Abstract
Objective: To investigate the protein expression of matrix metalloproteinase 2 (MMP2) and its clinical significance in laryngeal cancer. Methods: A comprehensive search for the related literature published in China and other countries was conducted in a variety of databases, including MEDLINE, Embase, China Academic Journals Full-text Database, Wanfang Data and VIP Database. A total of seven case-control studies were included in the final systematic assessment. A meta-analysis software program was used to statistically analyze the raw data from each study for the calculation of the pooled odds ratio (OR) and 95% confidence interval (95% CI). Results: The meta-analysis indicated that, compared with normal laryngeal tissue, the MMP2 protein was highly expressed in the laryngeal cancer tissue [OR=21.67; 95% CI: 11.61-40.43; P<0.001]. Compared with highly differentiated laryngeal cancer, the MMP2 protein expression level was higher in the moderately and poorly differentiated laryngeal cancers [OR=0.25; 95% CI: 0.13-0.46; P<0.001]. Compared with laryngeal cancers without lymph node metastasis, the laryngeal cancers with lymph node metastasis exhibited a greatly elevated MMP2 protein expression [OR=0.25; 95% CI: 0.14-0.46; P<0.001]. Conclusion: High protein expression levels of MMP2 may play an important role in the tumorigenesis, progression and prognosis of laryngeal cancer.
Keywords: Laryngeal cancer, matrix metalloproteinase 2 (MMP2), meta-analysis
Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of the common malignancies in the head and neck area [1]. Local infiltration and distant metastasis are crucial for the prognosis of laryngeal cancer patients. The invasion and metastasis of malignant tumors is a complex multi-stage and multi-step process. During this process, the most critical step is the degradation of the basal membrane and extracellular matrix, which involves the participation of the matrix metalloproteinase (MMP) family as the key enzymes [2,3]. Thus, the MMP family members play an extremely pivotal role in the process of tumor infiltration. The MMP2 protein is one of the most important proteinases in this protein family. Previous studies indicated that MMP2 is highly expressed in a variety of systemic and multi-loci malignant tumors. However, studies concerning its expression in LSCC are scarce [4-6]. In this study, the expression of the MMP2 protein in LSCC was investigated for the further elucidation of its roles in the tumorigenesis, progression and metastasis of LSCC.
Data and methods
Data source
We searched a variety of databases for independent case-control studies related to the “expression of MMP2 protein in laryngeal cancer and its significance” that were published in China and other countries between January 2004 and December 2013. The databases included MEDLINE, Embase, the China Academic Journals Full-text Database, Wanfang Data and VIP Database. The search terms included keywords “MMP2”, “laryngeal squamous cell carcinoma”, “laryngeal carcinoma”, “laryngeal cancer” and “laryngeal malignant tumor”.
Inclusion criteria
The inclusion criteria for the study selection were as follows: (1) an original independent study; (2) all enrolled patients had a primary malignant tumor in the larynx; (3) an independent case-control study on MMP2 expression and its significance in laryngeal cancer; (4) the diagnosis of laryngeal cancer in the patient group was confirmed by a pathological examination, and the control group consisted of individuals who had a normal laryngeal mucosa; and (5) the odds ratio (OR) and 95% confidence interval (CI) were calculated using the raw data in original reports.
Exclusion criteria
The exclusion criteria were as follows: (1) case studies without a control group; (2) immunohistochemistry was not used for the detection; (3) when multiple studies used the same study subjects and had similar study content, the study with the most comprehensive data was selected; (4) studies that had insufficient information or were of poor quality; and (5) reviews, abstracts and conference papers.
Data extraction and assessment of literature quality
The data were extracted independently by two researchers (JSH and SRY). A rigorous quality evaluation of all selected studies was conducted in accordance with the evidence grading criteria for evidence-based medicine [7]. All discrepancies were resolved through re-checking and discussion. For the articles with unclear or missing information, the corresponding authors were contacted through mail or email to obtain the information needed. An article would be excluded if there were no response after two contact attempts.
Statistical processing
The RevMan 5.1 software was used for the statistical analysis of the endpoints. The OR and 95% CI were used to present the statistical values derived from the efficacy analysis for dichotomous variables, and the significance level was defined as α=0.05. The fixed-effects model was adopted for the pooled analysis if a statistical homogeneity existed among the studies (P>0.05, I2<50%), and the random-effects model was utilized for the analysis if a statistical heterogeneity existed among the studies (P<0.05, I2>50%).
Results
Basic information of the studies
A total of 86 relevant records were identified and retrieved from the search, including 48 English reports and 38 Chinese reports. According to the inclusion and exclusion criteria, 7 records [8-14] were included in the meta-analysis. Among the included studies, 529 subjects were detected to perform this meta-analysis. Among the excluded records, 3 reported the same study, 63 reported a study without a control group, 6 reported studies without the use of immunohistochemical detection methods, and 7 reported studies with no apparent relevance to the present study.
Comparison of the cancerous laryngeal tissue and normal laryngeal tissue
A total of 7 papers [8-14] that reported studies with normal laryngeal tissue as the control group were included. The homogeneity test on these 7 studies resulted in P = 0.43 and I2 = 0%. An analysis using the fixed-effects model showed an OR of 21.67 (95% CI: 11.61-40.43) and a statistically significant difference (P<0.001), indicating a high expression of MMP2 protein in the laryngeal cancer tissue (Figure 1).
Figure 1.
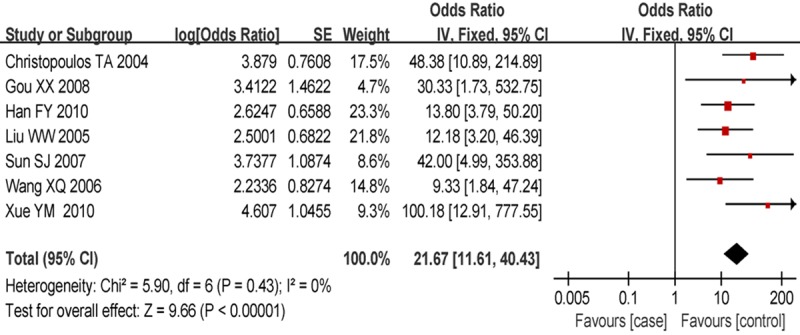
Forest plot of comparison of the cancerous laryngeal tissue and normal laryngeal tissue, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI.
Comparison of cancer tissues with different levels of differentiation
A total of 6 papers were included for the comparison of highly differentiated cancer tissues with moderately and poorly differentiated cancer tissues. The homogeneity test on these 6 studies showed P = 0.22 and I2 = 28%. The analysis using the fixed-effects model derived an OR of 0.25 (95% CI: 0.13-0.46) and a statistically significant difference (P<0.001). This result indicated that the MMP2 protein was highly expressed in moderately and poorly differentiated laryngeal cancers compared to well-differentiated laryngeal cancer (Figure 2).
Figure 2.
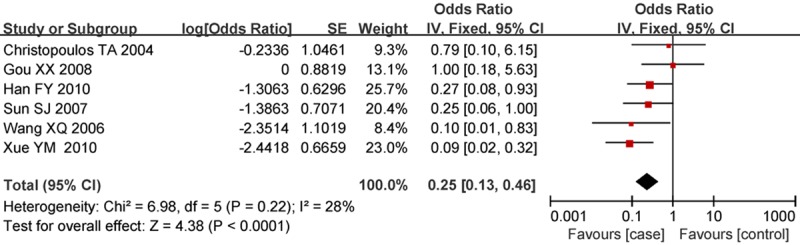
Forest plot of comparison of cancer tissues with different levels of differentiation, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI.
Comparison of laryngeal cancers with and without lymph node metastasis
Compared with laryngeal cancers without lymph node metastasis, the laryngeal cancers with lymph node metastasis exhibited a greatly elevated MMP2 protein expression [OR = 0.25; 95% CI: 0.14-0.46; P<0.001]. (Figure 3).
Figure 3.
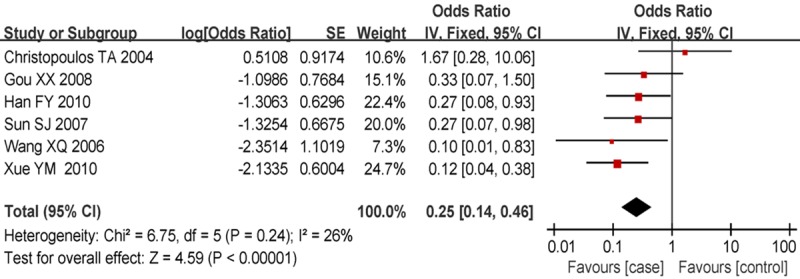
Forest plot of comparison of laryngeal cancers with and without lymph node metastasis, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95% CI.
Publication bias
Both Begg’s funnel plot and Egger’s test were performed to assess the publication bias of the literature. The shape of the funnel plots did not reveal any evidence of obvious asymmetry for the meta-analysis (Figure 4). Then, Egger’s test was used to provide statistical evidence of funnel plot symmetry. The results still did not present any obvious evidence of publication bias (P>0.05).
Figure 4.
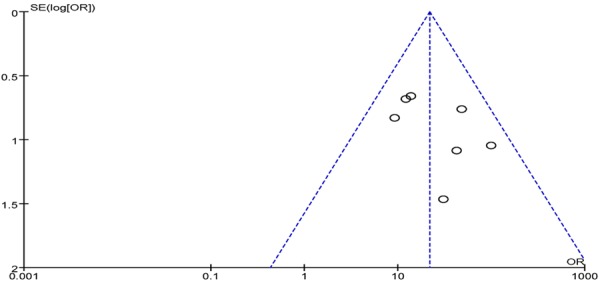
Begg’s funnel plot for publication bias tests. Each point represents a separate study for the indicated association. Log or represents natural logarithm of OR. Vertical line represents the mean effects size.
Sensitivity analysis
We deleted one single study from the overall pooled analysis each time to check the influence of the removed data set to the overall ORs. The pooled ORs and 95% CIs were not significantly altered when any part of the study was omitted, which indicated that any single study had little impact on the overall ORs.
Discussion
The results derived from a meta-analysis rely greatly on the quality of the selected reports and are thus substantially influenced by the results of previous studies. Therefore, a rigorous evaluation of the related literature and proper data processing are necessary to avoid bias and obtain reliable conclusions. A total of 7 case-control studies were included in this study, according to the inclusion criteria. The meta-analysis of the comprehensive data extracted from the selected papers derived statistically significant results, indicating a positive correlation between the MMP2 protein expression and laryngeal cancer. The sensitivity analysis indicated that the study results were stable and reliable.
One of the most distinct biological characteristics of malignant tumors is that the tumor can metastasize from the primary locus to adjacent or distant organs to form secondary tumors. Metastasis is an important factor influencing the therapeutic efficacy and prognosis of tumor patients [15-18]. The tumor cell infiltration is the first step of tumor metastasis. Cancer invasion initiates from the breaking of the barrier structures that are formed by the basal membrane and the extracellular matrix [19]. Zhang et al. [20] discovered that MMPs could degrade nearly all protein constituents of the extracellular matrix and damage the histological barrier that can impede cancer cell invasion, thereby playing a critical role in tumor invasion and metastasis. MMPs have received growing attention and are considered the primary proteinases that participate in the processes of tumor cell infiltration and metastasis. At present, 26 members of the MMP family have been identified, which are numbered as MMP1 to 26. According to their substrates and the homology of the generated fragments, MMPs are divided into six classes, as follows: collagenases, gelatinases, matrilysins, stromelysins, furin-activated MMPs and other secretory MMPs. Type IV collagenase is the most important class of MMP, and it mainly exists in the following two forms: the non-glycosylated MMP2, with a molecular weight of 72 ku, and the glycosylated MMP9, with a molecular weight of 92 ku. Currently, more in-depth studies are available on MMP2. The gene encoding MMP2 is located on human chromosome 16q21 and consists of 13 exons and 12 introns. The total length of the structural gene is 27 kb. The substrates of the MMP2 protein are type IV, V, VII and X collagens and elastic fibers [12]. Unlike other metalloproteinases, the enhancer in the 5’ flanking sequence of the MMP2 gene contains two GC boxes, instead of a TATA box. Activated MMP2 is located in the protruding part of the cell-penetrating matrix, suggesting that it may function as a “drilling bit” in the enzymatic lysis of intercellular matrix components and the major component of basal membrane type IV collagen. Thus, the activation of MMP2 can facilitate the passage of tumor cells through the extracellular matrix barrier and the basal membrane of the blood vessel wall to enter the host microenvironment, promoting the detachment of tumor cells from the primary tumor and their invasion and metastasis into adjacent and/or distant tissues. Christopoulos et al [14]. Believed that MMP2 could be clinically considered as an important pathological diagnostic parameter of laryngeal cancer because its protein expression emerges at the early stage of laryngeal cancer and gradually increases with disease progression. Significantly elevated expression levels of MMP2 protein with tumor progression and lymph node metastasis have been observed. Gorogh et al. [21] and Bogusiewicz et al. [22] reported that MMP2 played a critical role in the infiltration of laryngeal cancer cells and was closely related to lymph node metastasis of laryngeal cancer. The results from this study showed that the high expression of MMP2 protein in laryngeal cancer has a significant correlation with the tumor differentiation level and with the status of cervical lymph node metastasis. However, a specific understanding of the MMP2 protein requires further in-depth research.
Disclosure of conflict of interest
None.
References
- 1.Braakhuis BJ, Leemans CR, Visser O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol. 2014;50:670–5. doi: 10.1016/j.oraloncology.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Colović Z, Pesutić-Pisac V, Poljak NK, Racić G, Cikojević D, Kontić M. Expression of matrix metalloproteinase-9 in patients with squamous cell carcinoma of the larynx. Coll Antropol. 2013;37:151–5. [PubMed] [Google Scholar]
- 3.Bodnar M, Szylberg L, Kazmierczak W, Marszalek A. Differentiated expression of membrane type metalloproteinases (MMP-14, MMP-15) and pro-MMP2 in laryngeal squamous cell carcinoma. A novel mechanism. J Oral Pathol Med. 2013;42:267–74. doi: 10.1111/jop.12000. [DOI] [PubMed] [Google Scholar]
- 4.Bauvois B. New facets of matrix metalloprotienases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Uloza V, Liutkevičius V, Pangonytė D, Saferis V, Lesauskaitė V. Expression of matrix metalloproteinases (MMP-2 and MMP-9) in recurrent respiratory papillomas and laryngeal carcinoma: clinical and morphological parallels. Eur Arch Otorhinolaryngol. 2011;268:871–8. doi: 10.1007/s00405-011-1494-1. [DOI] [PubMed] [Google Scholar]
- 6.Uloza V, Liutkevičius V, Pangonytė D, Saferis V, Lesauskaitė V. Expression of matrix metalloproteinases (MMP-2 and MMP-9) in recurrent respiratory papillomas and laryngeal carcinoma: clinical and morphological parallels. Eur Arch Otorhinolaryngol. 2011;268:871–8. doi: 10.1007/s00405-011-1494-1. [DOI] [PubMed] [Google Scholar]
- 7.Purgato M, Cipriani A, Barbui C. Randomized trials, systematic reviews, meta-analyses: basic criteria in the world of scientific evidence. Riv Psichiatr. 2012;47:21–9. doi: 10.1708/1034.11288. [DOI] [PubMed] [Google Scholar]
- 8.Liu WW, Zeng ZY, Wu QL, Hou JH, Chen YY. Overexpression of MMP2 in laryngeal squamous cell carcinoma: a potential indicator for poor prognosis. Otolaryngol Head Neck Surg. 2005;132:395–400. doi: 10.1016/j.otohns.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 9.Gou XX, Jin F, He C. EMMPR IN/CD147 and MMP2 expression in laryngeal carcinoma and its relationship with prognosis. Zhong Liu Yu Fang Yu Zhi Liao. 2008;21:263–269. [Google Scholar]
- 10.Han FY, Liu AD. Significance of claudin-1, MMP-2, and E-cadherin expression in LSCC patients. Zhong Guo Wu Zhen Xue Za Zhi. 2010;10:1544–1545. [Google Scholar]
- 11.Wang XQ, Shan YC, Wang JQ. Matrix metalloproteinase 2 expression and clinical significance in laryngeal squamous cell carcinoma. Lin Chuang Er Bi Hou Ke Za Zhi. 2006;12:1109–1110. [PubMed] [Google Scholar]
- 12.Xu YM, Xie MQ, Liu T. The relationship between matrix metalloproteinases MMP-1 and MMP-2 with laryngeal cancer invasion and lymph node metastasis. Zhong Guo Xian Dai Yi Xue Za Zhi. 2010;20:1930–1934. [Google Scholar]
- 13.Sun SJ, Liu QM, Tian J. Expression and clinical significance of supraglottic laryngeal squamous cell carcinoma tissue VEGF, MMP2, eIF4E’s. Zhong Guo Er Bi Hou Lu Di Wai Ke Za Zhi. 2007;13:332–336. [Google Scholar]
- 14.Christopoulos TA, Papageorgakopoulou N, Theocharis DA, Aletras AJ, Tsiganos CP, Papadas TA, Mastronikolis NS, Goumas P, Vynios DH. Diagnostic and classification value of metalloproteinasesin squamous human laryngeal carcinoma. Int J Oncol. 2004;25:481–485. [PubMed] [Google Scholar]
- 15.Nakamura M, Onoda N, Noda S, Kashiwagi S, Aomatsu N, Kurata K, Kawajiri H, Takashima T, Ishikawa T, Hirakawa K. E-cadherin expression and cell proliferation in the primary tumor and metastatic lymph nodes of papillary thyroid microcarcinoma. Mol Clin Oncol. 2014;2:226–232. doi: 10.3892/mco.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Enomoto Y, Morikawa T, Matsushita H, Kume H, Fukayama M, Yamaguchi H, Kakimi K, Homma Y. High expression of heat shock protein 105 predicts a favorable prognosis for patients with urinary bladder cancer treated with radical cystectomy. Mol Clin Oncol. 2014;2:38–42. doi: 10.3892/mco.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei ZW, Xia GK, Wu Y, Schwarz RE, Smith DD, He YL, Zhang CH. Evaluation of skeletonization of the hepatoduodenal ligament for the lower third gastric cancer by propensity score analysis. Hepatogastroenterology. 2013;60:1789–96. [PubMed] [Google Scholar]
- 18.Huang YG, Li YF, Pan BL, Wang LP, Zhang Y, Lee WH, Zhang Y. Trefoil factor 1 gene alternations and expression in colorectal carcinomas. Tumori. 2013;99:702–7. doi: 10.1177/030089161309900610. [DOI] [PubMed] [Google Scholar]
- 19.Engbring JA, Kleinman HK. The basement membrane matrix in malignancy. J Pathol. 2003;200:465–470. doi: 10.1002/path.1396. [DOI] [PubMed] [Google Scholar]
- 20.Zhang QW, Liu L, Chen R, Wei YQ, Li P, Shi HS, Zhao YW. Matrix metalloproteinase-9 as a prognostic factor in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2012;13:2903–8. doi: 10.7314/apjcp.2012.13.6.2903. [DOI] [PubMed] [Google Scholar]
- 21.Gorogh T, Beier UH, Baumken J, Meyer JE, Hoffmann M, Gottschlich S, Maune S. Metalloproteinases and their inhibitors: Influence on tumor invasiveness and me-tastasis formation in head and neck squamous cell car-cinomas. Head Neck. 2006;28:31–39. doi: 10.1002/hed.20298. [DOI] [PubMed] [Google Scholar]
- 22.Bogusiewicz M, Stryjecka-zimmer M, Szymanski M, Rechberger T, Golabek W. Activity of matrix metalloproteinases-2 and-9 in advanced laryngeal cancer. Otolaryngol Head Neck Surg. 2003;128:132–136. doi: 10.1067/mhn.2003.8. [DOI] [PubMed] [Google Scholar]


