Abstract
Objective: This study aimed to confirm the potential of growth-related gene product β (GROβ) as a biomarker for colorectal cancer. We compared serum GROβ levels in patients with colorectal cancer, healthy individuals and individuals with non-tumor diseases. Methods: We measured serum GROβ levels with enzyme-linked immunosorbent assay in patients with colorectal cancer (123 preoperative samples and 66 postoperative samples), 88 healthy controls and 125 individuals with other diseases. Serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were measured in all samples with an immunoluminometric assay. Statistical analyses were performed to determine associations between serum GROβ levels and clinical parameters for colorectal cancer. A receiver operating characteristic (ROC) curve was analyzed for GROβ, CEA and CA19-9. Results: The serum GROβ levels were much higher in patients with colorectal cancer (median: 96.15 pg/ml) than those in healthy controls (median: 43.28 pg/ml, P < 0.01) and other disease controls (median: 57.30 pg/ml, P < 0.01). Serum GROβ levels in colorectal cancer were correlated positively with tumor-node-metastasis staging (P < 0.01) and the depth of infiltration (P < 0.05), but not with the histological grade, tumor embolus, lymph node metastasis, gross pathologic tumor type, or patient gender. The sensitivity and specificity of the assay for serum GROβ were 56.1% (69/123) and 95.31% (203/213), respectively. The area under the ROC curve constructed with GROβ (0.834) was larger than that constructed with CEA (0.739) or CA19-9 (0.676) for discriminating colorectal cancer from matched controls. Conclusion: These preliminary results suggested that the serum GROβ level could be a useful biomarker for colorectal cancer diagnoses.
Keywords: GROβ, colorectal cancer, biomarker, CEA, CA19-9
Introduction
Colorectal cancer is one of the most commonly diagnosed cancers. It is the fourth leading cause of cancer mortality worldwide. In China, there is very little governmental support for colorectal cancer screening, and we lack any kind of colorectal cancer screening initiative, including screening guidelines. Consequently, although China has historically displayed a low risk of colorectal cancer, the incidence rates are rapidly increasing [1,2]. Thus, despite recent improvements in surveillance and therapeutic approaches, approximately 1.2 million cases of colorectal cancer are reported annually, with over 608,700 deaths per year [3]. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are commonly used as tumor markers for the diagnosis and surveillance of colorectal cancer [4,5]. However, these two markers lack sensitivity for detecting potentially curable lesions [6]. Therefore, the development of novel diagnostic biomarkers for colorectal cancer is crucial for improving the prognosis and optimizing the efficiency of individualized therapies.
Chemokines are a group of small (8-14kDa) proteins that interact with cell-surface receptors during development of the host immune response [7]. Growing evidence has suggested that, in addition to their role in the immune system, chemokines are also involved in tumor development and progression [8,9]. Chemokines are produced by many cell types, including leukocytes, endothelial cells, fibroblasts, epithelial cells and tumor cells [10]. The growth-related oncogene (GRO) protein, a member of the CXC chemokine subfamily, plays a major role in inflammation and wound healing. CXC chemokines have been associated with angiogenesis, tumorigenesis, metastasis and tumor-leukocyte interactions [11,12]. GRO proteins include GROα, GROβ and GROγ subtypes [13,14]. GROβ is an angiogenic chemokine that belongs to the CXC chemokine family; it is also known as CXCL2 chemokine, MIP2-alpha protein and GRO-2 protein. CXCL2/GROβ was originally identified from culture supernatants from melanoma cell lines [15], and its expression was high in human melanomas [16]. An increasing number of reports have gradually revealed the roles of GROβ in tumorigenesis. GROβ attracts tumor infiltrating cells and may also directly contribute to esophageal cancer cell transformation and growth. When CXCL2 was depleted with small interfering RNA (siRNA), esophageal cancer cells showed reduced proliferation and colonization capacity [17]. cDNA microarray analysis showed that GROβ was highly expressed in esophageal squamous cell carcinoma tumor tissues and cultured tumor cells [11]. Thus, GROβ may act as a proangiogenic chemokine in esophageal cancer biology. Downregulation of CXCL1 and CXCL2 was associated with marked inhibition of metastasis-promoting genes [18]. Doll et al. used quantitative RT-PCR to show that GRO-2 expression was significantly increased in colon cancer and premalignant adenomas compared to normal colon tissue [19]. Oral squamous cell carcinomas synthesized CXCL2, which promoted osteoclast formation by stimulating RANKL expression in stromal cells [20]. However, GROβ suppressed the growth of Lewis lung tumors in immune-competent and immune-deficient mice by suppressing tumor vascularization [21]. When breast tumors with little or no GROβ mRNA expression were compared to matched, uninvolved tissue, the results suggested that impaired autocrine/paracrine chemokine signaling may contribute to breast tumorigenesis [22]. Thus, biological responses to GROβ vary, depending on the cellular context. Recently, one research group has reported that serum GROβ levels were much higher in patients with esophageal squamous cell carcinoma than in healthy controls [23]. However, no report has described serum GROβ levels in colorectal cancer.
In this study, we measured the serum levels of GROβ in patients with colorectal cancer, in patients with non-tumor diseases and in control healthy patients. We also analyzed correlations between the clinicopathological characteristics of colorectal cancer and serum GROβ levels. We evaluated whether the combination of preoperative levels of GROβ, CEA and CA19-9 had any diagnostic significance in patients with colorectal cancer. We reasoned that the combination of three tumor markers may provide a more sensitive biomarker for colorectal cancer. Furthermore, we considered the correlation between the preoperative and postoperative levels of GROβ; i.e., with and without a tumor burden.
Materials and methods
Clinical specimen collection and preparation
In this study, sera from 123 subjects newly diagnosed with colorectal cancer were provided by the Cancer Hospital of Peking Union Medical College (PUMC; 123 preoperative serum samples and 66 postoperative serum samples). The patients included 86 males and 37 females, with a mean age of 57.7 ± 10.5 y (range 24-80 y). All patients provided informed consent. The resected specimens were pathologically classified according to the seventh edition of the Union for International Cancer Control tumor-node-metastasis (TNM) classification of malignant tumors [24]. Sixty six patients had tumors located in the colon, and 57patients had tumors located in the rectum. Postoperative sera were obtained two weeks after surgery.
We also obtained sera from 88 matched healthy individuals with a mean age of 40.2 ± 9.6 y (range 26-59 y) from the Health Examinations Center, China Meitan General Hospital. All subjects provided informed consent. The healthy individuals had no obvious evidence of malignancy, based on abdominal ultrasound examinations, X-rays, routine blood tests and biochemistry tests.
The 125 samples from matched individuals with non-tumor diseases (controls) were obtained from inpatients in China Meitan General Hospital. All subjects provided informed consent. These patients had a mean age of 63.8 ± 14.5 y (range 25-94 y), and they showed no evidence of malignancy. Each subject had at least one organic disease, and we obtained records from regular physical and blood examinations and from mandatory screenings with colonoscopy and computed tomography.
The clinical characteristics of the serum samples are shown in Table 1. This study was approved by the ethics committees of PUMC and China Meitan General Hospital.
Table 1.
Clinical parameters of patients with colorectal cancer, patients with non-tumor diseases, and healthy controls; values represent n (%)
| Colorectal cancer (Preoperative Samples) n = 123 | Colorectal cancer (Postoperative Samples) n = 66 | Non-tumor diseases n = 125 | Healthy controls n = 88 | |
|---|---|---|---|---|
| Age (y) | ||||
| < 50 | 26 (21.1) | 10 (15.2) | 20 (16) | 67 (76.1) |
| ≥ 50 | 97 (78.9) | 56 (84.8) | 105 (84) | 21 (23.9) |
| Gender | ||||
| Male | 86 (69.9) | 45 (68.2) | 59 (47.2) | 54 (61.4) |
| Female | 37 (30.1) | 21 (31.8) | 66 (52.8) | 34 (38.6) |
| Histological grade | ||||
| Well | 12 (9.8) | 3 (1.5) | ||
| Moderate | 79 (64.2) | 48 (72.7) | ||
| Poor | 26 (21.1) | 15 (9.1) | ||
| Unknown | 6 (4.9) | 0 | ||
| TNM stage | ||||
| I | 18 (14.6) | 15 (22.7) | ||
| II | 39 (31.7) | 17 (25.8) | ||
| III | 57 (46.3) | 30 (45.5) | ||
| IV | 9 (7.3) | 4 (6.1) | ||
| Tumor embolus | ||||
| Positive | 39 (31.7) | 20 (30.3) | ||
| Negative | 80 (65.0) | 46 (69.7) | ||
| Unknown | 4 (3.3) | 0 | ||
| Lymph node metastasis | ||||
| Yes | 56 (45.5) | 27 (40.1) | ||
| No | 67 (54.5) | 39 (59.1) | ||
| Type | ||||
| Swelling | 50 (40.6) | 28 (42.4) | ||
| Ulcer | 61 (49.6) | 34 (51.5) | ||
| Other types | 12 (9.8) | 4 (6.1) | ||
| Depth of infiltration | ||||
| Mucosa | 10 (8.1) | 6 (9.1) | ||
| Muscular layer | 23 (18.7) | 11 (16.7) | ||
| Peritoneum | 64 (52.0) | 35 (53.0) | ||
| Lipid of pericolon | 22 (17.9) | 13 (19.7) | ||
| Unknown | 4 (3.3) | 1 (1.5) |
ELISA for GROβ
A human GROβ ELISA detection kit (Peprotech, Rocky Hill, NJ, USA) was used to measure GROβ in human serum. Briefly, 96-well ELISA microplates were coated with 100 μl of anti-GROβ antibody (Peprotech, Rocky Hill, NJ, USA) suspended in PBS buffer at a final concentration of 0.25 μg/ml. After an overnight incubation, the microplates were washed with PBS/0.05% (w/v) Tween-20 (PBST, pH 7.4). Then, the wells were blocked with blocking buffer at room temperature for at least 1 h. Next, 100 μl crude serum samples were added to the wells and incubated at room temperature for 2 h. Similarly, 100 μl PBST buffer lacking antibody was used as a negative control, and 100 μl pooled healthy serum was used as a quality control in one well of each plate. Following four washes with PBST, 100 μl biotinylated antigen-affinity purified rabbit anti-hGROβ antibody (diluted to a final concentration of 0.25 μg/ml) was added, and plates were incubated at room temperature for 2 h. Then, 100 μl of avidin-horseradish peroxidase-conjugated secondary antibody (1:2000) was added, and the plates were incubated at room temperature for 30 min. The excess conjugates were removed by washing the plates four times with PBST. The amount of bound conjugate was determined by adding 100 μl ABTS liquid substrate solution to each well, and allowing the plates to incubate at room temperature for color development. The absorbance was measured at 405 nm with wavelength correction set at 650 nm on a Stat Fax 2100 microplate reader (Awareness Technology, Inc., Palm City, FL, USA). Reliable standard curves were obtained by monitoring the plates at 5-min intervals for approximately 15 min. The O.D. readings did not exceed 0.2 units for the zero standard concentration or 1.4 units for the highest standard concentration.
Immunoluminometric assay for CEA and CA19-9
The serum CEA and CA19-9 levels were measured with an immunoluminometric assay on a random-access analyzer (Architect i2000, Abbott Diagnostics Division) [25]. The recommended cut-off limits for CA19-9 and CEA were 37 U/ml and 5 ng/ml, respectively. The within-run (intra-assay) analytical variation was minimized by using the same lot No. of reagent on the same day. Two quality control samples of two different concentrations of the analyses were included in each assay run, and Westgard Multi-rules were used to accept or reject runs [26]. A high CEA level was defined as exceeding 5 ng/ml, and a high CA19-9 level was defined as exceeding 37 U/ml, according to the guidelines defined by the manufacturer of the test kit.
Statistical analysis
Statistical significance was determined with the nonparametric Mann-Whitney U-test (for differences between two groups) and the Kruskal-Wallis nonparametric test (for differences among more than two groups) for the serum GROβ levels and clinicopathological characteristics. Analyses were performed with the SPSS 16.0 software package for Windows (Chicago, IL, USA). We used the χ2 or Fisher’s exact test to analyze the significance of the sensitivity between the preoperative serum GROβ and CEA or CA19-9 levels. Statistical significance was set at P < 0.05. The diagnostic significances of GROβ, CEA, and CA19-9 were analyzed with receiver operating characteristic curve (ROC) analyses.
Results
Serum GROβ levels in patients with colorectal cancer and controls
A total of 336 subjects were enrolled in this study. The serum levels of GROβ were measured with ELISA. Among patients with colorectal cancer, preoperative serum GROβ concentrations (range 25.56 to 721.09 pg/ml; median = 96.15 pg/ml) were significantly lower than the postoperative levels (range 30.35 to 542.44 pg/ml; median = 167 pg/ml; P < 0.01, Figure 1A). However, the preoperative GROβ levels in patients with colorectal cancer were significantly higher than those in healthy controls (range 29.67 to 185.01 pg/ml; median = 43.28 pg/ml; P < 0.001) and those in non-tumor disease controls (range 12.79 to 246.54 pg/ml; median = 57.30 pg/ml; P < 0.01, Figure 1A).
Figure 1.
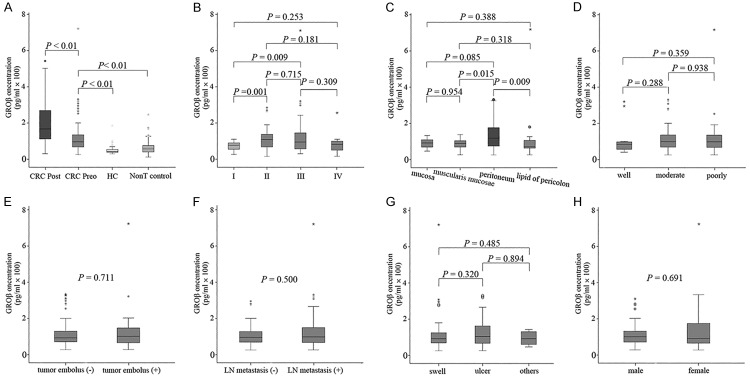
Comparisons between serum growth-related oncogene product β (GROβ) levels and clinicopathological parameters in colorectal cancer (CRC). (A) Enzyme-linked immunosorbent assay results show serum GROβ levels in patients with colorectal cancer (CRC), both preoperatively (CRC preo; n = 123) and postoperatively (CRC post: n = 66 samples), compared to healthy controls (HC: n = 88) and patients with non-tumor diseases (Non-T controls: n = 125). B-H. Serum GROβ levels were compared among samples with different (B) tumor-node-metastasis stages (I-IV), (C) depths of infiltration, (D) histological grades, (E) presence of tumor embolus, (F) presence of lymph node (LN) metastasis, (G) gross pathologic tumor types, and (H) patient gender. Boxes represent the distribution of data between the 25th and 75th percentiles (the internal horizontal line is the median, and the dots indicate outlying points).
Association between serum GROβ concentrations and clinical parameters in patients with colorectal cancer
We assessed potential correlations between serum GROβ levels and clinical parameters, including the TNM stage, lymph node metastasis, tumor embolus, histological grade, depth of infiltration, tumor type and patient gender. The median levels of GROβ in patients with colorectal cancer at different stages were: stage I: 77.45 pg/ml (range 27.18 to 110.71 pg/ml); stage II: 118.44 pg/ml (range 25.56 to 295.4 pg/ml); stage III: 105.37 pg/ml (range 40.86 to 721.09 pg/ml); and stage IV: 92.81 pg/ml (range 27.09 to 266.28 pg/ml). The serum GROβ levels were significantly different (Kruskal-Wallis nonparametric test, P = 0.014) among the four TNM stages. Compared to patients in stage I, serum GROβ levels were significantly higher in patients in stages II (Mann-Whitney U-test, P = 0.001) and III (Mann-Whitney U-test, P = 0.009, Figure 1B). However, there was no significant difference between GROβ levels in stages II and III (Mann-Whitney U-test, P = 0.715). Furthermore, high GROβ levels were significantly related to the depth of tumor infiltration. Patients with peritoneum infiltration had significantly higher serum GROβ levels (median = 119.78 pg/ml; range 25.56 to 330.89 pg/ml) than those with muscular layer infiltration (median = 90.15 pg/ml; range 27.18 to 266.28 pg/ml; Mann-Whitney U-test, P = 0.015) and those with pericolon lipid infiltration (median = 71.66 pg/ml; range 27.09 to 721.09 pg/ml, Figure 1C). However, there was no significant difference in serum GROβ levels among patients with well-differentiated tumors (median = 83.66 pg/ml; range 40.86 to 319.82 pg/ml), moderately-differentiated tumors (median = 99.18 pg/ml; range 25.56 to 330.89 pg/ml), and poorly-differentiated tumors (median = 97.83 pg/ml; range 27.09 to 721.09 pg/ml; Figure 1D). The serum GROβ levels were not related to the presence of tumor embolus (Mann-Whitney U-test, P = 0.711, Figure 1E), lymph node metastasis (Mann-Whitney U-test, P = 0.500, Figure 1F), the gross pathologic tumor type (Mann-Whitney U-test, P > 0.05, Figure 1G), or patient gender (Mann-Whitney U-test, P = 0.691, Figure 1H).
Diagnostic evaluation of GROβ, CEA, and CA19-9 for colorectal cancer
Out of 123 preoperative serum samples from patients with colorectal cancer, 42.3% (52/123) had high CEA levels and 17.1% (21/123) had high CA19-9 levels. Out of 213 control serum samples from 88 healthy individuals and 125 patients with non-tumor diseases, 9.4% (20/213) had high CEA levels and 5.2% (11/213) had high CA19-9 levels. The sensitivities of CEA for identifying stage I and stage II colorectal cancer were 16.7% (3/18) and 46.1% (18/39), respectively. The sensitivities of CA19-9 for identifying stage I and stage II colorectal cancer were 5.6% (1/18) and 12.8% (5/39), respectively. In this study, the GROβ values for the 213 control samples were not normally distributed. Therefore, we defined a cut-off value for GROβ of 105pg/ml, which represented the 95th percentile of 213 controls. This cutoff value was used to differentiate healthy and non-tumor disease control samples from colorectal malignant tumor samples. The sensitivity and specificity of serum GROβ were 56.1% (69/123) and 95.31% (203/213), respectively. The sensitivity of serum GROβ was significantly higher than those of CEA (P = 0.041) and CA199 (P < 0.01). The sensitivities of serum GROβ for identifying stage I and stage II colorectal cancer were 5.6% (1/18) and 41% (16/39), respectively. There was no significant difference between GROβ and CEA in the sensitivity for identifying stage I (P = 0.603) or II (P = 0.820) colorectal cancer. However, the sensitivity of GROβ was significantly higher than that of CA19-9 for identifying stage II colorectal cancer (P = 0.01). When the data for GROβ, CEA, and CA19-9 were combined into a panel and the samples positive for colorectal cancer were pooled, the sensitivity was 22.2% (4/18) for identifying stage I and 66.7% (26/39) for identifying stage II. We evaluated the diagnostic value of GROβ for identifying colorectal cancer in a receiver operating characteristic analysis. We compared the results with those for CEA and CA19-9. The areas under the curve (AUC) were 0.834, 0.739, and 0.676 for GROβ, CEA, and CA19-9, respectively (Figure 2).
Figure 2.
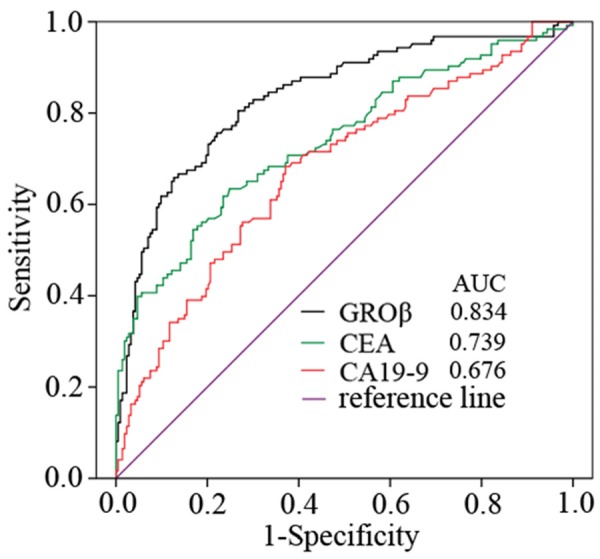
Receiver operator characteristics (ROC) curve analysis shows the performances of GROβ, CEA, and CA19-9. The areas under curve (AUC) for GROβ (black line), CEA (green line), and CA19-9 (red line) were 0.834, 0.739, and 0.676, respectively.
Discussion
The present study was the first to demonstrate that serum GROβ levels were significantly higher in patients with colorectal cancer than in healthy individuals and patients with non-tumor diseases. Furthermore, we found that serum GROβ levels were significantly correlated with two clinical parameters of colorectal cancer, the TNM stage and the depth of infiltration. Thus, serum GROβ may be useful for detecting colorectal cancer progression.
Currently, the TNM staging system is the main tool used by clinicians to estimate tumor burden and to predict prognosis and survival. We found that serum GROβ levels were significantly increased in stage II or III compared with stage I colorectal cancer (P < 0.01). The increase in serum GROβ concentrations observed when patients with colorectal cancer demonstrated peritoneum metastasis after intestinal wall penetration may reflect an enhancement in the potential of tumor cells to proliferate and metastasize. However, the serum GROβ levels in patients with tumors located in the muscularis mucosa were not significantly increased compared to the levels in patients with tumors limited to the mucosa. This finding suggested that the stage change related to tumor infiltration depth may not be related to serum GROβ levels. However, when tumor cells penetrate from the muscularis mucosa to the peritoneum, vascular growth is a very important molecular event. Interestingly, the serum GROβ level was increased significantly in this stage, which suggested that GROβ might play a key role in angiogenesis and vasculogenesis [10].
Patients with distant metastases out of the intestine exhibited significantly decreased GROβ levels compared to patients with metastases to the peritoneum. These results suggested that GROβ may initiate and promote the different stages of colorectal cancer metastasis by stimulating complicated cross-talk between immunocytes and tumor cells. The detailed interactions involved in this process must be further investigated.
The histological grade of tumors is also an important indicator associated with the development and prognosis of cancer [27]. However, the serum GROβ levels were not significantly different in colorectal cancer patients with different histological tumor grades (P > 0.05) in this study.
We found that, after surgery, patients with colorectal cancer displayed significant increases in serum GROβ levels compared to those measured before surgery. This increase suggested that GROβ may be important for tissue regeneration after resection, consistent with results from a previous study [28].
In the present study, we used a control group that comprised patients with non-tumor diseases, including benign neoplasms, ulcers, enteritis, obstructions, digestive system bleeding, and other common systemic diseases. This study design was different from those used in many other biomarker discovery studies. We used this strategy to challenge the reliability of the candidate biomarker in a differential diagnosis.
Several studies have reported correlations between GROβ expression and inflammation and angiogenesis related to tumor growth [7,11,15,29]. However, most of those studies focused on GROβ expression in tumor tissues and cells. No report has studied serum GROβ in patients with cancer, except for one study on esophageal squamous cell carcinoma [23]. Here, we analyzed serum GROβ levels in 123 patients with colorectal cancer and estimated the potential of serum GROβ for diagnosis compared to CA19-9 and CEA, two widely used serum biomarkers for colorectal cancer [30]. A ROC AUC value up to 0.7 is typically statistically significant for a biomarker [31]. The ROC AUC for GROβ was 0.834, larger than that for CEA (0.739), which was considered a statistically significant colorectal cancer predictor; in contrast, the ROC AUC for CA19-9 was 0.676. Although the quantity of samples in our study may have been insufficient for claiming superiority, we found that, under the same conditions, GROβ displayed a higher sensitivity for predicting colorectal cancer (56.1%; 69/123) than CEA (42.3%; 52/123) and CA19-9 (17.1%; 21/123).
The patients with colorectal cancer in stages I and II could undergo complete tumor resection, which provided an improved prognosis. Our data strongly suggested that, by analyzing the levels of GROβ, CEA, and CA19-9, successful diagnosis of early-stage colorectal cancer could be increased from 16.7% (3/18) to 22.2% (4/18) for stage I and from 46.1% (18/39) to 66.7% (26/39) for stage II; however, the sensitivities of GROβ alone in stages I and II were a bit inferior to those of CEA alone. Therefore, our results showed that combining GROβ with CEA and CA19-9 could increase the sensitivity of the diagnosis of colorectal cancer. In addition, our findings suggested that GROβ may be potentially useful for the early detection of colorectal cancer; however, this interpretation was based on only a limited number of patients with early stage colorectal cancer.
In conclusion, we found that serum GROβ levels were increased in patients with colorectal cancer. The GROβ levels in patients with colorectal cancer were positively correlated with TNM stage and depth of infiltration, but not with lymph node metastasis, histological grade, tumor embolus, histological grade, gross pathologic tumor type, or patient gender. Our results suggested that GROβ may function as an oncogene, with roles in tumor formation, progression, and metastasis.
However, the role and significance of GROβ in colorectal cancer remain uncertain, due to insufficient data. More advanced studies are therefore needed to clarify the roles of GROβ in tumorigenesis. Those studies may result in novel diagnostic and therapeutic applications for patients with colorectal cancer. Moreover, because our statistical data were limited, cross validations are needed to evaluate the sensitivity and specificity of GROβ in a large-scale validation study. In conclusion, GROβ may be useful as both a tumor marker and a target for new immunotherapies.
Acknowledgements
This study was supported by grants from the“128 talents project” awarded by the China Meitan General Hospital, PR China. We thank Professors Xiaohang Zhao, Fang Liu, and Lanping Zhou from the State Key Laboratory of The Oncology of Cancer Institute & Hospital, at the Chinese Academy of Medical Sciences, for providing the sample preparations used in this study. We also thank Professor Hui Xu from the China Meitan General Hospital for technical support.
Disclosure of conflict of interest
None.
References
- 1.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 2.Sung JJ, Lau JY, Young GP, Sano Y, Chiu HM, Byeon JS, Yeoh KG, Goh KL, Sollano J, Rerknimitr R, Matsuda T, Wu KC, Ng S, Leung SY, Makharia G, Chong VH, Ho KY, Brooks D, Lieberman DA, Chan FK. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008;57:1166–1176. doi: 10.1136/gut.2007.146316. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Hara M, Sato M, Takahashi H, Takayama S, Takeyama H. Accuracy of monitoring serum carcinoembryonic antigen levels in postoperative stage III colorectal cancer patients is limited to only the first postoperative year. Surg Today. 2011;41:1357–1362. doi: 10.1007/s00595-010-4519-2. [DOI] [PubMed] [Google Scholar]
- 5.Shibutani M, Maeda K, Nagahara H, Ohtani H, Sakurai K, Toyokawa T, Kubo N, Tanaka H, Muguruma K, Ohira M, Hirakawa K. Significance of CEA and CA19-9 combination as a prognostic indicator and for recurrence monitoring in patients with stage II colorectal cancer. Anticancer Res. 2014;34:3753–3758. [PubMed] [Google Scholar]
- 6.Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, Lamerz R, Peltomaki P, Sturgeon C, Topolcan O. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43:1348–1360. doi: 10.1016/j.ejca.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verbeke H, Struyf S, Laureys G, Van Damme J. The expression and role of CXC chemokines in colorectal cancer. Cytokine Growth Factor Rev. 2011;22:345–358. doi: 10.1016/j.cytogfr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Song IC, Yun HJ, Jo DY, Kim S. CXC chemokines and chemokine receptors in gastric cancer: from basic findings towards therapeutic targeting. World J Gastroenterol. 2014;20:1681–1693. doi: 10.3748/wjg.v20.i7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modi WS, Yoshimura T. Isolation of novel GRO genes and a phylogenetic analysis of the CXC chemokine subfamily in mammals. Mol Biol Evol. 1999;16:180–193. doi: 10.1093/oxfordjournals.molbev.a026101. [DOI] [PubMed] [Google Scholar]
- 14.Haskill S, Peace A, Morris J, Sporn SA, Anisowicz A, Lee SW, Smith T, Martin G, Ralph P, Sager R. Identification of three related human GRO genes encoding cytokine functions. Proc Natl Acad Sci U S A. 1990;87:7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richmond A, Thomas HG. Melanoma growth stimulatory activity: Isolation from human melanoma tumors and characterization of tissue distribution. J Cell Biochem. 1988;36:185–198. doi: 10.1002/jcb.240360209. [DOI] [PubMed] [Google Scholar]
- 16.Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins. Int J Cancer. 1997;73:94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Bruyere C, Lonez C, Duray A, Cludts S, Ruysschaert JM, Saussez S, Yeaton P, Kiss R, Mijatovic T. Considering temozolomide as a novel potential treatment for esophageal cancer. Cancer. 2011;117:2004–2016. doi: 10.1002/cncr.25687. [DOI] [PubMed] [Google Scholar]
- 18.Kavandi L, Collier MA, Nguyen H, Syed V. Progesterone and calcitriol attenuate inflammatory cytokines CXCL1 and CXCL2 in ovarian and endometrial cancer cells. J Cell Biochem. 2012;113:3143–3152. doi: 10.1002/jcb.24191. [DOI] [PubMed] [Google Scholar]
- 19.Doll D, Keller L, Maak M, Boulesteix AL, Siewert JR, Holzmann B, Janssen KP. Differential expression of the chemokines GRO-2, GRO-3, and interleukin-8 in colon cancer and their impact on metastatic disease and survival. Int J Colorectal Dis. 2010;25:573–581. doi: 10.1007/s00384-010-0901-1. [DOI] [PubMed] [Google Scholar]
- 20.Oue E, Lee JW, Sakamoto K, Iimura T, Aoki K, Kayamori K, Michi Y, Yamashiro M, Harada K, Amagasa T, Yamaguchi A. CXCL2 synthesized by oral squamous cell carcinoma is involved in cancer-associated bone destruction. Biochem Biophys Res Commun. 2012;424:456–461. doi: 10.1016/j.bbrc.2012.06.132. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y, Chen C, Weatherbee JA, Tsang M, Folkman J. Gro-beta, a -C-X-C- chemokine, is an angiogenesis inhibitor that suppresses the growth of Lewis lung carcinoma in mice. J Exp Med. 1995;182:2069–2077. doi: 10.1084/jem.182.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta V, Yeo G, Kawakubo H, Rangnekar V, Ramaswamy P, Hayashida T, MacLaughlin DT, Donahoe PK, Maheswaran S. Mullerian-inhibiting substance induces Gro-beta expression in breast cancer cells through a nuclear factor-kappaB-dependent and Smad1-dependent mechanism. Cancer Res. 2007;67:2747–2756. doi: 10.1158/0008-5472.CAN-06-2312. [DOI] [PubMed] [Google Scholar]
- 23.Dong QM, Zhang JQ, Li Q, Bracher JC, Hendricks DT, Zhao XH. Clinical significance of serum expression of GRObeta in esophageal squamous cell carcinoma. World J Gastroenterol. 2011;17:2658–2662. doi: 10.3748/wjg.v17.i21.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobin L, Gospodarowicz M, Wittekind C. UICC International Union Against Cancer. 7th edition. New Jersey: Wiley-Blackwell; 2009. TNM Classification of Malignant Tumors. [Google Scholar]
- 25.Erden G, Barazi AO, Tezcan G, Yildirimkaya MM. Biological variation and reference change values of CA 19-9, CEA, AFP in serum of healthy individuals. Scand J Clin Lab Invest. 2008;68:212–218. doi: 10.1080/00365510701601699. [DOI] [PubMed] [Google Scholar]
- 26.Westgard JO, Groth T. Design and evaluation of statistical control procedures: Applications of a computer “quality control simulator” program. Clin Chem. 1981;27:1536–1545. [PubMed] [Google Scholar]
- 27.Derwinger K, Kodeda K, Bexe-Lindskog E, Taflin H. Tumour differentiation grade is associated with TNM staging and the risk of node metastasis in colorectal cancer. Acta Oncol. 2010;49:57–62. doi: 10.3109/02841860903334411. [DOI] [PubMed] [Google Scholar]
- 28.Ren X, Carpenter A, Hogaboam C, Colletti L. Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am J Pathol. 2003;163:563–570. doi: 10.1016/S0002-9440(10)63684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 30.Mitsuyama Y, Shiba H, Haruki K, Fujiwara Y, Furukawa K, Iida T, Hayashi T, Ogawa M, Ishida Y, Misawa T, Kashiwagi H, Yanaga K. Carcinoembryonic antigen and carbohydrate antigen 19-9 are prognostic predictors of colorectal cancer with unresectable liver metastasis. Oncol Lett. 2012;3:767–771. doi: 10.3892/ol.2012.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin JK, Lin CC, Yang SH, Wang HS, Jiang JK, Lan YT, Lin TC, Li AF, Chen WS, Chang SC. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis. 2011;26:1135–1141. doi: 10.1007/s00384-011-1209-5. [DOI] [PubMed] [Google Scholar]


