Abstract
Objective: The levels of corticosterone and noradrenalin as the two nociception modulators modify after stress condition. The propose of current study was to investigate the effect of chrysin on formalin-induced nociceptive behaviors and serum levels of corticosterone and noradrenalin in rats. Materials and methods: Pain was induced by applying 20 μL of 5% formalin in distilled water in the subplantar of the right hind paw. Chrysin (50, 100 and 150 mg/kg, intraperitoneally (i.p.) was administered 60 min before formalin injection. Morphine (10 mg/kg, i.p.) was administered 30 min before formalin injection. The control group received the same volume of saline by i.p. injection 30 min before formalin injection. Results: Chrysin treatment can significantly decrease formalin-induced pain in rat in a dose-dependent manner. Chrysin (150 mg/kg) significantly inhibit the first phase (P < 0.01), whereas, the all concentration of chrysin were affected on the later phase of formalin-induced pain (P < 0.05). Chrysin could significantly attenuate the content of corticosterone and noradrenalin in the serum versus to the control rats (P < 0.01). Conclusion: The current study confirms that the chrysin decreased the nociceptive behaviors in the formalin test and indicate a correlation with decrease in serum corticosterone and noradrenalin levels.
Keywords: Chrysin, nociception, noradrenalin, corticosterone
Introduction
Pain is a general symptom and characterization of many diseases. Overall, analgesics reduce pain by acting on the central and peripheral nervous system of pain mechanisms, without significantly modifying consciousness [1]. Patients involving in this kind of pain include those with so-called neuropathic pain and nociceptive, hyperalgesia and allodynia [2,3]. However, pain control remains a main clinical problem, since; there is not an appropriate knowledge of the mechanisms underlying pain and effective treatments [4].
The current drugs for treatment of pain have limited safety and effectiveness [5]. The usage of non-steroidal anti-inflammatory drugs (NSAIDs) may induce adverse effects including liver and renal failure or gastrointestinal lesions [6]. In addition, available analgesic drugs, like the opioids, cannot prevent the pain easier of painful situation such as neuropathic pain [7]. Thus, it is essential to discover new, safe, effective and analgesics among the natural products derived from secondary metabolites. Natural products are focused as an incomparable source of molecular diversity that has led to the finding of drugs in new medicine, particularly for pain management [8-10]. Chrysin (5, 7-dihydroxyflavone) (CH) is a flavonoids contents extracted from propolis, honey and plants. Flavonoids are the major polyphenolic ingredients that express a wide range of biological activities, such as antiinflammatory, antithrombotic antioxidant, antiallergic, antibacterial, analgesic and vasodilatory effects [11,12]. Chrysin has been showed to have many potent pharmacological activities, such as antiestrogenic, antitumor, antidiabetogenic, antihypertensive, anti-inflammatory, antioxidant, anxiolytic and also involves in apoptosis in the variety of cancer cells [13,14]. The levels of corticosterone and noradrenalin as the two nociception modulators modify after stress situation [15]. Several reports proposed that flavonoids pass through the blood-brain-barrier and directly modify neuroendocrine system via influences the neuronal cells [16].
However, no studies have been done of the effect of chrysin on formalin-induced nociceptive behaviors. Thus, the current investigation was designed to search whether chrysin show anti-nociceptive through modification of corticosterone and noradrenalin in rat model.
Materials and methods
Chemicals
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Elisa kits were obtained from Cusabio Chemical Co.
Animals
48 Male Wistar rats were obtained from The Laboratory Animal Centre, Medical University of Mashhad. Rats were kept in their own cages at constant room temperature (21 ± 2°) under a normal 12 hr light: 12 hr dark cycle with free access to food and water. The animals were housed according to regulating the Walfare of experimented animals. The study was conducted in Mashhad Medical University Experimental Animal Research Laboratory. Protocols were approved by the Ethical Committee. After 14 days of acclimatization, rats were randomly allocated in to six experimental groups, (n = 8 per group) as follows: sham group (sham); 0.1 ml normal saline (9% NaCl) administrated in the rats, negative control group (C); formalin (20 μL of 5% formalin) was injected (i.p.) in the rats that treated with normal saline (9% NaCl), positive control group (M); formalin (20 μL of 5% formalin) was injected in the rats that treated (i.p.) with 10 mg/kg morphine and chrysin treatment groups (CH1-3); formalin (20 μL of 5% formalin) was injected in the rats that treated (i.p.) with 50, 100 and 150 mg/kg of chrysin.
Formalin test
The method used in our study was according to Tsung-Chun et al., study [17]. Pain was induced by administrating 20 μL of 5% formalin in distilled water in the subplantar of the right hind paw. Chrysin (50, 100 and 150 mg/kg, i.p.) was injected 60 min before formalin treatment. Morphine (10 mg/kg, i.p.) was administered 30 min before formalin injection. The control group received the same volume of saline by i.p. administration. The rats were placed in a transparent Plexiglas cage (30 × 20 × 20 cm). The time spent for licking the injected paw, as the indicators of pain, was registered separately at 0-5 min (first phase or neurogenic pain) and 20-30 min (second phase or inflammatory pain) [18,19].
Corticosterone and noradrenalin evaluation
Under deep anesthesia, blood was collected from the heart of rats (n = 8 for each group). Blood was allowed to clot and sera were separated using centrifugation at 5000 rpm for 5 min and stored at -80°C until use. Total serum level of corticosterone was measured by ELISA kits (CORT ELISA Kit CSB-E07014r) and also the total serum level of noradrenalin was measured using ELISA kit (NA ELISA Kit CSBE07022r).
Statistical analysis
All experiments were carried out at least in duplicate. Every group consisted of eight rats. The data were expressed as mean ± SEM and tested with One-way analysis of variance (ANOVA) followed by the post-hoc test of Tukey-Kramer. Statistical analyses were performed using the InStat 3.0 program. Linear correlation tests were also performed. Differences of P < 0.05 were considered significant.
Results
In the formalin test, formalin induced nociception in the control rats versus to the sham operated group (P < 0.001). Chrysin (50 and 100 mg/kg) could not significantly inhibit the first phase, but was active in the later phase of formalin-induced pain (P < 0.05, P < 0.001, respectively), whereas chrysin (150 mg/kg) showed notable activity in both phases versus to the control group (P < 0.01, P < 0.001, respectively). In the both phase, morphine (10 mg/kg) significantly reduced the nociception (P < 0.001). Chrysin demonstrated a dose dependent relationship in the formalin-induced pain test (Figure 1).
Figure 1.
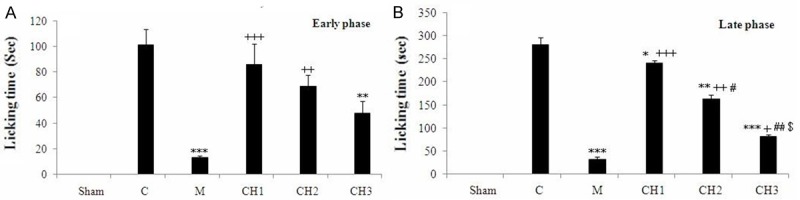
Analgesic effect of the chrysin (CH) on the (A) early phase and (B) late phase in formalin test in rats. Sham operated (Sham), Negative control group (C), positive control group (M), CH (50 mg/kg) - treated rats (CH1), CH (100 mg/kg) - treated rats (CH2) and CH (150 mg/kg) - treated groups (CH3) (n = 8, for each group). Values are the means ± SEM for eight rats in each group. Significantly different from Negative control group (C) rats (*: P < 0.05, **: P < 0.01, ***: P < 0.001). Significantly different from positive control group (M) rats (+: P < 0.05, ++: P < 0.01, +++: P < 0.001). Significant difference between CH1 vs CH2 and CH3 groups (#: P < 0.05, ##: P < 0.01). Significant difference between CH2 vs. CH3 groups (&: P < 0.05).
Under deep anesthesia, blood was collected from the heart of rats. The effect of chrysin, morphine and formalin on serum noradrenalin level are shown in Figure 2. The injection of formalin in the male rats significantly increased the concentration of serum noradrenalin in the control rat versus to the sham operated group (P < 0.001). Morphine decreased noradrenalin level in male rats, as compared with the control (P < 0.001). Chrysin (100 and 150 mg/kg) significantly reduced noradrenalin level in male rats, as compared with the control animals (P < 0.001). The injection of formalin in the male rats significantly increased the concentration of serum corticosterone in the control rats versus to the sham operated group (P < 0.001) (Figure 3). Morphine decreased corticosterone level in male rats, as compared with the control (P < 0.001). Chrysin significantly reduced corticosterone level in male rats, as compared with the control animals in dose dependent manner (P < 0.05, P < 0.001, respectively) (Figure 3). Although, there is significant difference in licking time and corticosterone serum between chrysin and morphine treatment groups, but there is no significant difference between the noradrenalin level in chrysin (150 mg/kg) and morphine treatment rats.
Figure 2.
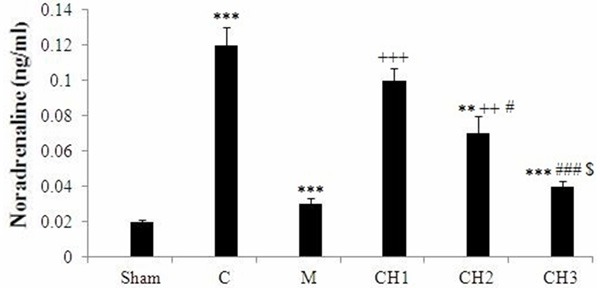
Effect of chrysin (CH) on serum noradrenalin concentration of rats. Sham operated (Sham), Negative control group (C), positive control group (M), CH (50 mg/kg) - treated rats (CH1), CH (100 mg/kg) - treated rats (CH2) and CH (150 mg/kg) - treated groups (CH3) (n = 8, for each group). Values are the means ± SEM for eight rats in each group. Significantly different from negative control group (C) rats (**: P < 0.01, ***: P < 0.001). Significantly different from positive control group (M) rats (++: P < 0.01, +++: P < 0.001). Significant difference between CH1 vs CH2 and CH3 groups (#: P < 0.05, ###: P < 0.001). Significant difference between CH2 vs. CH3 groups ($: P < 0.05).
Figure 3.
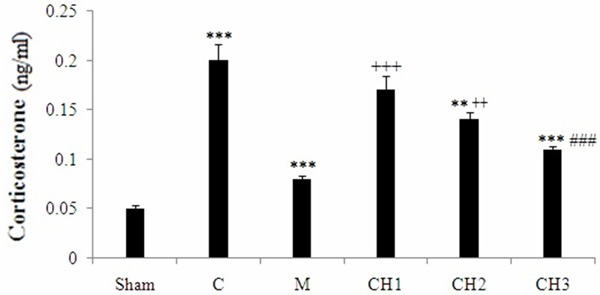
Effect of chrysin (CH) on serum corticosterone concentration of rats. Sham operated (Sham), Negative control group (C), positive control group (M), CH (50 mg/kg) - treated rats (CH1), CH (100 mg/kg) - treated rats (CH2) and CH (150 mg/kg) - treated groups (CH3) (n = 8, for each group). Values are the means ± SEM for eight rats in each group. Significantly different from negative control group (C) rats (**: P < 0.01, ***: P < 0.001). Significantly different from positive control group (M) rats (+: P < 0.05, ++: P < 0.01, +++: P < 0.001). Significant difference between CH1 vs CH2 and CH3 groups (###: P < 0.001).
Discussion
The advantage of the formalin-pain test of nociception is that it can determine pain in its central and peripheral components [20]. The model includes of the two different phases which can be separated in time: the first one is produced in the periphery via the activation of nociceptive neurons by the direct action of formalin and the second phase induces through the activation of the ventral horn neurons at the spinal cord level [20]. The present results indicate that chrysin treatment shows anti-nociceptive effect in the both phase of the formalin test and also decreased corticosterone and noradrenaline levels, therefore, chrysin might cause the analgesic effect as well as morphine injection. Chrysin (50, 100 mg/kg) significantly inhibit the first phase of formalin-induced pain, whereas, morphine (10 mg/kg) and chrysin (150 mg/kg) showed notable activity in the two phases. We observed that the injection of formalin in the male rats induced nociceptive effect and also increased the serum concentration of corticosterone and noradrenalin. This result is in consistent with Sajedianfard et al. who illustrated that after the injection of formalin, the noradrenaline level in the locus coeruleus enhanced [21]. They proposed that part of the increase in noradrenaline level in the locus coeruleus is mainly as the result of the pain caused by formalin administration [21]. Furthermore, noxious stimuli, including electrical stimulation of the locus coeruleus and foot shock, accelerated noradrenaline turnover in the brain cortex [21]. Our results also showed that morphine, a centrally acting analgesic drug, induced an inhibitory effect on the nociceptive response in the formalin-pain test and also decreased corticosterone and noradrenaline concentrations. Antinociceptive activity of opioid agonists, opioid partial agonists, and non-steroidal antiinflammatory agents can be determined by the formalin test [22].
In the present study, chrysin treatment decreased licking time in the both phase of the formalin test and decreased noradrenalin and corticosterone levels in male rats in the dose dependent manner. Similar with our result, it has been indicated that various natural flavonoids including quercetin, crocin, sfranal and carvacrol produced significant antinociceptive and/or anti-inflammatory activities [23-26].
Recently, it has been shown that chrysin illustrated inhibitory effects on corticosterone and catecholamine levels [27]. Noradernaline participates in reducing pain inhibitory system. Brain stem nuclei like, locus coeruleus in centrally and sympathetic nerves in peripherally are the major sources of noradernaline. Locus coeruleus has a key role in noradrenergic pain modulation [28,29]. Locus coeruleus stimulation releases noradernaline [30] and induces analgesia that is inhibited by alpha-2-adrenoceptor antagonist’s treatment [31]. In addition, noradernaline is released by peripheral noxious stimulation [32-34]. Destruction of noradrenergic system diminished formalin-induced nociceptive behaviors in phase2 [35]. Safari et al. showed that chemical inactivation or stimulation of lateral hypothalamus induced analgesia effect and injection of lidocaine into the Locus coeruleus blocked the carbachol-induced analgesia [36]. There are several documentations that indicated noradernaline is connected in pain modulation during formalin-pain test. In confirming, it has been indicated that alpha-1-adrenoceptor binding attenuated in the spinal dorsal horn during formalin-pain test in mice [37]. According to the previous studies, we also proposed that raised in noradrenalin level might induce the antinociceptive effect that happens after formalin-pain test.
Central analgesic drugs, including morphine, inhibited equally in both phases, whereas peripherally acting drugs, including steroids and NSAIDs inhibited mainly pain in the later phase [20]. Our data also indicated that the analgesic activity of high concentration of chrysin in the both phases of formalin-pain test was similar to the morphine effect.
These results suggest that chrysin could be useful to suppress pain in the both phase of the formalin test through modulating corticosterone and noradrenalin, but more pharmacological investigations are needed for finding the exact mechanism of action.
Disclosure of conflict of interest
None.
References
- 1.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among us workers with depression. JAMA. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 2.Koltzenburg M, Scadding J. Neuropathic pain. Curr Opin Neurol. 2001;14:641–647. doi: 10.1097/00019052-200110000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ, Doubell TP. The pathophysiology of chronic pain--increased sensitivity to low threshold a beta-fibre inputs. Curr Opin Neurol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 4.Dickenson AH. Recent advances in the physiology and pharmacology of pain: Plasticity and its implications for clinical analgesia. J Psychopharmacol. 1991;5:342–351. doi: 10.1177/026988119100500424. [DOI] [PubMed] [Google Scholar]
- 5.Gilron I, Coderre TJ. Emerging drugs in neuropathic pain. Expert Opin Emerg Drugs. 2007;12:113–126. doi: 10.1517/14728214.12.1.113. [DOI] [PubMed] [Google Scholar]
- 6.Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (nsaids): Cyclooxygenase (cox) inhibition and beyond. J Pharm Pharm Sci. 2008;11:81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 7.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects. Can Med Assn J. 2006;174:1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da Silva KA, Manjavachi MN, Paszcuk AF, Pivatto M, Viegas C Jr, Bolzani VS, Calixto JB. Plant derived alkaloid (-)-cassine induces anti-inflammatory and anti-hyperalgesics effects in both acute and chronic inflammatory and neuropathic pain models. Neuropharmacology. 2012;62:967–977. doi: 10.1016/j.neuropharm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Samarghandian S, Nezhad MA, Mohammadi G. Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in the A549 human lung adenocarcinoma epithelial cells. Anticancer Agents Med Chem. 2014;14:901–909. doi: 10.2174/1871520614666140209144042. [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Zhou L, Chen Z, Hu C. Analgesic effect of iridoid glycosides from paederia scandens (lour. ) merrill (rubiaceae) on spared nerve injury rat model of neuropathic pain. Pharmacol Biochem Behav. 2012;102:465–470. doi: 10.1016/j.pbb.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Yimam M, Brownell L, Hodges M, Jia Q. Analgesic effects of a standardized bioflavonoid composition from scutellaria baicalensis and acacia catechu. J Diet Suppl. 2012;9:155–156. doi: 10.3109/19390211.2012.708713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook NC, Samman S. Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7:66–76. [Google Scholar]
- 13.Samarghandian S, Borji A. Anticarcinogenic effect of saffron (Crocus sativus L. ) and its ingredients. Pharmacognosy Res. 2014;6:99–107. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin JS, Kim KS, Kim MB, Jeong IJ, Kim HBK. Synthesis and hypoglycemic effect of chrysin derivatives. Bioorg Med Chem Lett. 1999;9:869–74. doi: 10.1016/s0960-894x(99)00092-x. [DOI] [PubMed] [Google Scholar]
- 15.Bhunia S, Bharambe MS, Singh R, Premendran J, Pande S. Effect of microinjections of 5-hydroxytryptamine and adrenaline in central grey on pain responsiveness during acute food deprivation in conscious rats. Indian J Exp Biol. 2000;38:237–241. [PubMed] [Google Scholar]
- 16.Haleagrahara N, Radhakrishnan A, Lee N, Kumar P. Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in the hypothalamus of rats. Eur J Pharmacol. 2009;621:46–52. doi: 10.1016/j.ejphar.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Met. 1985;14:69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- 18.Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 19.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 20.Sajedianfard J, Khatami S, Semnanian S, Naghdi N, Jorjani M. In vivo measurement of noradrenaline in the locus coeruleus of rats during the formalin test: a microdialysis study. Eur J Pharmacol. 2005;512:153–156. doi: 10.1016/j.ejphar.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Singewald N, Kaehler ST, Philippu A. Noradrenaline release in the locus coeruleus of conscious rats is triggered by drugs, stress and blood pressure changes. Neuroreport. 1999;10:1583–1587. doi: 10.1097/00001756-199905140-00035. [DOI] [PubMed] [Google Scholar]
- 22.Mobasher MA, Sajedianfard J, Jamshidzadeh A, Naghdi N, Namvaran MM. The effects of tramadol on norepinephrine and MHPG releasing in locus coeruleus in formalin test in rats: a brain stereotaxic study. Iran J Basic Med. 2014;17:420. [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez AL, González-Trujano ME, Aguirre-Hernández E, Moreno J, Soto-Hernández M, López-Muñoz FJ. Antinociceptive activity of Tilia americana var. mexicana inflorescences and quercetin in the formalin test and in an arthritic pain model in rats. Neuropharmacol. 2009;56:564–571. doi: 10.1016/j.neuropharm.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Tamaddonfard E, Hamzeh-Gooshchi N. Effect of crocin on the morphine-induced antinociception in the formalin test in rats. Phytother Res. 2010;24:410–413. doi: 10.1002/ptr.2965. [DOI] [PubMed] [Google Scholar]
- 25.Tamaddonfard E, Farshid AA, Eghdami K, Samadi F, Erfanparast A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacol Rep. 2013;65:1272–1280. doi: 10.1016/s1734-1140(13)71485-3. [DOI] [PubMed] [Google Scholar]
- 26.Cavalcante Melo FH, Rios ER, Rocha NF, Citó Mdo C, Fernandes ML, de Sousa DP, de Vasconcelos SM, de Sousa FC. Antinociceptive activity of carvacrol (5-isopropyl-2-methylphenol) in mice. J Pharm Pharmacol. 2012;64:1722–1729. doi: 10.1111/j.2042-7158.2012.01552.x. [DOI] [PubMed] [Google Scholar]
- 27.Brown E’, Hurd NS, McCall S, Ceremuga TE. Evaluation of the anxiolytic effects of chrysin, a Passifloraincarnata extract, in the laboratory rat. AANA J. 2007;75:333–337. [PubMed] [Google Scholar]
- 28.Proudfit HK. Pharmacologic evidence for the modulation of nociception by noradrenergic neurons. Prog Brain Res. 1988;77:357–370. doi: 10.1016/s0079-6123(08)62802-2. [DOI] [PubMed] [Google Scholar]
- 29.Kwiat GC, Basbaum AI. The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somatosens Mot Res. 1992;9:157–173. doi: 10.3109/08990229209144768. [DOI] [PubMed] [Google Scholar]
- 30.Hentall ID, Mesigil R, Pinzon A, Noga BR. Temporal and spatial profiles of pontine-evoked monoamine release in the rat’s spinal cord. J Neurophysiol. 2003;89:2943–2951. doi: 10.1152/jn.00608.2002. [DOI] [PubMed] [Google Scholar]
- 31.Jones SL. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- 32.Takagi H, Shiomi H, Kuraishi Y, Fukui K, Ueda H. Pain and the bulbospinal noradrenergic system: pain-induced increase in normetanephrine content in the spinal cord and its modification by morphine. Eur J Pharmacol. 1979;54:99–110. doi: 10.1016/0014-2999(79)90412-6. [DOI] [PubMed] [Google Scholar]
- 33.Tyce GM, Yaksh TL. Monoamine release from cat spinal cord by somatic stimuli: an intrinsic modulatory system. J Physiol. 1981;314:513–529. doi: 10.1113/jphysiol.1981.sp013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaksh TL, Tyce GM. Release of norepinephrine and serotonin in cat spinal cord: direct in vivo evidence for the activation of descending monoamine pathways by somatic stimulation. J Physiol (Paris) 1981;77:483–487. [PubMed] [Google Scholar]
- 35.Martin WJ, Gupta NK, Loo CM, Rohde DS, Basbaum AI. Differential effects of neurotoxic destruction of descending noradrenergic pathways on acute and persistent nociceptive processing. Pain. 1999;80:57–65. doi: 10.1016/s0304-3959(98)00194-8. [DOI] [PubMed] [Google Scholar]
- 36.Safari MS, Haghparast A, Semnanian S. Effect of lidocaine administration at the nucleus locus coeruleus level on lateral hypothalamus-induced antinociception in the rat. Pharmacol Biochem Behav. 2009;92:629–634. doi: 10.1016/j.pbb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Nalepa I, Vetulani J, Borghi V, Kowalska M, Przewlocka B, Pavone F. Formalin hindpaw injection induces changes in the [3H] prazosin binding to alpha1-adrenoceptors in specific regions of the mouse brain and spinal cord. J Neural Transm. 2005;112:1309–1319. doi: 10.1007/s00702-005-0279-3. [DOI] [PubMed] [Google Scholar]


