a role in the development of neurodegenerative diseases, such as AD. In fact, accumulating evidence suggested a profound relationship between AD and glaucoma [14,40,41]. Animal experiments have shown apolipoprotein E could be synthesized by Muller cells, the important glial cell of retina [42,43]. The three polymorphisms in APOE promoter region, rs405509 (-219G/T), rs769446 (-427T/C) and rs449647 (-491A/T), were first analyzed in 1998 by Artiga, M. J. et al. [44] and rs405509 and rs449647 were suggested influence on transcriptional activity. Rs405509 and rs449647 also showed significant associations with the development of AD [45]. In 2002, Copin, B. et al. reported that rs405509 and rs449647 had influence on the POAG clinical features and rs449647 might have an interaction with rs12035719 (MYOC.mt1) [18]. This report revealed the potential role of APOE and MYOC promoter SNP in POAG and firstly linked them together. In the 2005 study by Fan, B. J. et al., another two polymorphism interaction between APOE and MYOC (rs2075648 and APOE ε2/ε3/ε4; MYOC IVS2+35A>G and rs405509) was identified significant in high tension glaucoma patients [28]. This meta-analysis failed to provide any evidence for the association between APOE promoter SNPs and the risk of POAG. The relatively small numbers of the included studies for the three SNPs might influence the evaluation.
Until now, eleven GWAS has been conducted to explore the genetic basis of POAG [46-56]. To our knowledge, the five SNPs discussed here were not reported in any of them except rs2075648. The study by Wael Osman et al. performed in 2012 revealed that rs2075648 was not associated with POAG in a Japanese cohort (OR=0.99, 95% CI=0.79-1.24). Due to the lack of information relating to the detailed genotypes, we cannot include this study in this meta-analysis. However, the conclusion of this study was consistent with the Japanese study. Although significant association was detected between POAG risk and rs2075648 in Caucasian, we didn’t detect association between POAG and Asian populations.
Our study contains some limitations. In promoter region of MYOC, we only selected two SNPs in consideration of sufficient studies included. The ethnicity stratification is also limited because it ignores the different genetic backgrounds within the Caucasian and Asian cohorts. We could not further examine the associations between these SNPs and POAG clinical features, which are very meaningful, because of limited data to extract.
Conclusion
This meta-analysis assessed the association between five SNPs in MYOC or APOE promoter regions and the risk of POAG and firstly provide the statistic evidence for the association between rs2075648 (GA+AA vs. GG) and POAG in Caucasian population, which might imply mechanisms in POAG pathogenesis and molecular diagnosis values. This result should be interpreted with caution because of some limitations. Well-designed studies with more ethnic groups are required to further validate the results.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81100645) and Postdoctoral Science Foundation of China (No. 2013M530324).
Disclosure of conflict of interest
None.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol. 1994;112:69–73. doi: 10.1001/archopht.1994.01090130079022. [DOI] [PubMed] [Google Scholar]
- 3.Gottfredsdottir MS, Sverrisson T, Musch DC, Stefansson E. Chronic open-angle glaucoma and associated ophthalmic findings in monozygotic twins and their spouses in Iceland. J Glaucoma. 1999;8:134–139. [PubMed] [Google Scholar]
- 4.Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 5.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 6.Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Heon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005;14:725–733. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- 7.Fingert JH. Primary open-angle glaucoma genes. Eye (Lond) 2011;25:587–595. doi: 10.1038/eye.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alward WL. The genetics of open-angle glaucoma: the story of GLC1A and myocilin. Eye (Lond) 2000;14:429–436. doi: 10.1038/eye.2000.127. [DOI] [PubMed] [Google Scholar]
- 9.Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002;47:547–561. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- 10.Colomb E, Nguyen TD, Bechetoille A, Dascotte JC, Valtot F, Brezin AP, Berkani M, Copin B, Gomez L, Polansky JR, Garchon HJ. Association of a single nucleotide polymorphism in the TIGR/MYOCILIN gene promoter with the severity of primary open-angle glaucoma. Clin Genet. 2001;60:220–225. doi: 10.1034/j.1399-0004.2001.600308.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Zeng D, Zeng C, He X. Association between MYOC. mt1 promoter polymorphism and risk of primary open-angle glaucoma: a systematic review and meta-analysis. Med Sci Monit. 2008;14:RA87–93. [PubMed] [Google Scholar]
- 12.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 13.Zhang MD, Gu W, Qiao SB, Zhu EJ, Zhao QM, Lv SZ. Apolipoprotein E gene polymorphism and risk for coronary heart disease in the Chinese population: a meta-analysis of 61 studies including 6634 cases and 6393 controls. PLoS One. 2014;9:e95463. doi: 10.1371/journal.pone.0095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsolaki F, Gogaki E, Tiganita S, Skatharoudi C, Lopatatzidi C, Topouzis F, Tsolaki M. Alzheimer’s disease and primary open-angle glaucoma: is there a connection? Clin Ophthalmol. 2011;5:887–890. doi: 10.2147/OPTH.S22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun G, Naj AC, Beecham GW, Wang LS, Buros J, Gallins PJ, Buxbaum JD, Ertekin-Taner N, Fallin MD, Friedland R, Inzelberg R, Kramer P, Rogaeva E, St George-Hyslop P, Alzheimer’s Disease Genetics C, Cantwell LB, Dombroski BA, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Lunetta KL, Martin ER, Montine TJ, Goate AM, Blacker D, Tsuang DW, Beekly D, Cupples LA, Hakonarson H, Kukull W, Foroud TM, Haines J, Mayeux R, Farrer LA, Pericak-Vance MA, Schellenberg GD. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch Neurol. 2010;67:1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Zhou M, Huang W, Chen S, Zhang X. Lack of association of apolipoprotein E (Apo E) epsilon2/epsilon3/epsilon4 polymorphisms with primary open-angle glaucoma: a meta-analysis from 1916 cases and 1756 controls. PLoS One. 2013;8:e72644. doi: 10.1371/journal.pone.0072644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Q, Chen P, Liu Q. Role of the APOE epsilon2/epsilon3/epsilon4 polymorphism in the development of primary open-angle glaucoma: evidence from a comprehensive meta-analysis. PLoS One. 2013;8:e82347. doi: 10.1371/journal.pone.0082347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copin B, Brezin AP, Valtot F, Dascotte JC, Bechetoille A, Garchon HJ. Apolipoprotein E-promoter single-nucleotide polymorphisms affect the phenotype of primary open-angle glaucoma and demonstrate interaction with the myocilin gene. Am J Hum Genet. 2002;70:1575–1581. doi: 10.1086/340733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantel N. Chi-square tests with one degree of freedom; extensions of the Mantel-Haenszel procedure. J Ame Statist Associa. 1963;58:690–700. [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A) N Engl J Med. 1998;338:1022–1027. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- 24.Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 25.Alward WL, Kwon YH, Khanna CL, Johnson AT, Hayreh SS, Zimmerman MB, Narkiewicz J, Andorf JL, Moore PA, Fingert JH, Sheffield VC, Stone EM. Variations in the myocilin gene in patients with open-angle glaucoma. Arch Ophthalmol. 2002;120:1189–1197. doi: 10.1001/archopht.120.9.1189. [DOI] [PubMed] [Google Scholar]
- 26.Lam DSC, Leung YF, Chua JKH, Baum L, Fan DSP, Choy KW, Pang CP. Truncations in the TIGR gene in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1386–1391. [PubMed] [Google Scholar]
- 27.Pang CP, Leung YF, Fan B, Baum L, Tong WC, Lee WS, Chua JK, Fan DS, Liu Y, Lam DS. TIGR/MYOC gene sequence alterations in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2002;43:3231–3235. [PubMed] [Google Scholar]
- 28.Fan BJ, Wang DY, Fan DS, Tam PO, Lam DS, Tham CC, Lam CY, Lau TC, Pang CP. SNPs and interaction analyses of myocilin, optineurin, and apolipoprotein E in primary open angle glaucoma patients. Mol Vis. 2005;11:625–631. [PubMed] [Google Scholar]
- 29.Lam CY, Fan BJ, Wang DY, Tam PO, Yung Tham CC, Leung DY, Ping Fan DS, Chiu Lam DS, Pang CP. Association of apolipoprotein E polymorphisms with normal tension glaucoma in a Chinese population. J Glaucoma. 2006;15:218–222. doi: 10.1097/01.ijg.0000212217.19804.a7. [DOI] [PubMed] [Google Scholar]
- 30.Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res. 2009;88:837–844. doi: 10.1016/j.exer.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karali A, Russell P, Stefani FH, Tamm ER. Localization of myocilin/trabecular meshwork--inducible glucocorticoid response protein in the human eye. Invest Ophthalmol Vis Sci. 2000;41:729–740. [PubMed] [Google Scholar]
- 32.Fingert JH, Ying L, Swiderski RE, Nystuen AM, Arbour NC, Alward WL, Sheffield VC, Stone EM. Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Res. 1998;8:377–384. doi: 10.1101/gr.8.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng JW, Cheng SW, Ma XY, Cai JP, Li Y, Lu GC, Wei RL. Myocilin Polymorphisms and Primary Open-Angle Glaucoma: A Systematic Review and Meta-Analysis. PLoS One. 2012;7:e46632. doi: 10.1371/journal.pone.0046632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutjen-Drecoll E, May CA, Polansky JR, Johnson DH, Bloemendal H, Nguyen TD. Localization of the stress proteins alpha B-crystallin and trabecular meshwork inducible glucocorticoid response protein in normal and glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 1998;39:517–525. [PubMed] [Google Scholar]
- 35.Abu-Amero KK, Azad TA, Spaeth GL, Myers J, Katz LJ, Moster M, Bosley TM. Unaltered myocilin expression in the blood of primary open angle glaucoma patients. Mol Vis. 2012;18:1004–1009. [PMC free article] [PubMed] [Google Scholar]
- 36.Fan BJ, Leung YF, Pang CP, Fan DS, Wang DY, Tong WC, Tam PO, Chua JK, Lau TC, Lam DS. Polymorphisms in the myocilin promoter unrelated to the risk and severity of primary open-angle glaucoma. J Glaucoma. 2004;13:377–384. doi: 10.1097/01.ijg.0000133149.37063.84. [DOI] [PubMed] [Google Scholar]
- 37.Kirstein L, Cvekl A, Chauhan BK, Tamm ER. Regulation of human myocilin/TIGR gene transcription in trabecular meshwork cells and astrocytes: role of upstream stimulatory factor. Genes Cells. 2000;5:661–676. doi: 10.1046/j.1365-2443.2000.00355.x. [DOI] [PubMed] [Google Scholar]
- 38.Jia LY, Tam PO, Chiang SW, Ding N, Chen LJ, Yam GH, Pang CP, Wang NL. Multiple gene polymorphisms analysis revealed a different profile of genetic polymorphisms of primary open-angle glaucoma in northern Chinese. Mol Vis. 2009;15:89–98. [PMC free article] [PubMed] [Google Scholar]
- 39.Hewitt AW, Samples JR, Allingham RR, Jarvela I, Kitsos G, Krishnadas SR, Richards JE, Lichter PR, Petersen MB, Sundaresan P, Wiggs JL, Mackey DA, Wirtz MK. Investigation of founder effects for the Thr377Met Myocilin mutation in glaucoma families from differing ethnic backgrounds. Mol Vis. 2007;13:487–492. [PMC free article] [PubMed] [Google Scholar]
- 40.McKinnon SJ. Glaucoma: ocular Alzheimer’s disease? Front Biosci. 2003;8:s1140–1156. doi: 10.2741/1172. [DOI] [PubMed] [Google Scholar]
- 41.Wostyn P, Audenaert K, De Deyn PP. Alzheimer’s disease: cerebral glaucoma? Med Hypotheses. 2010;74:973–977. doi: 10.1016/j.mehy.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 42.Shanmugaratnam J, Berg E, Kimerer L, Johnson RJ, Amaratunga A, Schreiber BM, Fine RE. Retinal Muller glia secrete apolipoproteins E and J which are efficiently assembled into lipoprotein particles. Mole Brain Res. 1997;50:113–120. doi: 10.1016/s0169-328x(97)00176-9. [DOI] [PubMed] [Google Scholar]
- 43.Amaratunga A, Abraham CR, Edwards RB, Sandell JH, Schreiber BM, Fine RE. Apolipoprotein E is synthesized in the retina by Müller glial cells, secreted into the vitreous, and rapidly transported into the optic nerve by retinal ganglion cells. J Biol Chem. 1996;271:5628–5632. doi: 10.1074/jbc.271.10.5628. [DOI] [PubMed] [Google Scholar]
- 44.Artiga MJ, Bullido MJ, Sastre I, Recuero M, Garcia MA, Aldudo J, Vazquez J, Valdivieso F. Allelic polymorphisms in the transcriptional regulatory region of apolipoprotein E gene. FEBS Lett. 1998;421:105–108. doi: 10.1016/s0014-5793(97)01543-3. [DOI] [PubMed] [Google Scholar]
- 45.Xin XY, Ding JQ, Chen SD. Apolipoprotein E promoter polymorphisms and risk of Alzheimer’s disease: evidence from meta-analysis. J Alzheimers Dis. 2010;19:1283–1294. doi: 10.3233/JAD-2010-1329. [DOI] [PubMed] [Google Scholar]
- 46.Gharahkhani P, Burdon KP, Fogarty R, Sharma S, Hewitt AW, Martin S, Law MH, Cremin K, Bailey JN, Loomis SJ, Pasquale LR, Haines JL, Hauser MA, Viswanathan AC, McGuffin P, Topouzis F, Foster PJ, Graham SL, Casson RJ, Chehade M, White AJ, Zhou T, Souzeau E, Landers J, Fitzgerald JT, Klebe S, Ruddle JB, Goldberg I, Healey PR, Mills RA, Wang JJ, Montgomery GW, Martin NG, Radford-Smith G, Whiteman DC, Brown MA, Wiggs JL, Mackey DA, Mitchell P, MacGregor S, Craig JE. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat Genet. 2014;46:1120–1125. doi: 10.1038/ng.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osman W, Low SK, Takahashi A, Kubo M, Nakamura Y. A genome-wide association study in the Japanese population confirms 9p21 and 14q23 as susceptibility loci for primary open angle glaucoma. Hum Mol Genet. 2012;21:2836–2842. doi: 10.1093/hmg/dds103. [DOI] [PubMed] [Google Scholar]
- 48.Blue Mountains Eye Study (BMES); Wellcome Trust Case Control Consortium 2 (WTCCC2) Genome-wide association study of intraocular pressure identifies the GLCCI1/ICA1 region as a glaucoma susceptibility locus. Hum Mol Genet. 2013;22:4653–4660. doi: 10.1093/hmg/ddt293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibson J, Griffiths H, De Salvo G, Cole M, Jacob A, Macleod A, Yang Y, Menon G, Cree A, Ennis S, Lotery A. Genome-wide association study of primary open angle glaucoma risk and quantitative traits. Mol Vis. 2012;18:1083–1092. [PMC free article] [PubMed] [Google Scholar]
- 50.Nakano M, Ikeda Y, Tokuda Y, Fuwa M, Omi N, Ueno M, Imai K, Adachi H, Kageyama M, Mori K, Kinoshita S, Tashiro K. Common variants in CDKN2B-AS1 associated with optic-nerve vulnerability of glaucoma identified by genome-wide association studies in Japanese. PLoS One. 2012;7:e33389. doi: 10.1371/journal.pone.0033389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakano M, Ikeda Y, Taniguchi T, Yagi T, Fuwa M, Omi N, Tokuda Y, Tanaka M, Yoshii K, Kageyama M, Naruse S, Matsuda A, Mori K, Kinoshita S, Tashiro K. Three susceptible loci associated with primary open-angle glaucoma identified by genome-wide association study in a Japanese population. Proc Natl Acad Sci U S A. 2009;106:12838–12842. doi: 10.1073/pnas.0906397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheetz TE, Fingert JH, Wang K, Kuehn MH, Knudtson KL, Alward WL, Boldt HC, Russell SR, Folk JC, Casavant TL, Braun TA, Clark AF, Stone EM, Sheffield VC. A genome-wide association study for primary open angle glaucoma and macular degeneration reveals novel Loci. PLoS One. 2013;8:e58657. doi: 10.1371/journal.pone.0058657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiggs JL, Yaspan BL, Hauser MA, Kang JH, Allingham RR, Olson LM, Abdrabou W, Fan BJ, Wang DY, Brodeur W, Budenz DL, Caprioli J, Crenshaw A, Crooks K, Delbono E, Doheny KF, Friedman DS, Gaasterland D, Gaasterland T, Laurie C, Lee RK, Lichter PR, Loomis S, Liu Y, Medeiros FA, McCarty C, Mirel D, Moroi SE, Musch DC, Realini A, Rozsa FW, Schuman JS, Scott K, Singh K, Stein JD, Trager EH, Vanveldhuisen P, Vollrath D, Wollstein G, Yoneyama S, Zhang K, Weinreb RN, Ernst J, Kellis M, Masuda T, Zack D, Richards JE, Pericak-Vance M, Pasquale LR, Haines JL. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 2012;8:e1002654. doi: 10.1371/journal.pgen.1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tam PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U, Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42:906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hysi PG, Cheng CY, Springelkamp H, Macgregor S, Bailey JN, Wojciechowski R, Vitart V, Nag A, Hewitt AW, Hohn R, Venturini C, Mirshahi A, Ramdas WD, Thorleifsson G, Vithana E, Khor CC, Stefansson AB, Liao J, Haines JL, Amin N, Wang YX, Wild PS, Ozel AB, Li JZ, Fleck BW, Zeller T, Staffieri SE, Teo YY, Cuellar-Partida G, Luo X, Allingham RR, Richards JE, Senft A, Karssen LC, Zheng Y, Bellenguez C, Xu L, Iglesias AI, Wilson JF, Kang JH, van Leeuwen EM, Jonsson V, Thorsteinsdottir U, Despriet DD, Ennis S, Moroi SE, Martin NG, Jansonius NM, Yazar S, Tai ES, Amouyel P, Kirwan J, van Koolwijk LM, Hauser MA, Jonasson F, Leo P, Loomis SJ, Fogarty R, Rivadeneira F, Kearns L, Lackner KJ, de Jong PT, Simpson CL, Pennell CE, Oostra BA, Uitterlinden AG, Saw SM, Lotery AJ, Bailey-Wilson JE, Hofman A, Vingerling JR, Maubaret C, Pfeiffer N, Wolfs RC, Lemij HG, Young TL, Pasquale LR, Delcourt C, Spector TD, Klaver CC, Small KS, Burdon KP, Stefansson K, Wong TY, Viswanathan A, Mackey DA, Craig JE, Wiggs JL, van Duijn CM, Hammond CJ, Aung T. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet. 2014;46:1126–1130. doi: 10.1038/ng.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y, Lin Y, Vithana EN, Jia L, Zuo X, Wong TY, Chen LJ, Zhu X, Tam PO, Gong B, Qian S, Li Z, Liu X, Mani B, Luo Q, Guzman C, Leung CK, Li X, Cao W, Yang Q, Tham CC, Cheng Y, Zhang X, Wang N, Aung T, Khor CC, Pang CP, Sun X, Yang Z. Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat Genet. 2014;46:1115–1119. doi: 10.1038/ng.3078. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki R, Hattori Y, Okano K. Promoter mutations of myocilin gene in Japanese patients with open angle glaucoma including normal tension glaucoma. Br J Ophthalmol. 2000;84:1078. doi: 10.1136/bjo.84.9.1075c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mabuchi F, Yamagata Z, Kashiwagi K, Tang S, Iijima H, Tsukahara S. Analysis of myocilin gene mutations in Japanese patients with normal tension glaucoma and primary open-angle glaucoma. Clin Genet. 2001;59:263–268. doi: 10.1034/j.1399-0004.2001.590408.x. [DOI] [PubMed] [Google Scholar]
- 59.Hulsman CA, De Jong PT, Lettink M, Van Duijn CM, Hofman A, Bergen AA. Myocilin mutations in a population-based sample of cases with open-angle glaucoma: the Rotterdam Study. Graefes Arch Clin Exp Ophthalmol. 2002;240:468–474. doi: 10.1007/s00417-002-0455-1. [DOI] [PubMed] [Google Scholar]
- 60.Mukhopadhyay A, Acharya M, Mukherjee S, Ray J, Choudhury S, Khan M, Ray K. Mutations in MYOC gene of Indian primary open angle glaucoma patients. Mol Vis. 2002;8:442–448. [PubMed] [Google Scholar]
- 61.Melki R, Belmouden A, Brezin A, Garchon HJ. Myocilin analysis by DHPLC in French POAG patients: increased prevalence of Q368X mutation. Hum Mutat. 2003;22:179. doi: 10.1002/humu.9165. [DOI] [PubMed] [Google Scholar]
- 62.Ozgul RK, Bozkurt B, Orcan S, Bulur B, Bagiyeva S, Irkec M, Ogus A. Myocilin mt1 promoter polymorphism in Turkish patients with primary open angle glaucoma. Mol Vis. 2005;11:916–921. [PubMed] [Google Scholar]
- 63.Saura M, Cabana M, Ayuso C, Valverde D. Mutations including the promoter region of myocilin/TIGR gene. Eur J Hum Genet. 2005;13:384–387. doi: 10.1038/sj.ejhg.5201299. [DOI] [PubMed] [Google Scholar]
- 64.Bhattacharjee A, Acharya M, Mukhopadhyay A, Mookherjee S, Banerjee D, Bandopadhyay AK, Thakur SK, Sen A, Ray K. Myocilin variants in Indian patients with open-angle glaucoma. Arch Ophthalmol. 2007;125:823–829. doi: 10.1001/archopht.125.6.823. [DOI] [PubMed] [Google Scholar]
- 65.Kumar A, Basavaraj MG, Gupta SK, Qamar I, Ali AM, Bajaj V, Ramesh TK, Prakash DR, Shetty JS, Dorairaj SK. Role of CYP1B1, MYOC, OPTN, and OPTC genes in adult-onset primary open-angle glaucoma: predominance of CYP1B1 mutations in Indian patients. Mol Vis. 2007;13:667–676. [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez-Martinez F, Lopez-Garrido MP, Sanchez-Sanchez F, Campos-Mollo E, Coca-Prados M, Escribano J. Role of MYOC and OPTN sequence variations in Spanish patients with primary open-angle glaucoma. Mol Vis. 2007;13:862–872. [PMC free article] [PubMed] [Google Scholar]
- 67.Yen YC, Yang JJ, Chou MC, Li SY. Identification of mutations in the myocilin (MYOC) gene in Taiwanese patients with juvenile-onset open-angle glaucoma. Mol Vis. 2007;13:1627–1634. [PubMed] [Google Scholar]
- 68.Sohn S, Hur W, Choi YR, Chung YS, Ki CS, Kee C. Little evidence for association of the glaucoma gene MYOC with open-angle glaucoma. Br J Ophthalmol. 2010;94:639–642. doi: 10.1136/bjo.2009.158261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasahara N, Caixeta-Umbelino C, Paolera MD, Rocha MN, Richeti F, Vasconcellos JP, Cohen R, Costa VP, Longui CA, Melo MR, Melo MB. Myocilin mt. 1 gene promoter single nucleotide polymorphism (-1000C>G) in Brazilian patients with primary open angle glaucoma. Ophthalmic Genet. 2011;32:18–23. doi: 10.3109/13816810.2010.535887. [DOI] [PubMed] [Google Scholar]
- 70.Whigham BT, Williams SE, Liu Y, Rautenbach RM, Carmichael TR, Wheeler J, Ziskind A, Qin X, Schmidt S, Ramsay M, Hauser MA, Allingham RR. Myocilin mutations in black South Africans with POAG. Mol Vis. 2011;17:1064–1069. [PMC free article] [PubMed] [Google Scholar]
- 71.Banerjee D, Bhattacharjee A, Ponda A, Sen A, Ray K. Comprehensive analysis of myocilin variants in east Indian POAG patients. Mol Vis. 2012;18:1548–1557. [PMC free article] [PubMed] [Google Scholar]
- 72.Buentello-Volante B, Elizondo-Olascoaga C, Miranda-Duarte A, Guadarrama-Vallejo D, Cabral-Macias J, Zenteno JC. Association study of multiple gene polymorphisms with the risk of adult-onset primary open-angle glaucoma in a Mexican population. Exp Eye Res. 2013;107:59–64. doi: 10.1016/j.exer.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 73.Saglar E, Bozkurt B, Irkec M. Association of apolipoprotein E-219T>G promoter polymorphism with primary open angle glaucoma in Turkish population. Int J Ophthalmol. 2014;7:426–430. doi: 10.3980/j.issn.2222-3959.2014.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]


