Abstract
Multiple studies had focused on the association between interleukin-1 (IL-1) rs1143634 polymorphism and aggressive periodontitis (AgP) susceptibility, but the results remained inconclusive. Therefore, this meta-analysis was conducted to explore its role in the development of AgP. PubMed and Embase databases were searched up to April 15, 2014. After study selection and data extraction form eligible studies, meta-analysis was performed. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the association. All the analysis was performed using Comprehensive Meta-Analysis software. Finally a total of 25 case-control studies were included. The pooled results showed non-association between AgP susceptibility and IL-1 rs1143634 polymorphism [for T vs. C: OR = 0.99, 95% CI = 0.79-1.23; for TT vs. CC: OR = 1.14, 95% CI = 0.78-1.66; for CT vs. CC: OR = 0.97, 95% CI = 0.70-1.36; for (CT + TT) vs. CC: OR = 1.02, 95% CI = 0.76-1.37; for TT vs. (CT + CC): OR = 1.22, 95% CI = 0.85-1.75]. Subgroup analyses remain did not find any association. No publication bias was detected. Hence, our meta-analysis showed that IL-1β rs1143634 polymorphism is not linked to AgP susceptibility, regardless of ethnicity.
Keywords: Interleukin-1, periodontitis, aggressive periodontitis, polymorphism, meta-analysis
Introduction
There are 200 possible connections between systemic diseases and periodontal disease have been highlighted by the American dental association in 2006 [1], such as chronic obstructive pulmonary diseases [2], head and neck cancer [3], cardiovascular diseases [4], diabetes [5]. Therefore, seek the risk factor of periodontal disease and prevent them is an important and interesting work for overall health. Periodontal disease is divided into two major forms, namely, chronic periodontitis (CP) and aggressive periodontitis (AgP) [6]. CP is widely regarded as one of the most common diseases with a prevalence of 10-15% [7] whereas AgP is less prevalent than CP. However, AgP shows more rapid attachment loss and bone destruction than CP [8]. Both CP and AgP were believed as multifactor diseases [9], environmental and genetic factors combines play a role to make individuals affected [10]. However, the susceptibility is not always the same to CP and AgP for the same genetic polymorphism, sometimes is linked to CP but not linked to AgP [11-13].
Interleukin-1 (IL-1) is considered to be one of the most active stimulators of osteoclastic activity and contributed to periodontal disease development [14]. The IL-1 gene family locates on chromosome 2q13-14 and encodes three proteins: IL-1α (alpha), IL-1β (beta), and IL-1RN (receptor antagonist); of them, IL-1β is believed as the most potent and pathogenic form [15,16]. The IL-1β gene is highly polymorphic and three polymorphisms that base on transitions between C and T at positions -511 (C→T, rs16944), -31 (T→C, rs1143627), and +3954/3953 (C→T, rs1143634) base pairs from the transcriptional site have been widely researched [16,17]. The IL-1β rs1143634 is a synonymous single nucleotide polymorphism and locates in exon 5, a published meta-analysis indicated that IL-1β rs1143634 polymorphism was associated with increased risk of CP [18]. For CP and AgP are different types of periodontal disease and the results of numerous epidemiological studies that investigated the association between IL-1β rs1143634 polymorphism and AgP were inconsistent, we conducted this meta-analysis for deriving a more precise estimation of the association between IL-1β rs1143634 polymorphism and AgP.
Materials and methods
We following the recommended Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (File S1) [19] to report this meta-analysis.
Eligibility criteria
The study was included if it met all the following criteria: (1) the design was a case-control study, (2) the topic was evaluated the association between IL-1β rs1143634 polymorphism and AgP susceptibility, (3) the AgP patient was not company with other systematic diseases and the control was healthy individuals or periodontitis-free; (4) reported odds ratios (ORs) and its 95% confidence intervals (CIs) or/and the number of genotypes in both case and control group, or the reported data can calculate them.
Search strategy
The PubMed and Embase databases were comprehensively searched using the search terms [(polymorphism OR mutation OR variant) AND (interleukin-1 OR IL-1) AND (periodontal disease OR periodontitis)] up to April 15, 2014. For each identified study, additional studies were manually searched from its references.
Data extraction
Two authors independently selected studies according to the criteria listed above and then extracted data from all eligible studies. The first author’s name, publication year, country of origin and ethnicity, source of control, genotyping method, number of cases and controls and genotype frequency, ORs and its 95% CIs, and HWE (Hardy Weinberg Equilibrium) for controls were gathered from each study. All disagreements were resolved by asking a third author.
Data analysis
First, the heterogeneity among included studies was detected using I 2 statistics [20]. The value of I 2 ≤ 40% was considered no substantive heterogeneity existed and we used the fixed effect model to pool the data; otherwise, the random-effects model was used [21]. The ORs and corresponding 95% CIs was used for estimating the association between IL-1β rs1143634 polymorphism and AgP using the five genetic models: allele comparison (T vs. C), homozygote comparison (TT vs. CC), heterozygote comparison (CT vs. CC), dominant model (TT + CT vs. CC), and recessive model (CC + CT vs. TT). The subgroups analysis based on the ethnicity, source of controls, and the HWE for controls were conducted to explore the potential source of heterogeneity among studies and test the effects of study characteristics on the overall estimation. Sensitivity analysis was applied by excluding each single study every time to explore the robust of pooled results. The publication bias was detected by funnel plot and the Egger linear regression test [22]. All the analysis was performed using the Comprehensive Meta-Analysis software, version 2.2 (Biostat, Englewood, New Jersey) [23], and all the p values were two-sided.
Results
Study section and characteristic
The primary search yielded 216 publications and finally 25 case-control studies involving 1594 AgP patients and 2483 healthy controls were included [24-48]. Figure 1 shows the study selection process.
Figure 1.
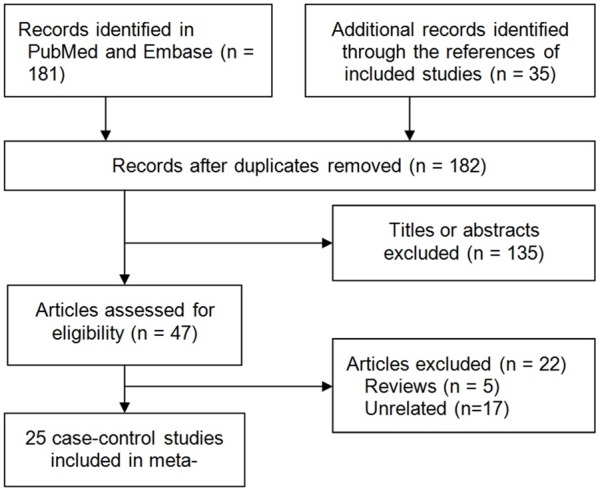
Flow chart from identification of eligible studies to final inclusion.
Of these studies, 16 studies were concerned about Caucasian origin [24,26,28,31,33,34,36-44,48], 7 were Asian origin [27,29,30,32,45-47], one was African-American origin [25], and one was Brazil (Mixed) origin [35]. The controls of five studies were out of HWE [24,40,44,45,48]. Two studies reported ORs and 95% CIs for the allele comparison (T vs. C) [39,41]. Table 1 shows the main characteristics of identified studies.
Table 1.
Characteristics of included studies in the meta-analysis
| Reference | Country (Ethnicity) | Sample size (case/control) | Source of control | Genotype method | HWE (P value) |
|---|---|---|---|---|---|
| Walker 2000 | USA (African-American) | 37/104 | PB | PCR | 0.89 |
| Parkhill 2000 | UK (Caucasian) | 70/72 | Mixed | PCR | < 0.05 |
| Hodge 2001 | UK (Caucasian) | 56/56 | HB | PCR | 0.34 |
| Duan 2002 | China (Asian) | 20/94 | HB | PCR-RFLP | 0.83 |
| Rogers 2002 | Australia (Caucasian) | 21/60 | PB | PCR | 0.21 |
| Tai 2002 | Japan (Asian) | 47/97 | HB | PCR | 0.63 |
| Anusaksathien 2003 | Thailand (Asian) | 26/43 | HB | PCR | 0.94 |
| Gonzales 2003 | Germany (Caucasian) | 44/47 | PB | PCR | 0.13 |
| Li 2004 | China (Asian) | 122/95 | Mixed | PCR-RFLP | 0.92 |
| Quappe 2004 | Chile (Caucasian) | 36/75 | HB | PCR | 0.07 |
| Moreira 2005 | Brazil (Mixed) | 31/46 | PB | PCR | 0.31 |
| Brett 2005 | UK (Caucasian) | 50/103 | PB | PCR | 0.39 |
| Scapoli 2005 | Italy (Caucasian) | 40/96 | PB | PCR | 0.99 |
| Sakellari 2006 | Greece (Caucasian) | 46/90 | Mixed | PCR | 0.73 |
| Havemose-Poulsen 2007 | Denmark (Caucasian) | 45/25 | HB | PCR-RFLP | 0.27 |
| Guzeldemir 2008 | Turkey (Caucasian) | 31/31 | PB | PCR | < 0.05 |
| Karasneh 2011 | Jordan (Caucasian) | 80/80 | PB | PCR | 0.86 |
| Schulz 2011 | Germany (Caucasian) | 85/88 | PB | PCR-SSP | 0.88 |
| Shibani 2011 | Syria (Caucasian) | 32/35 | PB | PCR | < 0.05 |
| Masamatti 2012 | India (Asian) | 30/30 | HB | PCR | < 0.05 |
| Ebadian 2013 | Iran (Iran) | 53/48 | HB | PCR-RFLP | 0.95 |
| Ayazi 2013 | Iran (Iran) | 26/26 | HB | PCR-RFLP | 0.09 |
| Yücel 2013 | Turkey (Caucasian) | 56/47 | PB | PCR-RFLP | < 0.05 |
| Fiebig 2008* | Germany and Netherlands (Caucasian) | 415/874 | PB | TaqMan | 0.58 |
| Scapoli 2010* | Italy (Caucasian) | 95/121 | PB | MassARRAY | > 0.05 |
HWE: Hardy Weinberg Equilibrium; Mixed: hospital and population based; PB: population based; HB, hospital based;
OR and its 95% CI for T vs. C;
PCR: polymerase chain reaction; PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism.
Meta-analysis
Table 2 presents the results of overall and subgroup analyses. All the genetic models provided evidence that there was no association between the IL-1β rs1143634 polymorphism and AgP susceptibility in overall populations [for T vs. C: OR = 0.99, 95% CI = 0.79-1.23, I 2 = 62.22%, Figure 2; for TT vs. CC: OR = 1.14, 95% CI = 0.78-1.66, I 2 = 35.68%; for CT vs. CC: OR = 0.97, 95% CI = 0.70-1.36, I 2 = 59.13%; for (CT + TT) vs. CC: OR = 1.02, 95% CI = 0.76-1.37, I 2 = 55.14%; for TT vs. (CT + CC): OR = 1.22, 95% CI = 0.85-1.75, I 2 = 35.18%, respectively]. In the subgroup analysis for ethnicity, source of controls, and HWE, we remain did not find any association. Sensitivity analysis showed that the conclusions remained similar when any single study was deleted each time (Figure 3).
Table 2.
Results of overall and subgroups analyses of pooled ORs and 95% CIs
| Genetic model | Subgroup | Number of studies | OR (95% CI) | I 2 (%) |
|---|---|---|---|---|
| T vs. C | Overall | 25 | 0.99 (0.79-1.23) | 62.2 |
| Caucasian | 16 | 0.89 (0.72-1.09) | 52.92 | |
| Asian | 7 | 1.99 (0.84-4.69) | 73.63 | |
| Other ethnic | 2 | 0.67 (0.34-1.31) | 0 | |
| HWE (yes) | 20 | 1.02 (0.82-1.27) | 51.3 | |
| HWE (no) | 5 | 0.83 (0.38-1.82) | 83.19 | |
| HB | 9 | 1.53 (0.89-2.64) | 65.78 | |
| PB | 13 | 0.82 (0.64-1.06) | 57.02 | |
| Mixed | 3 | 1.02 (0.53-1.96) | 57.81 | |
| TT vs. CC | Overall | 23 | 1.14 (0.78-1.66) | 35.7 |
| Caucasian | 14 | 0.99 (0.65-1.51) | 31.23 | |
| Asian | 7 | 1.58 (0.35-6.62) | 56.26 | |
| Other ethnic | 2 | 0.81 (0.03-20.41) | 0 | |
| HWE (yes) | 18 | 1.26 (0.82-1.94) | 23.2 | |
| HWE (no) | 5 | 0.58 (0.16-2.11) | 61.03 | |
| HB | 9 | 1.48 (0.77-2.86) | 38.86 | |
| PB | 11 | 0.70 (0.32-1.56) | 50.29 | |
| Mixed | 3 | 1.42 (0.53-3.76) | 0 | |
| CT vs. CC | Overall | 23 | 0.97 (0.70-1.36) | 59.1 |
| Caucasian | 14 | 0.87 (0.60-1.26) | 57.75 | |
| Asian | 7 | 1.57 (0.63-3.91) | 64.84 | |
| Other ethnic | 2 | 0.66 (0.32-1.36) | 0 | |
| HWE (yes) | 18 | 0.89 (0.71-1.11) | 39 | |
| HWE (no) | 5 | 0.98 (0.26-3.77) | 84.49 | |
| HB | 9 | 1.43 (0.77-2.66) | 58.68 | |
| PB | 11 | 0.79 (0.52-1.21) | 51.96 | |
| Mixed | 3 | 0.81 (0.33-2.02) | 65.39 | |
| (CT + TT) vs. CC | Overall | 23 | 1.02 (0.76-1.37) | 55.1 |
| Caucasian | 14 | 0.90 (0.66-1.23) | 50.66 | |
| Asian | 7 | 1.97 (0.85-4.53) | 63.16 | |
| Other ethnic | 2 | 0.65 (0.31-1.33) | 0 | |
| HWE (yes) | 18 | 0.94 (0.76-1.16) | 35.9 | |
| HWE (no) | 5 | 0.89 (0.29-2.75) | 82.18 | |
| HB | 9 | 1.56 (0.90-2.73) | 54.51 | |
| PB | 11 | 0.83 (0.58-1.19) | 47.83 | |
| Mixed | 3 | 0.90 (0.37-2.22) | 66.87 | |
| TT vs. (CT + CC) | Overall | 23 | 1.22 (0.85-1.75) | 35.2 |
| Caucasian | 14 | 1.11 (0.75-1.63) | 16.14 | |
| Asian | 7 | 1.11 (0.13-9.92) | 70.43 | |
| Other ethnic | 2 | 0.92 (0.04-23.08) | 0 | |
| HWE (yes) | 18 | 1.42 (0.93-2.17) | 37.6 | |
| HWE (no) | 5 | 0.80 (0.40-1.61) | 20.27 | |
| HB | 9 | 1.03 (0.27-3.88) | 59.09 | |
| PB | 11 | 1.04 (0.66-1.65) | 36.77 | |
| Mixed | 3 | 1.74 (0.67-4.52) | 0 |
HWE: Hardy Weinberg Equilibrium; Mixed: hospital and population based; PB: population based; HB: hospital based.
Figure 2.
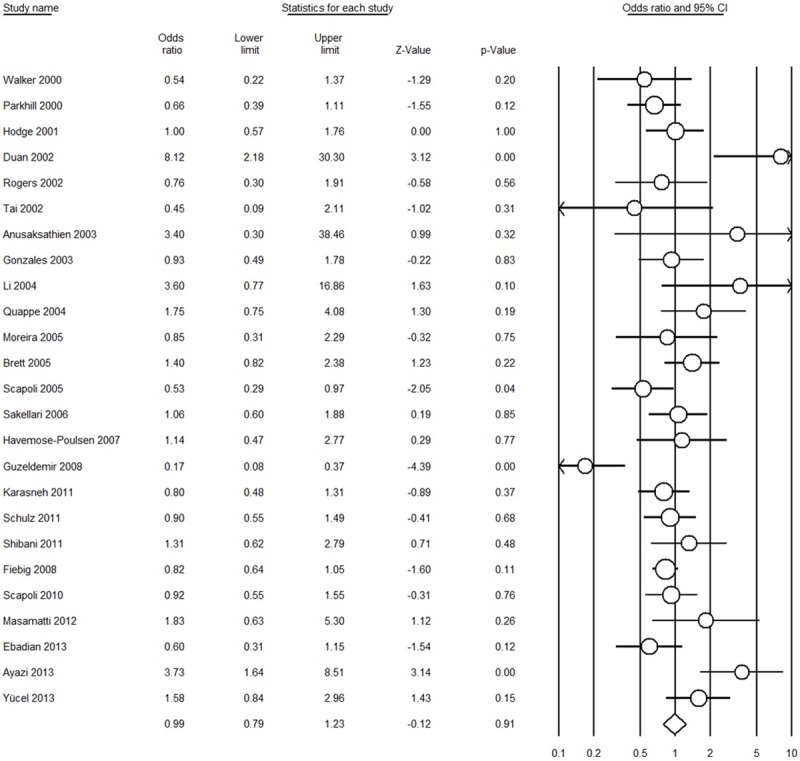
Forest plot for T vs. C comparison (random-effect model).
Figure 3.
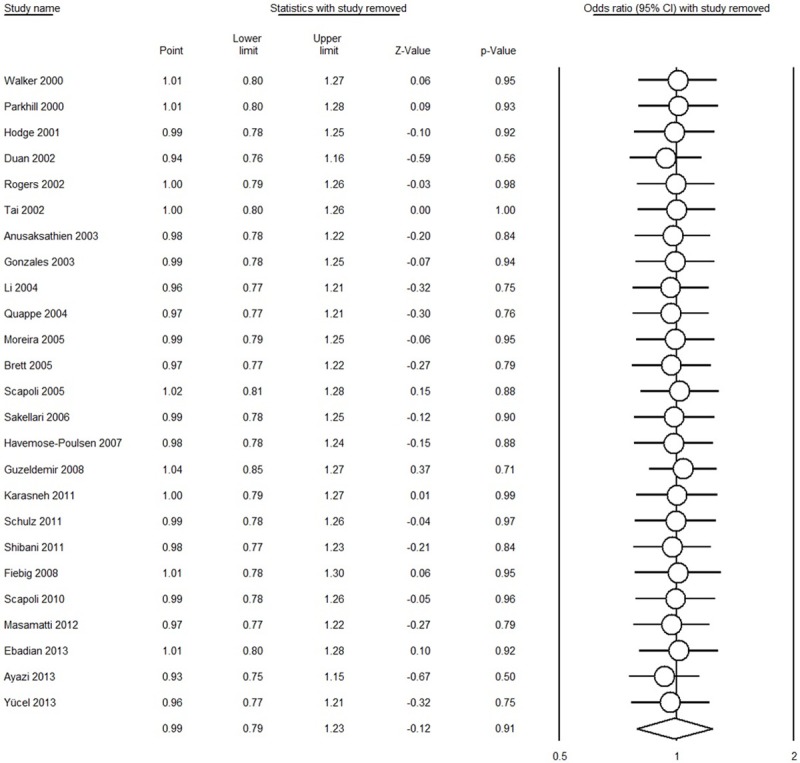
Sensitivity analysis by detecting any single study each time in T vs. C comparison (random-effect model).
Publication bias
Egger’s test showed that there was no bias in the T vs. C genetic model (P = 0.16), CT vs. CC (P = 0.58), (CT + TT) vs. CC (P = 0.37), or the TT vs. (CT + CC) (P = 0.09); but that bias was evident in the TT vs. CC (P = 0.03) model.
Discussion
To date, numerous studies evaluated the association between IL-1β rs1143634 polymorphism and AgP risk have been published, but the results were inconsistent. Moreover, the credibility of results from a single case-control study is limited due to relative small sample size. Meta-analysis has the benefit to overcome this limitation by increasing the sample size [49,50] and is being widely used in genetic association studies [11,12,21,51-54]. Therefore, we performed this meta-analysis to assess the association between IL-1β rs1143634 polymorphism and AgP risk based on pooled results. Of all included studies, two studies showed a significantly increased risk [27,46], two studies showed a significantly decreased risk [36,40], and the other 19 studies showed non-significant association; however, the results of present meta-analysis based on these 25 case-control studies obtained a negative association (Figure 2). The sensitivity analysis also proved that the overall results were not influenced by any single study. To make a comprehensive analysis between IL-1β rs1143634 polymorphism and AgP, we also conducted subgroup analyses according to the ethnicity, source of controls, and the HWE for controls. All the results were same with overall analysis (Table 2), indicating the genetic backgrounds and the environment they lived in did not play a role.
IL-1β is the secreted form of IL gene and can promote the movement of inflammatory cells from the blood to inflamed tissues and regulate the extracellular matrix and induce other cytokines [55,56]. Higher levels of IL-1β in gingival crevicular fluid were detected in the patients who with periodontal disease [57,58]. It suggested that IL-1β rs1143634 polymorphism might influence the levels of IL-1β and that was associated with periodontal disease. The published meta-analysis of Deng et al in 2013 suggested that IL-1β rs1143634 polymorphism is associated with CP [18]; however, our meta-analysis indicated IL-1β rs1143634 polymorphism is not associated with AgP. The reason maybe AgP is more like a genetically inherited disease [59] and the IL-1 gene is not belonged to the specify genes. For some scholars considered that AgP and CP shared some susceptibility genes, but not in all [60,61]; hence, our result also provided further evidence that AgP was different from CP in some aspects.
Some limitations should be demonstrated in our meta-analysis. First, the sample size is still large enough. Although we comprehensively searched relevant articles, however, due to the less prevalent of AgP, it is different to obtain large sample size. For lacking of accurate prevalence of AgP, we could not estimate the optimal sample size in this topic. Second, heterogeneity is a potential problem that may affect the interpretation of the results. Obviously, substantial heterogeneity existed of all the genetic models in our meta-analysis. The heterogeneity might due to the diversity in study design, sample size, inclusion and exclusion criteria, demographic background, etc; however, the heterogeneity of our meta-analysis could not be interpreted by ethnicity or source/HWE of controls. Third, due to the limited of right to use databases and languages, studies included in our meta-analysis were limited to English and Chinese published articles. Moreover, we did not track the unpublished articles. Although four genetic models indicated no publication bias existed, we could not ignore that publication bias may have distorted our results. Fourth, for smoking is the classical risk factors of periodontal disease [62], data were not stratified by gender, smoking, or other environmental variables because of insufficient data. Hence, we could not perform subgroup analysis based on adjusted information due to the limited data.
In summary, our meta-analysis suggested that IL-1β rs1143634 polymorphism does not contribute to the risk of AgP, and there is no genetic or ethnic background. In addition, there was no statistical evidence of publication bias among studies and the sensitivity analysis showed the overall results are stable, indicating that the pooled results may be unbiased. However, further studies are suggested to conduct multiple variables adjustment in order to explore the gene-gene, gene-environmental interactions.
Acknowledgements
This research was supported (in part) by the Foundation of Evidence-based Medicine Nursery Fund of Taihe Hospital (EBM2013028 and EBM2014007), without commercial or not-for-profit sectors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Disclosure of conflict of interest
None.
References
- 1.Loos BG. Systemic effects of periodontitis. Int J Dent Hyg. 2006;4(Suppl 1):34–38. doi: 10.1111/j.1601-5037.2006.00200.x. discussion 50-32. [DOI] [PubMed] [Google Scholar]
- 2.Zeng XT, Tu ML, Liu DY, Zheng D, Zhang J, Leng W. Periodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studies. PLoS One. 2012;7:e46508. doi: 10.1371/journal.pone.0046508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng XT, Deng AP, Li C, Xia LY, Niu YM, Leng WD. Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PLoS One. 2013;8:e79017. doi: 10.1371/journal.pone.0079017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leng WD, Zeng XT, Chen YJ, Zhan ZQ, Yang Y. Periodontal disease is associated with increased coronary heart disease risk: A meta-analysis based on 38 case-control studies. World J Meta-Anal. 2013;1:47–56. [Google Scholar]
- 5.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kebschull M, Guarnieri P, Demmer RT, Boulesteix AL, Pavlidis P, Papapanou PN. Molecular Differences between Chronic and Aggressive Periodontitis. J Dent Res. 2013;92:1081–1088. doi: 10.1177/0022034513506011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albandar JM, Rams TE. Global epidemiology of periodontal diseases: an overview. Periodontol 2000. 2002;29:7–10. doi: 10.1034/j.1600-0757.2002.290101.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandhu SP, Kakar V, Gogia G, Narula SC. Unilateral gingival fibromatosis with localized aggressive periodontitis (involving first molars): An unusual case report. J Indian Soc Periodontol. 2009;13:109–113. doi: 10.4103/0972-124X.55834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 10.Gemmell E, Seymour GJ. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol 2000. 2004;35:21–41. doi: 10.1111/j.0906-6713.2004.003557.x. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Cai Q, Ma L, Wang M, Ma J, Zhang W, Pan Y, Wang L. Association between MMP-1 g. -1607dupG polymorphism and periodontitis susceptibility: a meta-analysis. PLoS One. 2013;8:e59513. doi: 10.1371/journal.pone.0059513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LL, Li H, Zhang PP, Wang SM. Association between vitamin D receptor polymorphisms and periodontitis: a meta-analysis. J Periodontol. 2012;83:1095–1103. doi: 10.1902/jop.2011.110518. [DOI] [PubMed] [Google Scholar]
- 13.Dimou NL, Nikolopoulos GK, Hamodrakas SJ, Bagos PG. Fcgamma receptor polymorphisms and their association with periodontal disease: a meta-analysis. J Clin Periodontol. 2010;37:255–265. doi: 10.1111/j.1600-051X.2009.01530.x. [DOI] [PubMed] [Google Scholar]
- 14.Taylor JJ, Preshaw PM, Donaldson PT. Cytokine gene polymorphism and immunoregulation in periodontal disease. Periodontol 2000. 2004;35:158–182. doi: 10.1111/j.0906-6713.2004.003561.x. [DOI] [PubMed] [Google Scholar]
- 15.Tokoro Y, Yamamoto T, Hara K. IL-1 beta mRNA as the predominant inflammatory cytokine transcript: correlation with inflammatory cell infiltration into human gingiva. J Oral Pathol Med. 1996;25:225–231. doi: 10.1111/j.1600-0714.1996.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 16.Bird S, Zou J, Wang T, Munday B, Cunningham C, Secombes CJ. Evolution of interleukin-1beta. Cytokine Growth Factor Rev. 2002;13:483–502. doi: 10.1016/s1359-6101(02)00028-x. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Yin Z, Cao S, Gao W, Liu L, Yin Y, Liu P, Shu Y. Systematic review and meta-analysis on the association between IL-1B polymorphisms and cancer risk. PLoS One. 2013;8:e63654. doi: 10.1371/journal.pone.0063654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng JS, Qin P, Li XX, Du YH. Association between interleukin-1beta C (3953/4)T polymorphism and chronic periodontitis: evidence from a meta-analysis. Hum Immunol. 2013;74:371–378. doi: 10.1016/j.humimm.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 21.Zeng XT, Luo W, Geng PL, Guo Y, Niu YM, Leng WD. Association between the TP53 codon 72 polymorphism and risk of oral squamous cell carcinoma in Asians: a meta-analysis. BMC Cancer. 2014;14:469. doi: 10.1186/1471-2407-14-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng XT, Yao QS, Weng H, Li S, Huang JY, Wang XH. Meta-analysis of vitamin D receptor gene polymorphisms and benign prostatic hyperplasia risk. Mol Biol Rep. 2014;41:6713–6717. doi: 10.1007/s11033-014-3554-2. [DOI] [PubMed] [Google Scholar]
- 24.Parkhill JM, Hennig BJ, Chapple IL, Heasman PA, Taylor JJ. Association of interleukin-1 gene polymorphisms with early-onset periodontitis. J Clin Periodontol. 2000;27:682–689. doi: 10.1034/j.1600-051x.2000.027009682.x. [DOI] [PubMed] [Google Scholar]
- 25.Walker SJ, Van Dyke TE, Rich S, Kornman KS, di Giovine FS, Hart TC. Genetic polymorphisms of the IL-1alpha and IL-1beta genes in African-American LJP patients and an African-American control population. J Periodontol. 2000;71:723–728. doi: 10.1902/jop.2000.71.5.723. [DOI] [PubMed] [Google Scholar]
- 26.Hodge PJ, Riggio MP, Kinane DF. Failure to detect an association with IL1 genotypes in European Caucasians with generalised early onset periodontitis. J Clin Periodontol. 2001;28:430–436. doi: 10.1034/j.1600-051x.2001.028005430.x. [DOI] [PubMed] [Google Scholar]
- 27.Duan H, Zhang J, Zhang Y. [The association between IL-1 gene polymorphisms and susceptibility to severe periodontitis] . Hua Xi Kou Qiang Yi Xue Za Zhi. 2002;20:48–51. [PubMed] [Google Scholar]
- 28.Rogers MA, Figliomeni L, Baluchova K, Tan AE, Davies G, Henry PJ, Price P. Do interleukin-1 polymorphisms predict the development of periodontitis or the success of dental implants? J Periodontal Res. 2002;37:37–41. doi: 10.1034/j.1600-0765.2002.00651.x. [DOI] [PubMed] [Google Scholar]
- 29.Tai H, Endo M, Shimada Y, Gou E, Orima K, Kobayashi T, Yamazaki K, Yoshie H. Association of interleukin-1 receptor antagonist gene polymorphisms with early onset periodontitis in Japanese. J Clin Periodontol. 2002;29:882–888. doi: 10.1034/j.1600-051x.2002.291002.x. [DOI] [PubMed] [Google Scholar]
- 30.Anusaksathien O, Sukboon A, Sitthiphong P, Teanpaisan R. Distribution of interleukin-1beta (+3954) and IL-1alpha (-889) genetic variations in a Thai population group. J Periodontol. 2003;74:1796–1802. doi: 10.1902/jop.2003.74.12.1796. [DOI] [PubMed] [Google Scholar]
- 31.Gonzales JR, Michel J, Rodriguez EL, Herrmann JM, Bodeker RH, Meyle J. Comparison of interleukin-1 genotypes in two populations with aggressive periodontitis. Eur J Oral Sci. 2003;111:395–399. doi: 10.1034/j.1600-0722.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 32.Li QY, Zhao HS, Meng HX, Zhang L, Xu L, Chen ZB, Shi D, Feng XH, Zhu XL. Association analysis between interleukin-1 family polymorphisms and generalized aggressive periodontitis in a Chinese population. J Periodontol. 2004;75:1627–1635. doi: 10.1902/jop.2004.75.12.1627. [DOI] [PubMed] [Google Scholar]
- 33.Quappe L, Jara L, Lopez NJ. Association of interleukin-1 polymorphisms with aggressive periodontitis. J Periodontol. 2004;75:1509–1515. doi: 10.1902/jop.2004.75.11.1509. [DOI] [PubMed] [Google Scholar]
- 34.Brett PM, Zygogianni P, Griffiths GS, Tomaz M, Parkar M, D’Aiuto F, Tonetti M. Functional gene polymorphisms in aggressive and chronic periodontitis. J Dent Res. 2005;84:1149–1153. doi: 10.1177/154405910508401211. [DOI] [PubMed] [Google Scholar]
- 35.Moreira PR, de Sa AR, Xavier GM, Costa JE, Gomez RS, Gollob KJ, Dutra WO. A functional interleukin-1 beta gene polymorphism is associated with chronic periodontitis in a sample of Brazilian individuals. J Periodontal Res. 2005;40:306–311. doi: 10.1111/j.1600-0765.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- 36.Scapoli C, Trombelli L, Mamolini E, Collins A. Linkage disequilibrium analysis of case-control data: an application to generalized aggressive periodontitis. Genes Immun. 2005;6:44–52. doi: 10.1038/sj.gene.6364152. [DOI] [PubMed] [Google Scholar]
- 37.Sakellari D, Katsares V, Georgiadou M, Kouvatsi A, Arsenakis M, Konstantinidis A. No correlation of five gene polymorphisms with periodontal conditions in a Greek population. J Clin Periodontol. 2006;33:765–770. doi: 10.1111/j.1600-051X.2006.00983.x. [DOI] [PubMed] [Google Scholar]
- 38.Havemose-Poulsen A, Sorensen LK, Bendtzen K, Holmstrup P. Polymorphisms within the IL-1 gene cluster: effects on cytokine profiles in peripheral blood and whole blood cell cultures of patients with aggressive periodontitis, juvenile idiopathic arthritis, and rheumatoid arthritis. J Periodontol. 2007;78:475–492. doi: 10.1902/jop.2007.060135. [DOI] [PubMed] [Google Scholar]
- 39.Fiebig A, Jepsen S, Loos BG, Scholz C, Schafer C, Ruhling A, Nothnagel M, Eickholz P, van der Velden U, Schenck K, Schreiber S, Grossner-Schreiber B. Polymorphisms in the interleukin-1 (IL1) gene cluster are not associated with aggressive periodontitis in a large Caucasian population. Genomics. 2008;92:309–315. doi: 10.1016/j.ygeno.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Guzeldemir E, Gunhan M, Ozcelik O, Tastan H. Interleukin-1 and tumor necrosis factor-alpha gene polymorphisms in Turkish patients with localized aggressive periodontitis. J Oral Sci. 2008;50:151–159. doi: 10.2334/josnusd.50.151. [DOI] [PubMed] [Google Scholar]
- 41.Scapoli C, Borzani I, Guarnelli ME, Mamolini E, Annunziata M, Guida L, Trombelli L. IL-1 gene cluster is not linked to aggressive periodontitis. J Dent Res. 2010;89:457–461. doi: 10.1177/0022034510363232. [DOI] [PubMed] [Google Scholar]
- 42.Karasneh JA, Ababneh KT, Taha AH, Al-Abbadi MS, Ollier WE. Investigation of the interleukin-1 gene cluster polymorphisms in Jordanian patients with chronic and aggressive periodontitis. Arch Oral Biol. 2011;56:269–276. doi: 10.1016/j.archoralbio.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Schulz S, Stein JM, Altermann W, Klapproth J, Zimmermann U, Reichert Y, Glaser C, Schaller HG, Reichert S. Single nucleotide polymorphisms in interleukin-1gene cluster and subgingival colonization with Aggregatibacter actinomycetemcomitans in patients with aggressive periodontitis. Hum Immunol. 2011;72:940–946. doi: 10.1016/j.humimm.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Shibani K, Shhab R, Khattab R. Analysis of IL-1alpha (-889) and IL-1B (+3953) Gene Polymorphism in Syrian Patients with Aggressive Periodontitis: A Pilot Study. ISRN Dent. 2011;2011:682564. doi: 10.5402/2011/682564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masamatti SS, Kumar A, Baron TK, Mehta DS, Bhat K. Evaluation of interleukin -1B (+3954) gene polymorphism in patients with chronic and aggressive periodontitis: A genetic association study. Contemp Clin Dent. 2012;3:144–149. doi: 10.4103/0976-237X.96815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayazi G, Pirayesh M, Yari K. Analysis of interleukin-1beta gene polymorphism and its association with generalized aggressive periodontitis disease. DNA Cell Biol. 2013;32:409–413. doi: 10.1089/dna.2012.1905. [DOI] [PubMed] [Google Scholar]
- 47.Ebadian AR, Radvar M, Tavakkol Afshari J, Sargolzaee N, Brook A, Ganjali R, Tamizi M, Arab HR. Gene Polymorphisms of TNF-alpha and IL-1beta Are Not Associated with Generalized Aggressive Periodontitis in an Iranian Subpopulation. Iran J Allergy Asthma Immunol. 2013;12:345–351. [PubMed] [Google Scholar]
- 48.Yucel OO, Berker E, Mescil L, Eratalay K, Tepe E, Tezcan I. Association of interleukin-1 beta (+3954) gene polymorphism and gingival crevicular fluid levels in patients with aggressive and chronic periodontitis. Genet Couns. 2013;24:21–35. [PubMed] [Google Scholar]
- 49.Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu YM, Du L. The methodological quality assessment tools for pre-clinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015 doi: 10.1111/jebm.12141. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Jain V, Sharma R, Singh S. Doing meta-analysis in research: a systematic approach. Indian J Dermatol Venereol Leprol. 2012;78:242–250. doi: 10.4103/0378-6323.95438. [DOI] [PubMed] [Google Scholar]
- 51.Albuquerque CM, Cortinhas AJ, Morinha FJ, Leitao JC, Viegas CA, Bastos EM. Association of the IL-10 polymorphisms and periodontitis: a meta-analysis. Mol Biol Rep. 2012;39:9319–9329. doi: 10.1007/s11033-012-1738-1. [DOI] [PubMed] [Google Scholar]
- 52.Leng WD, He MN, Chen QL, Gong H, Zhang L, Zeng XT. Vascular endothelial growth factor (VEGF) gene polymorphisms and risk of head and neck cancer: a meta-analysis involving 2,444 individuals. Mol Biol Rep. 2013;40:5987–5992. doi: 10.1007/s11033-013-2708-y. [DOI] [PubMed] [Google Scholar]
- 53.Mao M, Zeng XT, Ma T, He W, Zhang C, Zhou J. Interleukin-1alpha -899 (+4845) C-->T polymorphism increases the risk of chronic periodontitis: evidence from a meta-analysis of 23 case-control studies. Gene. 2013;532:114–119. doi: 10.1016/j.gene.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 54.Yan Y, Weng H, Shen ZH, Wu L, Zeng XT. Association between interleukin-4 gene -590 c/t, -33 c/t, and 70 base-pair polymorphisms and periodontitis susceptibility: a meta-analysis. J Periodontol. 2014;85:e354–62. doi: 10.1902/jop.2014.140317. [DOI] [PubMed] [Google Scholar]
- 55.Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA Jr. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puhlmann M, Weinreich DM, Farma JM, Carroll NM, Turner EM, Alexande HR Jr. Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (TF) activity. J Transl Med. 2005;3:37. doi: 10.1186/1479-5876-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gore EA, Sanders JJ, Pandey JP, Palesch Y, Galbraith GM. Interleukin-1beta +3953 allele 2: association with disease status in adult periodontitis. J Clin Periodontol. 1998;25:781–785. doi: 10.1111/j.1600-051x.1998.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 58.Stashenko P, Fujiyoshi P, Obernesser MS, Prostak L, Haffajee AD, Socransky SS. Levels of interleukin 1 beta in tissue from sites of active periodontal disease. J Clin Periodontol. 1991;18:548–554. doi: 10.1111/j.1600-051x.1991.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 59.Hart TC, Pallos D, Bozzo L, Almeida OP, Marazita ML, O’Connell JR, Cortelli JR. Evidence of genetic heterogeneity for hereditary gingival fibromatosis. J Dent Res. 2000;79:1758–1764. doi: 10.1177/00220345000790100501. [DOI] [PubMed] [Google Scholar]
- 60.Yoshie H, Kobayashi T, Tai H, Galicia JC. The role of genetic polymorphisms in periodontitis. Periodontol 2000. 2007;43:102–132. doi: 10.1111/j.1600-0757.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 61.Vijayalakshmi R, Geetha A, Ramakrishnan T, Emmadi P. Genetic polymorphisms in periodontal diseases: an overview. Indian J Dent Res. 2010;21:568–574. doi: 10.4103/0970-9290.74226. [DOI] [PubMed] [Google Scholar]
- 62.Cesar Neto JB, Rosa EF, Pannuti CM, Romito GA. Smoking and periodontal tissues: a review. Braz Oral Res. 2012;26(Suppl 1):25–31. doi: 10.1590/s1806-83242012000700005. [DOI] [PubMed] [Google Scholar]


