Abstract
This study aims to observe the changes of myocardial injuries after the firearm wound-induced intestinal perforation in porcine abdomen. 42 healthy Landrace piglets were randomly divided into the control group and the injury group, which was then subdivided into the post-injury 1 h, 2 h, 4 h, 8 h, 12 h and 24 h subgroup. the LDH, CK and CK-MB levels of each group, as well as the plasma endotoxin, were determined and compared. The plasma endotoxin levels of the experimental groups were significantly higher than the control group, and the light microscope observation revealed that the 8 h, 12 h and 24 h subgroup appeared the gradually-aggravated myocardial cell edema and degeneration; the electron microscope revealed that the 4 h, 8 h, 12 h and 24 h subgroup appeared the mitochondrial swelling and dissolution gradually; the serum levels of LDH, CK and CK-MB of each experimental group were higher than the control group. The abdominal firearm wound-induced intestinal perforation would lead to the damaged changes of myocardial morphology and enzymes, which would aggravate as time went along.
Keywords: Abdominal injury, firearm wound, heart, endotoxin
Introduction
The abdominal firearm wound has the characteristic of high intestinal perforation rate and high multiple organ dysfunction syndrome (MODS) rate [1], the intestinal barrier dysfunction is the important cause of MODS, the bacterial translocation and enterogenic infection would induce the release of a variety inflammatory mediators, resulting in the injuries of remote organs [2]. The heart is the arteriovenous connection and the cardiovascular power pump, as well as the key organ of MODS-caused death [3]. There has not been reported about the changes of cardiac functions and morphology after the abdominal intestinal firearm wound at home and in aboard. This study was based on the established model of abdominal intestinal firearm wound [4], and then the post-injury myocardial morphology and enzymes changes were observed, aiming to explore its variation, and providing the theoretical basis towards the treatment of abdominal intestinal firearm wound.
Materials and methods
Experimental animals and grouping
42 healthy Landrace piglets were provided by the People’s Liberation Army, 2-3 months old, with the body weight as (31.20 ± 1.40) kg, and without gender restriction. The animals were randomly divided into seven groups: the control group (CG), and the post- injury 1 h, 2 h, 4 h, 8 h, 12 h and 24 h group, with 6 piglets in each group. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Xinxiang Medical College (Permit Number: 20060828001).
Firearms parameters
54 military pistol, with 7.62 mm handgun bullet, and calibrated by the professional institute: the initial velocity was 430-435 m/s, with the projectile weight as 5.5 g, and the impact energy was 508.48-520.37J.
Environment
The experiment was carried out at room temperature, and the animals were sent back to the lab within 15 min after the injury. The room temperature and the animal laboratory environments were: temperature 22-24°C, relative humidity 50%-60%.
Experimental abdominal gunshot injury
Before the injury, the animals were conventionally fasted for 12 h, while they could freely access to water. After intramuscularly anesthetized with 5% ketamine hydrochloride injection (20 mg/kg) (Gutian Pharmaceutical Group, Fujian, China) and 0.05% atropine sulfate injection (0.04 mg/kg) (Tianjin Pharmaceutical Group, Tianjin, China), the pig was hung on the porch of Gobi military firing range with the normal walking positions, with the 4 limbs drooping naturally to completely expose the abdomen, the shooting point was marked in the non-hair area of the right abdominal plica lower edge, 2 cm away behind the navel level, a professional shooter aimed at the point 30 cm from the porcine lateral abdominal wall, and shot the projectile vertically into the right abdominal wall, and the projectile was withdrew from the left corresponding position (Figure 1). The animals were transported back to the lab by truck within 15 min after the injury, and free for the glucose saline. Except for the injury, the control group were performed the same steps with the injured groups.
Figure 1.
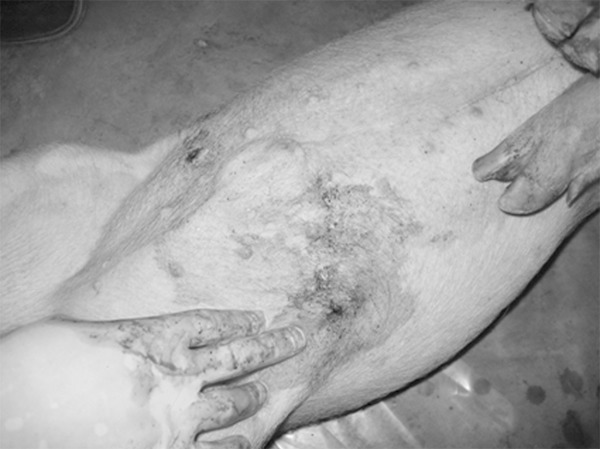
Exit of the abdominal trajectory.
Determination of biochemical indicators and endotoxin
The piglets of the injured groups were sampled the venous blood from the porcine ear vein at the post-injury 1 h, 2 h, 4 h, 8 h, 12 h and 24 h, while the control group was immediately sampled the blood when sent back to the lab. Certain part of blood sample was stood for 30 min, followed by the centrifugation, and then the Toshiba automatic biochemical analyzer was used to detect the serum levels of LDH, CK and CK-MB. The other part of blood sample was placed into the pyrogen-free heparin-coated tube for the centrifugation to extract the plasma, which was then placed into the pyrogen-free tube and stored at -80°C for the endotoxin detection. The detection was performed with the tachypleus amebocyte lysate (TAL) method, and operated according to the instructions. The TAL reagent was provided by the Xiamen TAL reagent industrial (Chinese Horseshoe Crab Reagent Manufactory, CO., Ltd., Xiamen, China).
Histopathological observation
The control group was performed the thoracotomy immediately after the blood sampling, while the injured groups were performed the thoracotomy at the corresponding time points, the apical myocardial tissues of each group were obtained, fixed with 10% neutral formalin, and performed the conventional paraffin section. After HE staining, the myocardial tissue changes were observed under the light microscope.
Electron microscopic observation
The myocardial tissues were immediately fixed with glutaraldehyde, then the fixed specimen was cut into 1 × 1 × 1 mm3 size; after performed the ultrathin section, the ultrastructural changes of myocardial tissues were observed by the electron microscopy.
Statistical analysis
The experimental data were expressed as X̅ ± s, and SPSS13.0 software was used for the data processing, the analysis of variance (ANOVA) and Newman-Keuls test were performed, with P < 0.05 considered as statistical significance.
Results
Animal’s survival and laparotomic investigation
The animals of the control group, the 1 h, 2 h, 4 h and 12 h subgroup all survived, while the 8 h and 24 h subgroup died 1 animal, respectively. All the animals were performed the laparotomy at the corresponding time points, and the exploration showed that no active bleeding and rupture of parenchymal organs, there existed about 50-300 ml yellow, light red to red bloody exudate intraperitoneally, and 2-9 sites of intestinal perforation rupture occurred at the primary injury area, with or without the mesenteric rupture at the edge of small intestine, there would exist 0-3 sites of perforation or rupture in the colon, accompanied with such intestinal contents as feces or pus moss, hematoma on the surface, the perforated intestinal wall would be valgus, with some necrosis (Figure 2). While in the 8 h, 12 h and 24 h subgroup, the animals’ abdominal pressure might be higher, the smell was much more obvious, and the peritonitis was much heavier. The sites of intestinal perforation of each group exhibited no significant difference by the analysis of variance (Table 1), thus the injury of each group could be considered as consistent.
Figure 2.
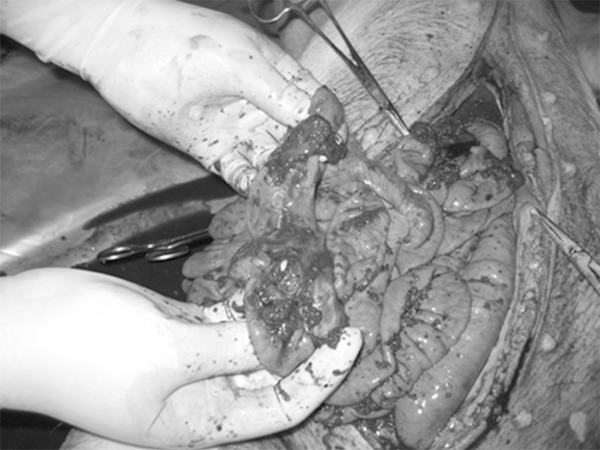
Intestinal multiple perforations.
Table 1.
Comparison of intestinal perforation sites of each group ( X̅± s)
| Group | N | Intestinal perforation sites | Colonic perforation sites | Total perforation sites |
|---|---|---|---|---|
| 1 h | 6 | 5.00 ± 2.28 | 1.33 ± 1.21 | 6.33 ± 2.80 |
| 2 h | 6 | 5.33 ± 2.42 | 1.67 ± 1.03 | 6.50 ± 3.33 |
| 4 h | 6 | 6.00 ± 2.37 | 1.50 ± 1.05 | 7.50 ± 1.76 |
| 8 h | 6 | 4.80 ± 2.59 | 1.40 ± 1.14 | 6.20 ± 2.16 |
| 12 h | 6 | 6.00 ± 1.79 | 1.33 ± 1.03 | 7.33 ± 2.50 |
| 24 h | 5 | 5.40 ± 2.30 | 1.60 ± 1.14 | 7.00 ± 2.55 |
Note: The intergroup comparisons of intestinal perforation sites aomng the experimental groups P > 0.05.
Changes of serum enzymes and plasma endotoxin
The serum levels of LDH, CK and CK-MB gradually increased along with the time after injury, and were all higher than the control group (P < 0.01), among which the indexes of the 1 h and 2 h group increased faster, while those of the 4 h, 8 h and 12 h group increased slower, but still exhibited a continuous gradual upward trend, the indexes of te 24 h group increased rapidly again. The plasma endotoxin of each experimental group was higher than the control group, and reached the peak 8 h after the injury, which still maintained at the peak level value 12 h after the injury (P < 0.05) (Table 2; Figure 3).
Table 2.
Comparison of LDH, CK, CK-MB and Endotoxin levels at different times after injury
| N | LDH (u/L) | CK (u/L) | CK-MB | Endotoxin (EU/ml) | |
|---|---|---|---|---|---|
| CG | 6 | 369.33 ± 37.76a | 398.83 ± 39.74a | 673.55 ± 14.30a | 0.135 ± 0.029 |
| 1 h | 6 | 501.17 ± 43.00a | 1089.17 ± 112.86a | 789.17 ± 32.03a | 0.274 ± 0.034 |
| 2 h | 6 | 655.83 ± 67.52 | 1657.33 ± 106.89a | 936.17 ± 82.57 | 0.352 ± 0.063 |
| 4 h | 6 | 708.00 ± 51.79b | 1808.50 ± 131.30c | 959.00 ± 103.54b | 0.474 ± 0.070 |
| 8 h | 5 | 742.80 ± 48.96b | 2123.80 ± 155.35a | 1121.40 ± 72.56b | 0.627 ± 0.083 |
| 12 h | 6 | 804.83 ± 68.54b | 2308.67 ± 194.28c | 1174 ± 125.15b | 0.612 ± 0.067 |
| 24 h | 5 | 1099.8 ± 80.67a | 4 002 ± 213.45a | 2035.8 ± 199.97a | 0.537 ± 0.046 |
P < 0.01, Compared with each experimental group;
P > 0.05, compared with the previous time point of the same group;
P < 0.05, compared with the previous time point of the same group.
Figure 3.
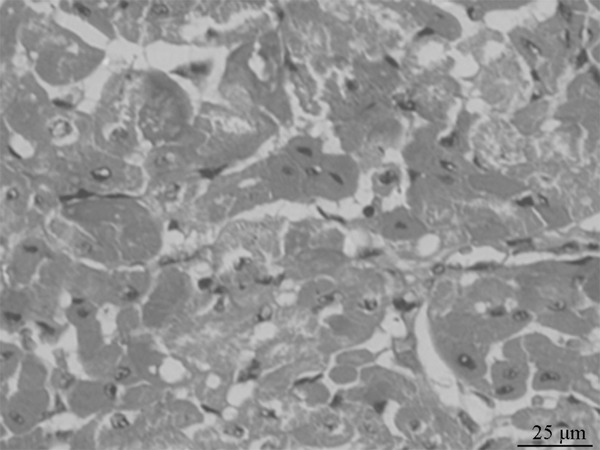
Light microscope observation of the myocardial cells of the 12 h group (×400).
Morphological changes
The appearances of the control group and the 1 h, 2 h and 4 h group were normal, under the light microscope, the myocardial cells were arranged in neat rows, with the consistent staining, and the arterial and venous endothelia were integrated. While in the 8 h, 12 h and 24 h group, the myocardium exhibited the progressively-aggravated widening of muscle bundle gaps, the myocardial cells exhibited the edema and eosinophilic changes, while no significant inflammatory cell infiltration and myocardial necrosis (Figure 3).
Ultrastructural changes
The myocardial cells of the control group, the 1 h and 2 h group: the sarcomeres were clear, the myofibrils were arranged neatly, the bright and dark bands were legible and recognizable, and the myocardial mitochondria were abundant inside the cytoplasm, which were larger and much more regularly arranged among the myofibrils. The 4 h, 8 h, 12 h and 24 h group exhibited the progressive focal dissolution of myocardial filaments, swelled mitochondria, focal cavitations, the myofibrils dissolved or disappeared flakily, and some mitochondria exhibited the flocculent dissolution and degeneration. (Figure 4).
Figure 4.
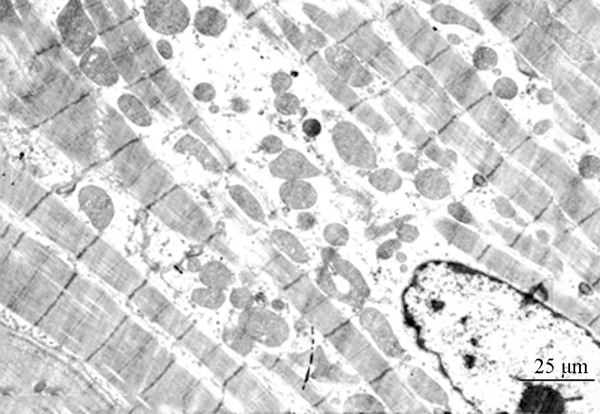
Electron microscope observation of the myocardial cells of the 4 h group (×5000).
Discussion
The heart is mainly composed of myocardium, which are the connection of arteries and veins and the “power pump” of the cardiovascular system. Through the normal operation of heart, the blood circulation is promoted normally, thus completing the body’s material transportation and continuous metabolism, achieving the humoral regulation, and maintaining the relatively constant physicochemical characteristics of internal environment. The cardiac function disorder would lead to the destruction of internal environment, even threatening the life [5,6].
The abdominal firearm wound-caused intestinal perforation would not be limited to the damaged intestine itself, it would also cause the long-period effects, the blood which contained a lot of toxins and inflammatory cytokines would cause the serious injuries of the distant organs [7,8].
In this study, the structural and functional damages caused by the abdominal firearm wound were observed through the animal experiments. The intestinal mucosa with full physiological functions would constitute the barrier against the intestinal bacteria and endotoxin.
Under the stress conditions such as trauma and infection, the visceral blood flow would reduce, especially the intestinal mucosa blood supply would be firstly involved, the oxygen saturation would thus reduce, resulting the hypoxia-induced intestinal mucosal injury, and the intestinal barrier functions would then be weakened or damaged, a lot of bacteria and LPS could thus invade the blood circulation via the portal vein and mesenteric lymph system, causing the enterogenic LPS-emia and bacterial translocation [9,10].
The experiments focused on the changes of cardiac injuries after abdominal firearm wound-causes intestinal perforations, providing the experimental basis for the further mechanism study of the secondary cardiac injury, as well as the prevention and treatment of MODS.
Our experiments showed that, 2 hours after the injury, such cardiac enzymes as LDH, CK and CK-MB increased rapidly, which might because of the acute damages caused by the stress, intestinal barrier damages and the long-distance effect of the firearm wound, the levels of LDH, CK and CK-MB rapidly increased within 2 h after the wound; 4 h, 8 h and 12 h after the injury, the levels of LDH, CK and CK-MB increased steadily, with slower increasing rate; while 24 h after the injury, LDH, CK and CK-MB increased rapidly again, reaching the peak value, the reason of this phenomenon might because the sepsis occurred, which was caused by the intestinal perforation-induced abdominal infection, based on the original acute injury, a lot of LPS entered the blood via the portal vein, which would induce the large amount generation of intracellular TNF-α, IL-6 and other inflammatory mediators [11,12].
The impacts of abdominal firearm wound towards the heart were mainly expressed in the following areas: 1) the trauma caused the transient myocardial ischemia-reperfusion, thus causing the minor damage of cardiac structures; 2) the sepsis process existed the high power cycle, resulting in the increases of wall tension and ventricular filling pressure, thereby damaging the myocardial cells; 3) the sepsis might cause the myocardial cell damages directly or through the actions of inflammatory cytokines.
LPS and these inflammatory cytokines might directly damage the cardiac cells and their ultrastructures, damage the cell membranes, interfere the ion transportation, and damage the mitochondria [13-16]. LPS could also increase the apoptotic activity, promote the myocardial stunning, so that the number of functional myocardial cells would reduce [17,18]. 24 h after the injury, the increasing rates of LDH, CK and CK-MB were slightly different, but the overall trend was rising, which was basically consistent with the gradually increased endotoxin after the injury, and it was also consistent with the trend of gradually increased cardiac structural damages.
Although the mechanisms of cardiac injury after the abdominal firearm wound-induced intestinal perforation still needed the further study, this experiment had exhibited the post-injury changes of cardiac structures and functions, and prompted that it would be really necessary to take the appropriate protective measures against heart damage as early as possible.
The intestinal perforation might cause the changes of cardiac functions and morphology, which would aggravate with the time’s going on, and it might also be one of the factors that led to the high incidence rate of abdominal firearm wound-caused MODS.
Mastering the mechanism of cardiac injury caused by the abdominal firearm wound-induced intestinal perforation might provide the appropriate measures to prevent and treat the myocardial injuries, as well as the theoretical basis for the protection and treatment of cardiac dysfunction.
Disclosure of conflict of interest
None.
References
- 1.Vertrees A, Wakefield M, Pickett C, Greer L, Wilson A, Gillern S, Nelson J, Aydelotte J, Stojadinovic A, Shriver C. Outcomes of primary repair and primary anastomosis in war-related colon injuries. J Trauma. 2009;66:1286–1291. doi: 10.1097/TA.0b013e31819ea3fc. discussion 1291-1293. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Yuan C, Hua G, Tong R, Luo X, Ying Z. Early gut barrier dysfunction in patients with severe acute pancreatitis: attenuated by continuous blood purification treatment. Int J Artif Organs. 2010;33:706–715. [PubMed] [Google Scholar]
- 3.Smeding L, Kuiper JW, Plötz FB, Kneyber MC, Groeneveld AJ. Aggravation of myocardial dysfunction by injurious mechanical ventilation in LPS-induced pneumonia in rats. Respir Res. 2013;14:92. doi: 10.1186/1465-9921-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu JW, Zhang YJ, Li ZX, Zhao XY, Long XP, Chang DC, Xu FL. Establishment of porcine models of firearm wound of intestine in normal temperature environment and dry heat environment. Trauma Surg. 2007;9:408–410. [Google Scholar]
- 5.Michler RE. Stem Cell Therapy for Heart Failure. Methodist Debakey Cardiovasc J. 2013;9:187–194. doi: 10.14797/mdcj-9-4-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrese V, Taglialatela M. New advances in beta-blocker therapy in heart failure. Front Physiol. 2013;4:323. doi: 10.3389/fphys.2013.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toft P, Andersen SK, Tønnesen EK. The systematic inflammatory response after major trauma. Ugeskr Laeger. 2003;165:669–672. [PubMed] [Google Scholar]
- 8.Fullerton JN, O’Brien AJ, Gilroy DW. Lipid mediators in immune dysfunction after severe inflammation. Trends Immunol. 2013;35:12–21. doi: 10.1016/j.it.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maynar J, Martínez-Sagasti F, Herrera-Gutiérrez M, Martí F, Candel FJ, Belda J, Castaño S, Sanchez-Izquierdo JÁ. Direct hemoperfusion with polymyxin B-immobilized cartridge in severe sepsis due to intestinal perforation: hemodynamic findings and clinical considerations in anticoagulation therapy. Rev Esp Quimioter. 2013;26:151–158. [PubMed] [Google Scholar]
- 10.Ito M, Kase H, Shimoyama O, Takahashi T. Effects of polymyxin B-immobilized fiber using a rat cecal ligation and perforation model. ASAIO J. 2009;55:246–250. doi: 10.1097/MAT.0b013e31819434ab. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Jia B, Wang F, Lv X, Peng X, Wang Y, Li H, Wang Y, Lu D, Wang H. α1 adrenoceptor activation by norepinephrine inhibits LPS-induced cardiomyocyte TNF-α production via modulating ERK1/2 and NF-κB pathway. J Cell Mol Med. 2014;18:263–273. doi: 10.1111/jcmm.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott MJ, Liu S, Su GL, Vodovotz Y, Billiar TR. Hepatocytes enhance effects of lipopolysaccharide on liver nonparenchymal cells through close cell interactions. Shock. 2005;23:453–458. doi: 10.1097/01.shk.0000160939.08385.f1. [DOI] [PubMed] [Google Scholar]
- 13.Tatsumi T, Akashi K, Keira N, Matoba S, Mano A, Shiraishi J, Yamanaka S, Kobara M, Hibino N, Hosokawa S, Asayama J, Fushiki S, Fliss H, Nakagawa M, Matsubara H. Cytokine-induced nitric oxide inhibits mitochondrial energy production and induces myocardial dysfunction in endotoxin-treated rat hearts. J Mol Cell Cardiol. 2004;37:775–784. doi: 10.1016/j.yjmcc.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Li YP, Huang J, Huang SG, Xu YG, Xu YY, Liao JY, Feng X, Zhang XG, Wang JH, Wang J. The compromised inflammatory response to bacterial components after pediatric cardiac surgery is associated with cardiopulmonary bypass-suppressed Toll-like receptor signal transduction pathways. J Crit Care. 2014;29:312.e7–312.e13. doi: 10.1016/j.jcrc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, Kalra PR, Buhner S, Herrmann R, Springer J, Doehner W, von Haehling S, Anker SD, Rauchhaus M. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157:80–85. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed LA. Protective effects of magnesium supplementation on metabolic energy derangements in lipopolysaccharide-induced cardiotoxicity in mice. Eur J Pharmacol. 2012;694:75–81. doi: 10.1016/j.ejphar.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Dong M, Hu N, Hua Y, Xu X, Kandadi MR, Guo R, Jiang S, Nair S, Hu D, Ren J. Chronic Akt activation attenuated lipopolysaccharide-induced cardiac dysfunction via Akt/GSK3β-dependent inhibition of apoptosis and ER stress. Biochim Biophys Acta. 2013;1832:848–863. doi: 10.1016/j.bbadis.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HY, Liu XY, Han G, Wang ZY, Li XX, Jiang ZM, Jiang CM. LPS induces cardiomyocyte injury through calcium-sensing receptor. Mol Cell Biochem. 2013;379:153–159. doi: 10.1007/s11010-013-1637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


