Abstract
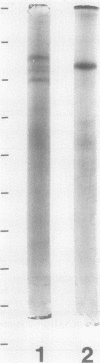
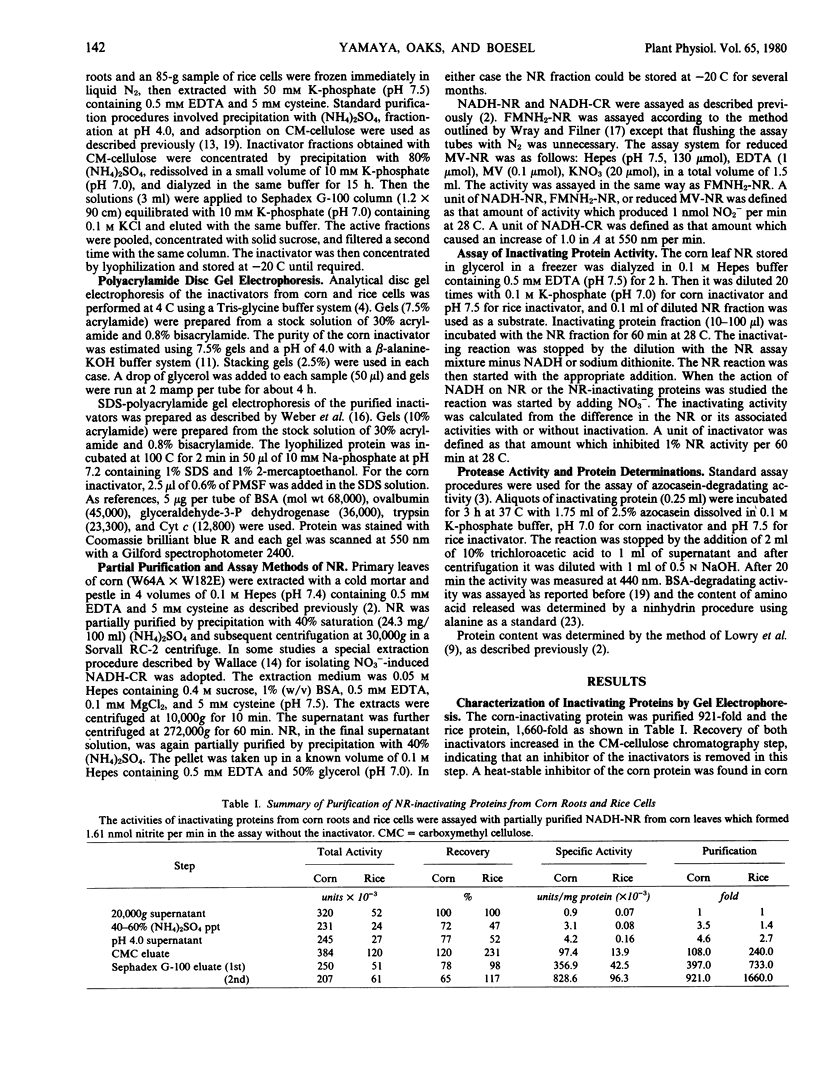
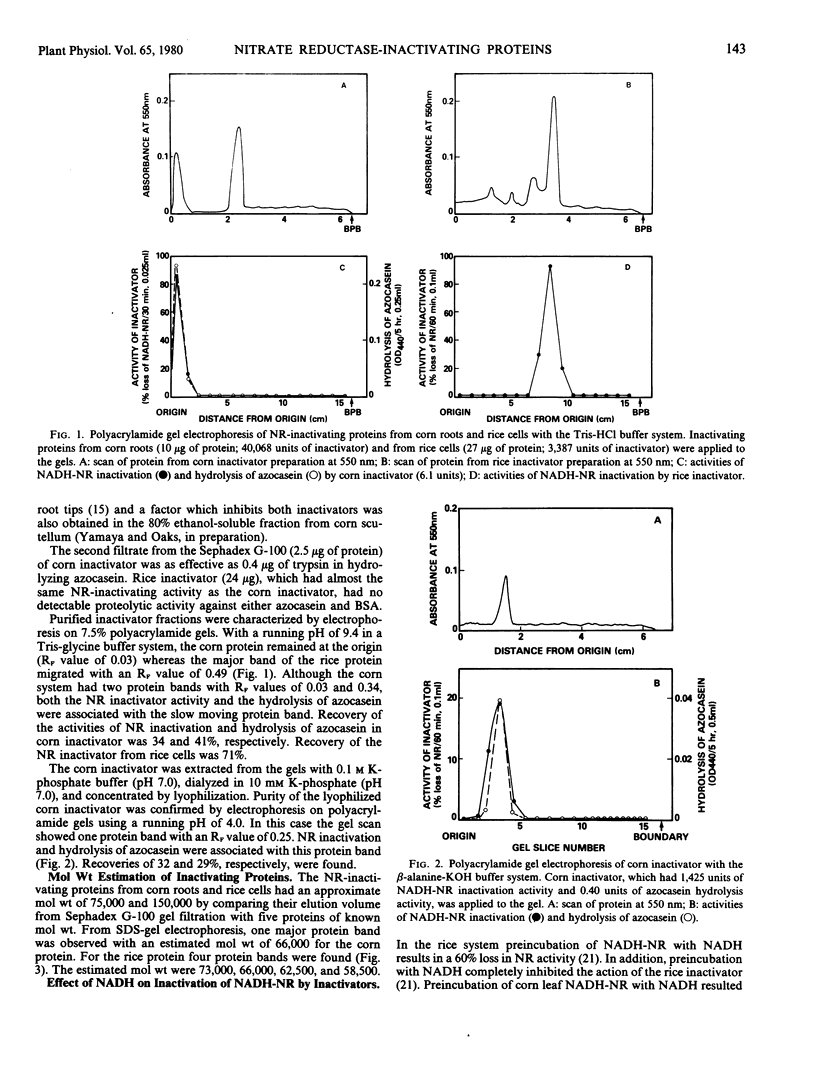
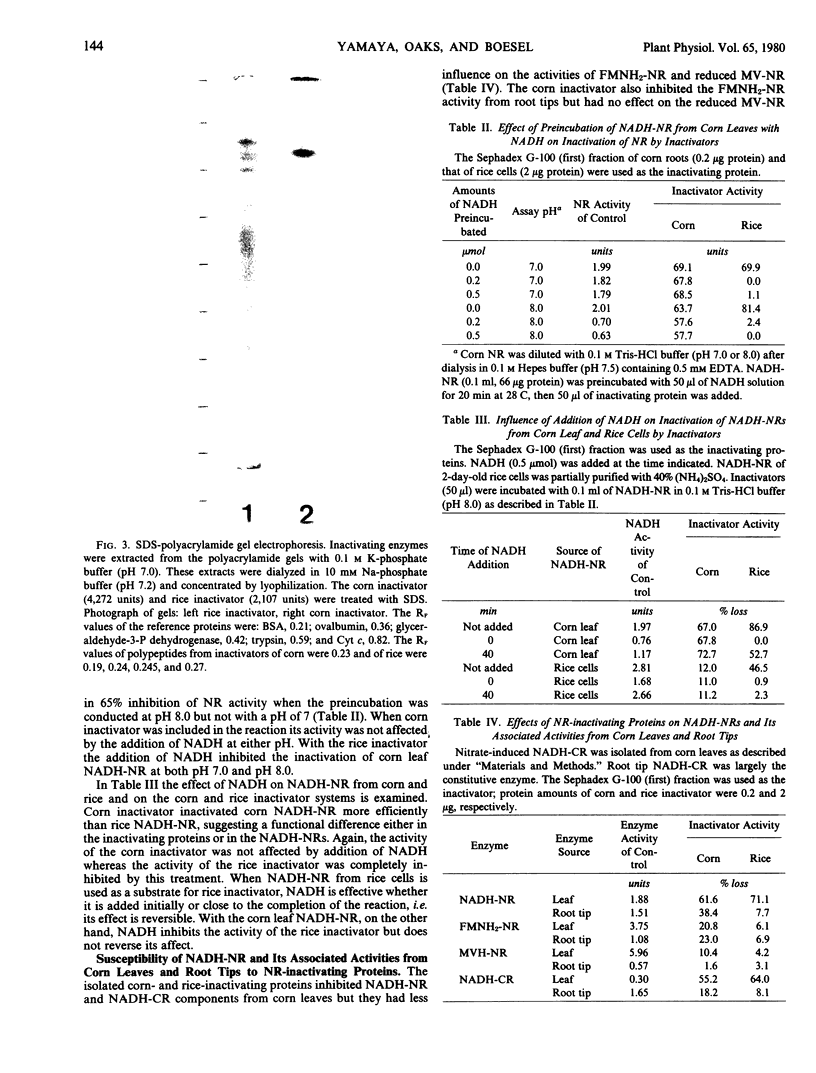
Nitrate reductase (NR)-inactivating proteins from corn roots (Wf-9 × 38-11) and rice cell suspension cultures were tested against a partially purified NR obtained from corn leaves (W64A × W182E). The corn protein was purified 921-fold and the rice protein, 1,660-fold using standard purification procedures. Approximate molecular weight values were 75,000 for the corn protein, and 150,000 for the rice protein as determined by Sephadex G-100 gel filtration. The Sephadex-treated proteins were characterized by electrophoresis on polyacrylamide gels. With a running pH of 9.4 the corn protein remained at the origin whereas the rice protein migrated with an RF value of 0.49. With a running pH of 4.0 the corn protein migrated with an RF value of 0.25. With the corn protein the activities of NR inactivation and hydrolysis of azocasein were detected in the same protein band. The rice protein, however, had no associated protease activity. From sodium dodecyl sulfate gel electrophoresis, there was one major protein band with an estimated molecular weight of 66,000 in corn protein. In rice protein four bands were observed with estimated molecular weights of 73,000, 66,000, 62,500, and 58,500, respectively.
Both inactivators had an inhibitory effect on NADH-NR and NO3− induced NADH-cytochrome c reductase activities but they had less influence on the activities of FMNH2-NR and reduced methylviologen-NR. Inactivation of rice cell NR by rice inactivator was reversed by addition of NADH. Inactivation of corn leaf NR by rice inactivator was inhibited by the simultaneous addition of NADH, but rice inactivator-inactivated corn leaf NR could not be reactivated by NADH.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Baki A. A. Pitfalls in using sodium hypochlorite as a seed disinfectant in C incorporation studies. Plant Physiol. 1974 May;53(5):768–771. doi: 10.1104/pp.53.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Oaks A. Comparative studies on the induction and inactivation of nitrate reductase in corn roots and leaves. Plant Physiol. 1976 Apr;57(4):572–576. doi: 10.1104/pp.57.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Jolly S. O., Tolbert N. E. NADH-Nitrate Reductase Inhibitor from Soybean Leaves. Plant Physiol. 1978 Aug;62(2):197–203. doi: 10.1104/pp.62.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S. S., Gandhi A. P., Sawhney S. K., Naik M. S. Inhibitor of nitrate reductase in the roots of rice seedlings and its effect on the enzyme activity in the presence of NADH. Biochim Biophys Acta. 1974 May 20;350(1):162–170. doi: 10.1016/0005-2744(74)90214-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oaks A., Wallace W., Stevens D. Synthesis and turnover of nitrate reductase in corn roots. Plant Physiol. 1972 Dec;50(6):649–654. doi: 10.1104/pp.50.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Wallace W. A nitrate reductase inactivating enzyme from the maize root. Plant Physiol. 1973 Sep;52(3):197–201. doi: 10.1104/pp.52.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. Comparison of a nitrate reductase-inactivating enzyme from the maize root with a protease form yeast which inactivates tryptophan synthase. Biochim Biophys Acta. 1978 Jun 9;524(2):418–427. doi: 10.1016/0005-2744(78)90179-1. [DOI] [PubMed] [Google Scholar]
- Wallace W. Effects of a nitrate reductase inactivating enzyme and NAD(P)H on the nitrate reductase from higher plants and Neurospora. Biochim Biophys Acta. 1975 Feb 19;377(2):239–250. doi: 10.1016/0005-2744(75)90306-x. [DOI] [PubMed] [Google Scholar]
- Wallace W. Purification and properties of a nitrate reductase-inactivating enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):265–276. doi: 10.1016/0005-2744(74)90087-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya T., Solomonson L. P., Oaks A. Action of Corn and Rice-inactivating Proteins on a Purified Nitrate Reductase from Chlorella vulgaris. Plant Physiol. 1980 Jan;65(1):146–150. doi: 10.1104/pp.65.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]