Abstract
Background:Scutellaria baicalensis is a Chinese herbal medicine that has been used for centuries to treat psoriasis. Baicalin is one of the major flavonoids and bioactive components of S. baicalensis and is responsible for the pharmacologic actions of the plant. Objective: This study aimed to investigate the anti-inflammatory effect and keratinocyte differentiation-inducing activity of baicalin in vivo. Methods: Baicalin was formulated into topical creams at concentrations of 1%, 3%, and 5%. The anti-inflammatory effect of baicalin cream was evaluated in 2,4-dinitrofluorobenzene (DNFB)-induced contact hypersensitivity (CHS) mice, and its keratinocyte-modulating action was assessed using the mouse tail model for psoriasis. Results: During the topical application of baicalin cream, no evidence of irritant effect was observed in both tests. In the inflammation model, mice exposed to baicalin cream displayed a reduction in DNFB-induced CHS responses compared with vehicle-treated animals, showing that the topical application of baicalin cream exerted an anti-inflammatory effect. In the second model, baicalin cream dose-dependently increased the orthokeratosis of granular layers and the relative epidermal thickness of mouse tail skin, indicative of the keratinocyte differentiation-inducing activity of this topical preparation. Conclusions: Taking the in vivo findings together, the present study indicated that baicalin cream may be a promising antipsoriatic agent worthy of further investigation for psoriasis treatment.
Keywords: Baicalin, psoriasis, antipsoriatic, contact hypersensitivity, mouse tail test
Introduction
Psoriasis is an incurable, chronic, recurrent and immune-mediated inflammatory skin disease affecting 2% to 3% of the global population [1]. Patients seldom die from psoriasis. However, the pruritic erythema and thick loose scales, as well as comorbidities such as arthritis, cardiovascular diseases, and metabolic disorders, significantly impair their quality of life [2]. Drug treatment remains the remedy for most patients with psoriasis. However, current antipsoriatic drugs do not completely meet the needs of sufferers mainly because of their side effects. A large proportion of patients also develop pharmacoresistance after long-term drug exposure [3]. Hence, the identification of new and effective antipsoriatic agents with few adverse effects remains a research hotspot in dermatology to date.
Scutellaria baicalensis, also known as Huangqin in Chinese, is the dried root of the Labiatae plant S. baicalensis Georgi. This Chinese herbal medicine, which has been used for centuries to treat psoriasis, is effective, safe, inexpensive, and widely available [4]. Among the most abundant constituents of S. baicalensis, baicalin is one of the major flavonoids responsible for the pharmacologic actions of the plant [5]. This kind of medicine has diverse pharmacologic actions such as liver protection, anti-inflammation, antiallergic reaction, and antitumor, it also has few toxic and side effects [6]. Nonrandomized clinical trials on psoriasis treatment have proven that the oral administration of baicalin capsule or intravenous injection of Chinese herbal preparations containing baicalin can effectively relieve a patient’s illness [7,8].
Current research has provided some theoretical basis for the clinical application of baicalin in treating psoriasis. For instance, baicalin can specifically inhibit polymorphonuclear leukocyte chemotaxis in response to leukotriene B4 [9] and also the cytokine-stimulated expression of inducible nitric oxide synthase in fibroblasts to reduce the production of nitric oxide [10]. Baicalin can also inhibit the growth of keratinocyte proliferation by blocking the fibroblast cell cycle [11]. Baicalin can further inhibit the abnormal proliferation of keratinocyte and its IL-8 secretion [12], thereby exerting an anti-inflammatory effect and antipsoriatic effect.
However, many questions remain unanswered despite progress in the application of baicalin in psoriasis treatment. For example, apart from several of the abovementioned studies, in vivo experiments on its antipsoriatic mechanism have not yet been reported. Moreover, except for oral administration and injection, the effectivity of a topical preparation of baicalin monomer for treating psoriasis has also not yet been studied. If it is effective, what are the proper dose and mechanisms underlying its antipsoriatic effects? Accordingly, we used 2,4-dinitrofluorobenzene (DNFB)-induced contact hypersensitivity (CHS) mice and a mouse tail model in the present study to simulate the inflammatory reaction and parakeratosis of keratinocytes in psoriasis, respectively. The in vivo effects of baicalin on inhibiting inflammatory reaction and inducing the differentiation of keratinocytes were observed, which can provide experimental basis for the application of baicalin in psoriasis treatment.
Materials and methods
Chemicals and materials
Experimental reagents were purchased from Sinopharm Chemical Reagent Co., Ltd. Vehicle cream and baicalin creams were prepared in our laboratory. Tazarotene cream (0.1%) was purchased from Chongqing Huapont Pharm Co., Ltd. (Lot 2011005). Tacrolimus ointment (0.03%) was purchased from Toyama Technology Center of Astellas Pharma Tech Co., Ltd. (Lot 025590). Corneal trephine with 7.0 mm diameter (MR-D101) was purchased from Suzhou Xiehe Medical Instruments. Electronic scale with a precision of 0.1 mg (JA2003) was purchased from Shanghai Liangping Instrument and Meter Co., Ltd. Digital vernier caliper with a precision of 0.01 mm was purchased from Guilin Measuring & Cutting Tool Co., Ltd. Optical microscope (BX60) was purchased from Olym-pus Company from Japan.
Preparation of baicalin cream
Hyaluronic acid was added to an adequate volume of water and heated at 85°C until complete dissolution. Ultrez 10 was soaked in the above solution to achieve complete swelling, and the mixture changed into a transparent non-mass gel after adding triethanolamine. Glycerol, Propylene glycol, and Tween 80 were gradually added and mixed well. The gel was weighed to calculate water loss. Sodium sulfite was dissolved by water with the volume equivalent to the water loss and slowly added to the gel. After complete mixing, the blank hydrogel was obtained, and its aqueous phase was prepared by heating to 85°C. Monoglyceride, Hexadecanol, Geroil GTCC, Silicone oil, Vitamin E, Solid paraffin, Albolene, Nipagin, and Laurocapram were heated in 85°C water and melted into the oil phase. The two phases were completely mixed, and then the vehicle cream matrix was prepared. An adequate amount of baicalin was added to dimethyl sulfoxide and heated in an 85°C water bath to dissolve. The mixture was added to the vehicle cream matrix and weighed to replenish the lost water. After stirring, the baicalin cream was prepared. The prepared vehicle cream and baicalin cream (1%, 3%, and 5%) were stored separately at low temperature.
Animals
Female BALB/c mice (6-8 weeks of age) and male ICR mice (22-25 g in weight) were purchased from the Shanghai Lab Animal Research Center. All mice were kept in a specific pathogen-free animal room and had free access to normal mouse food and water. An artificial light cycle (12 h light/12 h dark) and controlled temperature (23 ± 2°C) were maintained. Animals were allowed to acclimatize for 1 week prior to beginning experiments. The experimental protocols were approved by the Animal Care and Use Ethical Committee of Zhongshan Hospital, Fudan University.
DNFB-induced CHS
Fifty-six BALB/c mice were divided into seven groups composed of eight animals each. The first group served as a blank control and was left untreated. After CHS induction, the second group was left untreated and served as a negative control (CHS model group); the third group was treated with 0.03% tacrolimus ointment and served as a positive control. The remaining four groups were treated with the test samples, i.e., vehicle cream, 1% baicalin cream, 3% baicalin cream, and 5% baicalin cream.
According to Hotchkiss et al. [13], CHS to DNFB was induced with minor modifications. Except for the blank control group, the other mice had their abdominal hair removed at an approximate size of 2 cm × 2 cm one day prior to the start of experiment. The first sensitization was performed at the hair-removed area by external application of 25 μL of DNFB (0.5%, v/v in 4:1 acetone/olive oil) on the first experimental day. After 24 h, the procedure was repeated to intensify sensitization. On the sixth experimental day, 10 μL of DNFB (1% v/v in 1:1 acetone/olive oil) was smeared on the dorsal surface of the right ear of the mice to induce CHS, and 10 μL of the acetone/olive oil matrix was smeared on the left ear for comparison. The test drugs were first smeared on the dorsal surface of the right ear of the mice 6 h before challenge. Subsequently, they were performed twice a day (20:00 and 08:00), at 0.02 g each, for 2 consecutive days. The thicknesses of both ears were measured 24 h before challenge and then 24 h and 48 h after the challenge. The mice were weighed 6 h after the last administration of drugs and were sacrificed by cervical dislocation. Both ears were drilled with a corneal trephine to get a piece of tissue with a diameter of 7 mm. Meanwhile, the thymus gland as well as the spleen were removed and weighed. The ear samples were then fixed in 4% neutral-buffered paraformaldehyde and stained with hematoxylin and eosin (HE).
The anti-inflammatory effects of topical preparations were evaluated according to the following equations:
(1) Increased ear thickness (mm) = (right ear thickness - left ear thickness) after challenge - (right ear thickness - left ear thickness) before challenge.
(2) Increased ear weight (mg) = right ear weight – left ear weight.
(3) Thymus index (mg/g) = thymus gland weight/mice weight.
(4) Spleen index (mg/g) = spleen weight/mice weight.
(5) Tissue edema and inflammatory cell infiltration reflected by the HE-stained section of mice ear.
Mouse tail test
Forty-eight ICR mice were divided into six groups composed of eight animals each. The first group received normal saline and served as negative controls, whereas the second group was treated with standard 0.1% tazarotene cream and served as positive controls. The remaining four groups were treated with the test samples, that is, vehicle cream, 1% baicalin cream, 3% baicalin cream, and 5% baicalin cream.
In a typical procedure, approximately 0.1 g of the topical preparation was applied to the dorsal surface of mouse tails in a thin coating. To prevent the topical agents from rubbing off too quickly, mice were housed individually for 2 h and then placed back into their animal cages. The treatment was carried out twice a day (08:00 and 16:00) for four consecutive weeks. At the end of the entire treatment period, mice were sacrificed by cervical dislocation. Subsequently, the dorsal surface of tails, approximately 2 cm in length beginning 1 cm from the body, was removed from the underlying cartilage. The samples were then fixed in 4% neutral-buffered paraformaldehyde, cut longitudinally into histological sections of 5 μm in thickness, and stained with HE.
The specimens were inspected under a microscope and analyzed according to Bosman et al [14].
(A) The horizontal length of the fully developed granular layer within an individual scale lying at the turning point of the beginning and ending of two adjacent hair follicles (n = 10 scales per animal, n = 8 animals per group; i.e., a total of 80 measurements per group).
(B) The whole horizontal length of an individual scale lying at the turning point of the beginning and ending of two adjacent hair follicles (n = 10 scales per animal, n = 8 animals per group; i.e., a total of 80 measurements per group).
(C) The vertical epidermal thickness between the dermoepidermal borderline and the beginning of the horny layer (n = 5 measurements per scale, n = 10 scales per animal, n = 8 animals per treatment group, i.e., a total of 400 measurements per group).
With the raw data from (A-C) mentioned above, the antipsoriatic activities of topical preparations were further evaluated according to Bosman et al. [14]:
(1) The degree of orthokeratosis (OK) per scale was calculated as OK (%) = (A/B) × 100.
(2) The drug activity (DA) was calculated as Drug activity (DA) = (OK of treated group - mean OK of negative control group)/(100 - mean OK of control group) × 100.
(3) Out of the raw data from (C), the mean epidermal thickness of the negative controls was set to 100% and served as the baseline data. The relative epidermal thickness of the treated animals was defined as the percentage ratio of their mean epidermal thickness to that of the baseline data.
Statistical analysis
Data were analyzed using the statistical software SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and expressed as mean ± standard deviation (SD). To investigate the differences between groups in the mean values of Baicalin-derived variables, one-way analysis of variance (ANOVA) and subsequent Fisher’s least significant difference were done using PLSD. Values with P < 0.05 were considered to be significant.
Results
Inhibition of DNFB-induced CHS
Increased ear thickness, increased ear weight, thymus index, and spleen index of mice in each group are presented in Figure 1. As expected, the CHS model group produced the most obvious and most striking inflammatory response in the mouse tests. Increased ear thickness and ear weight, which reflect the degree of ear swelling, as well as thymus index and spleen index were all significantly higher than the blank control group (P < 0.05). Among the groups, 0.03% tacrolimus ointment strikingly inhibited the CHS reaction. Mice in this group showed a lower increase in ear thickness and ear weight compared with the CHS model group, their thymus index and spleen index were even lower than those in the blank control group (P < 0.05), thereby displaying excellent anti-inflammatory effect. Compared with the CHS model group, the inflammatory response showed no significant changes in the vehicle cream group (P > 0.05). This indicates that vehicle cream had no effect on CHS suppression and no skin irritation as well. As seen from the increase in ear thickness and ear weight, thymus index, and spleen index, baicalin cream could inhibit DNFB-induced CHS reaction, albeit at a less significant magnitude than that of 0.03% tacrolimus ointment. Additionally, the anti-inflammatory effect of baicalin cream was stronger not only at higher concentrations but also at 48 h than that at 24 h.
Figure 1.
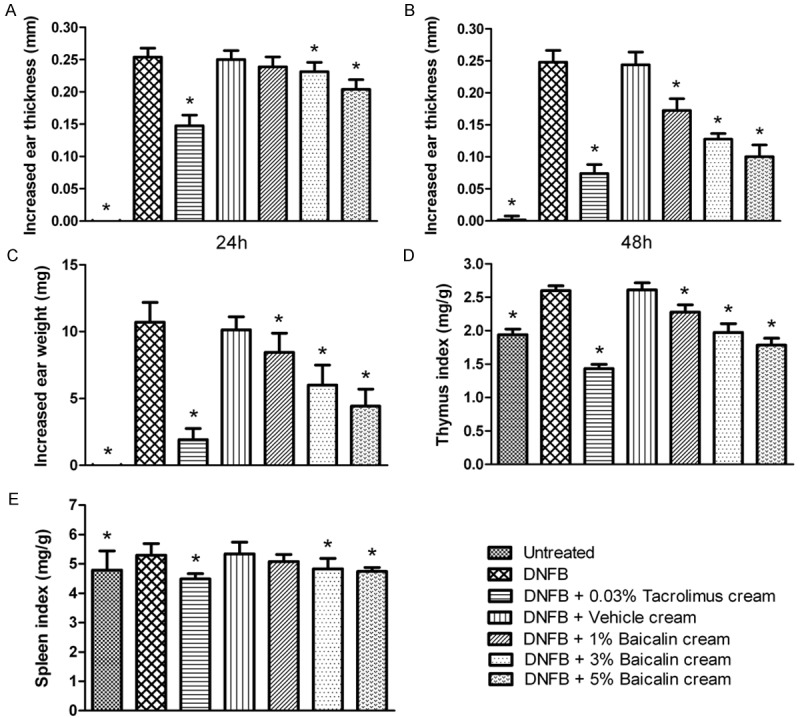
Baicalin cream inhibits DNFB-induced CHS reaction. Mouse were sensitized twice on the flank and challenged on the dorsal ear surfaces with DNFB. Topical treatments were performed twice daily for 2 consecutive days. Mouse exposed to baicalin cream displayed a reduced CHS response compared with negative control and vehicle-treated animals. Increased ear thickness, increased ear weight, thymus index, and spleen index were dose-dependently decreased. Data are expressed as mean ± SD (n = 8). *P < 0.05 as compared with the negative control group.
Consistent with the experimental outcome mentioned above, histomorphological changes in the mouse ear also revealed the same tendency. The typical pathology pictures of mouse ears are presented in Figure 2. Compared with the blank control group, mice in the CHS model group and the vehicle cream group both suffered from a striking tissue edema and inflammatory cell infiltration as well. After topical treatment with 0.03% tacrolimus ointment, inflammation of the mouse ears was notably relieved, and only mild tissue edema and inflammatory cell infiltration remained. As far as the baicalin cream groups were concerned, moderate inflammations were observed. Moreover, their tissue edema and inflammatory cell infiltration were alleviated as the concentration of baicalin increased.
Figure 2.
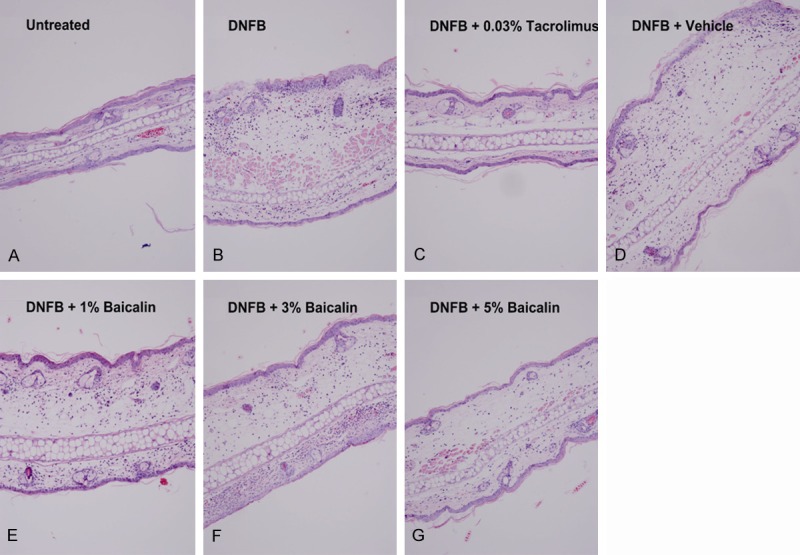
Histological sections of mouse ear stained with HE (original magnification, × 100). Notes: A. Tissue of normal mouse ear. B. Mice in CHS model group had notable tissue edema and massive inflammatory cell infiltration. C. Tissue edema and inflammatory cell infiltration were significantly relieved in the 0.03% tacrolimus ointment group. D-G. Tissue edema and inflammatory cell infiltration were alleviated as the concentration of baicalin increased.
Antipsoriatic action in mouse tail test
The keratinocyte differentiation-modulating action of baicalin cream in the mouse tail test is shown as Figure 3. During the 4 weeks of topical treatment, no visible erythema and/or edema was observed. As expected, 0.1% tazarotene cream produced the most obvious and striking increase in the degree of orthokeratosis, drug activity, and relative epidermal thickness in the mouse tail test. Although the relative epidermal thickness did not show any change, vehicle cream increased the degree of orthokeratosis as compared with the negative control. Additionally, 1% and 3% baicalin cream also produced significant differentiation in the epidermis, albeit at a less significant magnitude than that of 5% baicalin cream. As seen from the degree of orthokeratosis and drug activity, the keratinocyte differentiation-inducing activity of 5% baicalin cream was similar to that of the standard positive control.
Figure 3.
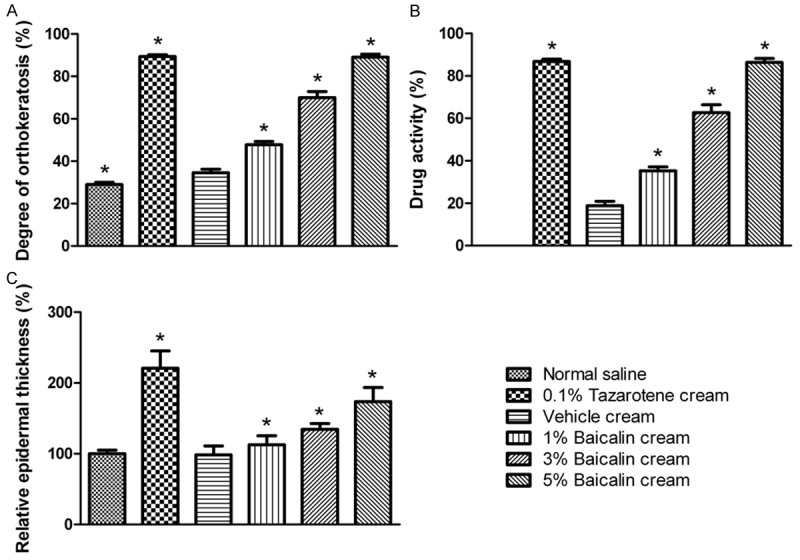
Keratinocyte differentiation-modulating action of baicalin cream in mouse tail test. Topical treatment were performed twice daily for 4 consecutive weeks. Mice exposed to baicalin cream displayed an increased orthokeratosis response compared with vehicle-treated animals, indicative of the keratinocyte differentiation-inducing activity. Their orthokeratosis of granular layer, drug activity, and relative epidermal thickness were dose-dependently increased. Data are expressed as mean ± SD (n = 8). *P < 0.05 compared with vehicle cream.
Consistent with measurement data, the histomorphological changes in the mouse tail also presented the same trend. The typical pathology pictures of mouse tail tests are presented in Figure 4. Compared with the negative control group, orthokeratosis of the epidermal granular layer in the vehicle cream group was slightly increased. After topical treatment with 0.1% tazarotene cream, a well-developed granular layer in the mouse tail skin was clearly seen. When treated with baicalin cream, a mild to moderate differentiation of the epidermal granular layer in mouse tail skins was observed. The degrees of orthokeratosis increased in a baicalin dose-dependent manner.
Figure 4.
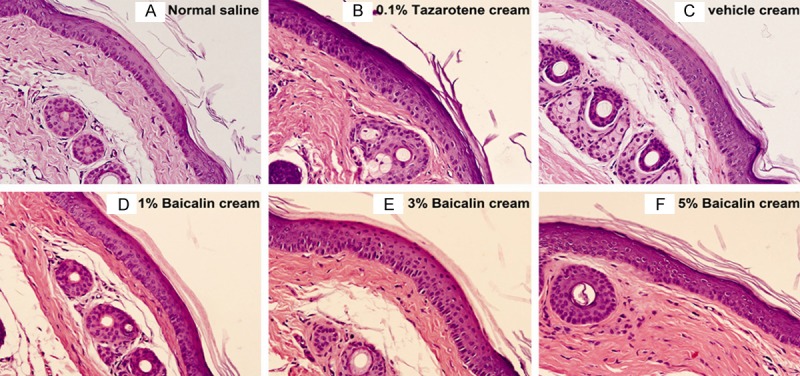
Histological sections of mouse tail skin treated topically for 4 consecutive weeks and stained with HE (original magnification, × 200). Note: A. The granular layer is less developed in most parts of the mouse tail skin. B. A well-developed granular layer was clearly seen in mouse tail skin treated with 0.1% tazarotene cream. C-F. The degrees of orthokeratosis increased in a baicalin dose-dependent manner.
Discussion
Psoriasis is an autoimmune disease with multiple abnormalities. The pathological changes include not only T cell-mediated inflammatory reaction but also excessive proliferation and abnormal differentiation of keratinocyte as well as subdermal vascular proliferation [15]. These abnormalities run through the whole process of psoriasis from occurrence, development, to outcome. Blocking these pathological changes can reduce the severity of a patient’s illness and ameliorate their symptoms. By using the DNFB-induced CHS model and mouse tail test for psoriasis, the present study proved for the first time that topical application of baicalin cream could not only relieve inflammatory reaction but also promote epidermal differentiation and normal keratization of keratinocyte in mouse skin. It provides in vivo findings for further application of baicalin in psoriasis treatment.
The DNFB-induced CHS mouse model belongs to a type IV immune response. Th1 cells, Th17 cells, and cytokines such as IFN-γ, IL-17, and TNF-α all participate in the immune response [16]. This kind of immune response exists in contact dermatitis and some inflammatory skin diseases such as psoriasis [17]. In this study, the most striking inflammatory reaction existed in the CHS model group at 24 to 48 h after DNFB challenge. Increased ear thickness, increased ear weight, thymus index, spleen index, and inflammatory cell infiltration were remarkably higher than in the blank control group, indicating the successful modeling in this experiment. Topical application of baicalin cream could inhibit DNFB-induced CHS reaction, and its anti-inflammatory effect was increased in a concentration- and time-dependent manner (Figures 1 and 2). These findings imply that baicalin cream could be used in treating inflammatory skin diseases associated with type IV immune response. Moreover, as the vehicle cream did not show any inhibitory effect on CHS reaction, this further proved that the anti-inflammatory activity of baicalin cream came from baicalin itself.
Abnormal keratinocyte level is one of the major pathological changes in psoriasis. Under the influence of proinflammatory cytokines, keratinocytes are not only highly proliferative and apoptosis-resistant but also express a plethora of cytokines and growth factors involved in the immune responses of psoriasis [18,19]. These disorders can further exacerbate the inflammatory reaction and drive angiogenesis in psoriasis [20,21]. Therefore, drugs that can block the pathological changes of keratinocytes, such as clinically used dithranol and vitamin D analogues, will be of benefit for patients with psoriasis [22,23]. As far as the baicalin cream is concerned, the keratinocyte differentiation-inducing activity thus becomes another key to its antipsoriatic mechanism.
The mouse tail model can partially mimic parakeratosis of psoriasis, and has the advantages of using easily obtained animals and having minimal technical requirements [24]. Many established and potential antipsoriatic agents have been evaluated using this method, and were found to have significant effects [25,26]. Hence, we also employed this model to evaluate the keratinocyte differentiation-inducing action of baicalin cream.
After 4 weeks of treatment with vehicle cream, the degree of orthokeratosis was increased when compared with that of negative controls. These findings suggest that long-term treatment with vehicle cream may benefit from inducing keratinocyte differentiation in mouse tail skin. Given that it contains no antipsoriatic ingredients, we hypothesized that albolene and paraffin in vehicle cream may be involved in the changes of epidermis. This may be related to the fact that humectant can reduce the transepidermal water loss and subsequently generate a normal keratotic response in the epidermis [27]. To delineate the mechanisms involved in the keratinocyte differentiation-inducing activity of vehicle cream, more experiments are needed in the future.
In comparison with vehicle control, the degree of orthokeratosis and relative epidermal thickness were significantly increased in mice treated with baicalin cream. These results not only indicated that baicalin cream is capable of inducing keratinocyte differentiation but also demonstrated that their drug activity was mainly derived from baicalin itself. Furthermore, with the concentration of baicalin elevated, their keratinocyte differentiation-modulating activities also increased in a dose-dependent manner. These findings, together with the results that its drug activity was similar to that of 0.1% tazarotene cream, indicate that 5% baicalin cream may be clinically useful in the treatment of psoriasis.
Although biological agents provide a new way for treating psoriasis, it is difficult to block a certain signaling pathway completely while the pathogenesis remains largely unclear. Worse, it may severely interfere with a patient’s normal physiological function [28]. By contrast, multitarget antipsoriatic drugs can relatively safely block the pathological process of psoriasis via multiple signaling pathways, and thus may meet clinical requirements better for long-term treatment. Hence, despite the anti-inflammatory effect being weaker than that of 0.03% tacrolimus ointment, baicalin cream is still a promising antipsoriatic drug because of its advantages such as promoting keratinocyte differentiation, high safety, and low price. Besides, extracting effective constituents from natural herbs remains an important method of developing new drugs [29]. This factor also makes treating psoriasis with baicalin deserve more consideration.
Conclusions
Our study revealed that baicalin cream possessed anti-inflammatory action in CHS response, as well as keratinocyte differentiation-modulating activity in mouse tail test. These findings partially explained the antipsoriatic effects of baicalin and suggested that 5% baicalin cream was a promising agent for psoriasis treatment worthy of further development. To better understand its antipsoriatic effects, more studies combined with other animal models are needed.
Acknowledgements
We gratefully acknowledge Dr. Xin Hong for the experiment design, as well as Dr. Weng Weiyu and Jiang Dongbo for formulating the topical preparations. We also thank Xu Yuansheng, Sheng Dexin, Sun Shuhui, Yang Lili, and Wei Yi for their technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong AW, Schupp C, Wu J, Bebo B. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PLoS One. 2012;7:e52935. doi: 10.1371/journal.pone.0052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nast A, Boehncke WH, Mrowietz U, Ockenfels HM, Philipp S, Reich K, Rosenbach T, Sammain A, Schlaeger M, Sebastian M, Sterry W, Streit V, Augustin M, Erdmann R, Klaus J, Koza J, Muller S, Orzechowski HD, Rosumeck S, Schmid-Ott G, Weberschock T, Rzany B Deutsche Dermatologische Gesellschaft (DDG); Berufsverband Deutscher Dermatologen (BVDD) S3 - Guidelines on the treatment of psoriasis vulgaris (English version). Update. J Dtsch Dermatol Ges. 2012;10(Suppl 2):S1–95. doi: 10.1111/j.1610-0387.2012.07919.x. [DOI] [PubMed] [Google Scholar]
- 4.Dong YQ, Qu X. Analysis of medication rules of corrective treatment for psoriasis with damp-heat syndrome. World Chinese Medicine. 2013;8:453–455. [Google Scholar]
- 5.Li C, Lin G, Zuo Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos. 2011;32:427–445. doi: 10.1002/bdd.771. [DOI] [PubMed] [Google Scholar]
- 6.Srinivas NR. Baicalin, an emerging multi-therapeutic agent: pharmacodynamics, pharmacokinetics, and considerations from drug development perspectives. Xenobiotica. 2010;40:357–367. doi: 10.3109/00498251003663724. [DOI] [PubMed] [Google Scholar]
- 7.Zheng MR, Xie Y, Zhang RZ. Preliminary study on baicalin treatment for psoriasis vulgaris. Chinese Journal of Dermatovenereology. 1990;4:217–218. [Google Scholar]
- 8.Shu HM. Effect evaluation of Qingkailing Injection for psoriasis vulgaris. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2011;20:61. [Google Scholar]
- 9.Zheng MR, Fang YM, Xie Y, Sun RZ, Jiang YH, Mou XL, Wang QZ, Liao SX. Effect of baicalin on the PMN and leukotrienes B4 chemotaxis in psoriasis patients. Journal of Clinical Dermatology. 1996;3:24–25. [Google Scholar]
- 10.Bi XL, Wang YN, Gu J, Gao SQ. Effect of baicalin on the iNOS expression in fibroblasts. Chinese Journal of Dermatology. 2004;37:112–113. [Google Scholar]
- 11.Wang YN, Bi XL, Gu J, Gao SQ. Study on the mechanism of baicalin in psoriasis treatment. Chinese Journal of Dermatovenereology of Integrated Traditional and Western Medicine. 2003;2:209–211. [Google Scholar]
- 12.Zhang J, Liu HY, Yu XJ. Efects of baicalin on proliferation and expression of IL-8 in culture HaCaT keratinocyte. Journal of Taishan Medical College. 2006;27:326–327. [Google Scholar]
- 13.Hotchkiss AK, Nelson RJ. An environmental androgen, 17beta-trenbolone, affects delayed-type hypersensitivity and reproductive tissues in male mice. J Toxicol Environ Health A. 2007;70:138–140. doi: 10.1080/15287390600755091. [DOI] [PubMed] [Google Scholar]
- 14.Bosman B, Matthiesen T, Hess V, Friderichs E. A quantitative method for measuring antipsoriatic activity of drugs by the mouse tail test. Skin Pharmacol. 1992;5:41–48. doi: 10.1159/000211016. [DOI] [PubMed] [Google Scholar]
- 15.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 16.McFadden JP, Puangpet P, Basketter DA, Dearman RJ, Kimber I. Why does allergic contact dermatitis exist? Br J Dermatol. 2013;168:692–699. doi: 10.1111/bjd.12145. [DOI] [PubMed] [Google Scholar]
- 17.Chiricozzi A, Zhang S, Dattola A, Gabellini M, Chimenti S, Nistico SP. Role of Th17 in the pathogenesis of cutaneous inflammatory diseases. J Biol Regul Homeost Agents. 2012;26:313–318. [PubMed] [Google Scholar]
- 18.Albanesi C, De Pità O, Girolomoni G. Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin Dermatol. 2007;25:581–588. doi: 10.1016/j.clindermatol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol. 2012;51:389–395. doi: 10.1111/j.1365-4632.2011.05154.x. quiz 395-398. [DOI] [PubMed] [Google Scholar]
- 20.Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, Chen CS, Fu W, Gudjonsson JE, McCormick TS, Ward NL. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol. 2013;190:2252–2262. doi: 10.4049/jimmunol.1201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013;34:174–181. doi: 10.1016/j.it.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sehgal VN, Verma P, Khurana A. Anthralin/dithranol in dermatology. Int J Dermatol. 2014;53:e449–460. doi: 10.1111/j.1365-4632.2012.05611.x. [DOI] [PubMed] [Google Scholar]
- 23.Mason A, Mason J, Cork M, Hancock H, Dooley G. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799–807. doi: 10.1016/j.jaad.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Jarrett A, Spearman RIG. Psoriasis. In: Taverner D, Trounce J, editors. Histochemistry of the Skin. London: University Press; 1964. [Google Scholar]
- 25.Dhanabal SP, Priyanka Dwarampudi L, Muruganantham N, Vadivelan R. Evaluation of the antipsoriatic activity of Aloe vera leaf extract using a mouse tail model of psoriasis. Phytother Res. 2012;26:617–619. doi: 10.1002/ptr.3589. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia A, Singh B, Wadhwa S, Raza K, Katare OP. Novel phospholipid-based topical formulations of tamoxifen: evaluation for antipsoriatic activity using mouse-tail model. Pharm Dev Technol. 2014;19:160–163. doi: 10.3109/10837450.2013.763260. [DOI] [PubMed] [Google Scholar]
- 27.Denda M, Sato J, Tsuchiya T, Elias PM, Feingold KR. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol. 1998;111:873–878. doi: 10.1046/j.1523-1747.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- 28.Jourabchi N, Adelzadeh L, Wu JJ. The risk of deep fungal infections during biologic therapy for psoriasis. J Eur Acad Dermatol Venereol. 2014;28:1277–1285. doi: 10.1111/jdv.12508. [DOI] [PubMed] [Google Scholar]
- 29.Itokawa H, Morris-Natschke SL, Akiyama T, Lee KH. Plant-derived natural product research aimed at new drug discovery. J Nat Med. 2008;62:263–280. doi: 10.1007/s11418-008-0246-z. [DOI] [PubMed] [Google Scholar]


