Abstract
Background: CD95 rs2234767 polymorphism in the promotor region of CD95 gene has been implicated in several studies of cervical cancer. However, the results have not been conclusively established. Objective: The main aim of this study was to deal with the controversy with respect to the correlation between CD95 rs2234767 polymorphism and risk of cervical cancer through a meta-analysis. Methods: Association studies that pertain to CD95 rs2234767 polymorphism and risk of cervical cancer were identified up to May 26, 2014. ORs and 95% CIs were calculated assuming AA versus GG, AA + AG versus GG, AA versus AG + GG, A versus G and AG versus GG genetic models. Results: A total of 5 case-control studies were included in this meta-analysis. Overall, no significant effect modification of cervical cancer risk was revealed either at the genotypic or the allelic level for CD95 rs2234767 polymorphism. This null association persisted in the stratified analysis of Asian population. Conclusions: These findings revealed that CD95 rs2234767 polymorphism may not act as a causative agent of cervical cancer. Further evidence is needed to confirm our findings.
Keywords: CD95, polymorphism, cervical cancer
Introduction
Following breast cancer and colorectal cancer, cervical cancer has been the third most commonly diagnosed cancer and the fourth major cause of cancer-related deaths among women worldwide, with 9% (529,800) of the total new cancer cases and 8% (275,100) of the total cancer deaths estimated in 2008 [1]. Human papillomavirus (HPV) infection and cigarette consumption have been recognized as independent risk factors for cervical cancer [2,3]. However, cervical cancer is considered as a genetic component disease attracting widespread attention since the genetic epidemiological studies based on biological relatives showed that cervical cancer is nearly two-fold more likely to favor the individuals with biological full-sisters of cervical cancer than the controls without [4,5]. Apoptosis is a regulated form of cell death involved in the development of organisms and pathophysiology of certain disorders [6,7]. The CD95 signaling system is essential for apoptotic signaling in cells of the immune response [8] and for the activation of a major extrinsic cell death pathway which plays a key role in the maintenance of immuno-privileged sites and fulfillment of other regulatory functions [9,10]. CD95 induces apoptosis in susceptible cells by interacting with the well-characterized death-inducing ligand CD95. Apoptosis caused by FASL leads to immune homeostasis and cell-mediated cytotoxicity [11]. CD95 has been shown to be commonly expressed in liver, spleen, cardiac, thymus and ovarian tissues [11,12].
A growing body of evidence has suggested the importance of the genetic polymorphisms of CD95 rs2234767 polymorphism death pathway genes in determining predisposition to human cancers, such as lung cancer, pancreatic cancer and esophageal squamous-cell carcinoma [13-15]. In the promoter region of the CD95 gene, there is a G to A substitution at nucleotide position-1377 (rs2234767) located in the stimulatory protein-1 (Sp1). This polymorphism deregulates cell death signaling, resulting in a consequent reduce in CD95 rs2234767 polymorphism expression [16,17], which is frequently reported in cancer occurrence, including cervical cancer. The role of CD95 rs2234767 polymorphism in cervical cancer development has once been frequently studied with uncertain results [18-21]. These results could be better convinced if a sufficiently large study is performed. In this investigation, therefore, we conducted a meta-analysis to identify the association between CD95 rs2234767 polymorphism and cervical cancer risk and to promote more studies to continually concern this association.
Materials and methods
Data sources
We carried out a search in electronic databases including Embase, PubMed and CNKI (China National Knowledge Infrastructure) up to May 26, 2014 to identify all case-control studies on the association between CD95 rs2234767 polymorphism and cervical cancer risk without using any restriction. The search strategy used was: (CD95) OR (TNFRSF6) OR (APO-1) AND (polymorphism) OR (genotype) OR (polymorphisms) AND (cervical cancer), in combination with (-1377 A/G) OR (rs2234767). We also conducted a manual review of the bibliographic references cited in the selected articles for additional data available for the present meta-analysis.
Study selection
For this meta-analysis, we selected the studies that: a) included both cases and well-matched controls; b) assessed the association of CD95 rs2234767 polymorphism and cervical cancer risk; c) contained clear genotype frequencies for odds ratios (ORs) and 95% confidence intervals (CIs) calculation; and d) no significant departure from Hardy-Weinberg equilibrium (HWE) for the genotype distribution of controls. When several studies selected the same cases series, we considered the most updated one.
Data extraction
Two independent investigators (Ping Liu, Xiaofeng He) extracted the following information from the selected studies: the last name of first authors, year of publication, study country, matching criteria, ethnicity, total counts of genotyped cases and controls, genotype frequency distributing between cases and controls, and genotyping method. Each of the extracted items was compared, and a senior investigator (Zibai Wei) was referred to when there was any disagreement.
Statistical analysis
Stata software (version 12.0; StataCorp LP, College Station, TX, USA) was chosen and utilized to perform all statistical analyses involved in this article. The risk of cervical cancer associated with CD95 rs2234767 polymorphism was indicated as ORs and 95% CIs. We calculated the ORs assuming AA versus GG and AG versus GG comparisons, as well as AA + AG versus GG, AA versus AG + GG, and A versus G genetic models. (Figures 1, 2).
Figure 1.
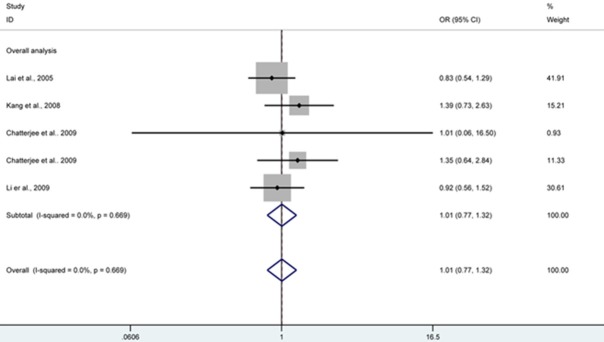
CD95 rs2234767 polymorphism and risk of cervical cancer by AA versus GG genetic model, forest plot shows no significant association between CD95 rs2234767 polymorphism and risk of cervical cancer under AA genotype compared with GG. CI confidence interval; OR odds risk.
Figure 2.
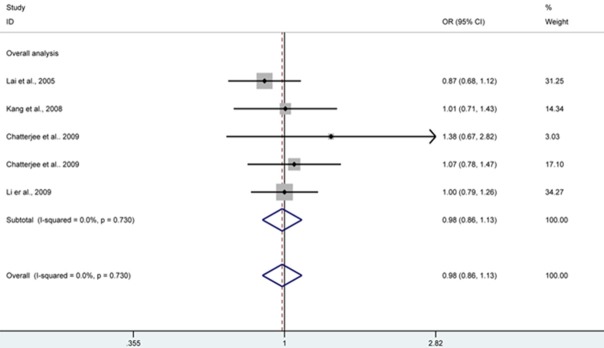
CD95 rs2234767 polymorphism and risk of cervical cancer by AA + AG versus GG genetic model, forest plot shows no significant association between CD95 rs2234767 polymorphism and risk of cervical cancer under AA + AG genotype compared with GG. CI confidence interval; OR odds risk.
Inter-study heterogeneity was tested using the Q-statistic and P < 0.10 was considered as representation of statistically significant heterogeneity [22]. In addition, I2 index was applied to quantify the inter-study variability, with larger values suggesting an increasing degree of heterogeneity (I2 < 25% low heterogeneity; I2 = 25-50% moderate heterogeneity; I2 > 50% large heterogeneity) [23]. Overall ORs with 95% CIs that indicated the association strength were pooled using the fixed effects model, when no heterogeneity presented (P > 0.10 and I2 < 50%) [24]; otherwise, the random effects model was more appropriate to pool the effect sizes [25]. HWE was tested by Pearson’s x2 test for controls in each study. Sensitivity analysis by removing a single study at a time was performed to assess if the pooled results were stable and robust. Publication bias was determined by Egger’s test which is a liner regression methodology to check the funnel plot asymmetry [26]. All studies were two-sided and P < 0.10 was considered statistically significant.
Results
Literature search and study selection
A total of 627 articles appeared to be potentially relevant to our study. By checking the titles, abstracts, and the full texts to examine whether available data were contained, we excluded 623 full-text articles and included 4 eligible articles [18-21], with 5 case-control studies (one record reported two populations) (Table 1), resulting in 1,172 cases and 1,476 well-matched controls. Among them, three studies conducted on Asian population [18,19,21], and one on both African and mixed populations [20]. Genotyping was completed by using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), real time-PCR (RT-PCR) and TaqMan. Control subjects of all studies were in agreement with HWE (P > 0.10) (Table 1).
Table 1.
Characteristics of studies included in the meta-analysis
| Study (author, year) | Population (country) | Genotyping method | Genotype frequency (case) | Genotype frequency (control) | Matching criteria | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| GG | GA | AA | GG | GA | AA | ||||
| Lai et al., 2005 | Asian (China) | RT-PCR | 127 | 138 | 53 | 99 | 165 | 54 | Age |
| Kang et al., 2008 | Asian (South Korea) | PCR-RFLP | 54 | 69 | 31 | 56 | 82 | 20 | Age |
| Chatterjee et al., 2009 | African (South Africa) | TaqMan | 74 | 21 | 1 | 75 | 14 | 1 | Age, ethnicity, Domicile status |
| Chatterjee et al., 2009 | Mixed (South Africa) | TaqMan | 186 | 87 | 17 | 196 | 86 | 13 | Age, ethnicity, Domicile status |
| Li et al., 2009 | Asian (China) | PCR-RFLP | 144 | 144 | 26 | 282 | 277 | 56 | Age, smoking status |
PCR: polymerase chain reaction; PCR-RFLP: PCR-restriction fragment length polymorphism; RT-PCR: real time-PCR.
Meta-analysis
Five studies with 4,271 genotyped subjects were included in the meta-analysis for the assessment of association between CD95 rs2234767 polymorphism and cervical cancer risk. Overall, no significant effect modification of cervical cancer risk was revealed either at the genotypic or allelic level of CD95 rs2234767 polymorphism. The null association persisted in the stratified analysis of Asian population. No additional analysis was conducted in the populations of African or mixed descents (Table 2).
Table 2.
Meta-analysis of the association between CD95 rs2234767 polymorphism and cervical cancer risk
| Studies (subjects) | OR (95% CI) | P value | Model | P-het | |
|---|---|---|---|---|---|
| Total studies | 5 (2648) | ||||
| AA versus GG | 1.01 (0.77, 1.32) | 0.971 | Fixed | 0.669 | |
| AA + AG versus GG | 0.98 (0.86, 1.13) | 0.814 | Fixed | 0.730 | |
| AA versus AG + GG | 1.09 (0.84, 1.41) | 0.512 | Fixed | 0.631 | |
| A versus G | 1.00 (0.89, 1.12) | 0.996 | Fixed | 0.594 | |
| AG versus GG | 0.97 (0.84, 1.12) | 0.670 | Fixed | 0.666 | |
| Asians | 3 (1871) | ||||
| AA versus GG | 0.96 (0.72, 1.29) | 0.788 | Fixed | 0.432 | |
| AA + AG versus GG | 0.95 (0.82, 1.11) | 0.518 | Fixed | 0.689 | |
| AA versus AG + GG | 1.06 (0.81, 1.40) | 0.672 | Fixed | 0.325 | |
| A versus G | 0.97 (0.85, 1.10) | 0.636 | Fixed | 0.504 | |
| AG versus GG | 0.93 (0.79, 1.10) | 0.411 | Fixed | 0.605 | |
| Others | 2 (777) | ||||
| AA versus GG | 1.32 (0.64, 2.72) | 0.449 | Fixed | 0.847 | |
| AA + AG versus GG | 1.11 (0.83, 1.49) | 0.465 | Fixed | 0.529 | |
| AA versus AG + GG | 1.30 (0.64, 2.66) | 0.472 | Fixed | 0.812 | |
| A versus G | 1.13 (0.87, 1.47) | 0.343 | Fixed | 0.582 | |
| AG versus GG | 1.10 (0.81, 1.50) | 0.538 | Fixed | 0.474 |
The heterogeneity for comparisons between cervical cancer subjects and controls was not significant (P > 0.10 and I2 < 50%). Similarly, no obvious indication of between-study heterogeneity was suggested in the analysis of Asian population. To further confirm the stability and credibility of our results, we also conducted sensitivity analysis and all studies turned out to be homogeneous. In addition, no significant publication bias was graphically or statistically suggested by performing the funnel plot and Egger’s test (AA versus GG: t = 0.25, P = 0.822) (Figure 3).
Figure 3.
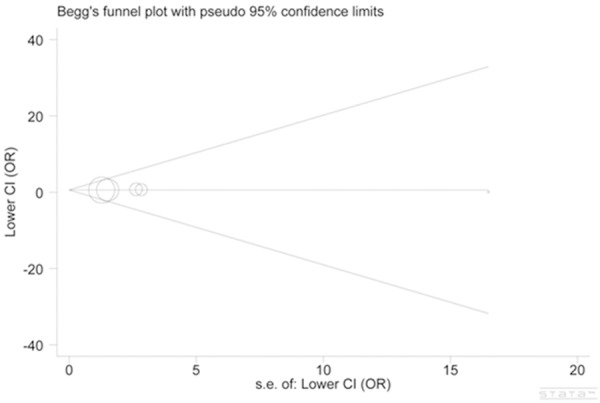
Begg funnel plot for publication bias test (AA versus GG: t = 0.25, P = 0.822). Each point represents a separate study for the indicated association. Log [OR], natural logarithm of odd ratio (OR). Horizontal line, mean effect size. s.e. = Standard error of the mean.
Discussion
CD95 rs2234767 polymorphism is a well-characterized genetic variant and has been widely studied in a number of cancers ranging from gastric cancer to lung cancer in various countries all over the world [13,27,28]. In contrast, only a small proportion of genetic association studies associate this extensively studied polymorphism with cervical cancer, especially in recent few years. Moreover, a relatively small number of investigations have concerned this point with the association inconclusively established. Considering these problems, we addressed two aims in our meta-analysis. The first aspect dealt with the controversy with respect to the correlation between CD95 rs2234767 polymorphism and risk of cervical cancer. The second goal related to encouraging more investigations to supply strong evidence for the association under dispute.
The principle findings of this meta-analysis suggested that the correlation between CD95 rs2234767 polymorphism and risk of cervical cancer was not statistically significant. In order to identify if the null results could be altered in Asian population, we performed a subsequent stratified analysis implicating similar nonsignificant association. African and mixed populations were not further analyzed, because the insufficient data (each dataset was provided by one single study) made it unnecessary to conduct such analysis.
It is well established that apoptosis plays a vital role in tumorigenesis. Biologically impaired apoptotic function is a prerequisite for cancer development, as more and more evidence implicating that neoplastic mutations appear to act by interfering with apoptosis [29]. The CD95 system is a mediator of apoptotic cell death. Somatic and germ line mutations within CD95 contribute to the development of various cancers and hematological malignancies [30-33]. CD95 rs2234767 polymorphism was revealed to regulate expression level of the CD95 gene. Patients positively expressed CD95 have a better prognosis compared with those negatively expressed [34].
Several investigators have correlated risk of cervical cancer with CD95 rs2234767 polymorphism. But the results were indecisive, even in the studies based on the subjects of the same descent. In an analysis of 318 cervical cancer cases, Lai et al. suggested that no association could be identified [18]. On the contrary, Li et al. (314 patients) believed CD95 rs2234767 polymorphism is significantly associated with the development of this cancer [21]. Relatively small sample size may be a possible explanation, but the major reason may attribute to the nonstandardized selection of subjects and misclassification of genotypes by using different method in genotyping CD95 rs2234767 polymorphism. In the meta-analysis performed in this study, the results showed cervical cancer risk was not associated with CD95 rs2234767 polymorphism. This could be explained by molecular role of the polymorphism in up regulation of the CD95 gene, precluding malignant transformation and progression.
The presence of significant heterogeneity and/or publication bias may influence the interpretation of the meta-analysis and lead to a potentially misleading conclusion. Corresponding analyses helped to quantitively confirm few effect from these factors in this study. However, several issues should be concerned. First, lack of sufficient data may have mistaken the susceptibility role of CD95 rs2234767 polymorphism, which can be accurately identified through a larger study. Second, we did not consider gene-gene and gene-environment interactions in this meta-analysis. Although CD95 rs2234767 polymorphism alone may not change cervical cancer risk, we cannot rule out the possibility that the risk will be influenced by the combination with other genes or environmental factors.
In conclusion, this meta-analysis suggested that no significant association existed between CD95 rs2234767 polymorphism and cervical cancer risk. The same was true for Asian population. Further large and well-designed studies with gene-gene and gene-environment interactions considered are needed to confirm our findings.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Hemminki K, Dong C, Vaittinen P. Familial risks in cervical cancer: is there a hereditary component? Int J Cancer. 1999;82:775–781. doi: 10.1002/(sici)1097-0215(19990909)82:6<775::aid-ijc1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Magnusson PK, Gyllensten UB. Cervical cancer risk: is there a genetic component? Mol Med Today. 2000;6:145–148. doi: 10.1016/s1357-4310(00)01685-3. [DOI] [PubMed] [Google Scholar]
- 6.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 7.Martin TR, Hagimoto N, Nakamura M, Matute-Bello G. Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc. 2005;2:214–220. doi: 10.1513/pats.200504-031AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krammer PH, Behrmann I, Daniel P, Dhein J, Debatin KM. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994;6:279–289. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 9.Los M, Wesselborg S, Schulze-Osthoff K. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity. 1999;10:629–639. doi: 10.1016/s1074-7613(00)80062-x. [DOI] [PubMed] [Google Scholar]
- 10.Rashedi I, Panigrahi S, Ezzati P, Ghavami S, Los M. Autoimmunity and apoptosis--therapeutic implications. Curr Med Chem. 2007;14:3139–3151. doi: 10.2174/092986707782793952. [DOI] [PubMed] [Google Scholar]
- 11.Poulaki V, Mitsiades CS, Mitsiades N. The role of Fas and FasL as mediators of anticancer chemotherapy. Drug Resist Updat. 2001;4:233–242. doi: 10.1054/drup.2001.0210. [DOI] [PubMed] [Google Scholar]
- 12.Lee HO, Ferguson TA. Biology of FasL. Cytokine Growth Factor Rev. 2003;14:325–335. doi: 10.1016/s1359-6101(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Miao X, Sun T, Tan W, Qu S, Xiong P, Zhou Y, Lin D. Functional polymorphisms in cell death pathway genes FAS and FASL contribute to risk of lung cancer. J Med Genet. 2005;42:479–484. doi: 10.1136/jmg.2004.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Sun T, Wang L, Yu D, Zhang X, Miao X, Liu J, Zhao D, Li H, Tan W, Lin D. Functional variants in cell death pathway genes and risk of pancreatic cancer. Clin Cancer Res. 2008;14:3230–3236. doi: 10.1158/1078-0432.CCR-08-0177. [DOI] [PubMed] [Google Scholar]
- 15.Sun T, Miao X, Zhang X, Tan W, Xiong P, Lin D. Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst. 2004;96:1030–1036. doi: 10.1093/jnci/djh187. [DOI] [PubMed] [Google Scholar]
- 16.Huang QR, Morris D, Manolios N. Identification and characterization of polymorphisms in the promoter region of the human Apo-1/Fas (CD95) gene. Mol Immunol. 1997;34:577–582. doi: 10.1016/s0161-5890(97)00081-3. [DOI] [PubMed] [Google Scholar]
- 17.Sibley K, Rollinson S, Allan JM, Smith AG, Law GR, Roddam PL, Skibola CF, Smith MT, Morgan GJ. Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 2003;63:4327–4330. [PubMed] [Google Scholar]
- 18.Lai HC, Lin WY, Lin YW, Chang CC, Yu MH, Chen CC, Chu TY. Genetic polymorphisms of FAS and FASL (CD95/CD95L) genes in cervical carcinogenesis: An analysis of haplotype and gene-gene interaction. Gynecol Oncol. 2005;99:113–118. doi: 10.1016/j.ygyno.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Kang S, Dong SM, Seo SS, Kim JW, Park SY. FAS -1377 G/A polymorphism and the risk of lymph node metastasis in cervical cancer. Cancer Genet Cytogenet. 2008;180:1–5. doi: 10.1016/j.cancergencyto.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee K, Engelmark M, Gyllensten U, Dandara C, van der Merwe L, Galal U, Hoffman M, Williamson AL. Fas and FasL gene polymorphisms are not associated with cervical cancer but differ among Black and Mixed-ancestry South Africans. BMC Res Notes. 2009;2:238. doi: 10.1186/1756-0500-2-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Guo HY, Sun T, Zhou YF, Lin DX, Zhang WH, Qiao J. [Association between Fas/Fas L genes promoter polymorphisms and pathogenic risk of cervical cancer] . Zhonghua Zhong Liu Za Zhi. 2009;31:38–41. [PubMed] [Google Scholar]
- 22.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672–3673. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupcinskas J, Wex T, Bornschein J, Selgrad M, Leja M, Juozaityte E, Kiudelis G, Jonaitis L, Malfertheiner P. Lack of association between gene polymorphisms of Angiotensin converting enzyme, Nod-like receptor 1, Toll-like receptor 4, FAS/FASL and the presence of Helicobacter pylori-induced premalignant gastric lesions and gastric cancer in Caucasians. BMC Med Genet. 2011;12:112. doi: 10.1186/1471-2350-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B, Sun T, Xue L, Han X, Lu N, Shi Y, Tan W, Zhou Y, Zhao D, Zhang X, Guo Y, Lin D. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis. 2007;28:1067–1073. doi: 10.1093/carcin/bgl250. [DOI] [PubMed] [Google Scholar]
- 29.Zornig M, Hueber A, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim Biophys Acta. 2001;1551:F1–37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Shin MS, Park WS, Kim SY, Dong SM, Pi JH, Lee HK, Kim HS, Jang JJ, Kim CS, Kim SH, Lee JY, Yoo NJ. Alterations of Fas (APO-1/CD95) gene in transitional cell carcinomas of urinary bladder. Cancer Res. 1999;59:3068–3072. [PubMed] [Google Scholar]
- 31.Lee SH, Shin MS, Park WS, Kim SY, Kim HS, Han JY, Park GS, Dong SM, Pi JH, Kim CS, Kim SH, Lee JY, Yoo NJ. Alterations of Fas (Apo-1/CD95) gene in non-small cell lung cancer. Oncogene. 1999;18:3754–3760. doi: 10.1038/sj.onc.1202769. [DOI] [PubMed] [Google Scholar]
- 32.Butler LM, Hewett PJ, Butler WJ, Cowled PA. Down-regulation of Fas gene expression in colon cancer is not a result of allelic loss or gene rearrangement. Br J Cancer. 1998;77:1454–1459. doi: 10.1038/bjc.1998.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 34.Koomagi R, Volm M. Expression of Fas (CD95/APO-1) and Fas ligand in lung cancer, its prognostic and predictive relevance. Int J Cancer. 1999;84:239–243. doi: 10.1002/(sici)1097-0215(19990621)84:3<239::aid-ijc7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]


