Abstract
Objective: The effect of radiotherapy on moderate and severe Thyroid associated ophthalmopathy (TAO) was evaluated by various objective and quantitative indexes including T2 signal intensity ratios of orbital MRI inferior rectus and ipsilateral temporal muscle (T2SIR), extraocular muscles (EOM) volume, and the degree of exophthalmos using clinical research with prospective, randomized, double blind, self controlled. Methods: The patients with TAO who in the moderate and severe active period and had similar double eyes condition in the Outpatient Department of Ophthalmology of Xiangya No. 2 Hospital of Central South University from 2011.2 to 2014.2 were selected as objects in this study. The related body check was finished after the research group was built. For the object, one eye of patient having random radiotherapy was chosen as the experimental eye. The other eye in the same patient with pseudo radiotherapy (merely known by operator, doctors in department of ophthalmology and patients were double blind) was selected as the control eye. The radiotherapy plan was made by the operator according to the CT results. The T2 signal intensity ratios of orbital MRI inferior rectus and ipsilateral temporal muscle (T2SIR), extraocular muscles (EOM) volume, and the exophthalmos degree was compared by MRI check to evaluate the effect of radiotherapy. Results: The T2 signal intensity ratios of orbital MRI inferior rectus and ipsilateral temporal muscle (T2SIR), extraocular muscles (EOM) volume, and the exophthalmos degree between both eyes (experimental and control eyes) had significant differences and these data had statistical significance. Conclusions: The treatment of radiotherapy is effective for the TAO in the moderate and severe active period.
Keywords: Moderate and severe thyroid associated ophthalmopathy, radiotherapy, T2SIR, EOM volume, exophthalmos degree
Introduction
Thyroid associated ophthalmopathy (TAO), also called as Graves’ Orbitopathy (GO) and Thyroid Eye Disease (TED), is one common disease in ophthalmology. Generally, its morbidity is biggest in adult orbital disease, and its male female ratio is about 1:5.3 [1]. This disease is easy to be happed for female, but the clinical symptom is more serious for male than female. Overall, TAO is one organ specific autoimmune diseases that is related to thyroid function but relatively independent. In adult hyperthyroidism, it is more than 90% of patients have increase of the extraocular muscles or fat by the detection of CT or MRI, but merely 20-50% of patients have obvious clinical manifestation [2,3].
The nosogenesis of TAO is complex: the inflammatory response behind eyeballs would lead to the accumulation of hydrophilic glycosaminoglycans (GAGs) and/or the increase to fat tissue behind eyeballs, and then results in the increase of orbital connective tissue and extraocular muscles (EOM) volume. Overall, TAO is one disease with self chronic immune dysfunction and it is related to the combination of different factors such as environmental and genetic factors [2].
The disease course to TAO could divide into active period and stationary phase. Usually, the active period would least for 18-24 months and then the stationary phase would come. During active period, a great amount of lymphocyte and macrophage are full of orbital tissues [4,5], leasing various cellular factors such as interleukin 1 (IL-1), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ), and finaly leading accumuation of GAGs. During stationary phase, extraocular muscles fibrosis and atrophy would lead to the disorder of clinical function.
The treatment of TAO is one difficulty of orbital disease and it depends on the definition of TAO in active or stationary phase. A great number of researches showed that medical treatment is effective for the TAO in active period but not effective for the TAO in stationary phase. The evaluation of TAO course have been developed from the past decades [6-8] and Clinical Activity Score (CAS) was undoubtedly the classical one. Recently, European Group on Graves’ Orbitopathy (EUGOGO) divided the TAO courses into three periods, namely light phrase, moderate and severe phrase, and visual damage phrase [9]. This evaluation of TAO courses is beneficial for further treatment of TAO.
Base on the disadvantages of CAS, various imaging techniques such as type-A ultrasonic, type-B ultrasonic, computed tomography (CT), and magnetic resonance imaging (MRI) are used for auxiliary examination of TAO. However, all these methods have their advantages and disadvantages. Overall, MRI is the most suitable method to evaluate the course of TAO [10,11]. Among MRI techniques, T2 signal intensity ratio (T2SIR) is obtained from the ratio of signal strength of the extraocular muscles and ipsilateral temporal muscle with the base of T2 weighted imaging. Generally, it is one objective, quantitative index, and easy to be compared.
The history of radiotherapy on TAO is more than 90 years, but its efficiency still lacked of related evidences to prove. To date, the retrospective study on TAO could reflect the efficiency of radiotherapy by some evidences, but its results are unreliable due to lacking standard factors. Some random pseudo control experiment could reflect that the vertical eye range of radiotherapy group had improvement compared with control group [12,13]. Some random control experiment used TAO patients as research object, but up to now, merely one study used the eyeballs of the same one patient as random control experiment. Undoubtedly, this random control experiment could keep the eyes of experimental group and pseudo radiotherapy in the same hormone and immune environment. To our knowledge, no work has used randomized prospective trial to show that orbital radiotherapy has more effective on improvement of proptosis, palpebral fissure width or soft tissue such as eyelid edema than pseudo radiotherapy [14,15].
In spite of that radiotherapy has been clinically applied widely, its effect is still in argument. To give objective and correct evaluation on the effect of radiotherapy on TAO patients, in this research, the effect of radiotherapy on moderate and severe TAO was evaluated by various objective quantitative indexes including T2 signal intensity ratios of orbital MRI inferior rectus and ipsilateral temporal muscle (T2SIR), extraocular muscles (EOM) volume, and the exophthalmos degree using clinical research with prospective, randomized, double blind, self controlled. Specially, in this study, both eyes of patients were in moderate severe active period, and one eye was treated by radiotherapy and the other was treated by pseudo radiotherapy. Thus, both eyes were in close activity and severity, and in the same hormone and immune environment.
Methods and materials
Experiment design
The TAO patients whose eyes were in similar moderate and severe period, and disease course was less than one year, were selected as research objects in this study. In the experiment, one eye was treated by radiotherapy and the other was treated by pseudo radiotherapy (merely known by operator, and doctor and patient were double blind). The radiotherapy plan was made by the operator according to the CT results. The T2 signal intensity ratios of orbital MRI inferior rectus and ipsilateral temporal muscle (T2SIR), extraocular muscles (EOM) volume, and the exophthalmos degree was compared by MRI check to evaluate the effect of radiotherapy after one month.
Evaluation index before radiotherapy
Before radiotherapy, the patients’ disease course, both eyes’ CAS score, triiodothyronine (TT3), thyroxine (TT4), Thyroid stimulating hormone (TSH), MRI T2SI5 of both eyes, EOM volume of both eyes, exophthalmos degree of both eyes, were evaluated.
Subjects underwent orbital MRI position
All patients would have MRI check before entering the group and after one month of radiotherapy. The check was finished by Philips Achieva 3.0T magnetic resonance scanner with a standard head coil. The patient was supine, and this head is placed in the coil, where the longitudinal axis of coil was in consistent with the patient’s craniocerebral midsagittal. Then, the orbital coronal and axial scan were finished respectively.
Scanning sequence
(1) Fast spin echo T2 weighted imaging with fat suppression sequence: repetition time, 3000 ms, echo time 80 ms, imaging vision, 200 mm×157 mm×59 mm, slice thickness, 3 mm/2 mm (coronal/transverse), scanning interval, 0.3 mm/0.2 mm (coronal/transverse), scanning number, 18, signal (superposition) average number 1.
(2) Fast spin echo T1 weighted imaging: Repetition time, 2000 ms, echo time, 20 ms, reverse time, 800 ms, imaging vision, 140 mm×164 mm×39 mm, slice thickness 3 mm/2 mm (coronal/transverse), scanning interval of 0.3 mm/0.2 mm (coronal/transverse), scanning number 18, signal (superposition) average number 1.
Measurement of T2SIR
The signal intensity of the inferior rectus and the ipsilateral temporalis was measured base on T2 weighted imaging with fat suppression sequence, and the ratio of both is SIR (Figure 1).
Figure 1.
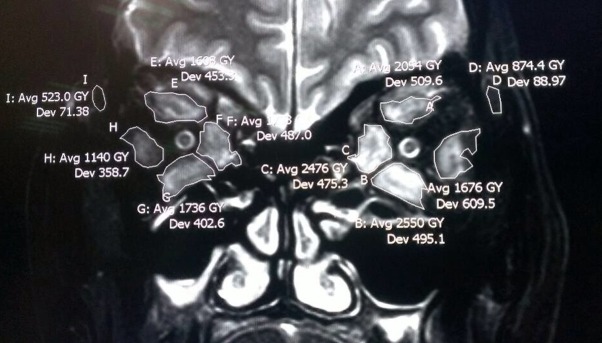
Signal strength of the extraocular muscles and ipsilateral temporal muscle.
Measurement of EOM volume
EOM three-dimensional image reconstruction was finished by using the marching cube method according to the magnetic resonance image data. And then the volume of four EOM was calculated (Figure 2).
Figure 2.
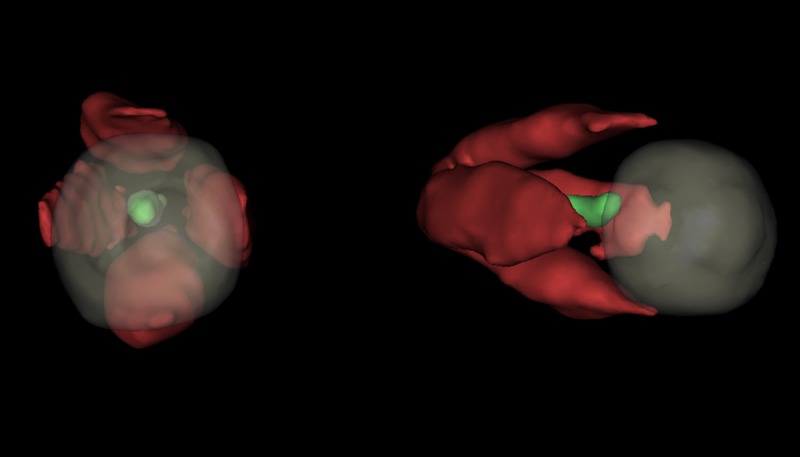
Reconstruction image of EOM three-dimensional image.
Measurement of the exophthalmos degree
In the T1WI section, the maximum level for eyeball and optic nerve showing was selected, and then one line was drawn between both sides of the front in the zygomatic arch. After that, the vertical distance from the corneal apex to bilateral zygomatic arch wires was measured and this value is the exophthalmos degree.
Method for radiotherapy
Fixing of patients and building of coordinate system
After loosing neck up, the patient’s neck and shoulder were fully exposed and he/she was lying in bed on the central axis of CT-Sim with standard. Head pillow was fixed on the bracket slot edge, and put under patient’s head, making the patient occipital natural fall in the headrest depression and horizontal position. And then, his/her head was fixed by hot plastic mesh memory head membrane. The laser light coronal was adjusted to get binocular lateral canthus, and the intersection of cross section of supraorbital cross and sagittal plane is point A, and its intersection with coronal section was left B and right C. One 3 cm×3 cm non-woven tape was affixed to the head membrane A, B, C three, noted “+” words with a pen. And dot markers were attached overlapping to the A, B, C three points, as the point of reference, providing the basis for the repeated positioning. Finally, the patient’s name, pillow type marker was recorded in the head film, for the further check for treatment.
CT image acquisition and set the scan range of orbital
The working conditions is voltage 130 kV, current 120 mA; the width of the window is 300 mm, windows 60 mm, slice thickness 3 mm, interval 3 mm, axial scanning, image via the network input radiotherapy treatment planning system (3D-TPS).
CT image reconstruction
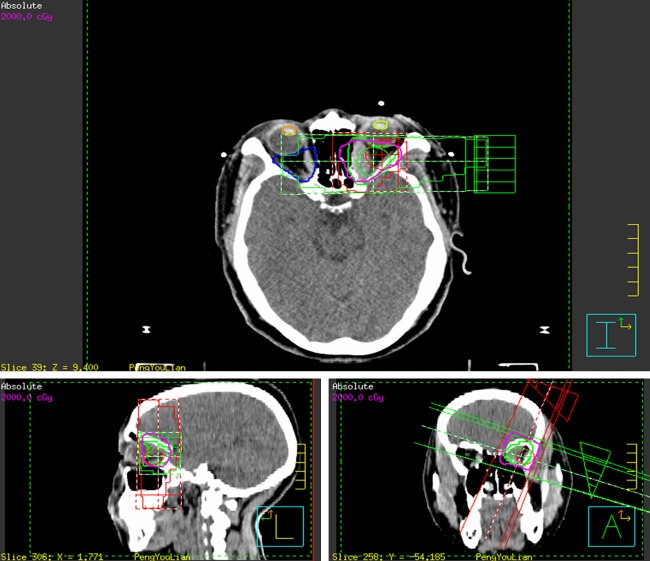
The lens of both eyes, clinical target volume (CTV) of experimental eyes, control eye retrobulbar tissue contour, and three-dimensional images of the clinical target volume and surrounding tissue was drawn by 3D-TPS with different colors (Figure 3).
Figure 3.
Three-dimensional reconstruction image of the left eye’s clinical target volume and its surrounding tissue.
Radiation field design
conformal irradiation with single field was applied and the planning target volume (PTV) was based on CTV with external 3 mm. At setting conditions (radiation field of bed rotation 70° and support 90° and radiation field of bed rotation 70° and support 90°) with field size 4 cm×4 cm and wedge plate adding, the radiation measurement (DT) was set with 6MV radiation source, 200 cGy every time, and once per day, 5 times per week. When the total dose was more than 95%, PTV dosage is 2000 cGy. The dose distribution curve and dose volume histogram (DVH) could be obtained by the calculation by setting a center beam using target area (Figure 4). Before radiotherapy, radiation treatment position was determined by the simulator, and compared with the anatomical target position obtained by 3D treatment planning, finally shooting for confirmation. The location verification was fulfilled by the real-time image verification system (iviewGT system) and the real-time image verification system in the course of treatment location verification, and when the error is greater than or equal to 3 mm, it is need to be repositioned. The results of DVH showed the dose distribution statistics, the total average dose on bilateral lens and the tissue behind the side ball throughout the treatment (Table 1), including the target volume with 100% dose (Table 2).
Figure 4.
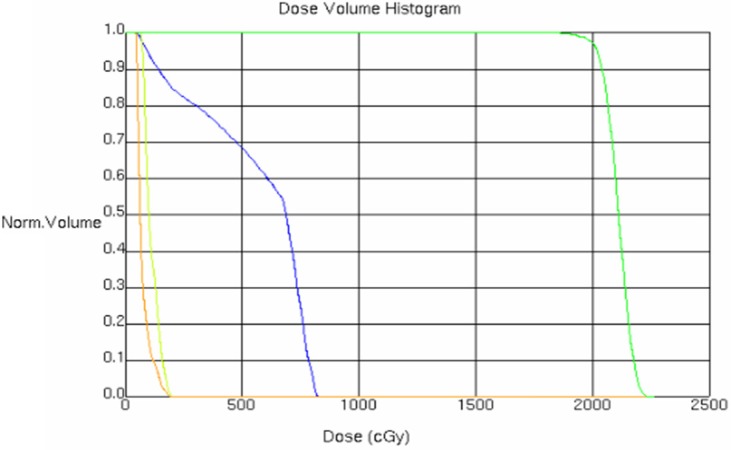
Histogram of dose distribution and dose-volume value. Yellow: the left eye lens; Orange: the right eye lens; Green: The target area of left eye; Blue: The side retrobulbar tissue of right eye.
Table 1.
Total average dose of binocular lens and the tissue of side retrobulbar tissue for the entire treatment (cGy)
| Left eye lens | Right eye lens | Tissue of side retrobulbar tissue | |
|---|---|---|---|
| Total average dose | 111.5 | 74.4 | 563.5 |
Table 2.
Target volume dose included by 100% dose
| Irradiation technique | Target volume of left eye |
|---|---|
| Single field conformal irradiation | >95% |
Special treatment
if the patient in treatment experienced type TAO visual injury, the current treatment should be terminated timely and the corticosteroid therapy should be applied (methylprednisolone 1000 mg intravenous drip and lasted for three days). If the corticosteroid therapy cannot improve the patient’s condition, orbital decompression should be carried out.
Evaluation index of experimental results
T2SIR
During the pathogenesis of TAO, the order of four rectus involvement was: inferior rectus, medial rectus, inferior rectus muscle, and lateral rectus. Because the inferior rectus muscle is the most commonly affected extraocular muscle signal intensity, its signal strength was chosen to compare with the signal strength of the ipsilateral temporalis. T2SI was obtained directly from the MRI image processor. T2SIR was the ratio of above two signals.
EOM volume
EOM three-dimensional reconstruction image was obtained by using marching cube method and base on the magnetic resonance image data.
Measurement of exophthalmos
On the T1WI cross section, the maximum level eyeball and nerve optic display were selected and one line was drawn on both sides of the front zygomatic arch. Then, the vertical distance from corneal apex to bilateral zygomatic arch wire was measured and this value was namely exophthalmos.
Statistical analysis
Data were analyzed using statistical software SPSS17.0. The comparison of T2SIR, EOM value, exophthalmos value for both experimental and control eyes before and after radiotherapy was finished by using t test (when P<0.05, the comparison had significant difference).
Results
In this work, totally 51 samples of patients (102 eyes) was included in the experiment and the information for patients is shown in Table 3. Among these patients, 33 ones had hyperthyroidism, 10 ones have eye symptoms with diagnosis of hyperthyroidism, and 2 ones had not been diagnosed of hyperthyroidism. To control hyperthyroidism, among 49 patients, 31 had taken anti thyroid drugs, 12 had 131I to treat the disease, while 6 had thyroid resection operation.
Table 3.
Normal information of patients in the experimental groups
| Male (n=15) | Female (n=36) | |
|---|---|---|
| Number of patients | 15 | 36 |
| Age (years old) | 51.13±9.58 | 51.78±8.87 |
| Course of disease (months) | 6.20±2.93 | 6.25±2.62 |
| CAS (pair) | 5±1.13 | 4.80±1.17 |
| TT3 (ng/ml) | 1.02±0.39 | 1.11±0.38 |
| TT4 (μg/dl) | 7.68±2.36 | 7.77±2.10 |
| TSH (μIU/ml) | 1.80±1.23 | 1.89±1.42 |
MRI T2SIR values of different groups
The MRI T2SIR values of different groups were shown in Tables 4 and 5. For the experimental eyes, the average value of T2SIR was 257.73±33.93%, while after one month of radiotherapy, the average value of T2SIR decreased to 191.69±28.82. As for the control eyes, the average value of T2SIR was close before and after treatment (257.73±33.93% and 231.25±33.80).
Table 4.
T2SIR value (%) of experimental and control group before and after radiotherapy
| Groups and condition | T2SIR (X±SD) | 95% credibility interval |
|---|---|---|
| Experimental group before radiotherapy | 257.73±33.93 | 248.18-267.27 |
| Experimental group after radiotherapy | 191.69±28.82 | 183.58-199.79 |
| Control group before radiotherapy | 259.61±32.46 | 250.48-268.74 |
| Control group before radiotherapy | 231.25±33.80 | 221.75-240.76 |
Table 5.
Decrease of T2SIR value (%) of experimental and control group before and after radiotherapy
| Group number (1) | Experimental group (2) | Control group (3) | Difference (d) (4)=(2)-(3) |
|---|---|---|---|
| 01 | 91 | 60 | 31 |
| 02 | 114 | 52 | 62 |
| 03 | 69 | 64 | 5 |
| 04 | 84 | 18 | 66 |
| 05 | 50 | 26 | 24 |
| 06 | 73 | 22 | 51 |
| 07 | 63 | 32 | 31 |
| 08 | 29 | 31 | -2 |
| 09 | 79 | 50 | 29 |
| 10 | 71 | 19 | 52 |
| 11 | 89 | 37 | 52 |
| 12 | 88 | 69 | 19 |
| 13 | 62 | 21 | 41 |
| 14 | 65 | 13 | 52 |
| 15 | 97 | 44 | 53 |
| 16 | 39 | 6 | 33 |
| 17 | 90 | 25 | 65 |
| 18 | 75 | 42 | 33 |
| 19 | 47 | 11 | 36 |
| 20 | 51 | 26 | 25 |
| 21 | 45 | 8 | 37 |
| 22 | 72 | 13 | 59 |
| 23 | 82 | 25 | 57 |
| 24 | 52 | 29 | 23 |
| 25 | 73 | 2 | 71 |
| 26 | 78 | 57 | 21 |
| 27 | 50 | 5 | 45 |
| 28 | 47 | 28 | 19 |
| 29 | 106 | 28 | 78 |
| 30 | 27 | 17 | 10 |
| 31 | 28 | 12 | 16 |
| 32 | 61 | 15 | 46 |
| 33 | 65 | 42 | 23 |
| 34 | 135 | 90 | 45 |
| 35 | 44 | 12 | 32 |
| 36 | 80 | 51 | 29 |
| 37 | 57 | 33 | 24 |
| 38 | 52 | 45 | 7 |
| 39 | 78 | 23 | 55 |
| 40 | 57 | 29 | 28 |
| 41 | 79 | 16 | 63 |
| 42 | 15 | -1 | 16 |
| 43 | 44 | 26 | 18 |
| 44 | 39 | 20 | 19 |
| 45 | 84 | 29 | 55 |
| 46 | 92 | 9 | 83 |
| 47 | 42 | 6 | 36 |
| 48 | 49 | 5 | 44 |
| 49 | 46 | 29 | 17 |
| 50 | 86 | 50 | 36 |
| 51 | 77 | 25 | 52 |
EOM volume values of different groups
The EOM volume of different eyes was shown in Tables 6 and 7. The decrease value of EOM volume of both experimental and control eyes before and after treatment are overall consistent with the normal distribution and obviously the decrease value of EOM volume of both experimental was more than that of control eyes, suggesting that it had effect for the radiotherapy.
Table 6.
EOM volume (ml) of experimental and control group before and after radiotherapy
| Groups and condition | EOM volume (X±SD) | 95% credibility interval |
|---|---|---|
| Experimental group before radiotherapy | 4.46±0.62 | 4.28-4.63 |
| Experimental group after radiotherapy | 3.82±0.54 | 3.67-3.97 |
| Control group before radiotherapy | 4.47±0.57 | 4.30-4.63 |
| Control group before radiotherapy | 4.26±0.56 | 4.10-4.42 |
Table 7.
Decrease of EOM volume (ml) of experimental and control group before and after radiotherapy
| Group number (1) | Experimental group (2) | Control group (3) | Difference (d) (4)=(2)-(3) |
|---|---|---|---|
| 01 | 0.71 | -0.03 | 0.74 |
| 02 | 1.58 | 0.65 | 0.93 |
| 03 | 0.43 | -0.02 | 0.45 |
| 04 | 0.98 | 0.57 | 0.41 |
| 05 | 0.67 | 0.28 | 0.39 |
| 06 | 0.79 | 0.1 | 0.69 |
| 07 | 0.46 | -0.02 | 0.48 |
| 08 | -0.05 | -0.04 | -0.01 |
| 09 | 0.73 | 0.02 | 0.71 |
| 10 | 0.29 | 0.01 | 0.28 |
| 11 | 0.41 | 0.07 | 0.34 |
| 12 | 0.49 | 0.14 | 0.35 |
| 13 | 0.62 | 0.28 | 0.34 |
| 14 | 0.6 | 0.06 | 0.54 |
| 15 | 0.52 | 0.21 | 0.31 |
| 16 | 0.57 | 0.06 | 0.51 |
| 17 | 0.34 | 0.13 | 0.21 |
| 18 | 0.74 | 0.33 | 0.41 |
| 19 | 1.05 | 0.11 | 0.94 |
| 20 | 0.61 | 0.14 | 0.47 |
| 21 | 0.56 | -0.02 | 0.58 |
| 22 | 0.7 | 0.17 | 0.53 |
| 23 | 0.61 | 0.13 | 0.48 |
| 24 | 0.51 | 0.03 | 0.48 |
| 25 | 0.72 | 0.44 | 0.28 |
| 26 | 0.71 | -0.05 | 0.76 |
| 27 | 0.86 | 0.15 | 0.71 |
| 28 | 0.32 | 0.24 | 0.08 |
| 29 | 0.76 | 0.46 | 0.3 |
| 30 | 0.82 | 0.48 | 0.34 |
| 31 | 0.92 | 0.45 | 0.47 |
| 32 | 0.67 | 0.11 | 0.56 |
| 33 | 0.61 | 0.08 | 0.53 |
| 34 | 0.67 | 0.23 | 0.44 |
| 35 | 0.43 | 0.21 | 0.22 |
| 36 | 1.01 | 0.48 | 0.53 |
| 37 | 0.57 | 0.06 | 0.51 |
| 38 | 0.66 | 0.17 | 0.49 |
| 39 | 0.38 | 0.09 | 0.29 |
| 40 | 0.8 | 0.34 | 0.46 |
| 41 | 0.96 | 0.42 | 0.54 |
| 42 | 0.71 | -0.04 | 0.75 |
| 43 | 0.31 | 0.33 | -0.02 |
| 44 | 0.93 | 0.55 | 0.38 |
| 45 | 0.33 | 0.44 | -0.11 |
| 46 | 0.67 | 0.15 | 0.52 |
| 47 | 0.54 | 0.58 | -0.04 |
| 48 | 0.74 | 0.22 | 0.52 |
| 49 | 0.63 | 0.06 | 0.57 |
| 50 | 0.51 | 0.05 | 0.46 |
| 51 | 0.54 | 0.22 | 0.32 |
Measurement of exophthalmos degree from different groups
The exophthalmos degree from both experimental and control eyes were measured (Tables 8 and 9). For the experimental eyes, the exophthalmos degree was 18.97±2.79 mm and 17.26±2.48 mm, respectively before and after radiotherapy. While for the control eyes, the corresponding values before and after radiotherapy was 18.92±2.92 mm and 18.39±2.85 mm, respectively.
Table 8.
Exophthalmos degree (mm) of experimental and control group before and after radiotherapy
| Groups and condition | EOM volume (X±SD) | 95% credibility interval |
|---|---|---|
| Experimental group before radiotherapy | 18.97±2.79 | 18.18-19.76 |
| Experimental group after radiotherapy | 17.26±2.48 | 16.57-17.96 |
| Control group before radiotherapy | 18.92±2.92 | 18.10-19.74 |
| Control group before radiotherapy | 18.39±2.85 | 17.59-19.20 |
Table 9.
Decrease of exophthalmos degree (mm) of experimental and control group before and after radiotherapy
| Group number (1) | Experimental group (2) | Control group (3) | Difference (d) (4)=(2)-(3) |
|---|---|---|---|
| 01 | 2.0 | 1.0 | 1.0 |
| 02 | 3.0 | 0 | 3.0 |
| 03 | 1.0 | 0 | 1.0 |
| 04 | 0 | 0.5 | -0.5 |
| 05 | 1.5 | 0 | 1.5 |
| 06 | 2.0 | 0.5 | 1.5 |
| 07 | 2.0 | 1.0 | 1.0 |
| 08 | 0.5 | 0 | 0.5 |
| 09 | 2.5 | 1.0 | 1.5 |
| 10 | 1.5 | 0.5 | 1.0 |
| 11 | 3.0 | 0 | 3.0 |
| 12 | 0.5 | 0 | 0.5 |
| 13 | 1.5 | 0.5 | 1.0 |
| 14 | 3.0 | 0 | 3.0 |
| 15 | 0 | 0.5 | -0.5 |
| 16 | 1.0 | 0 | 1.0 |
| 17 | 0.5 | 0.5 | 0 |
| 18 | 1.0 | 0 | 1.5 |
| 19 | 1.5 | 0 | 1.5 |
| 20 | 1.5 | 0 | 1.5 |
| 21 | 2.0 | 1.0 | 1.0 |
| 22 | 0 | -0.5 | 0.5 |
| 23 | 2.0 | 0.5 | 1.5 |
| 24 | 4.0 | 1.0 | 3.0 |
| 25 | 2.0 | 0.5 | 1.5 |
| 26 | 0.5 | 0.5 | 0 |
| 27 | 4.0 | 1.0 | 3.0 |
| 28 | 3.0 | 1.5 | 1.5 |
| 29 | 2.0 | 1.0 | 1.0 |
| 30 | 2.0 | 0.5 | 1.5 |
| 31 | 1.0 | 0 | 1.0 |
| 32 | 2.0 | 0 | 2.0 |
| 33 | 2.5 | 1.5 | 1.0 |
| 34 | 2.0 | 0.5 | 1.5 |
| 35 | 2.0 | 0 | 2.0 |
| 36 | 2.0 | 2.0 | 0 |
| 37 | 0.5 | 0 | 0.5 |
| 38 | 2.5 | 0.5 | 2.0 |
| 39 | 0.5 | 0.5 | 0 |
| 40 | 1.5 | 0 | 1.5 |
| 41 | 0 | 0.5 | -0.5 |
| 42 | 1.0 | 0 | 1.0 |
| 43 | 2.0 | 2.0 | 0 |
| 44 | 2.0 | 1.0 | 1.0 |
| 45 | 2.5 | 1.5 | 1.0 |
| 46 | 2.0 | 0 | 2.0 |
| 47 | 2.0 | 1.0 | 1.0 |
| 48 | 3.0 | 0.5 | 2.5 |
| 49 | 1.0 | 0.5 | 0.5 |
| 50 | 1.0 | 1.0 | 0 |
| 51 | 3.0 | 1.0 | 2.0 |
Statistical analysis
The T2SIR values, EOM volumes, and exophthalmos degree of experimental and control eyes before and after radiotherapy have been checked by normality, and all of these values followed normal distribution entirely. Thus, all the values were check by t test using software SPSS17.0. The t test showed that the P values of all indexes were less than 0.01, showing that the difference has statistical significance. Therefore, it could be considered that the decrease of T2SIR values, EOM volumes, and exophthalmos degree of experimental and control eyes before and after radiotherapy was different. Overall, the decrease of these values in the experimental eyes was larger than that in control eyes, indicating that it had efficiency for the treatment of radiotherapy.
Discussion
The happen of TAO is due to many reasons. After the activation T lymphocyte caused by polymorphisms of various genes, a great number of cytokine are produced, and then lead to fibrosis of tissue, extraocular muscule hypertrophy and lipotrophy of back eyeball. After these, activation and proliferation of fibroblast happen and the inflammatory reaction is aggravated [16]. The treatment of TAO by radiotherapy is base on the non-specific anti-inflammatory effect of ray and the hypersensitivity of T lymphocytes to ray [17]. As a result, the treatment by radiotherapy is effective to TAO in active stage but has not effect to the TAO in stationary stage.
20 Gy is the most common dose during orbital radiotherapy. Many studies [18,19] showed that more doses for radiotherapy would not increase the effect of radiotherapy. Thus, the same dose was applied in this work.
The complication of radiotherapy mainly includes cataract, radiation retinopathy, and second tumors. In most studies, no serious complication happens after radiotherapy. Marquez et al. [20] reported that 11 years (the median time) after radiotherapy, 12% of patients had cataract. Marcocci et al. [21] showed that about 10% of patients had cataract while 1% had radiation retinopathy. In this study, no complication happed after radiotherapy. However, it is still necessary to evaluate the possibility of complication in a longer period after treatment.
The efficiency of radiotherapy has been reported in many researches. For example, Donaldson et al. reported that after treatment of 23 patients with serious TAO by 4-6 MV linear accelerators, the efficiency of radiotherapy was about 65% including the effect on the patients had negative response to systemic steroid treatment. In another study, Marquez et al. [20] reported that 89% of patients had soft tissue improved, 70% of patients had exophthalmos improved, 85% of patients had extraocular muscle movement improved, 96% of patients had keratopathy improved and 67% of patients had visual impairment improvement after radiotherapy. Matthiesen et al. [22] found that 84.2% of patients had a better symptom using NOSPECS as evaluation parameters after radiotherapy. For the patients with poor response to hormone or who had taboo, 64% of them had efficiency in treatment after 20 Gy of radiotherapy [23]. Overall, in spite that the period of radiotherapy to TAO was long, but its effect was relatively stable, and also its effect could be shown merely after several weeks [24]. On the other hand, radiotherapy had some arguments in the treatment of soft tissue involvement, exophthalmos, eye movement, and optic nerve involvement [12-14].
Generally speaking, most studies merely used the clinical symptoms and change of body signs to evaluate the efficiency of radiotherapy on TAO, but these studies lacked the objective and quantitative evaluation index. In this study, extraocular muscle T2SIR, EOM volume, and the degree of exophthalmos could be the objective and quantitative evaluation index and thus made the experimental result more persuasion. In this work, after radiotherapy, the T2SIR decreased for 66.04±23.73% in the experimental eyes and 28.35±19.08% in the control eyes; the EOM volume decreased for 0.64±0.25 ml in the experimental eyes and 0.20±0.19 ml in the control eyes; the degree of exophthalmos decreased for 1.71±0.99 mm in the experimental eyes and 0.53±0.56 mm in the control eyes. Interestingly, in the control eyes, the T2SIR, EOM volume, and the degree of exophthalmos totally decreased, possibly due to the little dose of ray irradiation during radiotherapy, also the natural disease remission could be another reason. Overall, the P value of three groups are all less than 0.05, indicating the significant difference on the T2SIR, EOM volume, and the degree of exophthalmos before and after radiotherapy for TAO. Obviously, these three indexes decreased more quickly on the experimental eyes than that of control eyes, suggesting that the radiotherapy had efficiency.
In addition, the effect of radiotherapy on eyelid retraction and swelling was not included in this work. In fact, many factors such as apertor oculi, Miller’s muscle, musculi dormitator, and the influence of patient’s emotion and spirit status could affect the size of palpebral fissure. Thus, it is difficult to have a correct observation on the slight change of the size of palpebral fissure. Usually, the degree of eyelid swelling includes mild, moderate and severe and this was decided by human. Thus, for different people, the degree of eyelid swelling is various. Last but not least, the function of radiotherapy on reducing the course of TAO and preventing its recurrence is still need to be evaluated.
After comparison of T2SIR, EOM volume, and exophthalmos degree of experimental and control eye in the same patients after one month, all the patients were treated by intravenous steroid therapy according the method descried in the Part of Method and materials. Obviously, this is necessary in ethics.
In this work, it is shown that all index tested including T2SIR, EOM volume, and the exophthalmos degree between both experimental control eyes before and after radiotherapy had significant differences and these data had statistical significance. Thus, the treatment of radiotherapy is effective for the TAO in the moderate and severe active period.
Disclosure of conflict of interest
None.
References
- 1.Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, Gorman CA. The incidence of graves’ ophthalmopathy in olmsted county, minnesota. Am J Ophthalmol. 1995;120:511–517. doi: 10.1016/s0002-9394(14)72666-2. [DOI] [PubMed] [Google Scholar]
- 2.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes G, Gorman C, Brennan M, Gehring D, Ilstrup D, Earnest F. Ophthalmopathy of graves’ disease: Computerized volume measurements of the orbital fat and muscle. AJNR Am J Neuroradiol. 1986;7:651–656. [PMC free article] [PubMed] [Google Scholar]
- 4.Bartalena L, Pinchera A, Marcocci C. Management of graves’s Ophthalmopathy: Reality and perspectives 1. Endocrine Reviews. 2000;21:168–199. doi: 10.1210/edrv.21.2.0393. [DOI] [PubMed] [Google Scholar]
- 5.Matos K, Nos V, Manso PG, Ho RF, Marback E, Nakanami C, Nakanami D, Pares L, Stamato F. Correlation between clinical and histological analyses in retroocular connective tissues and extraocular muscles from patients with graves's Ophthalmopathy. Endocrine Pathology. 2000;11:185–194. doi: 10.1385/ep:11:2:185. [DOI] [PubMed] [Google Scholar]
- 6.Werner SC. Classification of the eye changes of graves’ disease. J Clin Endocrinol Metab. 1969;29:982–984. doi: 10.1210/jcem-29-7-982. [DOI] [PubMed] [Google Scholar]
- 7.Werner S. Modification of the classification of the eye changes of graves’ disease. Am J Ophthalmol. 1977;83:725–727. doi: 10.1016/0002-9394(77)90140-4. [DOI] [PubMed] [Google Scholar]
- 8.Mourits MP, Koornneef L, Wiersinga W, Prummel M, Berghout A, Van der Gaag R. Clinical criteria for the assessment of disease activity in graves’ ophthalmopathy: A novel approach. Br J Ophthalmol. 1989;73:639–644. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P, Boboridis K, Boschi A. Consensus statement of the european group on graves’ orbitopathy (eugogo) on management of go. Eur J Endocrinol. 2008;158:273–285. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 10.Bartalena L, Tanda ML. Graves’ ophthalmopathy. N Engl J Med. 2009;360:994–1001. doi: 10.1056/NEJMcp0806317. [DOI] [PubMed] [Google Scholar]
- 11.Lennerstrand G, Tian S, Isberg B, Landau Hgbeck I, Bolzani R, Tallstedt L, Schworm H. Magnetic resonance imaging and ultrasound measurements of extraocular muscles in thyroid associated ophthalmopathy at different stages of the disease. Acta Ophthalmologica Scandinavica. 2007;85:192–201. doi: 10.1111/j.1600-0420.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 12.Gorman CA, Garrity JA, Fatourechi V, Bahn RS, Petersen IA, Stafford SL, Earle JD, Forbes GS, Kline RW, Bergstralh EJ. A prospective, randomized, double-blind, placebo-controlled study of orbital radiotherapy for graves's Ophthalmopathy. Ophthalmology. 2001;108:1523–1534. doi: 10.1016/s0161-6420(01)00632-7. [DOI] [PubMed] [Google Scholar]
- 13.Prummel MF, Terwee CB, Gerding MN, Baldeschi L, Mourits MP, Blank L, Dekker FW, Wiersinga WM. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild graves’s Ophthalmopathy. J Clin Endocrinol Metab. 2004;89:15–20. doi: 10.1210/jc.2003-030809. [DOI] [PubMed] [Google Scholar]
- 14.Mourits MP, van Kempen-Harteveld ML, Garcia M, Koppeschaar HP, Tick L, Terwee CB. Radiotherapy for graves’ orbitopathy: Randomised placebo-controlled study. Lancet. 2000;355:1505–1509. doi: 10.1016/S0140-6736(00)02165-6. [DOI] [PubMed] [Google Scholar]
- 15.Gerling J, Kommerell G, Henne K, Laubenberger J, Schulte-Monting J, Fells P. Retrobulbar irradiation for thyroid-associated orbitopathy: Double-blind comparison between 2.4 and 16 gy. Int J Radiat Oncol Biol Phys. 2003;55:182–189. doi: 10.1016/s0360-3016(02)03795-1. [DOI] [PubMed] [Google Scholar]
- 16.Khalilzadeh O, Noshad S, Rashidi A, Amirzargar A. Graves’ ophthalmopathy: A review of immunogenetics. Curr Genomics. 2011;12:564. doi: 10.2174/138920211798120844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartalena L, Marcocci C, Manetti L, Tanda M, Dell’unto E, Rocchi R, Cartei F, Pinchera A. Orbital radiotherapy for graves’ ophthalmopathy. Thyroid. 1998;8:439–441. doi: 10.1089/thy.1998.8.439. [DOI] [PubMed] [Google Scholar]
- 18.Kahaly GJ, Rösler HP, Pitz S, Hommel G. Low- versus high-dose radiotherapy for graves’s Ophthalmopathy: A randomized, single blind trial. J Clin Endocrinol Metab. 2000;85:102–108. doi: 10.1210/jcem.85.1.6257. [DOI] [PubMed] [Google Scholar]
- 19.Petersen IA, Kriss JP, McDougall IR, Donaldson SS. Prognostic factors in the radiotherapy of graves’ ophthalmopathy. Int J Radiat Oncol Biol Phys. 1990;19:259–264. doi: 10.1016/0360-3016(90)90532-o. [DOI] [PubMed] [Google Scholar]
- 20.Marquez SD, Lum BL, McDougall IR, Katkuri S, Levin PS, MacManus M, Donaldson SS. Long-term results of irradiation for patients with progressive graves’s Ophthalmopathy. Int J Radiat Oncol Biol Phys. 2001;51:766–774. doi: 10.1016/s0360-3016(01)01699-6. [DOI] [PubMed] [Google Scholar]
- 21.Marcocci C, Bartalena L, Rocchi R, Marin M, Menconi F, Morabito E, Mazzi B, Mazzeo S, Sartini MS, Nardi M. Long-term safety of orbital radiotherapy for graves’s Ophthalmopathy. J Clin Endocrinol Metab. 2003;88:3561–3566. doi: 10.1210/jc.2003-030260. [DOI] [PubMed] [Google Scholar]
- 22.Matthiesen C, Thompson JS, Thompson D, Farris B, Wilkes B, Ahmad S, Herman T, Bogardus C Jr. The efficacy of radiation therapy in the treatment of graves's Orbitopathy. Int J Radiat Oncol Biol Phy. 2012;82:117–123. doi: 10.1016/j.ijrobp.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 23.Wiersinga W, Smit T, Schuster-Uittenhoeve A, Van der Gaag R, Koornneef L. Therapeutic outcome of prednisone medication and of orbital irradiation in patients with graves’ ophthalmopathy. Ophthalmologica. 1988;197:75–84. doi: 10.1159/000309924. [DOI] [PubMed] [Google Scholar]
- 24.Eckstein A, Quadbeck B, Mueller G, Rettenmeier A, Hoermann R, Mann K, Steuhl P, Esser J. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br J Ophthalmol. 2003;87:773–776. doi: 10.1136/bjo.87.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]