Abstract
Glucose transporter-1 (GLUT-1) plays critical roles in cancer development and progression. Warburg effect (aerobic glycolysis) contributes greatly to tumorigenesis and could be targeted for tumor therapy. However, published data on the relationship between GLUT-1 and Warburg effect are scarce. In this study, gastric cancer cell, MKN45, was transfected with GLUT-1 shRNA using Lipofectamine 2000. Oxygen consumption, LDH activity, lactate production and cytoplasmic pyruvate were detected after MKN45 cells with GLUT-1 knockdown. In the last, hexokinase 1 (HK1), HK2, and pyruvate kinase M2 (PKM2) expression were detected by using western blot. In this study, we showed that inhibition of GLUT-1 expression reversed Warburg effect in MKN45 cells, and induced apoptosis.
Keywords: GLUT-1, Warburg effect, gastric cancer, LDH, PKM2
Introduction
Although a trend of declining incidence has been observed, gastric cancer still represents a tremendous burden in China [1]. Gastric cancer is a heterogeneous, multifactorial disease [2]. It is crucial to understand the molecular mechanisms of cancer progression involved in gastric cancer and to provide novel promising therapy targets [3].
Glucose transporter-1 (GLUT-1), a member of glucose transporter family, is a basic high-affinity glucose transporter normally expressed in erythrocytes, endothelial cells, renal tubules, and placenta [4]. GLUT-1 has been identified as a hypoxic marker and plays a significant role in malignant glucose metabolism [5]. Previous studies have shown GLUT-1 expression was associated with tumor aggressiveness and poor prognosis in many cancers, such as lung, stomach, breast, and kidney [6-9]. The Warburg effect, also known as aerobic glycolysis, was firstly described by Otto Warburg in the 1920’s, cancer cells have increased conversion of glucose to lactic acid to produce ATP even under normoxic conditions [10]. Warburg effect contributes greatly to tumorigenesis and could be targeted for tumor therapy [11,12].
To our knowledge, published data on the relationship between GLUT-1 and Warburg effect are scarce. Therefore, the main objective of this study was to determine the role of GLUT-1 in regulation of the Warburg effect and its mechanism regarding this role in gastric cancer cells. In the last, we conclude that inhibition of GLUT-1 inhibits Warburg effect and induces apoptosis in gastric cancer cell, MKN45.
Materials and methods
Cell culture
Gastric cancer cell, MKN45, was obtained from American Type Culture Collection (Manassas, VA) and grown in RPMI 1640 medium (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) in a humidified incubator with 5% CO2 at 37°C.
GLUT-1 shRNA transfection
GLUT-1 shRNA (sc-35493-SH) plasmid was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). For gene transfection, MKN45 cells were grown overnight and transfected with GLUT-1 shRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Control shRNA Plasmid-A (sc-108060) was used as a control.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells using an RNeasy Mini Kit (Biomed, Beijing, China). cDNA was reverse transcribed with 2 μg of total RNA using a TaKaRa Reverse Transcription Kit (TaKaRa, Dalian, China) and was amplified using the following primers. GLUT-1 primers were 5’-CCGCAACGAGGAGAACC-3’ (sense) and 5’-GTGACCTTCTTCTCCCGCATC-3’ (antisense). GAPDH primers were 5’-TCCTTCCTGGGCATGGAGT-3’ (sense) and 5’-CAGGAGGAGCAATGATCTTGAT-3’ (antisense) and used as an internal control. The PCR products were electrophoresed on a 2% agarose gel, and visualized by ethidium bromide staining under a UV imaging system (UVP, LLC, Upland, CA). The lengths of the PCR products were 123 bp (GLUT-1) and 208 bp (GAPDH).
Immunofluorescence staining of GLUT-1 proteins
To assess expression of GLUT-1 protein in cells, the immunofluorescence technique with specific antibodies against GLUT-1 (rabbit IgG, sc-7903, Santa Cruz Biotechnology) was performed. Anti-rabbit Alexa Fluor® 488 IgG (Invitrogen) was used as the secondary antibody. Photographic images were taken using an Olympus CX71 fluorescence microscope (Olympus, Tokyo, Japan).
Apoptosis assays
Apoptosis was determined using an apoptosis detection kit (Keygen, Nanjing, China). Briefly, cells after 48 h of gene transfection were collected, washed twice in ice-cold PBS, and then resuspended in the binding buffer at a density of 1 × 106 cells/ml. The cells were simultaneously incubated with fluorescein-labeled Annexin V and PI for 20 min. The mixture was then analyzed using a FACSCalibur (Becton Dickinson Medical Devices, Shanghai, China).
Glucose uptake assay
As the methods of Zhang et al. [13], glucose uptake was detected by measuring the uptake of 3H-2-deoxyglucose by cells. Cells cultured in 12-well plates were pre-incubated in glucose-free media for 30 min. 3H-2-deoxyglucose (1 μCi/well) was then added to the cells and incubated for 30 min before cells were washed with PBS and lysed in 1% SDS. The radioactivity of cell lysates was determined in a liquid scintillation counter and normalized to the protein concentrations of cell lysates.
LDH, pyruvate, lactate, and oxygen consumption assays
LDH activity was determined by manual instructions of the CytoTox 96® Non-Radioactive cytotoxicity assay (Promega, Beijing, China). Concentrations of cytoplasmic pyruvate and lactate in the culture medium were respectively determined with a Pyruvate Assay Kit and Lactate Assay Kit (BioVision, Milpitas, CA). The oxygen consumption rate was measured with MitoCell (MT200, Strathkelvin Instruments, North Lanarkshire, Scotland).
Western blot
Aliquots of the lysates (30 μg of protein) from cells were boiled for 5 min and electrophoresed using a 10% sodium dodecysulfate-polyacrylamide (SDS) gel. The blots in the gels were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA), which were then incubated with GLUT-1 (sc-7903, Santa Cruz Biotechnology), hexokinase 1 (HK1) (sc-6517), HK2 (sc-6521), or pyruvate kinase M2 (PKM2) (sc-292640). β-actin (sc-47778) was used as an internal control. The nitrocellulose membranes were further incubated with secondary immunoglobulin-G-horseradish peroxidase conjugates. Immunostaining was detected using an enhanced chemiluminescence (ECL) system (Amersham Biosciences, Westborough, MA).
Statistical analysis
Densitometric quantification for RT-PCR, western-blot, and Immunofluorescence staining was performed using Scion Image software. Statistical analysis was performed using a one-tailed Student’s t-test (unilateral and unpaired) and the results were expressed as the mean ± standard deviation (SD). The level of significance was set to P less than 0.05. All statistical tests were performed using the software package, SPSS for Windows, version 16.0 (Chicago, IL).
Results
The mRNA and protein levels of GLUT-1 were evaluated in MKN45 cells after GLUT-1 shRNA transfection
After GLUT-1 shRNA transfection, GLUT-1 mRNA and protein levels were significantly lower than that in untransfected ones by using RT-PCR and western blot, respectively (Figure 1A, 1B, P < 0.05). The results of immunofluorescence analysis showed that GLUT-1 protein was localized in the membrane of MKN45 cells. However, GLUT-1 protein was disappeared in transfected ones (Figure 1C, P < 0.05). These results collectively indicated that the transfection was successful.
Figure 1.
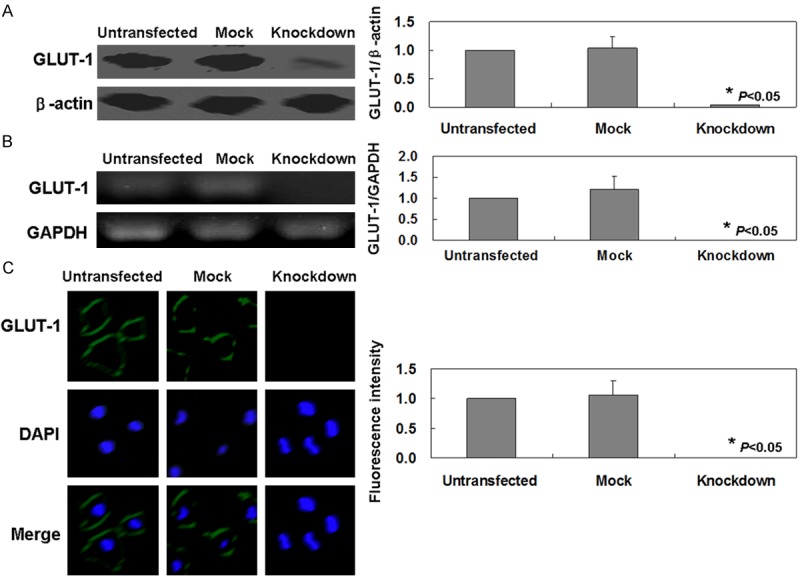
Detection of GLUT-1 in MKN45 cells following GLUT-1 shRNA transfection. GLUT-1 mRNA and protein levels in cells detected using western blot analysis (A) and RT-PCR (B), respectively. (C) Detection of GLUT-1 in transfected and untransfected MKN45 cells by immunofluorescence. Nuclei were stained with DAPI. Untransfected: Untransfected MKN45 cells; Mock: MKN45 cells transfected with control shRNA Plasmid-A; Knockdown: MKN45 cells transfected with GLUT-1 shRNA.
Inhibition of GLUT-1 induced apoptosis and suppressed the Warburg effect in MKN45 cells
As shown in Figure 2, we determined that the percentage of apoptosis in GLUT-1 knockdown cells was 3.5%, higher compared to MKN45 (1.2%) and mock control (1.4%) cells (P < 0.05). To determine whether GLUT-1 knockdown exerts any effects on Warburg effect in MKN45 cells, glucose uptake, LDH activity, and oxygen consumption were determined. We found that GLUT-1 knockdown could induce oxygen consumption in MKN45 cells (Figure 3A, P < 0.05). In contrast, GLUT-1 knockdown significantly reduced glucose uptake and LDH activity in MKN45 cells (Figure 3B, 3C, P < 0.05). Furthermore, we found that lactate production was inhibited in GLUT-1 knockdown MKN45 cells (Figure 3D, P < 0.05). In the aspect of pyruvate, the cytoplasmic pyruvate level was decreased in MKN45 cells without GLUT-1 expression (Figure 3E, P < 0.05). While total levels of HK1 showed no changes, the levels of HK2 and PKM2 were observed to be significantly lower in GLUT-1 knockdown MKN45 cells versus untransfected and mock ones (Figure 3F). In combination, these results suggest that GLUT-1 knockdown in MKN45 cells is related with Warburg effect.
Figure 2.
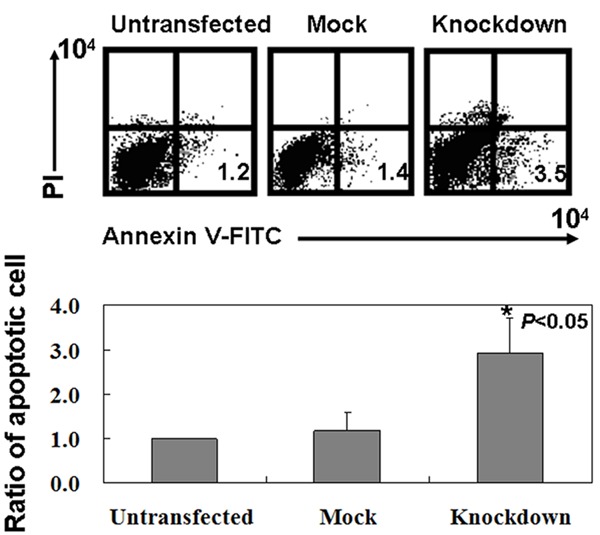
The proportion of apoptotic cells (early apoptosis) was determined by double-staining with Annexin-V/FITC and PI. Untransfected: Untransfected MKN45 cells; Mock: MKN45 cells transfected with control shRNA Plasmid-A; Knockdown: MKN45 cells transfected with GLUT-1 shRNA.
Figure 3.
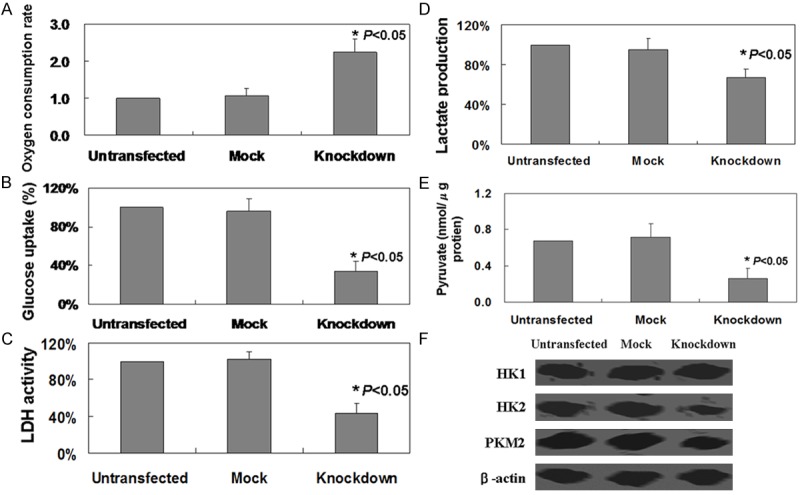
GLUT-1 knockdown reversed Warburg effect in MKN45 cells. A. Oxygen consumption. B. Glucose uptake. C. LDH activity. D. Lactate production. E. cytoplasmic pyruvate were detected in MKN45 cells with GLUT-1 knockdown. F. HK1, HK2 and PKM2 expression were detected by using western blot. Untransfected: Untransfected MKN45 cells; Mock: MKN45 cells transfected with control shRNA Plasmid-A; Knockdown: MKN45 cells transfected with GLUT-1 shRNA.
Discussion
In this study, we report a strong association of GLUT-1 expression and Warburg effect. GLUT-1 is responsible for basal glucose transport across the plasma membrane into the cytosol in many cancer cells [5]. Elevated GLUT-1 expression may provide more energy to malignant tumors [14]. GLUT-1 deficient B-cell acute lymphoblastic leukemia cells (B-ALL) showed low cell proliferation and a limited degree of apoptosis [15]. Shin et al. [16] also found that ciglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) agonist, induces apoptosis in ovarian cancer cells by the inhibition of GLUT-1. Consistent with previous studies, we confirmed the apoptotic rate in MKN45 cells was increased by GLUT-1 knockdown. Liu et al. [17] demonstrated that downregulation of GLUT-1 protein inhibits glucose uptake and glycolysis partially in HepG2 cells. We also found inhibition of GLUT-1 could inhibit glucose uptake in MKN45 cells.
Furthermore, we found that lactate production was inhibited by GLUT-1 knockdown in MKN45 cells. Lactate, the final product of the Warburg effect, is shown to impact various aspects of tumorigenesis, such as immune escape [18] and cell migration [19]. The expression of hexokinase 2 (HK2) and the M2 isoform of pyruvate kinase (PKM2) are considered to be of central functional importance for aerobic glycolysis and cell proliferation owing to their intracellular localization and kinetic properties [20]. Nemazanyy et al. [21] found that knockdown of PKM2 by shRNA significantly inhibited cell proliferation. Activity of HK2 and PKM2 could be the therapeutic target to limit the anabolic capacity of cancer cells [22]. In this study, we found inhibition of GLUT-1 could inhibit HK2 and PKM2 expression and followed with apoptosis in MKN45 cells.
In summary, this study for the first time provided critical insight into the role of GLUT-1 is significantly correlated with the Warburg effect and might be a potential therapeutic target for gastric cancer, and this warrants further investigation.
Acknowledgements
This work was supported by the Science and Technology Department Foundation of Liaoning Provincial (#2013225021).
Disclosure of conflict of interest
None.
References
- 1.Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei WQ, Qiao YL, Inoue M. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol. 2011;17:4421–4428. doi: 10.3748/wjg.v17.i39.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643–655. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasui W, Oue N, Sentani K, Sakamoto N, Motoshita J. Transcriptome dissection of gastric cancer: identification of novel diagnostic and therapeutic targets from pathology specimens. Pathol Int. 2009;59:121–136. doi: 10.1111/j.1440-1827.2009.02329.x. [DOI] [PubMed] [Google Scholar]
- 4.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 5.Han MW, Lee HJ, Cho KJ, Kim JS, Roh JL, Choi SH, Nam SY, Kim SY. Role of FDG-PET as a biological marker for predicting the hypoxic status of tongue cancer. Head Neck. 2012;34:1395–1402. doi: 10.1002/hed.21945. [DOI] [PubMed] [Google Scholar]
- 6.Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT. Overexpression of GLUT1 and GLUT3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80:1046–1051. doi: 10.1002/(sici)1097-0142(19970915)80:6<1046::aid-cncr6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, Honma K, Suzuki T. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–641. doi: 10.1002/1097-0142(20010801)92:3<634::aid-cncr1364>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895–2898. [PubMed] [Google Scholar]
- 9.Lidgren A, Bergh A, Grankvist K, Rasmuson T, Ljungberg B. Glucose transporter-1 expression in renal cell carcinoma and its correlation with hypoxia inducible factor-1α. BJU Int. 2008;101:480–484. doi: 10.1111/j.1464-410X.2007.07238.x. [DOI] [PubMed] [Google Scholar]
- 10.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Lin M, Wu R, Wang X, Yang B, Levine AJ, Hu W, Feng Z. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci U S A. 2011;108:16259–16264. doi: 10.1073/pnas.1113884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan SX, Luo XM, Zhou SH, Bao YY, Fan J, Lu ZJ, Liao XB, Huang YP, Wu TT, Wang QY. Effect of antisense oligodeoxynucleotides glucose transporter-1 on enhancement of radiosensitivity of laryngeal carcinoma. Int J Med Sci. 2013;10:1375–1386. doi: 10.7150/ijms.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Kishton RJ, Macintyre AN, Gerriets VA, Xiang H, Liu X, Dale Abel E, Rizzieri D, Locasale JW, Rathmell JC. Glucose transporter 1-mediated glucose uptake is limiting for B-cell acute lymphoblastic leukemia anabolic metabolism and resistance to apoptosis. Cell Death Dis. 2014;5:e1470. doi: 10.1038/cddis.2014.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin SJ, Kim JY, Kwon SY, Mun KC, Cho CH, Ha E. Ciglitazone enhances ovarian cancer cell death via inhibition of glucose transporter-1. Eur J Pharmacol. 2014;743:17–23. doi: 10.1016/j.ejphar.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu TQ, Fan J, Zhou L, Zheng SS. Effects of suppressing glucose transporter-1 by an antisense oligodeoxynucleotide on the growth of human hepatocellular carcinoma cells. Hepatobiliary Pancreat Dis Int. 2011;10:72–77. doi: 10.1016/s1499-3872(11)60010-6. [DOI] [PubMed] [Google Scholar]
- 18.Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 19.Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39:453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- 20.Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, Annicotte JS, Fajas L, Foretz M, Verdeguer F, Pontoglio M, Ferré P, Scoazec JY, Birnbaum MJ, Ricci JE, Pende M. PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat Commun. 2012;3:672. doi: 10.1038/ncomms1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemazanyy I, Espeillac C, Pende M, Panasyuk G. Role of PI3K, mTOR and Akt2 signalling in hepatic tumorigenesis via the control of PKM2 expression. Biochem Soc Trans. 2013;41:917–922. doi: 10.1042/BST20130034. [DOI] [PubMed] [Google Scholar]
- 22.Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, Yang H, Mattaini KR, Metallo CM, Fiske BP, Courtney KD, Malstrom S, Khan TM, Kung C, Skoumbourdis AP, Veith H, Southall N, Walsh MJ, Brimacombe KR, Leister W, Lunt SY, Johnson ZR, Yen KE, Kunii K, Davidson SM, Christofk HR, Austin CP, Inglese J, Harris MH, Asara JM, Stephanopoulos G, Salituro FG, Jin S, Dang L, Auld DS, Park HW, Cantley LC, Thomas CJ, Vander Heiden MG. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]


