Abstract
miR-210 has been found consistently induced by hypoxia and implicated in cancer progression. Despite widespread exploration on miR-210 function, little is known about its action on invasion and metastasis of ovarian cancer. In this study, miR-210 was induced by hypoxia in SKOV3 ovarian cancer cells and then suppressed with its specific inhibitor. Repression of miR-210 in hypoxic cells led to upregulation of E-cadherin, downregulation of vimentin and Snail, and attenuation of wound healing capability. On the other hand, miR-210 was overexpressed in normoxic SKOV3 cells, which resulted in decrease of E-cadherin, increase of vimentin and Snail, and facilitation of wound healing capability. These results revealed that miR-210 promoted ovarian cancer cell mobility by acting as a modulator of epithelial-mesenchymal transition (EMT), highlighting the importance of miR-210 in ovarian cancer progression.
Keywords: miR-210, ovarian cancer, epithelial-mesenchymal transition
Introduction
Ovarian cancer is the most lethal gynaecologic tumor [1]. The poor prognosis of patients with ovarian cancer closely relates to cancer cell invasion and metastasis that are profoundly affected by tumor microenvironment. Hypoxia is one of the critical contributors to the tumor microenvironment [2]. Hypoxia functions mainly by stabilizing the oxygen-sensitive α-subunit of hypoxia inducible factor (HIF) which heterodimerizes with the constitutively expressed β-subunit and gives rise to the transcription factor HIF. HIF then transcriptionally activates a complex set of intracellular molecules to prepare cell metabolism and processes for adaptation to hypoxia condition.
A wide array of protein-coding genes have been demonstrated as HIF-modulated molecules [3]. Besides, non-coding microRNA (miR) emerges as a new class of hypoxia-responsive element [4,5]. Among the hypoxic miRs, miR-210 captures a great deal of attention since it is consistently induced in a variety of tumor cells under hypoxic conditions [6].
In response to hypoxia, miR-210 is upregulated in epithelial ovarian cancer specimens as well as cell lines [7]. Of note, higher level of miR-210 exists in ovarian cancer cells in the malignant effusions compared to their counterparts in primary tumor tissues [8], documenting its potential participation in the metastatic dissemination. However, the exact role that miR-210 plays and the mechanisms that miR-210 functions by in ovarian cancer metastasis remain largely undefined, although its biological functions have been linked to DNA repair [9], cell cycle regulation [10], apoptosis, stem cell survival [11], angiogenesis [12] and so on. s Epithelial-mesenchymal transition (EMT) is a crucial mechanism hijacked by hypoxic cancer cells for metastasis [13,14]. The term EMT has been coined to define the conversion process of epithelial cells into migratory and invasive fibroblastoid cells, mostly characterized by loss of epithelial cell properties, acquisition of mesenchymal cell phenotypes, and enhancement of cell migration and invasion. E-cadherin is a classic epithelial cell marker and is the most studied target of EMT inducers [15]. Loss of E-cadherin during EMT process is typically connected with the action of certain transcription repressors including Snail, Slug and Twist that inhibit transcription of E-cadherin gene [16]. In tumor cells that reside in hypoxic niche, HIF encourages these EMT-inducing transcription factors to repress E-cadherin and trigger EMT [13,17]. Since hypoxia is a significant EMT initiator and miR-210 is a master hypoxia-transducer, it is of interest to investigate the relevance of miR-210 to hypoxia-triggered EMT process in cancer cells.
In this study, we found miR-210 modulated hypoxia-induced EMT process by showing that suppression of hypoxia-upregulated miR-210 could reverse hypoxia-induced EMT process, while forced expression of miR-210 could induce EMT in ovarian cancer cells. These results highlighted the regulatory role of miR-210 in ovarian cancer progression.
Material and methods
Cell lines and culture
The human ovarian cancer cell line SKOV3 was obtained from the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China).Cells were grown in RPMI 1640 supplemented with 10% newborn bovine serum (GIBCO, Grand Island, NY, USA) under normoxic conditions (21% O2, 5% CO2, 37°C) for 24h prior to further treatment. For hypoxia induction, cells were incubated in 1% O2, 5% CO2, 94% N2, at 37°C in a HF100 hypoxia chamber (Heal Force, Hong Kong, China) for another 48 h.
miR mimic or inhibitor transfection
miR mimic and inhibitor were designed by Ribo-Bio Co. Ltd. (Guangzhou, China). Both miR mimic and inhibitor were resuspended in nuclease-free water (20 μM). Transfection of mimic or inhibitor specific for miR-210, or a negative control was performed with X-tremeGENE siRNA transfection reagent (Roche, Indianapolis, IN, USA) following the manufacturer’s protocol. Briefly, the cells were seeded in 6-well plates at 40% confluence one day before transfection. 5 μl of transfection reagent and a final concentration of 50 nM of mimic or 100 nM of inhibitor were used for each transfection.
RNA isolation and reverse transcription
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Quality and concentration of total RNA were assessed on a UV spectrophotometer (BioRad Inc., Hercules, CA, USA) by 260/280 nm absorbance ratio and the 260 nm absorbance, respectively. Total RNA was reverse-transcribed using RevertAid first strand cDNA synthesis Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer’s instructions: 2 μg of RNA was mixed with 1 μl of random hexamer primer or stem-loop reverse transcription primers for miR-210 and U6 in a total volume of 12 μl, and incubated at 65°C for 5 minutes followed by chilled on ice, then mixed with 4 μl of 5 × reaction buffer, 2 μl of dNTP mixture, 1 μl of RNase Inhibitor, and 1 μl of M-MuLV reverse transcriptase in a final volume of 20 μl. The reactions were performed at 25°C for 5 min, followed by 42°C for 60 min and 70°C for 5 min. The cDNAs were stored at -80°C for later use.
Real-time PCR
Quantitative real-time RT-PCR was performed on a CFX-96 real-time PCR system (Bio Rad, Hercules, CA, USA) using SYBR Green Master Mix (Takara Biotechnology Co. Ltd., Dalian, China). For normalization of miR-210, snRNA U6 was used. For normalization of E-cadherin, vimentin, Snail. Slug. Twist1 and Twist2, the gene β-actin was used. The 25 μl PCR reaction mixture included 12.5 μl of 2 × SYBR Premix Ex Taq, 1 μl of each primer (10 μM), and 1 μl of cDNA. The reactions were incubated at 95°C for 30 sec, followed by 40 cycles of 95°C, 5 sec and 60°C, 30 sec. Each measurement was performed in triplicate, and no-template controls were included for each assay. After PCR, a dissociation curve analysis was done. The relative quantity of gene expression was automatically calculated using the 2-ΔΔCt method. Primers for miR-210 and U6 were designed and synthesized by Ribo-Bio Co. Ltd. (Guangzhou, China). Oligonucleotide primers were designed and synthesized by Shanghai Shenggong Biotechnological Ltd. (Shanghai, China). The following primer sequences were used: E-cadherin-forward, 5’-GCTGCTCTTGCTGTTTCTTCG-3’; E-cadherin-reverse, 5’-CCGCCTCCTTCTTCATCATAG-3’; vimentin-forward, 5’-AAGTTTGCTGACCTCTCTGAGGCT-3’; vimentin-reverse, 5’-CTTCCATTTCACGCATCTGGCGTT-3’; Snail-forward, 5’-TCCAGAGTTTACCTTCCAGCA-3’; Snail-reverse, 5’-CTTTCCCACTGTCCTCATCTG-3’; Slug-forward, 5’-CTACAGCGAACTGGACACACA-3’; Slug-reverse, 5’-GCCCCAAAGATGAGGAGTATC-3’; Twist1-forward, 5’-GTCCGCAGTCTTACGAGGAG-3’; Twist1-reverse, 5’-GTCTGAATCTTGCTCAGCTTGT-3’; Twist2-forward, 5’-ACAAGCTGAGCAAGATCCAGAC-3’; Twist2-reverse, 5’-GCTGGTCATCTTATTGTCCATC-3’; β-actin-forward, 5’-TCCCTGGAGAAGAGCTACGA-3’; β-actin-reverse, 5’-AGCACTGTGTTGGCGTACAG-3’.
Western blot
Total protein was isolated from cells in RIPA lysis buffer on ice. Protein concentration was quantified using Bradford Protein Assay kit (Bio-Rad, Hercules, CA, USA). Proteins were boiled before being separated by electrophoresis on SDS-PAGE gels and transferred onto nitrocellulose membranes (Pall Life Science, NY, USA). The membranes were blocked with 5% non-fat milk at room temperature for 1 h, probed overnight for E-cadherin, vimentin, Snail,and β-actin (Cell Signaling Technology, Beverly, MA, USA). Horse radish peroxidase (HRP) conjugated goat anti-rabbit immunoglobulin (IgG) (Pierce, Rockford, IL, USA) was used to detect E-cadherin, vimentin and Snail. A goat anti-mouse IgG secondary antibody (Pierce, Rockford, IL, USA) conjugated with HRP was used to detect β-actin. Immunodetection was performed using ECL reagent (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The protein amounts were semi-quantitatively quantified by analyzing blot intensity with BandScan software using β-actin as loading control.
Wound healing assay
When cells reached 90% confluence in 6-well plates, wounds were generated by scratching the monolayers with a 200 μl pipette tip. Cells were washed to remove the detached cells and then maintained in media without serum. The wounded areas were photographed after incubation for an indicated period.
Statistical analysis
Statistical differences were determined by two-tailed t-test. All statistical analyses were performed using SPSS software (Chicago, IL, USA). Differences were considered significant (*) at P < 0.05 and highly significant (**) at P < 0.01 for all comparison.
Results
Repression of miR-210 blocked hypoxia-driven EMT
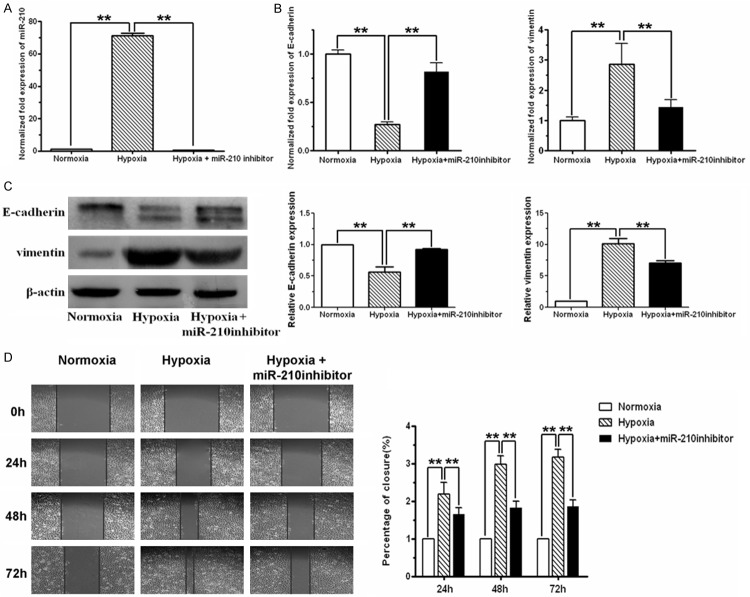
As shown by real-time PCR results, miR-210 was expressed at rather low level in normoxically cultured SKOV3 cells. Compared with normoxic cells, miR-210 level was significantly increased in cells exposed to 1% O2 (P < 0.01, t-test). Meanwhile, the epithelial marker E-cadherin was significantly down-regulated and the mesenchymal marker vimentin was up-regulated in hypoxic cells, as revealed by real-time PCR and western blot analysis.
To examine the role that miR-210 plays in hypoxia-driven EMT process, miR-210 was induced by hypoxia and then inhibited by specific inhibitor. Repression of miR-210 with its specific inhibitor decreased hypoxia-induced miR-210 expression (Figure 1A). Hypoxia triggered E-cadherin reduction and vimentin elevation in SKOV3 cells, which were reversed by treatment of miR-210 inhibitor (Figure 1B and 1C). In parallel, hypoxia-induced enhancement of wound healing capability was impeded by simultaneous miR-210 inhibitor treatment in a time-dependent manner (Figure 1D).
Figure 1.
miR-210 inhibitor blocked hypoxia-induced EMT in SKOV3 ovarian cancer cells. A. miR-210 level in SKOV3 cells exposed to 21% O2 (normoxia), 1% O2 (hypoxia) or 1% O2 plus miR-210 inhibitor, respectively, was analyzed using real-time RT-PCR assay. Compared with normoxic cells, miR-210 level was significantly increased in hypoxic cells. Transfection of miR-210 inhibitor into hypoxic cells substantially reduced hypoxia-induced miR-210. B. mRNA level of E-cadherin and vimentin in cells cultured for 48h under normoxia, hypoxia or hypoxia plus miR-210 inhibitor, respectively, were monitored by real-time RT-PCR. Hypoxia-induced E-cadherin decrease and vimentin increase were effectively reversed by miR-210 inhibitor. C. Protein levels of E-cadherin and vimentin in cells exposed to normoxia, hypoxia and hypoxia plus miR-210 inhibitor were examined by western blot, using β-actin as a loading control. Hypoxia decreased E-cadherin protein and increased vimentin protein, which was reversed by miR-210 inhibitor co-treatment. D. Cell mobility was detected by wound healing assay. Cells were incubated under normoxic condition for 24 h followed by being scratched and exposed to normoxia, hypoxia or hypoxia plus miR-210 inhibitor for indicated period, respectively. The closure of the scratch was monitored and photographed (100 ×). All of the treatments in this figure were carried out in triplicate, and the results were displayed as the means ± SD. *P < 0.05, **P < 0.01, t-test.
Over-expression of miR-210 induced EMT
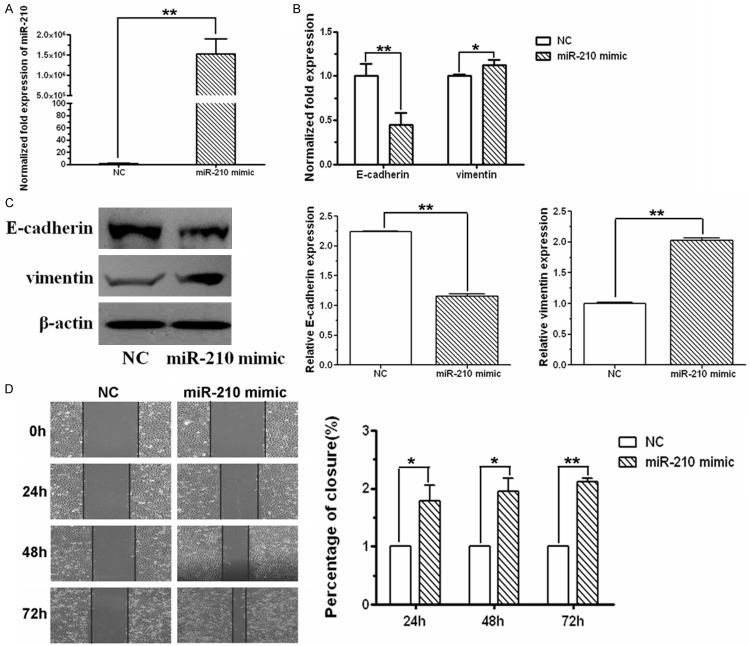
To verify the effect of miR-210 on EMT, miR-210 mimic was transfected into normoxic SKOV3 cells. Compared with control cells, more than 106-fold increase of miR-210 expression was observed in cells transfected with miR-210 mimic (Figure 2A). With the overexpression of miR-210, E-cadherin was substantially diminished at both mRNA and protein level, while vimentin was aggrandized (Figure 2B). Simultaneously, accelerated wound healing ability was observed over 72 h of miR-210 mimic transfection (Figure 2C).
Figure 2.
miR-210 mimic induced EMT in SKOV3 ovarian cancer cells. A. miR-210 level in SKOV3 cells transfected with miR-210 mimic or negative control, respectively, was analyzed using real-time RT-PCR assay. Compared with negative control cells, miR-210 level was significantly increased in cells transfected with miR-210 mimic. B. mRNA level of E-cadherin and vimentin in cells transfected with miR-210 mimic or negative control, respectively, was monitored by real-time RT-PCR. Compared with negative control cells, E-cadherin decreased and vimentin increased in cells transfected with miR-210 mimic. C. E-cadherin and vimentin protein levels in cells transfected with miR-210 mimic or negative control were examined by western blot. E-cadherin protein was decreased and vimentin protein was increased when miR-210 was overexpressed. D. Cell mobility was detected by wound healing assay. miR-210 mimic promoted the closure of the scratch. The photographs were captured using a phase contrast microscopy (100 ×). All of the treatments in this figure were carried out in triplicate, and the results were displayed as the means ± SD. *P < 0.05, **P < 0.01, t-test.
miR-210 modulated EMT by regulating Snail expression
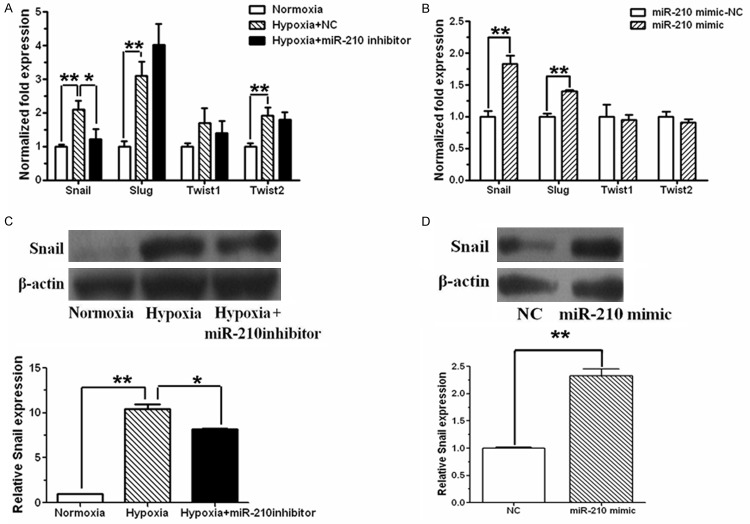
To examine the mechanism of miR-210 in downregulating E-cadherin, transcription repressors of E-cadherin were detected both in miR-210-suppressed hypoxic SKOV3 cells and miR-210-overexpressed normoxic SKOV3 cells. mRNA level of Snail, Slug, Twist1 and Twist2 was increased under hypoxia condition, but only Snail mRNA was slightly decreased by miR-210 inhibitor in hypoxic cells (Figure 3A). Additionally, 1.8-fold increase of Snail mRNA level was observed in normoxic cells transfected with miR-210 mimic, while other transcriptional repressors of E-cadherin including Slug, Twist1 and Twist2 showed insignificant change (Figure 3B). Consistently, Snail protein level was elevated in hypoxic cells, which was opposed by miR-210 inhibitor (Figure 3C). And overexpression of miR-210 increased Snail protein in normoxic cells (Figure 3D).
Figure 3.
Effect of miR-210 on Snail expression. A. SKOV3 cells were cultured in normal condition for 24 h, and then maintained in normoxia, hypoxia or hypoxia plus miR-210 inhibitor for another 48 h. Snail, Slug, Twist1 and Twist2 mRNA was determined by real time RT-PCR with β-actin as an inner control, and standardized against the level present in normoxically cultured cells. Snail and slug were increased at mRNA level in SKOV3 cells after hypoxia stimulation. Transfection of miR-210 inhibitor abrogated Snail upregulation caused by hypoxia. B. Real time RT-PCR showed Snail and Slug were increased at mRNA level in SKOV3 cells after miR-210 mimic transfection. C. Cell extracts were subjected to immunoblot analysis to detect Snail protein level, and β-actin was used as a loading control. Snail protein was increased in hypoxic cells, while miR-210 inhibitor decreased hypoxia-induced Snail expression. D. After miR-210 mimic transfection, protein level of Snail was examined by western blot with β-actin as an inner control, and standardized against the level present in negative control cells. Snail expression was increased in SKOV3 cells after miR-210 mimic transfection. All of the treatments in this figure were carried out in triplicate, and values are presented as the means ± SD of three experiments. *P < 0.05, **P < 0.01, for t-test.
Discussion
miR-210 is upregulated in multiple tumors and associated with poor patients prognosis [18-20]. However, inconsistency exists about transcript level of mature miR-210 in clinical ovarian cancer tissues. miR-210 has been reported diminished resulting from gene copy number loss in more than 50% of ovarian cancer tissues [21]. Contrarily, Li et al found miR-210 was upregulated in epithelial ovarian cancer specimens [7]. Nevertheless, elevated level of miR-210 existed in effusion-derived ovarian cancer cells compared to their counterparts in primary tumor tissues [8], suggesting its potential role in ovarian cancer metastasis. In view of the inconsistency, laboratory evidence is needed to define the exact role that miR-210 plays in ovarian cancer metastasis.
Little focus has been placed on the regulatory action of miR-210 on cancer cell invasion and metastasis until recently its mediation of hypoxia-induced hepatocellular carcinoma cell metastasis by direct downregulation of vacuole membrane protein 1 (VMP1) has been found [22]. The result has been later verified in colorectal cancer that miR-210 contributes to cell migration and invasion by directly inhibiting VMP1 [23]. These findings added a previously unexplored dimension to miR-210’s circle of influence on cancer cell biology, highlighting the detailed mechanism worth further investigation.
miR-210 is proposed as a master hypoxia sensor. As a crucial feature of tumor microenvironment, hypoxia sustains cancer progression and aids cancer metastasis by modulating different processes among which EMT is the one receiving intensive inquiry and wide acceptance. We therefore investigate the possibility of miR-210 as a transducer in hypoxia-triggered EMT process in ovarian cancer cells. Our results showed that miR-210 mediated hypoxia-induced EMT by promoting Snail expression to inhibit E-cadherin transcription. Moreover, miR-210 itself could induce EMT under normoxic condition, indicating the involvement of miR-210 in migration and invasion of ovarian cancer cells. In parallel with these results, hypoxia-induced miR-210 was able to convert fibroblasts into cancer associated fibroblast-like cells and promote prostate cancer cells EMT [24]. Additionally, miR-210 was found to result in an increase in transcriptional activity of HIF, suggesting a positive feedback may exist between miR-210 and HIF that reciprocally modulates miR-210 release [25] and thus sustains miR-210 function under hypoxia. These results underscored the mechanistic complexity and multiplicity of miR-210 in the context of cancer.
The present study provides a better insight into the mechanisms underlying the role that miR-210 plays in ovarian cancer progression. It is necessary to determine whether miR-210 directly targets Snail or other molecules to modulate EMT. Further functional analysis of miR-210 and identification its target molecules in EMT are essential to understand the adaptive mechanism triggered by miR-210, and may ultimately yield information on novel prognostic markers or targets for treatment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China: No. 30973429.
Disclosure of conflict of interest
None.
References
- 1.Cho KR, Shih IeM. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 3.Tsai YP, Wu KJ. Hypoxia-regulated target genes implicated in tumor metastasis. J Biomed Sci. 2012;19:102. doi: 10.1186/1423-0127-19-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivan M, Harris AL, Martelli F, Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12:1426–1431. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greco S, Martelli F. MicroRNAs in Hypoxia Response. Antioxid Redox Signal. 2014;21:1164–1166. doi: 10.1089/ars.2014.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Huang K, You Y, Fu X, Hu L, Song L, Meng Y. Hypoxia-induced miR-210 in epithelial ovarian cancer enhances cancer cell viability via promoting proliferation and inhibiting apoptosis. Int J Oncol. 2014;44:2111–2120. doi: 10.3892/ijo.2014.2368. [DOI] [PubMed] [Google Scholar]
- 8.Vaksman O, Stavnes HT, Kaern J, Trope CG, Davidson B, Reich R. miRNA profiling along tumour progression in ovarian carcinoma. J Cell Mol Med. 2011;15:1593–1602. doi: 10.1111/j.1582-4934.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Wu J, Xu N, Xie W, Li M, Li J, Jiang Y, Yang BB, Zhang Y. MiR-210 disturbs mitotic progression through regulating a group of mitosis-related genes. Nucleic Acids Res. 2013;41:498–508. doi: 10.1093/nar/gks995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL, Marszalek JR, Bartz SR, Carleton M, Cleary MA, Linsley PS, Grandori C. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009;8:2756–2768. doi: 10.4161/cc.8.17.9387. [DOI] [PubMed] [Google Scholar]
- 11.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 15.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 16.Kurrey NK, K A, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97:155–165. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 17.Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J, Sun T, Wang J, Sun R, Liu Y. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol Oncol. 2014;133:575–583. doi: 10.1016/j.ygyno.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- 19.Greither T, Grochola LF, Udelnow A, Lautenschlager C, Wurl P, Taubert H. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer. 2010;126:73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- 20.Greither T, Wurl P, Grochola L, Bond G, Bache M, Kappler M, Lautenschlager C, Holzhausen HJ, Wach S, Eckert AW, Taubert H. Expression of microRNA 210 associates with poor survival and age of tumor onset of soft-tissue sarcoma patients. Int J Cancer. 2012;130:1230–1235. doi: 10.1002/ijc.26109. [DOI] [PubMed] [Google Scholar]
- 21.Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O’Brien-Jenkins A, Katsaros D, Weber BL, Simon C, Coukos G, Zhang L. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7:255–264. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying Q, Liang L, Guo W, Zha R, Tian Q, Huang S, Yao J, Ding J, Bao M, Ge C, Yao M, Li J, He X. Hypoxia-inducible microRNA-210 augments the metastatic potential of tumor cells by targeting vacuole membrane protein 1 in hepatocellular carcinoma. Hepatology. 2011;54:2064–2075. doi: 10.1002/hep.24614. [DOI] [PubMed] [Google Scholar]
- 23.Qu A, Du L, Yang Y, Liu H, Li J, Wang L, Liu Y, Dong Z, Zhang X, Jiang X, Wang H, Li Z, Zheng G, Wang C. Hypoxia-inducible MiR-210 is an independent prognostic factor and contributes to metastasis in colorectal cancer. PLoS One. 2014;9:e90952. doi: 10.1371/journal.pone.0090952. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Taddei ML, Cavallini L, Comito G, Giannoni E, Folini M, Marini A, Gandellini P, Morandi A, Pintus G, Raspollini MR, Zaffaroni N, Chiarugi P. Senescent stroma promotes prostate cancer progression: The role of miR-210. Mol Oncol. 2014;8:1729–46. doi: 10.1016/j.molonc.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal R, Pandey P, Jha P, Dwivedi V, Sarkar C, Kulshreshtha R. Hypoxic signature of microRNAs in glioblastoma: insights from small RNA deep sequencing. BMC Genomics. 2014;15:686. doi: 10.1186/1471-2164-15-686. [DOI] [PMC free article] [PubMed] [Google Scholar]