Abstract
Objective: Several published literatures investigated the relation between a polymorphism (Leul25Val) in platelet endothelial cell adhesion molecule-1 (PECAM-1) gene and risk of coronary heart disease (CHD) and did not reach the same conclusion. To shed light on these inconclusive findings, we performed a meta-analysis of studies relating the PECAM-1 genetic polymorphism (Leul25Val) to the risk of CHD. Methods: We identified literatures by searching PubMed, EMBASE, Chinese National Knowledge Infrastructure databases (CNKI) and Wanfang database in China. Data from eligible studies were extracted for meta-analysis. CHD risk associated with PECAM-1 genetic polymorphism (Leul25Val) was estimated by pooled odds ratios (ORs) and 95% confidence intervals (95% CIs). The software Review Manager (Version 5.2) was used for meta-analysis. Publication bias was tested by funnel plot. Results: A total of 15 studies comprising 3696 cases and 3940 controls fulfilled the inclusion criteria. Our results did not show that Leul25Val polymorphism in PECAM-1 gene was associated with the risk of CHD [(LL+LV) vs VV, OR = 1.15, 95% CI: 0.84-1.56, P = 0.38; (VV+LV) vs LL, OR = 0.96, 95% CI: 0.79-1.17, P = 0.69; V vs L, OR = 1.08, 95% CI: 0.92-1.27, P = 0.80, respectively] by a meta-analysis. Conclusion: The results of our meta-analysis suggested that Leul25Val polymorphism in PECAM-1 gene is not a susceptibility marker of CHD.
Keywords: PECAM-1, coronary heart disease, polymorphism
Introduction
Coronary heart disease (CHD) is a leading cause of death and disability worldwide. CHD is usually caused by atherosclerosis that is characterized by the formation of cholesterol and fatty deposits on the inner walls of the arteries and inflammation [1,2]. Eventually, the narrowing or blockage of the coronary arteries [3,4] can lead to acute myocardial infarction, even sudden death. CHD is a complex disease resulting from the interactions between a number of genetic factors and various environmental factors [5,6].
Inflammation plays a critical role in the initiation and progression of atherosclerosis and subsequent CHD [7,8]. Platelet activation, aggregation and thrombus formation are regarded as key steps in the pathogenesis of atherosclerosis and CHD [9]. Previous studies indicated that platelet endothelial cell adhesion molecule-1 (PECAM-1), also known as CD31, is a membrane glycoprotein (MW: 130 KDa) and is an immunoglobulin (Ig) and involves inflammation [10,11]. PECAM-1 is constitutively expressed on the surface of circulating monocytes, platelets, neutrophils, specific classes of T cells, and endothelial cells [12-15]. Several publications suggested that genetic polymorphism (Leu125Val, rs668) of PECAM-1 were associated with the risk of CHD [16-23]. However, some studies did not verify this conclusion [24-31]. In view of the discrepancies in the findings of previous published studies, we aimed to perform a meta-analysis to clarify the association between rs668 in PECAM-1 and CHD.
Methods
Search strategy
We carried out a publication search in PubMed, EMBASE, Chinese National Knowledge Infrastructure (CNKI) and Wanfang database in China, with the following search terms: “platelet endothelial cell adhesion molecule-1” or “PECAM-1” or “CD31”, and “SNP” or “polymorphism” or “polymorphic” or “gene” or “genetic” or “genotype” or “variant” or “mutation” or “mutant”, and “coronary artery disease” or “coronary heart disease” or “cardiovascular” or “unstable angina” or “myocardial infarction” or “acute coronary syndrome”. The search followed the guidelines of the 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement. Searches were limited to papers published in the English and Chinese language.
Selection criteria
Inclusion criteria are as follows: (1) independently published case-control or cohort studies on the relation between PECAM-1 gene polymorphism and CHD; (2) with the genotype data of LL, LV and VV or with comprehensive statistical indicators directly or indirectly: OR or RR (relative risk) values and 95% CI (confidence interval); and (3) similar themes and methods, that is, case-control or cohort studies about the relation of the PECAM-1 gene polymorphism and CHD. The literatures were excluded if relevant data are not available or there is heterogeneity of gene polymorphism in the control population. And studies in which participants did not have documented symptoms or diagnostic evidence of CHD were excluded. For the heterogeneity test method, we use the Q-test and I2 test of the RevMan 5.2 software.
Data extraction
Two investigators independently extracted the following data with a standard protocol: 1) first author’s name; 2) year of publication; 3) study design; 2) number of participants in the case and control groups; 3) patient characteristics of each group; 4) ethnicity of the study population; 4) method of screening for CHD; and 5) odds ratio (OR) and 95% confidence interval for association with CHD. Discrepancies were resolved through a discussion between the two reviewers. In case of incomplete data on the genotype frequencies, the corresponding author of the respective article was contacted.
Statistical analysis
For each study, we first examined whether the genotype distribution in controls was consistent with Hardy-Weinberg equilibrium (HWE) by x2 test. Meta-analysis was performed using RevMan 5.2 software provided by the Cochrane Collaboration. We used Q-test and I2 test to examine the heterogeneity between each study. Using the heterogeneity test, if P > 0.05, we selected the fixed effects model, and if P < 0.05, we selected the random effects model to merge the OR. P < 0.05 was considered as a significant difference. Sensitivity analyses were performed using the one-study remove approach to assess the impact of each study on the combined effect as previously described [32]. Assessment of publication bias was carried out by inspection of funnel plot asymmetry.
Results
Flow of included studies
102 literatures were preliminarily detected, which includes 76 Chinese literatures and 36 English literatures; 67 literatures were excluded because of duplicate publication and nonclinical-based research literature. 35 studies appeared to be potentially relevant for inclusion in our study. 18 studies were further excluded because of not to detect the rs668 genotype. Therefore, 17 full-text articles were reviewed. 2 studies were further excluded for no control population. Therefore, a total of 15 articles met the inclusion criteria [16-31].
Characteristics of included studies
The overall study population included in the current meta-analysis included 7636 subjects, of which 3696 were cases and 3940 were controls. The characteristics of included studies are summarized in Table 1. The 15 included studies were published between 2000 and 2010. Genotyping of Leu125Val in the included studies was performed by either polymerase chain reaction (PCR) sequencing or PCR restriction fragment length polymorphism technique using restriction enzymes PvuII. The genotype distributions among the controls of all studies were in agreement with HWE.
Table 1.
The characteristics of included studies
| Publication Year | Author | Ethnicity | Case/Control | VV | LV | LL | V | L |
|---|---|---|---|---|---|---|---|---|
| 2000 | Gardemann et al. | Germany | 1170/1330 | 335/380 | 563/630 | 272/320 | 1233/1390 | 1107/1270 |
| 2001 | Sasaoka et al. | Japanese | 136/235 | 29/70 | 72/120 | 35/45 | 130/260 | 142/210 |
| 2003 | Song et al. | Chinese | 156/75 | 40/10 | 82/37 | 34/28 | 162/57 | 150/93 |
| 2004 | Listì et al. | Italian | 96/118 | 19/31 | 38/49 | 39/38 | 76/111 | 116/125 |
| 2004 | Wei et al. | Chinese | 144/150 | 54/29 | 63/86 | 27/35 | 171/144 | 117/156 |
| 2005 | Fang et al. | Indian | 137/110 | 32/47 | 83/52 | 22/11 | 147/127 | 146/74 |
| 2006 | Pan et al. | Chinese | 90/115 | 36/27 | 34/57 | 20/31 | 106/111 | 74/119 |
| 2007 | Chang et al. | Chinese | 600/725 | 223/396 | 252/147 | 125/182 | 698/939 | 502/511 |
| 2007 | Xu et al. | Chinese | 72/80 | 29/18 | 27/40 | 16/22 | 85/76 | 59/84 |
| 2008 | Kang et al. | Chinese | 95/89 | 25/26 | 54/50 | 16/13 | 104/102 | 86/76 |
| 2008 | Wei et al. | Chinese | 265/280 | 83/58 | 136/158 | 46/64 | 302/274 | 228/286 |
| 2009 | Listì et al. | Italian | 431/119 | 121/21 | 208/63 | 102/35 | 450/105 | 412/133 |
| 2009 | Reschner et al. | Slovenia | 142/310 | 35/91 | 67/166 | 40/53 | 137/348 | 147/272 |
| 2010 | Huang et al. | Chinese | 62/88 | 19/13 | 32/47 | 11/28 | 70/73 | 54/103 |
| 2010 | Shalia et al. | India | 100/116 | 21/26 | 44/63 | 35/27 | 86/115 | 114/113 |
Meta-analysis
The association between rs668 polymorphism and susceptibility to CHD was analyzed in 15 independent studies. Results of the meta-analysis are shown in Figures 1, 2 and 3. These three figures showed the result of meta-analysis of studies on the correlation between CHD and PECAM-1 polymorphism in 15 case-control studies. The systematic reviews of the included studies can be seen from these three figures, which include the number of case and control groups, weight, OR value, and 95% CI.
Figure 1.
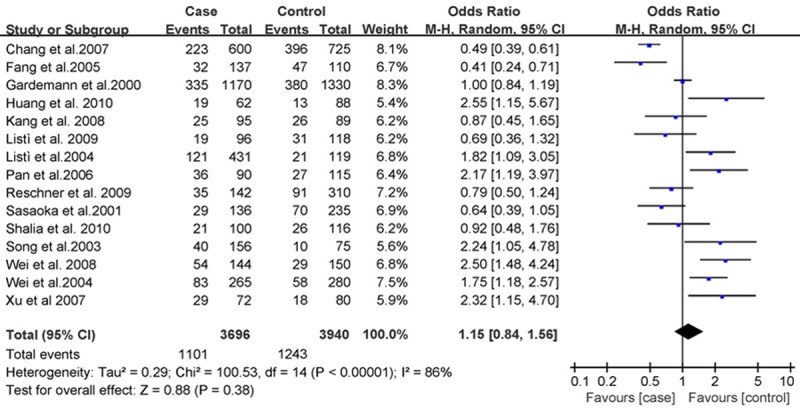
Forest plot of CHD and Leu125Val in a dominant model, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI.
Figure 2.
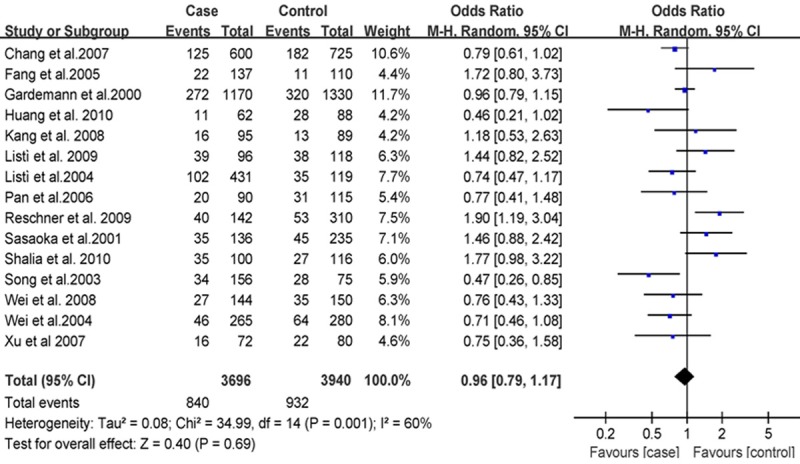
Forest plot of CHD and Leu125Val in a recessive model, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI.
Figure 3.
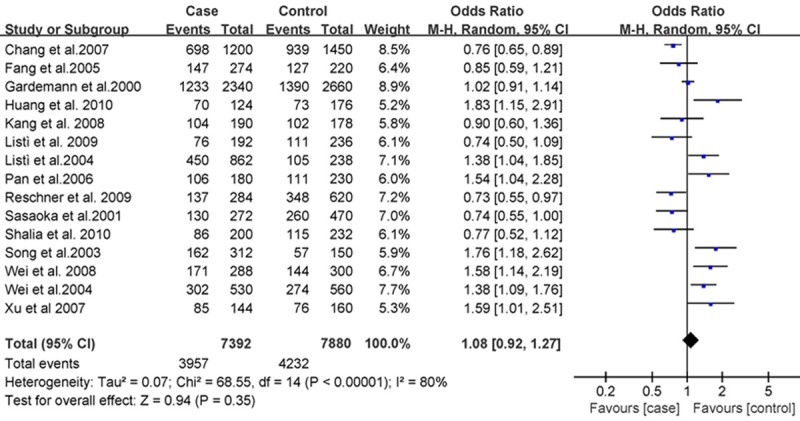
Forest plot of CHD and Leu125Val in an allele model, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI.
The heterogeneity test of the various studies revealed heterogeneous results (P < 0.001, I2 = 86%; P = 0.001, I2 = 60%; and P < 0.001, I2 = 80%, respectively); therefore, we used the random effects model in the analysis. We did not find associations of PECAM-1 Leu125Val with CHD risk in dominant model [(LL+LV) vs VV, OR = 1.15, 95% CI: 0.84-1.56, P = 0.38, Figure 1], recessive model [(VV+LV) vs LL, OR = 0.96, 95% CI: 0.79-1.17, P = 0.69, Figure 2], and allele model (V vs L, OR = 1.08, 95% CI: 0.92-1.27, P = 0.80, Figure 3), respectively, by a meta-analysis.
Test of sensitivity
For the sensitivity analysis, we deleted one single study from the overall pooled analysis each time to check the influence of the removed data set to the overall ORs. The pooled ORs and 95% CIs were not significantly altered when any part of the study was omitted, which indicated that any single study had little impact on the overall ORs.
Publication bias
We utilized RevMan 5.2 software to analyze the publication bias; the funnel plot (Figure 4) showed that the points are evenly distributed and symmetrical, and most of the points are within the 95% confidence interval. And the shape of funnel plots showed no obvious asymmetry. It indicates that there is no publication bias, and the result of the study is credible.
Figure 4.
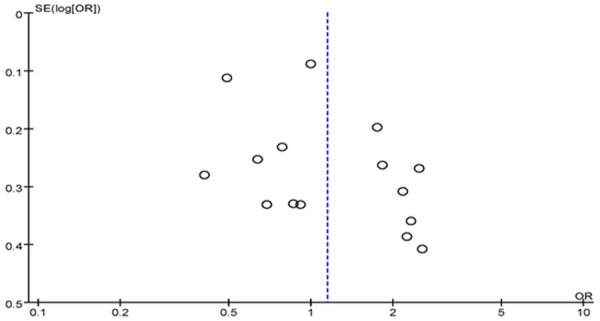
Begg’s funnel plot for publication bias tests. Each point represents a separate study for the indicated association. Log or represents natural logarithm of OR. Vertical line represents the mean effects size.
Discussion
In this meta-analysis, we found a polymorphism Leu125Val in PECAM-1 gene was not associated with CHD by the pooled results from 15 published studies. Current evidences indicate that a large number of genetic variations of low effect size are likely to contribute to overall risk. Pooled odds ratios calculated from meta-analysis of individual studies represent a means to generate sample sizes to examine with sufficient power whether candidate gene polymorphisms are associated with risk of a particular disease.
It has been suggested that PECAM-1 phosphorylation plays a pivotal role in platelet function and collagen-mediated activation, thereby affecting the risk of thrombus formation and potentially subsequent development of CHD. And polymorphisms of the PECAM-1 have been shown to be closely related to CHD. In the present study, we combined the results of 15 studies to pool analyze the relation between Leu125Val polymorphism and CHD. The result showed that there was not association of CHD with rs668 polymorphism of PECAM-1 gene.
The characteristic of meta-analysis is to combine comparable studies to increase the sample size and statistical power and draw a more compelling result. However, meta-analysis confounds factors such as publication bias, method of sampling, different genetic backgrounds of subjects, different protocols and quality of analysis. In the present study, we did not found the publication bias. All of the studies checked genotypes for quality control. Genotype distribution of controls in all studies was consistent with HWE. In addition, sensitivity analysis also showed that omission of any single study did not have significant impact on the combined ORs. This made the results of this meta-study more reliable to some extent.
However, there remained some limitations in this meta-analysis. Firstly, in the present study, we were unable to perform gender-specific analysis for the candidate SNP as genotype frequencies in most of included studies were not stratified for individual genders. Secondly, the included studies were relatively heterogenous regarding ethnicity and age. Finally, distribution of cardiovascular risk factors such as plasma lipids, blood pressure and prevalence of diabetes and obesity were not uniformly expressed in all studies, hence hindering the possibility of adjusting combined ORs for the respective parameters.
In conclusion, evidence from the current meta-analysis suggested that rs668 SNP were not found to be significantly associated with the presence of CHD. Further large studies taking into account the effects of gender, ethnicity and age are warranted to obtain a more robust assessment of the association between PECAM-1 SNP and risk of CHD.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2012HM063) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (20090131110059).
Disclosure of conflict of interest
None.
References
- 1.Li S, Guo YL, Xu RX, Zhang Y, Zhu CG, Sun J, Qing P, Wu NQ, Jiang LX, Li JJ. Association of plasma PCSK9 levels with white blood cell count and its subsets in patients with stable coronary artery disease. Atherosclerosis. 2014;234:441–5. doi: 10.1016/j.atherosclerosis.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Bhanushali AA, Das BR. Promoter variants in interleukin-6 and tumor necrosis factor alpha and risk of coronary artery disease in a population from Western India. Indian J Hum Genet. 2013;19:430–6. doi: 10.4103/0971-6866.124371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bidulescu A, Liu J, Chen Z, Hickson DA, Musani SK, Samdarshi TE, Fox ER, Taylor HA, Gibbons GH. Associations of adiponectin and leptin with incident coronary heart disease and ischemic stroke in african americans: the jackson heart study. Front Public Health. 2013;1:16. doi: 10.3389/fpubh.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Yi W, Cai Y, Fang S, Jiang X, Wen A, Wu Q. Percutaneous transluminal radiofrequency closure of the coronary artery in animal studies. Exp Ther Med. 2013;6:1044–1048. doi: 10.3892/etm.2013.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu SH, Neale MC, Acton J Jr, Considine RV, Krasnow RE, Reed T, Dai J. Genetic and environmental influences on the prospective correlation between systemic inflammation and coronary heart disease death in male twins. Arterioscler Thromb Vasc Biol. 2014;34:2168–74. doi: 10.1161/ATVBAHA.114.303556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polfus LM, Smith JA, Shimmin LC, Bielak LF, Morrison AC, Kardia SL, Peyser PA, Hixson JE. Genome-wide association study of gene by smoking interactions in coronary artery calcification. PLoS One. 2013;8:e74642. doi: 10.1371/journal.pone.0074642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo W, Liu H, Li L, Yang M, Du A. Regulation of lovastatin on a key inflammation-related microRNA in myocardial cells. Chin Med J (Engl) 2014;127:2977–81. [PubMed] [Google Scholar]
- 8.O’Sullivan JF, Neylon A, McGorrian C, Blake GJ. MicroRNA Expression in Coronary Artery Disease. Microrna. 2014;2:205–11. doi: 10.2174/22115366113026660018. [DOI] [PubMed] [Google Scholar]
- 9.Williams MS, Rogers HL, Wang NY, Ziegelstein RC. Do platelet-derived microparticles play a role in depression, inflammation, and acute coronary syndrome? Psychosomatics. 2014;55:252–60. doi: 10.1016/j.psym.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moraes LA, Vaiyapuri S, Sasikumar P, Ali MS, Kriek N, Sage T, Gibbins JM. Antithrombotic actions of statins involve PECAM-1 signaling. Blood. 2013;122:3188–96. doi: 10.1182/blood-2013-04-491845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan CC, Chennazhi KP, Menon D. In vitro hemocompatibility and vascular endothelial cell functionality on titania nanostructures under static and dynamic conditions for improved coronary stenting applications. Acta Biomater. 2013;9:9568–77. doi: 10.1016/j.actbio.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Busch R, Strohbach A, Rethfeldt S, Walz S, Busch M, Petersen S, Felix S, Sternberg K. New stent surface materials: the impact of polymer-dependent interactions of human endothelial cells, smooth muscle cells, and platelets. Acta Biomater. 2014;10:688–700. doi: 10.1016/j.actbio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Mohan CC, Chennazhi KP, Menon D. In vitro hemocompatibility and vascular endothelial cell functionality on titania nanostructures under static and dynamic conditions for improved coronary stenting applications. Acta Biomater. 2013;9:9568–77. doi: 10.1016/j.actbio.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Xu Y, Thomas V, Bellis SL, Vohra YK. Engineering an antiplatelet adhesion layer on an electrospun scaffold using porcine endothelial progenitor cells. J Biomed Mater Res A. 2011;97:145–51. doi: 10.1002/jbm.a.33040. [DOI] [PubMed] [Google Scholar]
- 15.Ming Z, Hu Y, Xiang J, Polewski P, Newman PJ, Newman DK. Lyn and PECAM-1 function as interdependent inhibitors of platelet aggregation. Blood. 2011;117:3903–6. doi: 10.1182/blood-2010-09-304816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardemann A, Knapp A, Katz N, Tillmanns H, Haberbosch W. No evidence for the CD31 C/G gene polymorphism as an independent risk factor of coronary heart disease. Thromb Haemost. 2000;83:629. [PubMed] [Google Scholar]
- 17.Sasaoka T, Kimura A, Hohta SA, Fukuda N, Kurosawa T, Izumi T. Polymorphisms in the platelet-endothelial cell adhesion molecule-1 (PECAM-1) gene, Asn563Ser and Gly670Arg, associated with myocardial infarction in the Japanese. Ann N Y Acad Sci. 2001;947:259–69. doi: 10.1111/j.1749-6632.2001.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 18.Song FC, Chen AH, Tang XM, Zhang WX, Qian XX, Li JQ, Lu Q. Association of platelet endothelial cell adhesion molecule-1 gene polymorphism with coronary heartdisease. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:156–8. [PubMed] [Google Scholar]
- 19.Listì F, Candore G, Lio D, Cavallone L, Colonna-Romano G, Caruso M, Hoffmann E, Caruso C. Association between platelet endothelial cellular adhesion molecule 1 (PECAM-1/CD31) polymorphisms and acute myocardial infarction: a study in patients from Sicily. Eur J Immunogenet. 2004;31:175–8. doi: 10.1111/j.1365-2370.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 20.Wei H, Fang L, Chowdhury SH, Gong N, Xiong Z, Song J, Mak KH, Wu S, Koay E, Sethi S, Lim YL, Chatterjee S. Platelet-endothelial cell adhesion molecule-1 gene polymorphism and its soluble level are associated with severe coronary artery stenosis in Chinese Singaporean. Clin Biochem. 2004;37:1091–7. doi: 10.1016/j.clinbiochem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Fang L, Wei H, Chowdhury SH, Gong N, Song J, Heng CK, Sethi S, Koh TH, Chatterjee S. Association of Leu125Val polymorphism of platelet endothelial cell adhesion molecule-1 (PECAM-1) gene & soluble level of PECAM-1 with coronary artery disease in Asian Indians. Indian J Med Res. 2005;121:92–9. [PubMed] [Google Scholar]
- 22.Pan M, Jiang WP, Liu ZH, Yang XJ, Zhu JH, Yuan J, Wu X. Relation between polymorphism of platelet endothelial cell adhesion molecule-1 (PECAM-1) gene and coronary artery disease in Chinese population. Jiang Su Yi Yao. 2006;32:366–368. [Google Scholar]
- 23.Xu Y, Shi YY. Association of polymorphism of platelet endothelial cell adhesion molecule-1 (PECAM-1) gene with coronary artery disease. Qiqhar Yi Xue Yuan Xue Bao. 2007;28:2712–2714. [Google Scholar]
- 24.Kang AC, Ao CH, Guo HY, Qi LT, Huo Y. Association of platelet endothelial cellular adhesion molecule-1 gene polymorphisms and its plasma level with severe coronary atherosclerosis. Zhonghua Lao Nian Duo Qi Guan Ji Bing Za Zhi. 2008;7:84–86. [Google Scholar]
- 25.Chang ZT, Chen J, Cheng LX, Zhao F, Mao XB, Yang Y, Hu P. Relation between polymorphism of platelet endothelial cell adhesion molecule-1 gene Leul25Val and coronary heart disease. Chin J Geriatr Heart Brain Vessel Dis. 2007;9:664–667. [Google Scholar]
- 26.Wei YS, Lan Y, Liu YG, Meng LQ, Xu QQ, Xie HY. Platelet-endothelial cell adhesion molecule-1 gene polymorphism and its soluble level are associated with ischemic stroke. DNA Cell Biol. 2009;28:151–8. doi: 10.1089/dna.2008.0817. [DOI] [PubMed] [Google Scholar]
- 27.Listì F, Caruso C, Balistreri CR, Grimaldi MP, Caruso M, Caimi G, Hoffmann E, Lio D, Candore G. PECAM-1/CD31 in infarction and longevity. Ann N Y Acad Sci. 2007;1100:132–9. doi: 10.1196/annals.1395.011. [DOI] [PubMed] [Google Scholar]
- 28.Listì F, Caruso C, Di Carlo D, Falcone C, Boiocchi C, Cuccia M, Candore G. Association between platelet endothelial cellular adhesion molecule-1 polymorphisms and atherosclerosis: results of a study on patients from northern Italy. Rejuvenation Res. 2010;13:237–41. doi: 10.1089/rej.2009.0940. [DOI] [PubMed] [Google Scholar]
- 29.Reschner H, Milutinovic A, Petrovič D. The PECAM-1 gene polymorphism - a genetic marker of myocardial infarction. Cent Eur J Biol. 2009;4:515–20. [Google Scholar]
- 30.Shalia KK, Mashru MR, Soneji SL, Shah VK, Payannavar S, Walvalkar A, Mokal RA, Mithbawkar SM, Kudalkar KV, Abraham A, Thakur PK. Leucine125Valine (Leu125Val) Gene Polymorphism of Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) and Myocardial Infarction in Indian Population. Indian J Clin Biochem. 2010;25:273–9. doi: 10.1007/s12291-010-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Tian Y, Dong J, Li L, Dong Z, Deng X. Application of a multiplex SNP genotyping system in predicting genetic susceptibility to CAD in Chinese people of Han ethnicity. Med Sci Monit. 2010;16:BR38–95. [PubMed] [Google Scholar]
- 32.Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of Microvascular Obstruction and Intramyocardial Hemorrhage by CMR on LV Remodeling and Outcomes After Myocardial Infarction: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging. 2014;7:940–952. doi: 10.1016/j.jcmg.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


