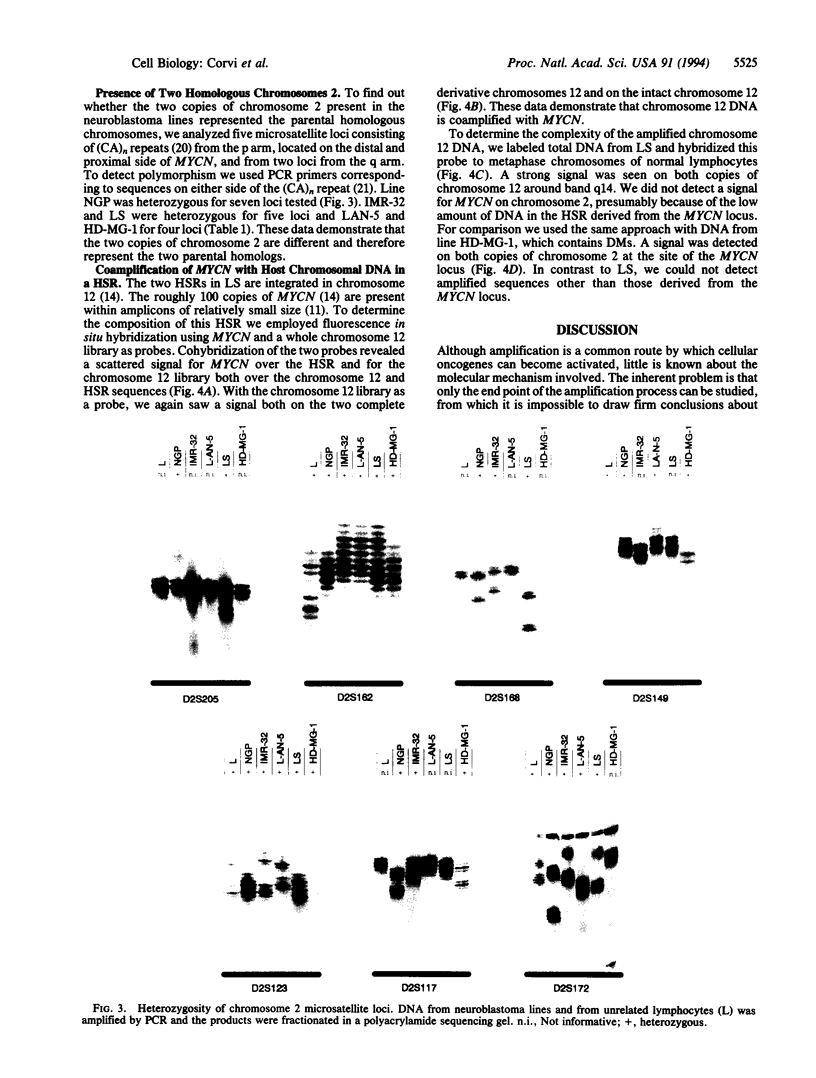
Abstract
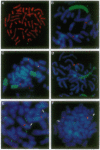
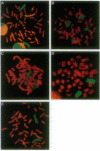
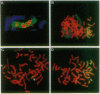
Amplification of the human N-myc protooncogene, MYCN, is frequently seen either in extrachromosomal double minutes or in homogeneously staining regions of aggressively growing neuroblastomas. MYCN maps to chromosome 2 band p23-24, but homogeneously staining regions have never been observed at this band, suggesting transposition of MYCN during amplification. We have employed fluorescence in situ hybridization to determine the status of MYCN at 2p23-24 in five human neuroblastoma cell lines. All five lines carried, in addition to amplified MYCN in homogeneously staining regions or double minutes, single-copy MYCN at the normal position. In one line there was coamplification of MYCN together with DNA of the host chromosome 12, to which MYCN had been transposed. Our results suggest a model of amplification where MYCN is retained at its original location. They further sustain the view that either the initial events of MYCN amplification or the further evolution of amplified MYCN copies follow mechanisms different from those leading to amplification of drug-resistance genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M. Oncogene amplification in tumor cells. Adv Cancer Res. 1986;47:235–281. doi: 10.1016/s0065-230x(08)60201-8. [DOI] [PubMed] [Google Scholar]
- Amler L. C., Schwab M. Amplified N-myc in human neuroblastoma cells is often arranged as clustered tandem repeats of differently recombined DNA. Mol Cell Biol. 1989 Nov;9(11):4903–4913. doi: 10.1128/mcb.9.11.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Green A. A., Hayes F. A., Williams K. J., Williams D. L., Tsiatis A. A. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981 Nov;41(11 Pt 1):4678–4686. [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Sekhon G., Goldstein M. N. Chromosomal aberrations in human neuroblastomas. Cancer. 1977 Nov;40(5):2256–2263. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- COX D., YUNCKEN C., SPRIGGS A. I. MINUTE CHROMATIN BODIES IN MALIGNANT TUMOURS OF CHILDHOOD. Lancet. 1965 Jul 10;1(7402):55–58. doi: 10.1016/s0140-6736(65)90131-5. [DOI] [PubMed] [Google Scholar]
- Ford M., Davies B., Griffiths M., Wilson J., Fried M. Isolation of a gene enhancer within an amplified inverted duplication after "expression selection". Proc Natl Acad Sci U S A. 1985 May;82(10):3370–3374. doi: 10.1073/pnas.82.10.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Feo S., Heard E. The role of inverted duplication in the generation of gene amplification in mammalian cells. Biochim Biophys Acta. 1991 Oct 8;1090(2):143–155. doi: 10.1016/0167-4781(91)90095-4. [DOI] [PubMed] [Google Scholar]
- Hunt J. D., Valentine M., Tereba A. Excision of N-myc from chromosome 2 in human neuroblastoma cells containing amplified N-myc sequences. Mol Cell Biol. 1990 Feb;10(2):823–829. doi: 10.1128/mcb.10.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyrien O., Debatisse M., Buttin G., de Saint Vincent B. R. The multicopy appearance of a large inverted duplication and the sequence at the inversion joint suggest a new model for gene amplification. EMBO J. 1988 Feb;7(2):407–417. doi: 10.1002/j.1460-2075.1988.tb02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi A., Kallioniemi O. P., Sudar D., Rutovitz D., Gray J. W., Waldman F., Pinkel D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992 Oct 30;258(5083):818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- Kanda N., Schreck R., Alt F., Bruns G., Baltimore D., Latt S. Isolation of amplified DNA sequences from IMR-32 human neuroblastoma cells: facilitation by fluorescence-activated flow sorting of metaphase chromosomes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4069–4073. doi: 10.1073/pnas.80.13.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl N. E., Kanda N., Schreck R. R., Bruns G., Latt S. A., Gilbert F., Alt F. W. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983 Dec;35(2 Pt 1):359–367. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr Novel amplification and transcriptional activity of chorion genes in Drosophila melanogaster follicle cells. Cell. 1983 Jun;33(2):543–553. doi: 10.1016/0092-8674(83)90435-x. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph G., Schilbach-Stückle K., Handgretinger R., Kaiser P., Hameister H. Cytogenetic and molecular characterization of a newly established neuroblastoma cell line LS. Hum Genet. 1991 Apr;86(6):562–566. doi: 10.1007/BF00201542. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schneider S. S., Hiemstra J. L., Zehnbauer B. A., Taillon-Miller P., Le Paslier D. L., Vogelstein B., Brodeur G. M. Isolation and structural analysis of a 1.2-megabase N-myc amplicon from a human neuroblastoma. Mol Cell Biol. 1992 Dec;12(12):5563–5570. doi: 10.1128/mcb.12.12.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Amler L. C. Amplification of cellular oncogenes: a predictor of clinical outcome in human cancer. Genes Chromosomes Cancer. 1990 Jan;1(3):181–193. doi: 10.1002/gcc.2870010302. [DOI] [PubMed] [Google Scholar]
- Schwab M., Varmus H. E., Bishop J. M., Grzeschik K. H., Naylor S. L., Sakaguchi A. Y., Brodeur G., Trent J. Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature. 1984 Mar 15;308(5956):288–291. doi: 10.1038/308288a0. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Danon Y. L., Rayner S. A., Hoover F. Definition of a Thy-1 determinant on human neuroblastoma, glioma, sarcoma, and teratoma cells with a monoclonal antibody. J Immunol. 1982 Feb;128(2):983–989. [PubMed] [Google Scholar]
- Shiloh Y., Shipley J., Brodeur G. M., Bruns G., Korf B., Donlon T., Schreck R. R., Seeger R., Sakai K., Latt S. A. Differential amplification, assembly, and relocation of multiple DNA sequences in human neuroblastomas and neuroblastoma cell lines. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3761–3765. doi: 10.1073/pnas.82.11.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Gorman P. A., Stark M. B., Groves R. P., Stark G. R. Distinctive chromosomal structures are formed very early in the amplification of CAD genes in Syrian hamster cells. Cell. 1990 Dec 21;63(6):1219–1227. doi: 10.1016/0092-8674(90)90417-d. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Toledo F., Le Roscouet D., Buttin G., Debatisse M. Co-amplified markers alternate in megabase long chromosomal inverted repeats and cluster independently in interphase nuclei at early steps of mammalian gene amplification. EMBO J. 1992 Jul;11(7):2665–2673. doi: 10.1002/j.1460-2075.1992.tb05332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo F., Smith K. A., Buttin G., Debatisse M. The evolution of the amplified adenylate deaminase 2 domains in Chinese hamster cells suggests the sequential operation of different mechanisms of DNA amplification. Mutat Res. 1992 May;276(3):261–273. doi: 10.1016/0165-1110(92)90012-x. [DOI] [PubMed] [Google Scholar]
- Trask B. J., Hamlin J. L. Early dihydrofolate reductase gene amplification events in CHO cells usually occur on the same chromosome arm as the original locus. Genes Dev. 1989 Dec;3(12A):1913–1925. doi: 10.1101/gad.3.12a.1913. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Padgett R. A., Stark G. R. Single-copy and amplified CAD genes in Syrian hamster chromosomes localized by a highly sensitive method for in situ hybridization. Mol Cell Biol. 1982 Mar;2(3):308–319. doi: 10.1128/mcb.2.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Windle B., Draper B. W., Yin Y. X., O'Gorman S., Wahl G. M. A central role for chromosome breakage in gene amplification, deletion formation, and amplicon integration. Genes Dev. 1991 Feb;5(2):160–174. doi: 10.1101/gad.5.2.160. [DOI] [PubMed] [Google Scholar]