Abstract
Background: Epidemiological studies evaluating the association between sunscreens use and malignant melanoma risk have produced inconsistent results. Thus, we conducted a meta-analysis to summarize the evidence from epidemiological studies of sunscreens use with the risk of malignant melanoma. Methods: Pertinent studies were identified by a search in PubMed and Web of Knowledge up to October 2014. Random-effect model was used to combine the results. Publication bias was estimated using Egger’s regression asymmetry test. Results: Twenty-one studies including 7150 malignant melanoma cases about sunscreens use with the risk of malignant melanoma were included in this meta-analysis. The combined relative risk (RR) of malignant melanoma associated with sunscreens use was 1.145 (95% CI=0.912-1.438). The association was significant neither in the case-control studies nor in the cohort studies. No publication biases were found. Conclusions: Our analysis indicated that sunscreens use is not associated with the risk of malignant melanoma.
Keywords: Sunscreens, malignant melanoma, meta-analysis
Introduction
The sustained increase in malignant melanoma incidence over the past few decades highlights the fact that this disease represents a major public health management issue worldwide [1]. Exogenous sun exposure and several host features such as light complexions, skin reactivity to sun exposure, presence of dysplastic nevi, family history of melanoma, history of cancer, and immunosuppression have been identified as major risk factors for this malignancy [2-4]. An understanding of other factors, in particular behavioral factors associated with melanoma risk is however less clear. Behavioral factors are modifiable, and so it is of particular importance to study their role in the etiology of cancer.
If solar radiation is a primary risk factor for malignant melanoma, it is reasonable to conclude that reducing sun exposure via topical sunscreen use would be associated with reduced disease risk. However, the available epidemiological data are contradictory. In fact, the majority of studies suggest that sunscreen use is associated with an increased melanoma risk [5-8]. To address this uncertainty, we designed the present study to systematically evaluate the available data using rigorous meta-analytic techniques.
Methods
Search strategy and study selection
Studies were identified by a literature search of PubMed and Web of Knowledge up to October 2014. The following search terms were used: (melanoma OR skin neoplasm OR skin cancer) AND (sunscreens OR sun OR sunblock) AND (cohort OR prospective OR nested OR case-control). Moreover, we reviewed the reference lists from retrieved articles to search for further relevant studies. Two investigators searched articles and reviewed of all retrieved studies independently.
For inclusion, studies had to fulfill the following criteria: (1) have a prospective or case-control study design; (2) the exposure of interest was sunscreens use; (3) the outcome of interest was malignant melanoma; and (4) relative risk (RR) or odds ratio (OR) with 95% confidence interval (CI) was provided (or data available to calculate them).
Data extraction
Two researchers independently extracted the following data from each study that met the criteria for inclusion: the first author’s last name, year of publication, geographic locations, study design, sample source, the age range of study participants, the number of cases and participants. From each study, we extracted the RR that reflected the greatest degree of control for potential confounders.
Statistical analysis
The pooled measure was calculated as the inverse variance-weighted mean of the logarithm of RR with 95% CI, to assess the association between sunscreens use and risk of malignant melanoma. Random-effects model was used to combine study-specific RR (95% CI), which considers both within-study and between-study variation [9]. The I2 was used to assess heterogeneity, and I2 values of 0, 25, 50 and 75% represent no, low, moderate and high heterogeneity [10], respectively. Meta-regression with restricted maximum likelihood estimation was performed to assess the potentially important covariates that might exert substantial impact on between-study heterogeneity [11]. Publication bias was evaluated using Egger regression asymmetry test [12]. A study of influence analysis [13] was conducted to describe how robust the pooled estimator was to removal of individual studies. An individual study was suspected of excessive influence if the point estimate of its omitted analysis lay outside the 95% CI of the combined analysis. All statistical analyses were conducted with STATA version 11.0 (StataCorp LP, College Station, Texas, USA). Two-tailed p-value ≤ 0.05 was accepted as statistically significant.
Results
Search results and study characteristics
The search strategy identified 585 articles from PubMed and 708 from the Web of Knowledge, and 45 articles were reviewed in full after reviewing the title/abstract. Twenty-four of these 45 articles were subsequently excluded from the meta-analysis for various reasons. Hence, 21 articles (2 prospective studies and 19 case-control studies) [5-8,14-30] involving 7150 malignant melanoma cases and 23434 participants were used in this meta-analysis. The detailed steps of our literature search are shown in Figure 1. The characteristics of these studies are presented in Table 1. Ten studies come from Europe, 6 from America and 5 from Oceania.
Figure 1.
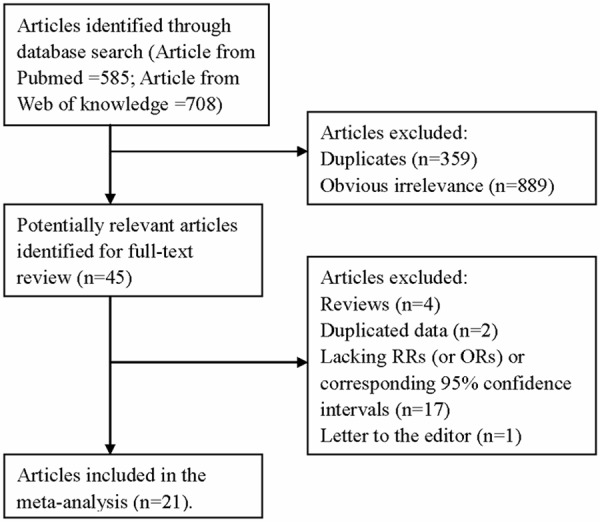
The flow diagram of screened, excluded, and analyzed publications.
Table 1.
Characteristics of studies on sunscreens use and risk of malignant melanoma
| First author, year | Country | Study design | Cases, age | Frequency of Sunscreen Use | RR (95% CI) | Adjustment or matched for |
|---|---|---|---|---|---|---|
| Autier et al. 1995 | Germany, Belgium and France | Case-control | 418, ≥20 | Regular use vs. never | 1.50 (1.09-2.06) | Age, sex, hair color, no. of holiday weeks spent in sunny climate. |
| Bakos et al. 2002 | Brazil | Case-control | 102, 20-84 | SPF15+ vs. never | 0.2 (0.1-0.8) | Age, sex, race, and residence. |
| Beitner et al. 1990 | Sweden | Case-control | 523, Na | Very often/often vs. never | 1.80 (1.20-2.70) | Age, sex, hair colour. |
| Espinoza Arranz et al. 1999 | Spain | Case-control | 116, 21-87 | Ever vs. never | 0.48 (0.34-0.71) | Skin type, nevi count, age. |
| Gandini et al. 2014 | Italy | Prospective | 139, Na | Ever vs. never | 0.82 (0.70-0.96) | Place of residence, interview location, age, sex, socio-economic features. |
| Graham et al. 1985 | United States | Case-control | 404, Na | Use vs. never used | 2.20 (1.20-4.10) | Na. |
| Green et al. 2011 | Australia | Prospective | 33, 25-75 | Use vs. never used | 0.50 (0.24-1.02) | Age, sex, phenotype, sun exposure, and history of skin cancer. |
| Herzfeld et al. 1993 | United States | Case-control | 324, ≥18 | Always vs. never | 2.6 (1.4-4.7) | Age, sex, race, and residence. |
| Holly et al. 1995 | United States | Case-control | 452, 25-59 | Almost always vs. never | 0.48 (0.33-0.67) | Age, complexion, maternal ethnicity, history of skin cancer, and sunburns up to 12 yrs of age, skin reaction to sun, host factors. |
| Holman et al. 1986 | Australia | Case-control | 507, <80 | Ever vs. never | 1.15 (0.78-1.68) | Host factors, age at arrival in Australia, ethnic origin. |
| Klepp et al. 1979 | Norway | Case-control | 78, ≥20 | Often vs. rarely or never | 2.27 (1.26-4.12) | Na. |
| Klug et al. 2010 | United States | Case-control | 349, Na | Ever vs. never | 0.90 (0.70-1.19) | Ambient residential UV intensity, number of hours outdoors, tan type, number of sunburns, gender, age group, and study site. |
| Lazovich et al. 2011 | United States | Case-control | 1167, 25-59 | High vs. no used | 1.10 (0.77-1.57) | Gender, age at interview, phenotypic risk score, moles, high income, college education, family history of melanoma, lifetime sunburns, routine sun exposure, activity sun exposure, and ever use of indoor tanning. |
| Naldi et al. 2000 | Italy | Case-control | 542, Na | Often vs. never used | 0.80 (0.54-1.17) | Age, sex, geographic area, education, skin, eye and hair colour, number of freckles and naevi ≥2 mm, history of sunburns, tanning pattern and sunny holiday weeks per year. |
| Osterlind et al. 1988 | Denmark | Case-control | 474, 20-79 | >10 yrs vs. never | 1.2 (0.9-1.5) | Constitutional factors, sex, age. |
| Rodenas et al. 1996 | Spain | Case-control | 105, 20-79 | Always vs. never | 0.6 (0.26-1.42) | Age, skin color, skin type, total number of hours of recreational sun exposure, total number of hours of occupational sun exposure, and total number of nevi. |
| Westerdahl et al. 1995 | Sweden | Case-control | 400. 15-75 | Almost always vs. never | 1.80 (1.10-2.80) | History of sunburn, history of sunbathing, employment, host factors. |
| Westerdahl et al. 2000 | Sweden | Case-control | 571, 16-80 | Often vs. never used | 1.8 (1.1-2.9) | Hair colour, history of sunburns, frequency of sunbathing during the summer and the duration of each sunbathing occasion. |
| Whiteman et al. 1997 | Australia | Case-control | 52, <21 | Always vs. never | 2.2 (0.4-11.6) | Sex, school, grade, tanning ability, freckling and number of naevi. |
| Wolf et al. 1998 | Australia | Case-control | 193, 18-83 | Often vs. never | 3.34 (1.81-6.64) | Age, sex, sunbathing, host factors. |
| Youl et al. 2002 | Australia | Case-control | 201, Na | Often vs. never | 2.2 (0.7-7.1) | Age, sex, total nevi, hair color, eye color, tanning ability, facial freckling, family history. |
Abbreviations: RR: relative risk; CI: confidence interval; SPF: solar protection factor; Na: not available; vs.: versus.
High versus low analyses
Data from 21 articles including 7150 malignant melanoma cases were used in this meta-analysis. Eight studies reported that sunscreens use could increase the risk of malignant melanoma, while no significant association was reported in 9 studies. However, four studies reported that sunscreen is a protective factor for malignant melanoma. Pooled results indicating no association between sunscreen use and development of malignant melanoma [summary RR=1.145, 95% CI=0.912-1.438, I2=83.7%] (Figure 2).
Figure 2.
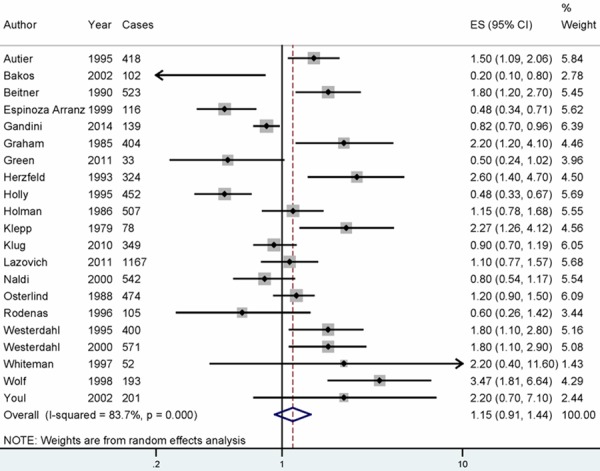
The forest plot between sunscreens use and malignant melanoma risk. White diamond denotes the pooled RR. Black squares indicate the RR in each study, with square sizes inversely proportional to the standard error of the RR. Horizontal lines represent 95% CI.
Meta-regression and subgroup analysis
As seen in the pooled results, high heterogeneity (I2=83.7%, P heterogeneity=0.000) was found in the analysis. In order to explore the high between-study heterogeneity founded in several analysis, univariate meta-regression with the covariates of publication year, location where the study was conducted, study design (case-control or prospective), number of cases and source of controls was performed. No significant findings were found in the above-mentioned analysis.
For the subgroup analyses by study design, the association was significant neither in the case-control studies [RR=1.219, 95% CI=0.942-1.576], nor in the cohort studies [RR=0.730, 95% CI=0.484-1.101] for the sunscreen use and risk of malignant melanoma. In subgroup analyses of geographic locations, when we restricted the analysis to America, Europe and Oceania, no significant associations were found in the subgroup analysis. The main results are summarized in Table 2.
Table 2.
Summary risk estimates of the association between sunscreens use and risk of malignant melanoma
| Sub-groups | Cases | Studies | RR (95% CI) | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|
| All studies | 7150 | 21 | 1.145 (0.912-1.438) | 83.7 | 0.000 |
| Study design | |||||
| Case-control | 6978 | 19 | 1.219 (0.942-1.576) | 83.3 | 0.000 |
| Prospective | 172 | 2 | 0.730 (0.484-1.101) | 41.7 | 0.190 |
| Geographic locations | |||||
| America | 6 | 2798 | 0.958 (0.567-1.618) | 87.9 | 0.000 |
| Europe | 10 | 3366 | 1.162 (0.870-1.552) | 84.8 | 0.000 |
| Oceania | 5 | 986 | 1.477 (0.727-2.998) | 76.5 | 0.002 |
Influence analysis and publication bias
Influence analysis showed that no individual study had excessive influence on the association of sunscreens use and risk of malignant melanoma (Figure 3). Egger’s test (P=0.192) showed no evidence of significant publication bias between sunscreens use and malignant melanoma risk.
Figure 3.
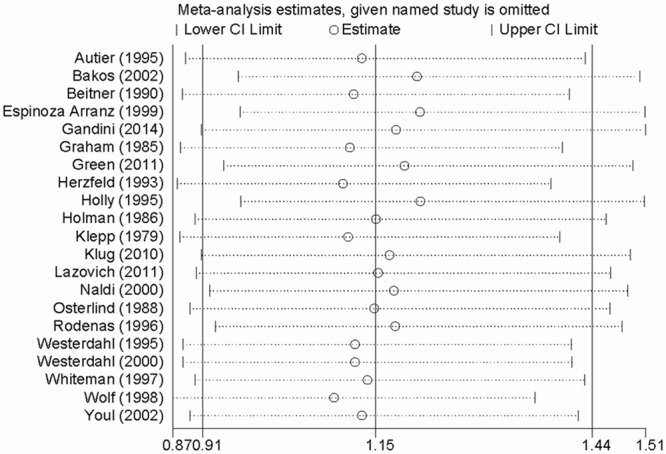
Analysis of influence of individual study on the pooled estimate in sunscreens use and malignant melanoma risk. Open circle, the pooled OR, given named study is omitted. Horizontal lines represent the 95% CIs.
Discussion
Finding from this meta-analysis suggested that sunscreens use is not associated on malignant melanoma risk. The associations were not significant both in cohort studies and in case-control studies.
Sunscreens are able to delay sunburns and to reduce some ultraviolet-induced skin lesions, such as non-melanoma tumors in rodents, local immunological depression, and the incidence of actinic keratoses in humans. As a consequence, sunscreen use is often recommended as a sun protection method, although its true impact on melanoma prevention remains obscure. Despite uncertainties in the available epidemiological data, experimental evidence using both animal models and humans suggests that sunscreen preparations capable of reducing exposure to ultraviolet-B radiation from the sun can prevent melanoma [31]. Regrettably, this finding has not been universal. In fact, some investigators suggest that sunscreen use could be a risk rather than a protective factor for malignant melanoma [32]. Although it is considered unlikely that available sunscreen preparations contain compounds with carcinogenic effects, other factors may account for this observed relationship; they include uncontrolled confounding caused by host factors and behavioral factors, such as increased sun exposure among patients who use sunscreen preparations. By pooling data from 21 studies meeting protocol inclusion criteria (yielding a statistically non-significant summary RR of 1.145), we demonstrated that sunscreen use is not associated with an increased risk of developing malignant melanoma. Unfortunately, further evaluation showed the data to be highly heterogeneous.
Between-study heterogeneity is common in meta-analysis [33], and exploring the potential sources of between-study heterogeneity is the essential component of meta-analysis. For sunscreens use on the risk of malignant melanoma, evidence of heterogeneity was found in the pooled results. The between-study heterogeneity might arise from publication year, location where the study was conducted, study design (case-control or prospective), number of cases and source of controls. Thus, we used meta-regression to explore the causes of heterogeneity for covariates. However, no covariate having a significant impact on between-study heterogeneity for the above mentioned covariates. Considering the pooled meta-analysis was fraught with the problem of heterogeneity, subgroup analyses by the type of study design and geographic locations were performed to explore the source of heterogeneity. However, the between-study heterogeneity persisted in some subgroups.
This is a comprehensive meta-analysis between sunscreens use and malignant melanoma risk. Our study included a larger number of participants and cases, allowing a much greater possibility of reaching reliable conclusions about the association between sunscreens use and malignant melanoma risk. However, our study has some limitations. First, most studies included in this meta-analysis were case-control studies. Overstated association may be expected from the case-control studies because of recall or selection bias, and early symptoms in patients may have resulted in a change in dietary habits. Further studies with cohort design are wanted to confirm this association between sunscreens use and malignant melanoma risk. Second, although we combined the results with sunscreens use and malignant melanoma risk, we did not do a dose-response analysis because of the limited data in the reported articles. Third, as a meta-analysis of observational studies, we cannot rule out that individual studies may have failed to control for potential confounders, which may introduce bias in an unpredictable direction. Fourth, between-study heterogeneity was found in some analysis in this meta-analysis, but the between-study heterogeneity was not successfully explained by the subgroup analysis and meta-regression. However, other genetic and environment variables, as well as their possible interaction may be potential contributors to this disease-effect unconformity.
Conclusion
In summary, results from this meta-analysis suggested that sunscreens use is not associated with the risk of malignant melanoma. Further studies with more participants and more cases are wanted to confirm this result.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41:28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, Masini C, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Autier P, Dore JF, Schifflers E, Cesarini JP, Bollaerts A, Koelmel KF, Gefeller O, Liabeuf A, Lejeune F, Lienard D, et al. Melanoma and use of sunscreens: an Eortc case-control study in Germany, Belgium and France. The EORTC Melanoma Cooperative Group. Int J Cancer. 1995;61:749–755. doi: 10.1002/ijc.2910610602. [DOI] [PubMed] [Google Scholar]
- 6.Beitner H, Norell SE, Ringborg U, Wennersten G, Mattson B. Malignant melanoma: aetiological importance of individual pigmentation and sun exposure. Br J Dermatol. 1990;122:43–51. doi: 10.1111/j.1365-2133.1990.tb08238.x. [DOI] [PubMed] [Google Scholar]
- 7.Graham S, Marshall J, Haughey B, Stoll H, Zielezny M, Brasure J, West D. An inquiry into the epidemiology of melanoma. Am J Epidemiol. 1985;122:606–619. doi: 10.1093/oxfordjournals.aje.a114140. [DOI] [PubMed] [Google Scholar]
- 8.Herzfeld PM, Fitzgerald EF, Hwang SA, Stark A. A case-control study of malignant melanoma of the trunk among white males in upstate New York. Cancer Detect Prev. 1993;17:601–608. [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobias A. Assessing the in fluence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15–17. [Google Scholar]
- 14.Bakos L, Wagner M, Bakos RM, Leite CS, Sperhacke CL, Dzekaniak KS, Gleisner AL. Sunburn, sunscreens, and phenotypes: some risk factors for cutaneous melanoma in southern Brazil. Int J Dermatol. 2002;41:557–562. doi: 10.1046/j.1365-4362.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa Arranz J, Sanchez Hernandez JJ, Bravo Fernandez P, Gonzalez-Baron M, Zamora Auñon P, Espinosa Arranz E, Jalon Lopez JI, Ordoñez Gallego A. Cutaneous malignant melanoma and sun exposure in Spain. Melanoma Res. 1999;9:199–205. doi: 10.1097/00008390-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Gandini S, Stanganelli I, Magi S, Mazzoni L, Medri M, Agnoletti V, Lombi L, Falcini F. Melanoma attributable to sunbed use and tan seeking behaviours: an Italian survey. Eur J Dermatol. 2014;24:35–40. doi: 10.1684/ejd.2013.2214. [DOI] [PubMed] [Google Scholar]
- 17.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J. Clin. Oncol. 2011;29:257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- 18.Holly EA, Aston DA, Cress RD, Ahn DK, Kristiansen JJ. Cutaneous melanoma in women. I. Exposure to sunlight, ability to tan, and other risk factors related to ultraviolet light. Am J Epidemiol. 1995;141:923–933. doi: 10.1093/oxfordjournals.aje.a117359. [DOI] [PubMed] [Google Scholar]
- 19.Holman CD, Armstrong BK, Heenan PJ, Blackwell JB, Cumming FJ, English DR, Holland S, Kelsall GR, Matz LR, Rouse IL, et al. The causes of malignant melanoma: results from the West Australian Lions Melanoma Research Project. Recent Results Cancer Res. 1986;102:18–37. doi: 10.1007/978-3-642-82641-2_3. [DOI] [PubMed] [Google Scholar]
- 20.Klepp O, Magnus K. Some environmental and bodily characteristics of melanoma patients. A case-control study. Int J Cancer. 1979;23:482–486. doi: 10.1002/ijc.2910230407. [DOI] [PubMed] [Google Scholar]
- 21.Klug HL, Tooze JA, Graff-Cherry C, Anver MR, Noonan FP, Fears TR, Tucker MA, De Fabo EC, Merlino G. Sunscreen prevention of melanoma in man and mouse. Pigment Cell Melanoma Res. 2010;23:835–837. doi: 10.1111/j.1755-148X.2010.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazovich D, Vogel RI, Berwick M, Weinstock MA, Warshaw EM, Anderson KE. Melanoma risk in relation to use of sunscreen or other sun protection methods. Cancer Epidemiol Biomarkers Prev. 2011;20:2583–2593. doi: 10.1158/1055-9965.EPI-11-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naldi L, Gallus S, Imberti GL, Cainelli T, Negri E, La Vecchia C. Sunscreens and cutaneous malignant melanoma: an Italian case-control study. Int J Cancer. 2000;86:879–882. doi: 10.1002/(sici)1097-0215(20000615)86:6<879::aid-ijc19>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. II. Importance of UV-light exposure. Int J Cancer. 1988;42:319–324. doi: 10.1002/ijc.2910420303. [DOI] [PubMed] [Google Scholar]
- 25.Rodenas JM, Delgado-Rodriguez M, Herranz MT, Tercedor J, Serrano S. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: a case-control study in a Mediterranean population. Cancer Causes Control. 1996;7:275–283. doi: 10.1007/BF00051303. [DOI] [PubMed] [Google Scholar]
- 26.Westerdahl J, Olsson H, Masback A, Ingvar C, Jonsson N. Is the use of sunscreens a risk factor for malignant melanoma? Melanoma Res. 1995;5:59–65. doi: 10.1097/00008390-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Westerdahl J, Ingvar C, Masback A, Olsson H. Sunscreen use and malignant melanoma. Int J Cancer. 2000;87:145–150. doi: 10.1002/1097-0215(20000701)87:1<145::aid-ijc22>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Whiteman DC, Valery P, McWhirter W, Green AC. Risk factors for childhood melanoma in Queensland, Australia. Int J Cancer. 1997;70:26–31. doi: 10.1002/(sici)1097-0215(19970106)70:1<26::aid-ijc4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Wolf P, Quehenberger F, Mullegger R, Stranz B, Kerl H. Phenotypic markers, sunlight-related factors and sunscreen use in patients with cutaneous melanoma: an Austrian case-control study. Melanoma Res. 1998;8:370–378. doi: 10.1097/00008390-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Youl P, Aitken J, Hayward N, Hogg D, Liu L, Lassam N, Martin N, Green A. Melanoma in adolescents: a case-control study of risk factors in Queensland, Australia. Int J Cancer. 2002;98:92–98. doi: 10.1002/ijc.10117. [DOI] [PubMed] [Google Scholar]
- 31.Osterlind A. Epidemiology on malignant melanoma in Europe. Acta Oncol. 1992;31:903–908. doi: 10.3109/02841869209089727. [DOI] [PubMed] [Google Scholar]
- 32.Donawho C, Wolf P. Sunburn, sunscreen, and melanoma. Curr Opin Oncol. 1996;8:159–166. doi: 10.1097/00001622-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]


