Abstract
The objective of this study was to perform a meta-analysis and literature review on the predictive role of vascular endothelial growth factor (VEGF) in prostate cancer. A detailed literature search was performed using PubMed and Embase databases for related research publications written in English. Methodological quality of the studies was also evaluated. Data was collected from studies comparing overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), biomedical failure (BF) and cancer-specific survival (CSS) in patients with elevated VEGF levels and those having lower levels. The hazard ratio (HR) and its 95% confidence interval (CI) were used to assess the strength of associations. A total of 12 studies (n = 1,737) were included in this meta-analysis (4 for OS, 3 for CSS, 2 for DFS, 4 for BF, and 4 for PFS). For OS, DFS and PFS, the pooled HR for VEGF was not statistically significant at 1.30 (95% CI, 0.74-2.29), 0.80 (95% CI, 0.57-1.13) and 1.04 (95% CI, 0.93-1.16), respectively. However, for CSS and BF, the pooled HR was 2.32 (95% CI, 1.20-4.46) and 1.30 (95% CI, 1.06-1.59), respectively. Our results demonstrate that VEGF may have a critical prognostic value in patients with prostatic cancer.
Keywords: Vascular endothelial growth factor, meta-analysis, prognosis, prostate cancer
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer and second leading cause of death in men [1]. While many men present with localized and potentially curable disease, the majority of deaths from PCa result from lymphatic and hematogenous dissemination of tumor cells, which gives rise to metastases. It is difficult for clinicians to estimate survival in patients with newly diagnosed PCa, and uncertainty therefore exists about the optimal treatment [2], especially for men with localized disease. Therefore, more accurate PCa prognosis and predictive markers should be used to guide therapy and monitor disease progress in individual patients.
Although current clinical strategies for evaluating PCa prognosis include Gleason grade, TNM stage, surgical margin status, performance status, hemoglobin (Hgb), weight loss and serum prostate-specific antigen (PSA) levels [3,4], better prognostic markers are required to identify both high- and low-risk patients for whom therapy could be more specifically tailored.
Angiogenesis plays an important role in the development and recruitment of new blood vessels, and it is necessary for tumor growth and metastasis [5,6]. Vascular endothelial growth factor (VEGF), originally known as vascular permeability factor, is one of the most potent and well-characterized proangiogenic factors. It plays a crucial role in tumor neovascularization by increasing blood vessel permeability and endothelial cell growth, proliferation, migration, and differentiation [7-9]. There is some epidemiologic evidence suggesting that higher a VEGF level is associated with a poorer prognosis in PCa patients, but previous studies investigating the relationship between higher VEGF levels and PCa patients have reported inconsistent findings [10-12]. Therefore, it is essential to carry out a systematic meta-analysis to summarize the global results and address the inconsistencies in the literature. We conducted a systematic review and meta-analysis to evaluate the overall risk of elevated VEGF and survival in PCa patients.
Materials and methods
This meta-analysis was performed according to the Meta-analysis of Observational Studies in Epidemiology Group (MOOSE) guidelines [13].
Publication search and inclusion criteria
We conducted a comprehensive literature search using PubMed and Embase databases from their inception through July, 2014. The following terms were used to identify relevant studies: “VEGF or vascular endothelial growth factor”, “prognosis”, and “prostate cancer”. References of retrieved articles and reviews were manually screened for additional studies.
The following inclusion criteria were used to identify eligible studies: (1) VEGF expression was measured; (2) full text articles in English; (3) the potential association between pretreatment VEGF and the survival outcome of PCa patients was evaluated; (4) the risk point was estimated and reported as a hazard ratio (HR) that compared a higher VEGF level to a lower VEGF level. Reviews, comments, laboratory studies, duplicated studies and irrelevant articles were excluded.
Two independent researchers (ZQL and JMF) screened and retrieved all of the publications that met the inclusion criteria. The identified studies were double-checked by both researchers. When discrepancies existed between the two investigators, another investigator (Xu) was invited to discuss until a consensus was reached.
A flow diagram of the study selection process is shown in Figure 1.
Figure 1.
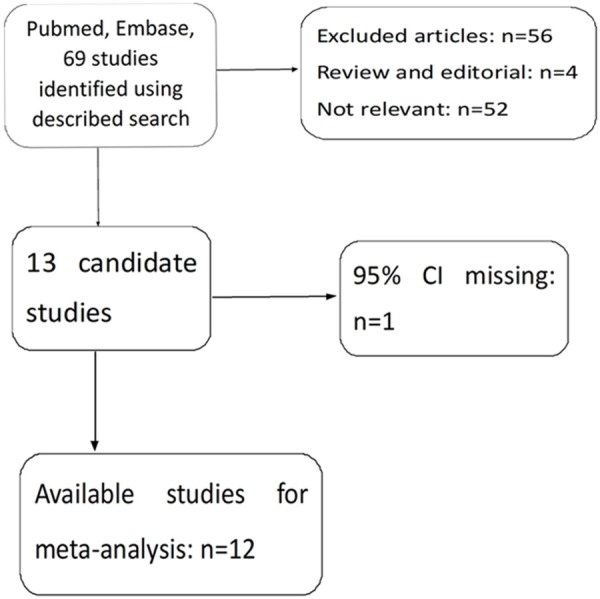
Flow diagram of the study selection process. HR = hazard ratio.
Data extraction and conversion
The following items were collected from each study: first author’s name, publication year, country where the study was performed, study design, number of patients, total sample size, age, years of follow up and HR of elevated VEGF for overall survival (OS), cancer-specific survival (CSS), disease-free survival (DFS), biomedical failure (BF) and progression-free survival (PFS) and their 95% CIs. We collected the HR and their 95% CIs from the original literature directly, and an HR of > 1 was associated with a poorer outcome. When these data were not directly reported, we extracted them from the graphical survival plots when data was available as Kaplan-Meier curves, and HR was estimated, as previously described [14].
Statistical analysis
Cochran’s Q test and Higgins’ I-squared statistics were used to verify the heterogeneity of the combined HRs. A P value of < 0.05 suggested significant heterogeneity among studies. We used the random effects model (Der Simonian and Laird method) if heterogeneity was observed (P < 0.05). The random effects model was also used in the absence of between-study heterogeneity (P ≥ 0.05). To validate the credibility of outcomes in this meta-analysis, sensitivity analysis was performed by sequential omission of individual studies. We evaluated the potential publication bias using a funnel plot with the Egger’s bias indicator test [15]. Statistical analyses were carried out using the statistical software Stata version 12.0 (Stata Corp LP, College Station, TX, USA).
Results
Data retrieval
Sixty-nine potentially relevant records for VEGF were initially identified after a search of the PubMed and Embase databases. After carefully reading the articles, 57 studies were excluded because they were review articles, letters, non-English studies, laboratory studies, studies lacking some data or key information or studies irrelevant to the current analysis. One study was excluded because the HR was missing [16]. There were 12 studies [10-12,17-25] included in this meta-analysis (Figure 1). Notably, there were two publications that involved three studies [12,20] and one publication that involved two studies [10].
Study characteristics
The characteristics of selected studies are listed in Table 1. We collected the data from 12 studies, which involved a total of 1,737 patients. Of these studies, 1 originated from Japan [18], 4 from the United States [11,12,17,22], 1 from China [23], 1 from France [21], 1 from Canada [20], 3 from UK [10,19,24] and 1 from Switzerland [25]. The follow-up period was stated in 10 studies and clarified the median follow-up period. The median follow-up period in all the studies ranged from 14 to 146.4 months. In each of the 12 articles (n = 1,737), VEGF values were analyzed in different ways. The VEGF level was measured by immunohistochemistry (IHC) in 8 studies, by enzyme-linked immunosorbent assay (ELISA) in 3 studies and by polymerase chain reaction (PCR) in 1 study. All articles related to IHC assessed and scored the VEGF intensity. However, positive VEGF staining in IHC was defined differently in various studies. The treatments used in the 12 studies were also different. For example, in 7 studies, patients were treated with prostatectomy, 4 studies used radiotherapy, and in the other 2 studies, patients were treated with hormone therapy. Seven of the studies were prospective analyses and 5 were retrospective analyses. Ten of the selected studies presented HRs. In the remaining 2 studies, we calculated the HRs from the survival curves.
Table 1.
Summery table of the meta-analysis
| Author | Year | Country | Study design | Recruitment period | Age (median) | Case | Treatment setting | Measurement | Cutoff | High expression of VEGF | Survival analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang Q | 2013 | China | Retrospective | 1982-2007 | 34-85 (65.83) | 148 | Prostatectomy | IHC | Low VS high | 98 | CSS |
| Vergis R | 2008 | UK | Prospective | 1995-2002 | 50-80 (67) | 201 | Radiotherapy | IHC | Low VS high | 34 | BF |
| Vergis R | 2008 | UK | Prospective | 1995-2005 | 45-78 (61) | 278 | Prostatectomy | IHC | Low VS high | 14 | BF |
| West AF | 2001 | UK | Prospective | 1989-1994 | 49-90 | 57 | Prostatectomy | IHC | Low VS high | 32 | CSS |
| Shariat SF | 2004 | USA | Prospective | 1994-1995 | 40-80 (62.6) | 215 | Prostatectomy | ELISA | 9.9 pg/ml | _ | PFS |
| Pan L | 2013 | Canada | Retrospective | -1991 | 55-81 (71) | 103 | Radiotherapy | IHC | 0-1 vs. 2-3 | 54 | OS DFS BF |
| George DJ | 2001 | USA | Prospective | 1996-1998 | 62-75 (68) | 197 | Hormone therapy | ELISA | 260 pg/ml | 16 | OS |
| Bok RA | 2001 | USA | Prospective | _ | 63-76 (70) | 100 | Hormone therapy | urine ELISA | 28 pg/ml | 50 | OS |
| Green MM | 2007 | UK | Retrospective | 1995-2000 | _ | 50 | Radiotherapy | IHC | Low VS high | 28 | CSS |
| Peyromaure M | 2007 | France | Prospective | 2005.06-11 | 57-68 (62) | 89 | Prostatectomy | IHC | Low VS high | 36 | PFS |
| Fukuda H | 2007 | Japan | Retrospective | 1997-2003 | 5-83 (71) | 58 | Prostatectomy | IHC | Low VS high | _ | PFS |
| Weber DC | 2012 | Switzerland | Prospective | 1994-2004 | 56-81 (69.1) | 103 | Radiotherapy | IHC | Low VS high | 76 | PFS |
| Mori R | 2010 | USA | Retrospective | 1972-1999 | _ | 138 | Prostatectomy | PCR | 10.35 | 44 | OS DFS BF |
Abbreviations: CSS, cancer-specific survival; PFS, progression-free survival; OS, overall survival; DFS, disease-free survival; BF, biomedical failure; IHC, immunohistochemistry; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; (-), not reported.
Overall survival
Because there was some evidence for heterogeneity in the studies evaluating the OS (I2 = 78.5%, P = 0.003), the random model was used to calculate the pooled HR and its 95% CI. We found that the increased VEGF level did not predict a worse outcome for OS with a pooled HR estimate of 1.30 (95% CI, 0.74-2.29) (Figure 2). We then conducted a sensitivity analysis to explore the heterogeneity among the studies by omitting the studies one by one and repeating the meta-analysis. Heterogeneity became insignificant with the removal of Mori et al. [12] (I2 = 20.2%; P = 0.286), and the results were substantially changed with an OR of 1.66 (95% CI, 1.21-2.29).
Figure 2.
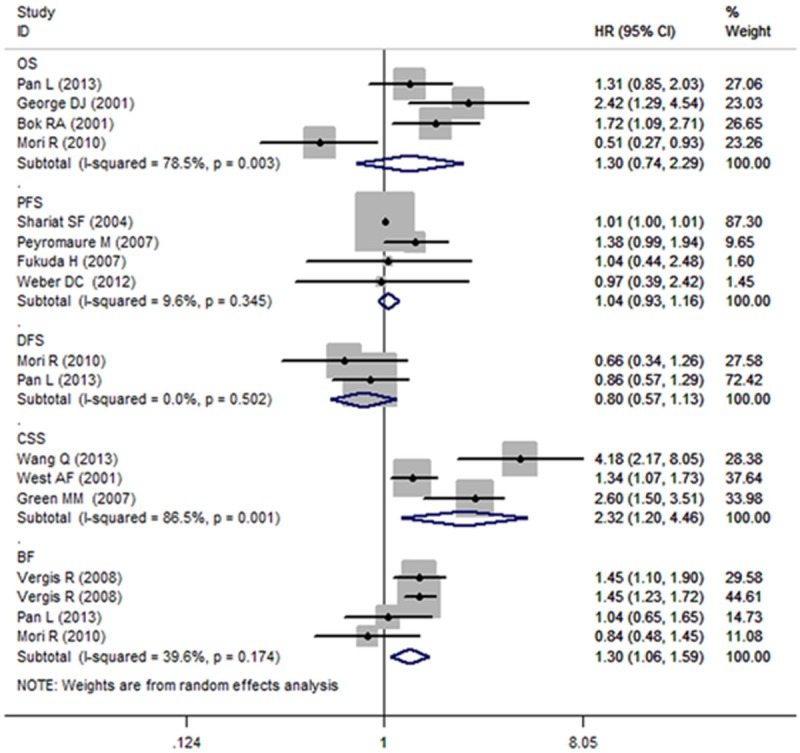
Forest plots of studies evaluating hazard ratios (HR) with 95% confidence interval (95% CI) for high vascular endothelial growth factor (VEGF) expression as compared with low expression. Survival data are reported as overall survival, cancer-specific survival, disease-free survival, progression-free survival and biomedical failure.
Disease-free survival and progression-free survival
A random effects model was also used for DFS and PFS, although the P values of between-study heterogeneity were 0.502 and 0.345 for DFS and PFS analyses, respectively. As illustrated in Figure 2, the combined HR of 0.80 (95% CI, 0.57-1.13) showed that the high VEGF level did not predict a worse outcome for DFS in PCa patients. In addition, the pooled HR was 1.04 (95% CI, 0.93-1.16) for the ability to evaluate PFS.
From the above data, VEGF was shown not to be a prognostic biomarker for OS, DFS and PFS in PCa patients.
Cancer-specific survival and biomedical failure
For CSS and BF, a random effects model was also used. The P values for between-study heterogeneity were 0.001 and 0.174 for CSS and BF analyses, respectively. As illustrated in Figure 2, the combined HR of 2.32 (95% CI, 1.20-4.46) showed that high VEGF levels had a significant relationship with CSS in PCa patients. A sensitivity analysis was thus conducted to explore the heterogeneity among the studies. When we omitted the studies one by one and repeated the meta-analysis, heterogeneity became insignificant with the removal of West et al. [24] (I2 = 29.3%; P = 0.234), and the results were substantially changed with an OR of 3.08 (95% CI, 1.97-4.80). The pooled HR was 1.30 (95% CI, 1.06-1.59) for the ability to evaluate BF.
From the above data, VEGF was shown to be a prognostic biomarker for CSS and BF in PCa patients.
Publication bias
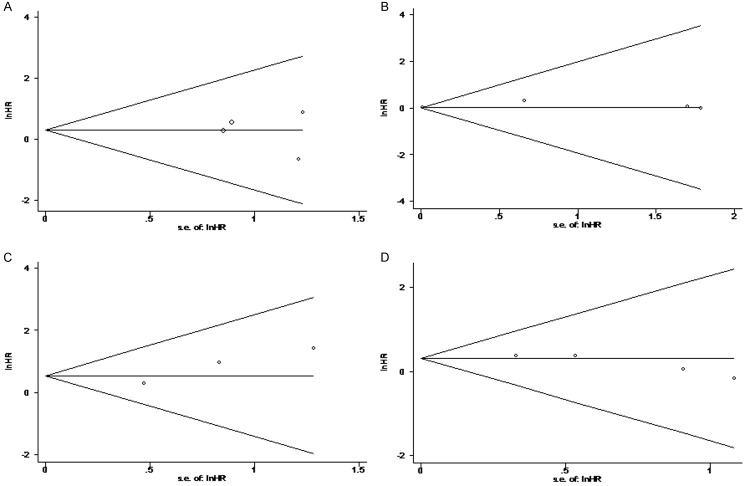
Funnel plots and Egger’s test were used to assess the publication bias of the studies that were included in this meta-analysis. As shown in Figure 3, four of the funnel plots were symmetrical. There was no evidence for significant publication bias for OS, CSS, PFS and BF because the P value for Egger’s regression intercepts was greater than 0.05 (P = 0.757, P = 0.096, P = 0.434 and P = 0.080, respectively). However, because there were only two studies for DFS, we did not perform a funnel plot to display publication bias.
Figure 3.
Funnel plots of studies included in the 4 meta-analyses: A. Overall survival. B. Progression-free survival. C. Cancer-specific survival. D. Biomedical failure.
Discussion
To date, clinically approved biomarkers have been identified to guide treatment and predict outcomes in several solid tumors. PSA, prostate-specific membrane antigen and prostate stem cell antigen are currently used in routine pathological assessment of PCa patients. Many clinical studies have indicated that C-reactive protein (CRP), pAKT, nuclear factor-κB (NF-κB), macrophage inhibitory cytokine-1 (MIC-1), matrix metalloproteinase (MMP)-1 and MMP-9 are associated with survival in PCa patients [26-28]. In addition, autophagy defects have been shown to be closely associated with survival in PCa patients [29]. A recent study showed that higher VEGF and MMP-9 expression predicts the presence and extent of axillary lymph node metastasis in breast cancer [30]. Using immunohistochemical staining and rapid colorimetric in situ hybridization, Kuniyasu et al. [30] found that VEGF expression and the PCa Gleason score were closely linked. However, previous studies have reported that a higher VEGF level was significantly associated with a worse outcome in PCa patients [10,11], but other studies did not show any significant link between VEGF and survival in these patients [12]. Therefore, it was essential to analyze the combined data to obtain acceptable results.
Wang et al. [31] systematically reviewed the data and found that VEGF showed a promising association with PCa outcomes. In our meta-analysis, a high VEGF-expression in PCa was shown to be a poor prognostic factor, with statistical significance for OS but not PFS. However, the number of studies included in these analyses was relatively small and the results might not have sufficient power. In addition, Wang et al. assessed only the relationship between VEGF expression and OS and PFS in PCa patients. Thus, we performed this updated meta-analysis that included 12 publications, and demonstrated that high a VEGF expression level was a significant marker for predicting OS, CSS and BF in PCa patients.
VEGF is a multifunctional cytokine that can increase microvascular permeability [32] and stimulate endothelial cell growth and angiogenesis [33-36]. Several factors can influence VEGF expression, including hypoxia [37] and transforming growth factor-β [38]. Once VEGF binds to VEGF receptors, receptor dimerization and autophosphorylation is induced and downstream signaling via several secondary messengers, including several protein kinases and phosphatases, is activated. This supports a proangiogenic phenotype [39].
VEGF polymorphism increases the risk of epithelial ovarian cancer [40]. Two meta-analyses showed the associations between three VEGF polymorphisms and the risk of PCa. In one meta-analysis, VEGF rs833061, rs3025039 and rs2010963 were not associated with risk of PCa [41]; in the other study, VEGF 1154G/A and 2578C/A polymorphisms were also not associated with PCa risk [42].
Our systematic review found that higher VEGF expression was not associated with OS, PFS and DFS in PCa patients. Conversely, a higher VEGF expression level was associated with CSS and BF. The OS indicates that a higher VEGF expression is unlikely to have a substantial association with PCa. However this overall result was heterogeneous, and a sensitivity analysis found that the heterogeneity was driven by the study by Mori et al. [12]. This study was methodologically different from the other studies included in the OS meta-analysis (VEGF expression in surgical specimens detected using RT-PCR), and the HR was found to be less than 1.
Our DFS meta-analysis also found no significant association between higher VEGF expression and the PCa prognosis. However, this meta-analysis had only two studies, so further research on DFS and PCa patient prognosis is required. Our data showed that the VEGF level was not associated with PFS. Although the data was not significantly heterogeneous, the treatment methods used in the four studies were all different. For example, in one study, patients were treated with radiotherapy [25] and in three studies, prostatectomy was used [17,21,22].
High VEGF levels were found to be associated with CSS and BF in our study. However, this CSS result was heterogeneous, and a sensitivity analysis found that the heterogeneity was driven by West et al. [25]. After removing West et al. [25], this result became more significant.
Several limitations of this study should be considered. First, the publications retrieved in our study were limited to those written in English, suggesting that there may be a language bias. Second, heterogeneity among some of these studies were relatively large, which might be caused by baseline characteristics of the patients, such as age, different countries, histological type of cancer, adjuvant treatment, the duration of follow-up and adjustments for other cofactors. Third, a key limitation is retrospective study design. In the current meta-analysis, only seven studies used a prospective design. Forth, in the absence of directly-reported HR values, HR extrapolation from survival curves introduced an element of decreased reliability.
In conclusion, the evidence from the meta-analysis indicated that high VEGF expression was somewhat associated with worse PCa survival. VEGF was a strong predictor of OS, CSS and BF outcomes. However, our results should be considered with caution due to the limitations listed above. To better understand and use VEGF as biomarkers in the clinical, further research with standardized, unbiased methods and larger, worldwide sample sizes are required.
Acknowledgements
This article is supported by the National Natural Science Foundation of China (NO: 81372749).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Holmboe ES, Concato J. Treatment decisions for localized prostate cancer: asking men what’s important. J Gen Intern Med. 2000;15:694–701. doi: 10.1046/j.1525-1497.2000.90842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George DJ, Kantoff PW. Prognostic indicators in hormone refractory prostate cancer. Urol Clin North Am. 1999;26:303–310. doi: 10.1016/s0094-0143(05)70070-7. [DOI] [PubMed] [Google Scholar]
- 4.Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, Scardino PT, Pearson JD. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445–1451. [PubMed] [Google Scholar]
- 5.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 7.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 9.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 10.Vergis R, Corbishley CM, Norman AR, Bartlett J, Jhavar S, Borre M, Heeboll S, Horwich A, Huddart R, Khoo V, Eeles R, Cooper C, Sydes M, Dearnaley D, Parker C. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9:342–351. doi: 10.1016/S1470-2045(08)70076-7. [DOI] [PubMed] [Google Scholar]
- 11.George DJ, Halabi S, Shepard TF, Vogelzang NJ, Hayes DF, Small EJ, Kantoff PW Cancer and Leukemia Group B 9480. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res. 2001;7:1932–1936. [PubMed] [Google Scholar]
- 12.Mori R, Dorff TB, Xiong S, Tarabolous CJ, Ye W, Groshen S, Danenberg KD, Danenberg PV, Pinski JK. The relationship between proangiogenic gene expression levels in prostate cancer and their prognostic value for clinical outcomes. Prostate. 2010;70:1692–1700. doi: 10.1002/pros.21204. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svatek RS, Jeldres C, Karakiewicz PI, Suardi N, Walz J, Roehrborn CG, Montorsi F, Slawin KM, Shariat SF. Pre-treatment biomarker levels improve the accuracy of post-prostatectomy nomogram for prediction of biochemical recurrence. Prostate. 2009;69:886–894. doi: 10.1002/pros.20938. [DOI] [PubMed] [Google Scholar]
- 17.Bok RA, Halabi S, Fei DT, Rodriquez CR, Hayes DF, Vogelzang NJ, Kantoff P, Shuman MA, Small EJ. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: a cancer and leukemia group B study. Cancer Res. 2001;61:2533–2536. [PubMed] [Google Scholar]
- 18.Fukuda H, Tsuchiya N, Narita S, Kumazawa T, Horikawa Y, Inoue T, Saito M, Yuasa T, Matsuura S, Satoh S, Ogawa O, Habuchi T. Clinical implication of vascular endothelial growth factor T-460C polymorphism in the risk and progression of prostate cancer. Oncol Rep. 2007;18:1155–1163. [PubMed] [Google Scholar]
- 19.Green MM, Hiley CT, Shanks JH, Bottomley IC, West CM, Cowan RA, Stratford IJ. Expression of vascular endothelial growth factor (VEGF) in locally invasive prostate cancer is prognostic for radiotherapy outcome. Int J Radiat Oncol Biol Phys. 2007;67:84–90. doi: 10.1016/j.ijrobp.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 20.Pan L, Baek S, Edmonds PR, Roach M 3rd, Wolkov H, Shah S, Pollack A, Hammond ME, Dicker AP. Vascular endothelial growth factor (VEGF) expression in locally advanced prostate cancer: secondary analysis of radiation therapy oncology group (RTOG) 8610. Radiat Oncol. 2013;8:100. doi: 10.1186/1748-717X-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peyromaure M, Badoual C, Camparo P, Grabar S, Goulvestre C, Fulla Y, Vieillefond A, Mao K, Dinh-Xuan AT. Plasma levels and expression of vascular endothelial growth factor-A in human localized prostate cancer. Oncol Rep. 2007;18:145–149. [PubMed] [Google Scholar]
- 22.Shariat SF, Anwuri VA, Lamb DJ, Shah NV, Wheeler TM, Slawin KM. Association of preoperative plasma levels of vascular endothelial growth factor and soluble vascular cell adhesion molecule-1 with lymph node status and biochemical progression after radical prostatectomy. J. Clin. Oncol. 2004;22:1655–1663. doi: 10.1200/JCO.2004.09.142. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Diao X, Sun J, Chen Z. Stromal cell-derived factor-1 and vascular endothelial growth factor as biomarkers for lymph node metastasis and poor cancer-specific survival in prostate cancer patients after radical prostatectomy. Urol Oncol. 2013;31:312–317. doi: 10.1016/j.urolonc.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 24.West AF, O’Donnell M, Charlton RG, Neal DE, Leung HY. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br J Cancer. 2001;85:576–583. doi: 10.1054/bjoc.2001.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber DC, Tille JC, Combescure C, Egger JF, Laouiti M, Hammad K, Granger P, Rubbia-Brandt L, Miralbell R. The prognostic value of expression of HIF1alpha, EGFR and VEGF-A, in localized prostate cancer for intermediate- and high-risk patients treated with radiation therapy with or without androgen deprivation therapy. Radiat Oncol. 2012;7:66. doi: 10.1186/1748-717X-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mimeault M, Johansson SL, Batra SK. Pathobiological implications of the expression of EGFR, pAkt, NF-kappaB and MIC-1 in prostate cancer stem cells and their progenies. PLoS One. 2012;7:e31919. doi: 10.1371/journal.pone.0031919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozden F, Saygin C, Uzunaslan D, Onal B, Durak H, Aki H. Expression of MMP-1, MMP-9 and TIMP-2 in prostate carcinoma and their influence on prognosis and survival. J Cancer Res Clin Oncol. 2013;139:1373–1382. doi: 10.1007/s00432-013-1453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ZQ, Chu L, Fang JM, Zhang X, Zhao HX, Chen YJ, Xu Q. Prognostic role of C-reactive protein in prostate cancer: a systematic review and meta-analysis. Asian J Androl. 2014;16:467–471. doi: 10.4103/1008-682X.123686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X, Zhong W, Huang H, He H, Jiang F, Chen Y, Yue F, Zou J, Li X, He Y, You P, Yang W, Lai Y, Wang F, Liu L. Autophagy defects suggested by low levels of autophagy activator MAP1S and high levels of autophagy inhibitor LRPPRC predict poor prognosis of prostate cancer patients. Mol Carcinog. 2014 doi: 10.1002/mc.22193. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng XH, Ou ZL, Yu KD, Feng LY, Yin WJ, Li J, Shen ZZ, Shao ZM. Absence of multiple atypical chemokine binders (ACBs) and the presence of VEGF and MMP-9 predict axillary lymph node metastasis in early breast carcinomas. Med Oncol. 2014;31:145. doi: 10.1007/s12032-014-0145-y. [DOI] [PubMed] [Google Scholar]
- 31.Wang K, Peng HL, Li LK. Prognostic value of vascular endothelial growth factor expression in patients with prostate cancer: a systematic review with meta-analysis. Asian Pac J Cancer Prev. 2012;13:5665–5669. doi: 10.7314/apjcp.2012.13.11.5665. [DOI] [PubMed] [Google Scholar]
- 32.Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50:1774–1778. [PubMed] [Google Scholar]
- 33.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 34.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 35.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 36.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 38.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 40.Rinck-Junior JA, Oliveira C, Lourenco GJ, Sagarra RA, Derchain SF, Segalla JG, Lima CS. Vascular endothelial growth factor (VEGF) polymorphism and increased risk of epithelial ovarian cancer. J Cancer Res Clin Oncol. 2015;141:69–73. doi: 10.1007/s00432-014-1786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen GQ, Luo JB, Wang GZ, Ding JE. Assessment of the associations between three VEGF polymorphisms and risk of prostate cancer. Tumour Biol. 2014;35:1875–1879. doi: 10.1007/s13277-013-1250-9. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Zhu S. Associations between vascular endothelial growth factor polymorphisms and prostate cancer risk: a meta-analysis. Tumour Biol. 2014;35:1307–1311. doi: 10.1007/s13277-013-1173-5. [DOI] [PubMed] [Google Scholar]