Abstract
Background: Advanced glycation end-products have been involved in the pathogenesis of proximal tubule dysfunction which characterizes diabetic tubulopathy. Methods: A total of 76 Type 2 diabetes mellitus patients and 28 healthy controls were evaluated concerning a potential association of glycated peptides with proximal tubule dysfunction by assessing urine albumin:creatinine ratio, urinary alpha1-microglobulin, urinary neutrophil gelatinase-associated lipocalin, plasma and urinary advanced glycation end-products, plasma asymmetric dimethyl-arginine, serum cystatin C. Fully automated chip-nanoelectrospray ionization and high-capacity ion trap multistage mass spectrometry characterized the urinary proteomic profile. Results: The urinary glycated proteins displayed a molecular weight of 15,121.4 Da in normoalbuminuric patients and of 30,180.4 Da in microalbuminuric patients. Urinary alpha1-microglobulin and neutrophil gelatinase-associated lipocalin correlated with urinary advanced glycation end-products (R2=0.586; R2=0.415), urine albumin: creatinine ratio (R2=0.292; R2=0.116), estimated glomerular filtration rate (R2=0.172; R2=0.135), serum cystatin C (R2=0.146; R2=0.129), but not with asymmetric dimethyl-arginine. In multivariable regression analysis models, the correlations for urinary alpha1-microglobulin and neutrophil gelatinase-associated lipocalin remained significant with urine albumin: creatinine ratio, urinary advanced glycation end-products, estimated glomerular filtration rate (P<0.0001, R2=0.674; P<0.0001, R2=0.551; P<0.0001, R2=0.482). Conclusions: In patients with Type 2 diabetes mellitus urinary glycated peptides are associated with proximal tubule dysfunction. The proteomic patterns of urinary glycated peptides could differentiate normo- from microalbuminuric patients and may explain a potential relation between the size and the glycation status of glycated peptides, and the extent of proximal tubule dysfunction. The lack of correlation between parameters of endothelial dysfunction and proximal tubule dysfunction cannot exclude glomerular involvement in early diabetic nephropathy.
Keywords: Glycated peptides, proximal tubule, normoalbuminuria, Type 2 diabetes mellitus
Introduction
Diabetes mellitus (DM) is now the leading cause of end-stage renal disease worldwide and may be attributed up to 20-40% of cases referred to renal replacement therapies, both in developed and emerging countries [1].
Currently, there is a debate as to whether early diabetic nephropathy (DN) in Type 2 DM may be attributed to the glomerulus or to the proximal tubule (PT). It is assumed that albuminuria is caused primarily by impaired tubular uptake of intact albumin rather than by an increased leakiness of the glomerular filtration barrier [2,3].
In previous works performed by us in normoalbuminuric patients with Type 2 DM we demonstrated that PT dysfunction precedes the occurrence of albuminuria and is dissociated from endothelial dysfunction [4,5]. Multiple markers of endothelial dysfunction have also been documented in normoalbuminuric subjects with Type 2 DM, suggesting that the vasculopathy occurs before the development of microalbuminuria [5,6].
Amongst other causative factors related to PT dysfunction, advanced glycation end-products (AGE) have been involved in the pathogenesis of diabetic tubulopathy, an emerging entity [7]. Low-molecular weight AGE are filtered by renal glomeruli and then reabsorbed and metabolized by the PT. AGE activate PT cells [8], and severely modified albumin molecules after binding to advanced glycation adducts display high labelling in the urinary space and endocytic compartments of PT cells. Most likely, PT dysfunction is initiated by various types of peptide fragments, among which glycated peptides are of major importance [8,9]. There is an ongoing debate as to what extent albumin per se is nephrotoxic and whether modification of albumin and its altered molecules, such as glycated albumin, induce a degree of nephrotoxicity [10]. Recently, it has been stated that plasma albumin fragments have a different pattern from urinary albumin fragments, which do not originate from intracellular degeneration in the kidney PT, but they may be filtered from plasma into the urine and become later a tubulotoxic component [11].
The aim of our study was to investigate a potential association of urinary glycated peptides with PT dysfunction in patients with Type 2 DM. Also, we hypothesized that the expression of glomerular endothelial dysfunction and the onset of microalbuminuria may be delayed by the PT albumin processing in early DN, a phenomenon which could be impaired by glycated peptides involvement.
Materials and methods
A total of 76 patients with Type 2 DM attending the Outpatient Department of Diabetes and Metabolic Diseases and 28 healthy control subjects were enrolled in a cross-sectional study from January 2013 through July 2013. The inclusion criteria were duration of DM higher than 5 years, normoalbuminuria [urine albumin:creatinine ratio (UACR) <30 mg/g] or microalbuminuria (UACR between 30 and 300 mg/g), therapy with oral antidiabetic drugs, angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers, and statins. Patients were divided in two groups: 40 patients with normoalbuminuria (group 1) and 36 patients with microalbuminuria (group 2).
The County Emergency Hospital Ethics Committee (Board of Human Studies) approved the protocol (approval number 4/11.01.2013), and every patient provided written informed consent before enrolment.
All patients were assessed concerning UACR; urinary alpha1-microglobulin and urinary neutrophil gelatinase-associated lipocalin (NGAL), as biomarkers of PT dysfunction; plasma and urinary AGE; plasma asymmetric dimethyl-arginine (ADMA), as a biomarker of endothelial dysfunction; and serum cystatin C. Fully automated chip-nanoelectrospray ionization and high-capacity ion trap multistage mass spectrometry characterized the urinary proteomic profile.
Serum and urinary biomarkers were determined in specimens frozen at -80°C and thawed before assay. CKD was defined according to the KDIGO Guideline for the Evaluation and Management of CKD (2009 CKD-EPI creatinine equation) [12].
Plasma ADMA was evaluated by the ELISA method with K7828 ADMA ELISA Kit (Immu-nodiagnostik AG, Bensheim, Germany). The reference interval was 0.45±0.19 μmol/l. The intra-assay precision was 6.2-10.5% (0.27±0.021 μmol/l; 0.78±0.041 μmol/l), while the inter-assay precision was 6.2-7.9% (0.33±0.029 μmol/l; 0.79±0.045 μmol/l).
Cystatin C was assessed in serum with N Latex Cystatin C Kit (Siemens Healthcare Diagnostics, Marburg, Germany) through particle-enhanced immunonephelometry using the BNProSpec System. The reference interval was calculated nonparametrically and was determined to be 0.53-0.95 mg/l. The intra-assay precision was 2.5% coefficient of variation (CV) and inter-assay precision was 2.0% CV with a total of 2.8% CV. Analytical sensitivity was calculated as two standard deviations above the mean signal of 20 replicates of N diluent and was determined to be 0.005 mg/l. A typical detection for N Latex Cystatin C is 0.05 mg/l.
Alpha1-microglobulin was evaluated in the second morning urine specimen with N α1-Microglobulin Kit (Siemens Healthcare Diagnostics, Marburg, Germany) through particle-enhanced immunonephelometry using the BNProSpec System. The reference interval was 12 mg/l or 0.07-5 mg/g creatinine. The intra-assay precision was 2.9-5.2% CV, while the inter-assay precision was 7.4-13.2% CV.
Albuminuria was measured in the second morning urine specimen through immunonephelometry on the BNProSpec System, with N Antiserum to Human Albumin (Siemens Healthcare Diagnostics, Marburg, Germany). Microalbuminuria was defined by UACR between 30 and 300 mg/g, and normoalbuminuria by UACR <30 mg/g. The N Antiserum to Human Albumin was evaluated for the assay of urine on a BN System and yielded a Within-Run CV of 2.2% and a total CV of 2.6% with a mean of 79 mg/l. The results (ten runs, four determinations per run) were evaluated by analysis of variance. Urine cultures were negative for bacteriuria in all patients.
Urinary NGAL was assessed in the second morning urine specimen by the ELISA method with the NGAL Rapid Elisa Kit 037 (BioPorto Diagnostics, Gentofte, Denmark). The reference interval was 4.3-12.0 ng/ml. Intra-assay variation was determined by measurement of urinary NGAL in two 24-hour urine samples with 8 replicates (CV=3.4-4.3%). Inter-assay variation was determined by measurement of urinary NGAL in two diluted 24-hour urine samples with 2 replicates in 4 separate assays (CV=4.7-22.7%).
Plasma and urinary AGE peptides were assessed in a 24-hour urine sample by the ELISA method with Human Advanced Glycosylation End-Products ELISA Kit (E01A0002), Shanghai Blue Gene Biotech Co., Shanghai, China. The sensitivity in this assay measured in two 24-hour urine samples was 1.0 pg/ml. This assay has high sensitivity and excellent specificity of AGE, and contains polyclonal antibodies which assess protein-bound AGE. The system utilized only allows the assessment of both high and low molecular AGE species. No significant cross-reactivity or interference between AGE and analogues was observed.
Proteomic analysis of urinary glycated peptides
Protein isolation and electrophoresis
0.5 ml of urine was centrifuged at 3500 rpm for 5 min at 4°C to pellet out the cells/debris if any. Proteins from 0.5 ml of the supernatant were isolated using Acetone Precipitation overnight. The pellet was vacuum dried. 10 μg protein sample was loaded on 12% SDS-PAGE protein gel and electrophoresis was carried out on an SE260 Mighty Small II device from Hoefer, Inc. The electrophoresis running buffer was prepared from a 10x Tris-glycine-SDS solution. Electrophoresis was carried out at 120 V and an initial current of ~32 mA using a PowerPac 1000 power supply (Bio-Rad; Hercules, CA) and was terminated when the dye front reached close to the bottom of the gel. Typical run times were 1.5-2 h.
Gel staining
Immediately following electrophoresis, the gels were stained with 0.25% Comassie Blue R-250 (Sigma) overnight, followed by destaining for 4H in 5% MeOH, 7.5% HOAC, 87.5% H2O. Coomassie removal was carried out using Blue-Out reagent from G-Biosciences according to manufacturer instructions. Protein bands of 64, 55, 50, 40, 36, and 30 kDa, respectively, were obtained. Due to the fact that urinary glycated peptides (most likely their fragments), potentially involved in PT dysfunction, display low-molecular weight, further analysis was only performed in the bands of 30 kDa, which were excised from the stained gel with a clean, sharp straight-edge razor. The gel slices were excised as close as possible to the boundaries of the protein band.
Protein extraction was performed immediately after destaining according to Cohen and Chait [13]. Briefly the gel slices were soaked with a lint-free tissue, crushed manually at the bottom of the microtube for a few seconds with a sharp-pointed dental tool. Extraction was performed using a solution of formic acid/water/2-propanol (1:3:2 v/v/v) (FWI). The tubes were shaken overnight, centrifuged and the supernatant was transferred in a new tube.
Mass spectrometry
Reagents and materials
Methanol and formic acid were from Merck (Darmstadt, Germany). All reagents and solvents were of highest available purity. Prior to analysis, the sample solution eluted from the 30 kDa bands was centrifuged for 2 hours in a Sigma 2-16 model centrifuge from Sartorius (Göttingen, Germany). The supernatant was collected and submitted to chip-nanoelectrospray ionization analysis.
Mass spectrometry experiments were conducted on a High Capacity Ion Trap Ultra (HCT Ultra, PTM discovery) mass spectrometer (Bruker Daltonics, Bremen, Germany). Spectra were acquired in the 100-3000 m/z range in positive ion mode detection with a scan speed of 8000 m/z per second. G2421A “tuning mix” (Agilent Technologies, Santa Rosa, CA, USA) was used for calibration.
For Nano Mate-MS experiments the protein was dissolved in Me OH/formic acid (1:1 v:v) to approximately 0.5 pmol/mL concentration. The sample was infused by fully automated chip-based nanoelectrospray conducted on a Nano Mate robot incorporating ESI 400 Chip technology (Advion BioSciences, Ithaca, USA), controlled and manipulated by Chip Soft 8.1.0 Software operating under Windows system. The robot was coupled to the HCT via an in-laboratory made mounting system which allows the whole robot positioning on O-xyz with respect to MS inlet, as described by us previously [14]. The Nano Mate was mounted on the HCT mass spectrometer, by the aid of an in-laboratory made bracket. To initiate the electrospray in the positive ion mode, a voltage of 1.3-1.5 kV was imposed on the pipette tip, while the chip was grounded. The spray was enhanced by the application of 0.3-0.40 psi nitrogen back pressure, and 50 psi pressure of nitrogen nebulizer on MS. For optimal dissolution of the sample droplets, the source block was maintained at a constant temperature of 100°C. The in-source fragmentation was prevented by keeping a potential difference between the ends of the transfer capillary and the skimmer (capillary exit) at 50 V.
For all experiments a glass-coated micro titer plate was used, thus preventing any risk of cross-contamination. Aliquots of the working sample solutions were loaded onto the 96-well glass plate. The robot was programmed to aspirate the sample and then deliver it to the inlet side of the microchip. The unused sample was returned into its original well after each measurement. Disposable conductive pipette tips were used throughout all experiments. After each measurement the pipette tip was ejected and a fresh tip and nozzle were used for the next experiment.
Statistical analysis
Clinical and biological data are presented as means, standard deviations, and proportions. We used ANOVA test and the Bonferroni multiple-comparison test. Univariable regression analyses were carried out to evaluate the significance of the relation between continuous variables for all 3 groups together (pooled data of normo- and microalbuminuric patients, and healthy controls). Only significant variables yielded by univariable regression analysis were introduced in the models for multivariable regression analysis. The p values for all hypothesis tests were two-sided, and statistical significance was set at P<0.05. All analyses were conducted with Stata 9.2 (Statacorp, Texas, USA).
Results
All patients and controls were included in the analysis.
Characteristics of the study subjects are presented in Table 1. The results of the univariable regression analysis concerning ADMA are presented in Table 2. Potential confounders for ADMA, such as age, hypertension, and the lipid profile have been included in the analysis.
Table 1.
Clinical and biological data of the patients studied
| Parameter | Group 1 | Group 2 | Controls | p* | p |
|---|---|---|---|---|---|
| Number of subjects | 40 | 36 | 28 | – | – |
| Age (years) | 57.16±7.83 | 59.04±9.33 | 57.18±4.95 | 0.3834 | 0.8396 |
| DM duration (years) | 10.08±4.04 | 10.45±5.14 | – | 0.7448 | – |
| Body mass index (kg/m2) | 32.67±6.19 | 31.38±6.62 | 24.22±0.45 | 0.4320 | 0.0175 |
| SBP (mmHg) | 131.56±12.59 | 132.5±9.72 | 121.36±7.44 | 0.7583 | 0.0018 |
| DBP (mmHg) | 76.87±8.22 | 75.22±8.08 | 65.90±6.25 | 0.437 | 0.0048 |
| Hb (g/dl) | 12.90±1.15 | 12.82±0.74 | 13.90±0.75 | 0.252 | 0.0186 |
| Serum creatinine (mg/dl) | 0.95±0.20 | 0.98±0.17 | 0.80±0.09 | 0.5055 | 0.0095 |
| eGFR (ml/min/1.73 m2) | 76.51±16.43 | 71.80±15.14 | 90.00±5.76 | 0.3321 | <0.0001 |
| Glycaemia (mg/dl) | 146.79±37.21 | 147.54±57.36 | 92±7.07 | 0.9476 | <0.0001 |
| HbA1c (%) | 6.97±0.62 | 7.11±1.05 | 5.99±0.17 | 0.4863 | <0.0001 |
| Serum cholesterol (mg/dl) | 216.20±49.19 | 220.13±49.29 | 157.18±23.47 | 0.7576 | 0.0014 |
| Serum triglycerides (mg/dl) | 144.54±47.50 | 151.95±48.03 | 96.45±25.61 | 0.3124 | <0.0001 |
| hsCRP (mg/dl) | 6.68±4.05 | 13.29±5.54 | 0.93±0.18 | <0.0001 | <0.0001 |
| UACR (mg/g) | 16.8±6.34 | 70.09±34.02 | 15.71±4.11 | <0.0001 | 0.0002 |
| Urinary alpha1/creat (mg/g) | 3.31±1.34 | 5.72±2.13 | 2.07±0.62 | <0.0001 | <0.0001 |
| Urinary NGAL/creat (μg/g) | 229.11±139.06 | 444.28±205.83 | 78.59±6.58 | <0.0001 | <0.0001 |
| Cystatin C (mg/l) | 0.82±0.19 | 0.96±0.27 | 0.61±0.07 | 0.0157 | <0.0001 |
| Urinary AGE (pg/ml) | 44.33±17.42 | 70.97±37.12 | 31.54±1.43 | <0.0001 | <0.0001 |
| ADMA (μmol/l) | 0.44±0.19 | 0.60±0.23 | 0.26±0.01 | 0.0026 | <0.0001 |
| Plasma AGE (pg/ml) | 442.64±175.69 | 655.22±152.03 | 272.39±22.59 | <0.0001 | <0.0001 |
DM-diabetes mellitus; SBP-systolic blood pressure; DBP-diastolic blood pressure; Hb-haemoglobin; eGFR-estimated glomerular filtration rate; HbA1c-glycated haemoglobin; hsCRP-high sensitive C reactive protein; UACR-urinary albumin:creatinine ratio; alpha1/creat- alpha1-microglobulin:creatinine ratio; urinary NGAL/creat- neutrophil gelatinase associated lipocalin: creatinine ratio; AGE-advanced glycation end-products; ADMA-asymmetric dymethyl-arginine; parameters are presented as means, standard deviations, and proportions;
Bonferroni’s test for the differences between groups 1 and 2;
p-ANOVA analysis of variance to compare groups 1, 2, and controls.
Table 2.
Univariable regression analysis for ADMA
| Variable | Parameter | ||
|---|---|---|---|
|
| |||
| ADMA | |||
|
| |||
| R squared | Coef β | p | |
| hsCRP | 0.241 | 0.018 | <0.0001 |
| Duration of DM | 0.112 | 0.016 | 0.005 |
| eGFR (CKD-EPI) | 0.084 | -0.003 | 0.008 |
| Cystatin C | 0.290 | 0.507 | <0.001 |
| HbA1c | 0.035 | 0.050 | 0.093 |
| pAGE | 0.620 | 0.0008 | <0.0001 |
| UACR | 0.029 | 0.001 | 0.124 |
| u alpha1/creat | 0.032 | 0.019 | 0.110 |
| uNGAL/creat | 0.023 | 0.0001 | 0.173 |
| uAGE | 0.007 | 0.0006 | 0.444 |
| Cholesterol | 0.002 | 0.0002 | 0.679 |
| Triglycerides | 0.016 | 0.0005 | 0.247 |
| SBP | 0.005 | -0.001 | 0.523 |
| DBP | 0.001 | 0.001 | 0.701 |
| Age | 0.002 | 0.001 | 0.680 |
ADMA: asymmetric dymethyl-arginine; hsCRP: high sensitive C-reactive protein; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; HbA1c: glycated haemoglobin; pAGE: plasma advanced glycation end-products; UACR: urinary albumin to creatinine ratio; u alpha1/creat: urinary alpha1-microglobulin to creatinine ratio; uNGAL/creat: urinary neutrophil gelatinase-associated lipocalin to creatinine ratio; uAGE: urinary advanced glycation end-products; SBP: systolic blood pressure; DBP: diastolic blood pressure.
A significant correlation was found between urinary alpha1-microglobulin and urinary NGAL, and UACR, plasma and urinary AGE, estimated glomerular filtration rate (eGFR), and cystatin C, respectively (Table 3). After adjustment in multivariable regression analysis, the models showed that the correlations for urinary alpha1-microglobulin and urinary NGAL remained significant with UACR, urinary AGE, and GFR (P<0.0001, R2=0.674; P<0.001, R2=0.551; P<0.0001, R2=0.482) (Table 4).
Table 3.
Univariable regression analysis for the tubular biomarkers
| Variable | Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| u alpha1/creat | uNGAL | uAGE | |||||||
|
|
|||||||||
| R-squared | Coef β | p | R-squared | Coef β | p | R-squared | Coef β | p | |
| eGFR | 0.178 | -0.051 | <0.001 | 0.074 | -2.477 | 0.014 | 0.130 | -0.602 | 0.001 |
| Cystatin C | 0.146 | 3.240 | <0.001 | 0.101 | 262.687 | 0.004 | 0.110 | 38.515 | 0.002 |
| pAGE | 0.132 | 36.69 | 0.001 | 0.089 | 4.60 | 0.007 | 0.058 | 1.777 | 0.03 |
| UACR | 0.292 | 0.035 | <0.001 | 0.282 | 3.398 | <0.0001 | 0.177 | 0.378 | <0.001 |
| u alpha1/creat | 0.542 | 0.112 | <0.001 | 0.586 | 0.055 | <0.001 | |||
| uNGAL/creat | 0.482 | 4.943 | <0.0001 | ||||||
u alpha1/creat: urinary alpha1-microglobulin to creatinine ratio; uNGAL/creat: urinary neutrophil gelatinase-associated lipocalin to creatinine ratio; uAGE: urinary advanced glycation end-products; eGFR: estimated glomerular filtration rate; pAGE: plasma advanced glycation end-products; UACR: urinary albumin to creatinine ratio.
Table 4.
Multivariable regression analysis for the tubular biomarkers
| Parameter | Variable | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Coef β | p | 95% CI | F | Prob>F | R-squared | ||
| u alpha1/creat | Constant | 0.863 | 0.002 | 0.323 to 1.403 | 53.14 | <0.0001 | 0.674 |
| eGFR | -0.025 | 0.003 | -0.042 to -0.008 | ||||
| uNGAL/creat | 0.002 | 0.009 | 0.0006 to 0.004 | ||||
| UACR | 0.012 | 0.016 | 0.002 to 0.022 | ||||
| uAGE | 0.036 | <0.0001 | 0.023 to 0.050 | ||||
| uNGAL/creat | Constant | 6.405 | <0.001 | 54.946 to 67.756 | 48.00 | <0.0001 | 0.551 |
| eGFR | -0.285 | 0.001 | -0.442 to -0.128 | ||||
| u alpha1/creat | 0.076 | <0.001 | 0.148 to 0.404 | ||||
| UACR | 1.857 | 0.001 | 0.793 to 2.921 | ||||
| uAGE | 4.071 | <0.0001 | 2.886 to 5.256 | ||||
| uAGE | Constant | 82.222 | <0.001 | 57.060 to 107.383 | 43.58 | <0.0001 | 0.482 |
| eGFR | -0.578 | <0.001 | -0.894 to -0.263 | ||||
| UACR | 0.367 | <0.001 | 0.198 to 0.537 | ||||
| u alpha1/creat | 2.348 | <0.001 | 1.236 to 3.876 | ||||
| uNGAL/creat | 4.943 | <0.0001 | 3.796 to 6.090 | ||||
u alpha1/creat: urinary alpha1-microglobulin to creatinine ratio; uNGAL/creat: urinary neutrophil gelatinase-associated lipocalin to creatinine ratio; uAGE: urinary advanced glycation end-products; eGFR: estimated glomerular filtration rate; UACR: urinary albumin to creatinine ratio.
The protein sample of 30 kDa analysed by fully automated chip-nanoelectrospray ionization and high-capacity ion trap multistage mass spectrometry provided a particular proteomic pattern of urinary protein-bound glycated peptides in both groups. We found that in the case of normoalbuminuric patients the urinary glycated protein displayed a molecular weight of 15,121.4 Da (Figure 1), while in the case of microalbuminuric patients, the molecular weight was almost two times this value, i.e. 30,180.4 Da (Figure 2). This difference in size indicates a significantly higher glycation status of the urinary glycoproteins in microalbuminuric patients.
Figure 1.
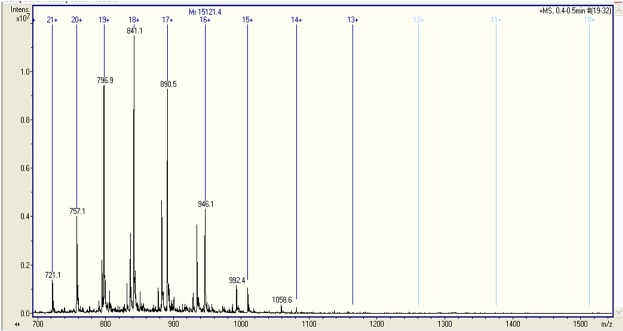
Fully automated chip-nanoelectrospray mass spectrometry of urinary glycated peptide in a normoalbuminuric patient with Type 2 DM. Acquisition time 1 min; solvent: HCOOH/MeOH (1:1 v:v); ChipnanoESI: 1.3 kV; EndCap: 50 V; Skimmer: 40 V; Nitrogen back pressure: 0.30 p.s.i.; Nebulizer on MS at 50 psi.
Figure 2.
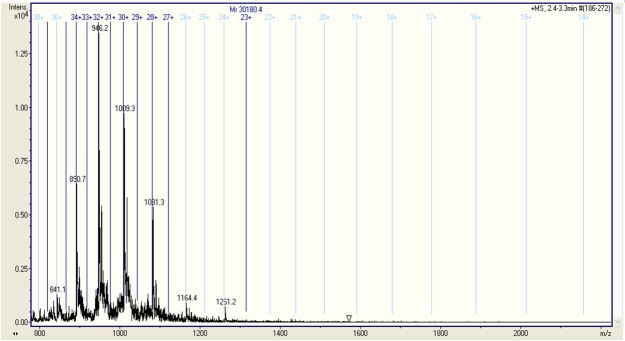
Fully automated chip-nanoelectrospray mass spectrometry of urinary glycated peptide in a microalbuminuric patient with Type 2 DM. Acquisition time 1 min; solvent: HCOOH/MeOH (1:1 v:v); ChipnanoESI: 1.3 kV; EndCap: 50 V; Skimmer: 40 V; Nitrogen back pressure: 0.30 p.s.i.; Nebulizer on MS at 50 psi.
Discussion
The results of our study showed increased levels of PT dysfunction biomarkers in both normo- and microalbuminuric patients. Urinary AGE correlated with the biomarkers of PT dysfunction studied, but not with plasma ADMA, a fact which documented a potential association of AGE with PT functional defects, even in the normoalbuminuria stage, before expression of glomerular endothelial dysfunction.
Proteomic mass spectrometry analysis revealed a particular pattern of urinary glycated peptides, the size of which differed in normoalbuminuric patients as compared to microalbuminuric patients. There occurred a slight decline in renal function as assessed by serum cystatin C, a fact which proves that patients with Type 2 DM might experience a steady decrease in their renal function, while remaining normoalbuminuric.
PT dysfunction is dissociated from and does not parallel glomerular endothelial dysfunction in early DN
The classical concept concerning albuminuria states that albuminuria is related to the severity of diabetic glomerular lesions, but this correlation is not strict in Type 2 DM due to the fact that these lesions may precede the onset of albuminuria [15]. Endothelial dysfunction may also occur in patients with Type 2 DM even when the patients are still normoalbuminuric, a fact demonstrated by markers of endothelial dysfunction which may be elevated years before any evidence of microangiopathic complications in both types of DM [16].
Nitric oxide (NO) is synthesized by the vascular endothelium from the aminoacid L-arginine by constitutive and inducible NO-synthase and plays an important role in the maintenance of vascular homeostasis. The endogenous L-arginine metabolite, ADMA, inhibits cellular L-arginine uptake and NO-synthase activity. Circulating ADMA is elevated in patients with overt DN, even when the GFR is still within normal range [17].
It has been stated that in both normo- and microalbuminuric patients with DM, plasma ADMA levels have prognostic implications for the transition to a more advanced stage of albuminuria [18].
In our study, ADMA levels were increased in a small number of the normoalbuminuric patients and did not correlate with UACR and PT dysfunction biomarkers. Plasma ADMA correlated with inflammation, plasma AGE, serum cystatin C, GFR, and DM duration. These results are in contrast to near similar data found in microalbuminuric patients with Type 2 DM [19], thus giving rise to the speculation that an inflammatory state at the glomerular level may be found in normoalbuminuric patients with Type 2 DM.
Decreased peritubular capillary flow due to impaired levels of plasma ADMA may induce tubulointerstitial ischemia and subsequent modifications in the function of PT cells [20]. In patients with early DN diagnosed on PT abnormalities and markers of tubular dysfunction, the levels of ADMA may remain within the normal (or high-to-normal) range [18], as was the case with our patients.
Glycated peptides are associated with PT dysfunction biomarkers in early DN
The tubular theory concerning mechanisms of albuminuria in early DN states that there is a reduction in the retrieval pathway of albumin in the PT, and this impairment precedes glomerular damage [3]. Albumin per se is considered nephrotoxic [21] and modifications of albumin increase its capacity to exert nephrotoxic properties and to cause PT dysfunction and peritubular fibrosis [10].
AGE have been shown to modulate NO-synthase in endothelial cells [22]. In our study, urinary AGE did not correlate with ADMA, a biomarker of endothelial dysfunction, but only with biomarkers of PT dysfunction, thus showing that the expression of glomerular endothelial dysfunction is not conditional on the onset of albuminuria in early DN.
AGE-modified peptides show tubulotoxic effects and lead to pathophysiologic responses described as diabetic tubulopathy [7]. The data provided by our study lead to the assumption that urinary glycated peptides may be associated with PT function impairment, even in normoalbuminuric patients. The proteomic patterns of urinary glycated peptides found in our patients could differentiate normo- from microalbuminuric patients and may explain a potential relation between the size of glycated peptides and the extent of PT dysfunction. The difference in size of the glycated peptides between normo- and microalbuminuric patients indicates a significantly higher glycation status of the urinary glycoproteins in microalbuminuric patients.
Biomarkers of PT dysfunction are strongly associated to the levels of plasma and urinary AGE [10]. In our study we found a significant relation of plasma and urinary AGE with biomarkers of PT dysfunction, such as urinary alpha1-microglobulin and urinary NGAL, even in normoalbuminuric patients. This association raises the possibility that renal tubular function defects precede the onset of microalbuminuria. Similar data have been provided by several studies performed in normoalbuminuric patients with Type 2 DM, in who increased levels of urinary alpha1-microglobulin [4,5,23] and urinary NGAL [24] show that tubular functional defects precede the onset of albuminuria.
In our study, urinary alpha1-microglobulin, urinary NGAL, plasma and urinary AGE-peptides correlated directly with serum cystatin C. Similarly, it has been revealed that urinary PT damage markers are associated with GFR and cystatin C, independently of albuminuria [24,25].
It has been shown that reduced GFR may correlate with the degree of albuminuria within the normal range [4,5,26]. Subtle changes in renal function in patients with Type 2 DM, as assessed by serum cystatin C, may parallel the degree of albuminuria, even in high-to-normal albuminuria [27,28], data which is similar to our findings. In our cross-sectional study, the declined GFR in normoalbuminuric patients should be regarded as an association with AGE-induced PT dysfunction, observation which does not necessarily prove causality. In a study performed in Type 1 and Type 2 DM patients it has been shown that urinary protein-bound AGE concentrations closely paralleled changes in albuminuria, reflecting the severity of DN [29]. This data is consistent with results from studies which support the hypothesis that renal function may show a steady decline in normoalbuminuric patients with Type 2 DM [30,31].
Our study has some limitations. First, the small study cohort could affect the statistical power of the study. Second, the glycated peptides found in mass spectrometry require further identification. Finally, this is a cross-sectional study which requires validation by a longitudinal study in order to prove causality between glycated peptides and PT dysfunction.
To sum up, urinary glycated peptides may be associated with PT dysfunction in patients with Type 2 DM. The proteomic patterns of urinary glycated peptides found in our patients could differentiate normo- from microalbuminuric patients and may explain a potential relation between the size of glycated peptides and the extent of PT dysfunction. This difference in size of the glycated peptides indicates a significantly higher glycation status of the urinary glycoproteins in microalbuminuric patients. We found a significant relation of plasma and urinary AGE with biomarkers of PT dysfunction in both normo- and microalbuminuric patients, association which raises the possibility that AGE-induced renal tubular function defects precede the onset of microalbuminuria. The expression of glomerular endothelial dysfunction and the onset of microalbuminuria may be delayed by the PT albumin processing in early DN, until AGE could induce PT dysfunction. Thus, the lack of correlation between parameters of endothelial dysfunction and of PT dysfunction cannot exclude glomerular involvement in early DN. Further prospective studies on larger cohorts are warranted in order to support these data.
Acknowledgements
This research received funding from an Internal Grant of “Victor Babes” University of Medicine and Pharmacy Timisoara, PIII-C1-PCFI-2014/2015. The supporting source had no involvement in study design, in collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the manuscript for publication.
Disclosure of conflict of interest
None.
References
- 1.Schernthaner G. Kidney disease in diabetology: lessons from 2010. Nephrol Dial Transplant. 2011;26:454–457. doi: 10.1093/ndt/gfq837. [DOI] [PubMed] [Google Scholar]
- 2.Comper WD, Haraldsson B, Deen WM. Resolved: normal glomeruli filter nephrotic levels of albumin. J Am Soc Nephrol. 2008;19:427–432. doi: 10.1681/ASN.2007090997. [DOI] [PubMed] [Google Scholar]
- 3.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20:489–494. doi: 10.1681/ASN.2008050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrica L, Petrica M, Vlad A, Jianu DC, Gluhovschi G, Ianculescu C, Dumitrascu V, Giju S, Gluhovschi C, Bob F, Ursoniu S, Gadalean F, Velciov S, Bozdog G, Marian R. Nephro- and neuroprotective effects of rosiglitazone versus glimepiride in normoalbuminuric patients with type 2 diabetes mellitus: a randomized controlled trial. Wien Klin Wochenschr. 2009;121:765–775. doi: 10.1007/s00508-009-1279-3. [DOI] [PubMed] [Google Scholar]
- 5.Petrica L, Petrica M, Vlad A, Jianu DC, Gluhovschi G, Ianculescu C, Firescu C, Dumitrascu V, Giju S, Gluhovschi C, Bob F, Gadalean F, Ursoniu S, Velciov S, Bozdog G, Milas O. Proximal tubule dysfunction is dissociated from endothelial dysfunction in normoalbuminuric patients with type 2 diabetes mellitus: a cross-sectional study. Nephron Clin Pract. 2011;118:c155–c164. doi: 10.1159/000320038. [DOI] [PubMed] [Google Scholar]
- 6.Lim SC, Caballero AE, Smakowski P, Logerfo FW, Horton ES, Veves A. Soluble intercellular adhesion molecule, vascular cell adhesion molecule, and impaired microvascular reactivity are early markers of vasculopathy in type 2 diabetic individuals without microalbuminuria. Diabetes Care. 1999;22:1865–1870. doi: 10.2337/diacare.22.11.1865. [DOI] [PubMed] [Google Scholar]
- 7.Tang SC, Leung JC, Lai KN. Diabetic tubulopathy: an emerging entity. Contrib Nephrol. 2011;170:124–134. doi: 10.1159/000325647. [DOI] [PubMed] [Google Scholar]
- 8.Morcos M, Sayed AA, Bierhaus A, Yard B, Waldherr R, Merz W, Kloeting I, Schleicher E, Mentz S, Abdel Baki RF, Tritschler H, Kasper M, Schwenger V, Hamann A, Dugi KA, Schmidt AM, Stern D, Ziegler R, Haering HU, Andrassy M, van der Woude F, Nawroth PP. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes. 2002;51:3532–3544. doi: 10.2337/diabetes.51.12.3532. [DOI] [PubMed] [Google Scholar]
- 9.Ozdemir AM, Hopfer U, Erhard P, Monnier VM, Weiss MF. Processing advanced glycation end product-modified albumin by the renal proximal tubule and the early pathogenesis of diabetic nephropathy. Ann N Y Acad Sci. 2005;1043:625–636. doi: 10.1196/annals.1338.071. [DOI] [PubMed] [Google Scholar]
- 10.Gross ML, Piecha G, Bierhaus A, Hanke W, Henle T, Schirmacher P, Ritz E. Glycated and carbamylated albumin are more “nephrotoxic” than unmodified albumin in the amphibian kidney. Am J Physiol Renal Physiol. 2011;301:F476–F485. doi: 10.1152/ajprenal.00342.2010. [DOI] [PubMed] [Google Scholar]
- 11.Weyer K, Nielsen R, Christensen EI, Birn H. Generation of urinary albumin fragments does not require proximal tubular uptake. J Am Soc Nephrol. 2012;23:591–596. doi: 10.1681/ASN.2011101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int (Suppl) 2013;3:1–150. [Google Scholar]
- 13.Cohen SL, Chait BT. Mass spectrometry of whole proteins eluted from sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Analytical Biochem. 1997;247:257–267. doi: 10.1006/abio.1997.2072. [DOI] [PubMed] [Google Scholar]
- 14.Zamfir AD, Serb A, Vukeli Ž, Flangea C, Schiopu C, Fabris D, Kalanj-Bognar S, Capitan F, Sisu E. Assessment of the molecular expression and structure of gangliosides in brain metastasis of lung adenocarcinoma by an advanced approach based on fully automated chip-nanoelectrospray mass spectrometry. J Am Soc Mass Spectrom. 2011;22:2145–2159. doi: 10.1007/s13361-011-0250-5. [DOI] [PubMed] [Google Scholar]
- 15.Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007;27:195–207. doi: 10.1016/j.semnephrol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 17.Tarnow L, Hovind P, Teerlink T, Stehouwer CD, Parving HH. Elevated plasma asymmetric dimethylarginine as a marker of cardiovascular morbidity in early diabetic nephropathy in type 1 diabetes. Diabetes Care. 2004;27:765–769. doi: 10.2337/diacare.27.3.765. [DOI] [PubMed] [Google Scholar]
- 18.Hanai K, Babazono T, Nyumura I, Toya K, Tanaka N, Tanaka M, Ishii A, Iwamoto Y. Asymmetric dimethylarginine is closely associated with the development and progression of nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant. 2009;24:1884–1888. doi: 10.1093/ndt/gfn716. [DOI] [PubMed] [Google Scholar]
- 19.Krzyzanowska K, Mittermayer F, Shnawa N, Hofer M, Schnabler J, Etmüller Y, Kapiotis S, Wolzt M, Schernthaner G. Asymmetrical dimethylarginine is related to renal function, chronic inflammation and macroangiopathy in patients with type 2 diabetes and albuminuria. Diabetic Med. 2007;24:81–86. doi: 10.1111/j.1464-5491.2007.02018.x. [DOI] [PubMed] [Google Scholar]
- 20.Shibata R, Ueda S, Yamagishi S, Kaida Y, Matsumoto Y, Fukami K, Hayashida A, Matsuoka H, Kato S, Kimoto M, Okuda S. Involvement of asymmetric dimethylarginine (ADMA) in tubulointerstitial ischaemia in the early phase of diabetic nephropathy. Nephrol Dial Transplant. 2009;24:1162–1169. doi: 10.1093/ndt/gfn630. [DOI] [PubMed] [Google Scholar]
- 21.Kern EF, Erhard P, Sun W, Gennth S, Weiss MF. Early urinary markers of diabetic kidney disease: a nested case-control study from the Diabetes Control and Complications Trial (DCCT) Am J Kidney Dis. 2010;55:824–834. doi: 10.1053/j.ajkd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amore A, Cirina P, Mitola S, Peruzzi L, Gianoglio B, Rabbone I, Sacchetti C, Cerutti F, Grillo C, Coppo R. Non-enzymatically glycated albumin (Amadori adducts) enhances nitric oxide synthase activity and gene expression in endothelial cells. Kidney Int. 1997;51:27–35. doi: 10.1038/ki.1997.4. [DOI] [PubMed] [Google Scholar]
- 23.Hong CY, Hughes K, Chia KS, Ng V, Ling SL. Urinary alpha1-microglobulin as a marker of nephropathy in type 2 diabetic Asian subjects in Singapore. Diabetes Care. 2003;26:338–342. doi: 10.2337/diacare.26.2.338. [DOI] [PubMed] [Google Scholar]
- 24.Fu WJ, Xiong SL, Fang YG, Wen S, Chen ML, Deng RT, Zheng L, Wang SB, Pen LF, Wang Q. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study. Endocrine. 2012;41:82–88. doi: 10.1007/s12020-011-9509-7. [DOI] [PubMed] [Google Scholar]
- 25.Nauta FL, Boertien WE, Bakker SJ, van Goor H, van Oeveren W, de Jong PE, Bilo H, Gansevoort RT. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care. 2011;34:975–981. doi: 10.2337/dc10-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritz E, Viberti GC, Ruilope LM, Rabelink AJ, Izzo JL Jr, Katayama S, Ito S, Mimran A, Menne J, Rump LC, Januszewicz A, Haller H. Determinants of urinary albumin excretion within the normal range in patients with type 2 diabetes: the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study. Diabetologia. 2010;53:49–57. doi: 10.1007/s00125-009-1577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kravaritou M, Thanopoulou A, Karamanos B, Kofinis A, Noutsou M, Spanou E, Varsou A, Archimandritis A. Evidence that even “normal” albuminuria may denote incipient GFR reduction in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;85:317–321. doi: 10.1016/j.diabres.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Jeon YK, Kim MR, Huh JE, Mok JY, Song SH, Kim SS, Kim BH, Lee SH, Kim YK, Kim IJ. Cystatin C as an early biomarker of nephropathy in patients with type 2 diabetes. J Korean Med Sci. 2011;26:258–263. doi: 10.3346/jkms.2011.26.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coughlan MT, Patel SK, Jerums G, Penfold SA, Nguyen TV, Sourris KC, Panagiotopoulos S, Srivastava PM, Cooper ME, Burrell LM, Macisaac RJ, Forbes JM. Advanced glycation urinary protein-bound biomarkers and severity of diabetic nephropathy in man. Am J Nephrol. 2011;34:347–355. doi: 10.1159/000331064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerums G, Panagiotopoulos S, Premaratne E, Mac Isaac RJ. Integrating albuminuria and GFR in the assessment of diabetic nephropathy. Nat Rev Nephrol. 2009;5:397–406. doi: 10.1038/nrneph.2009.91. [DOI] [PubMed] [Google Scholar]
- 31.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27:195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]


