Abstract
Aim: Coronary artery disease (CAD) and its serious clinical form, ST segment elevated myocardial infarction (STEMI) has been the leader within the death causes around the world and in our country. In STEMI, the main objective is providing the myocardial reperfusion. In our study, it was aimed to investigate the predictive value of tenascin-C level for the degree of myocardial reperfusion in patients with STEMI. Methods: In our study, 58 patients admitted to our hospital with acute anterior STEMI were included. All the patients had underwent primary percutaneous intervention for the single-vessel disease at left anterior descending coronary artery. After admission to coronary care unit tenascin-C levels were measured. Subjects were classified according to their myocardial blush grades (MBG); MBG 0, MBG 1 and MBG 2 were groupped as Group I, MBG 3 was groupped as Group II. The groups were compared according to their tenascin-C levels and other parameters. Results: Between group I (n = 31, mean age 55 ± 12.5) and group II (n = 27, mean ages 49.3 ± 11.1); tenascin-C, troponin I and CK-MB levels were significantly higher in group I compared to the group-II (P < 0.001; P < 0.001 and P < 0.05; respectively). In group I, left ventricular ejection fraction (LVEF) was significantly lower (P < 0.001), left ventricular end-diastolic volume and left ventricular end-systolic volume were significantly higher (P = 0.03) as compared to group II. In group I, ST-segment resolution at ECG was worse (P = 0.003). In correlation analyzes, tenascin-C was significantly positively correlated with troponin-I (r = 0.596; P < 0.001) and CRP (r = 0.615, P < 0.001). Tenascin-C was significantly negatively correlated with MBG, LVEF and ST-segment resolution (r = -0.626, P < 0.001, r = -0.411, P = 0.002 and r = -0.631; P < 0.001, respectively). Conclusion: Based on our study, it can be estimated that in patients with high tenascin-C levels myocardial reperfusion was inadequate, even underwent successfull PCI. In this context, increased tenascin-C may help predict not only left ventricular remodelling and prognosis but also the effectiveness of primary PCI.
Keywords: Myocardial infarction, tenascin-C, myocardial blush grade
Introduction
Acute ST elevated myocardial infarction (STEMI) is one of the main causes of death all over the world. The primary goal in the treatment of STEMI is to open infarct-related artery as soon as possible [1]. The best way to achieve this goal is rapid determination and reperfusion of infarct related artery by means of percutaneous coronary intervention (PCI). In 90% of patients, epicardial blood flow is provided by PCI [2,3]. However, a significant proportion of patients may suffer impaired myocardial reperfusion and an associated poor prognosis despite providing of epicardial coronary blood flow [4,5].
Many clinical and angiographic parameters are used to assess procedural success after primary angioplasty. Among them, the most commonly used one is the myocardial blush grade (MBG). First defined by Van’t Hof et al., MBG is a simple visual angiographic assessment of myocardial perfusion in the region of infarct [6]. MBG also reflects the extent of the damage in the microvascular bed.
Tenascin-C is an oligomeric extracellular matrix glycoprotein with a dynamic release pattern. It is not expressed in healthy adult cardiac tissue except for chorda tendinea of papillary muscles and bases of valve leaflets [7-9]. On the other hand, it starts to be expressed in pathological conditions such as acute coronary syndrome, myocarditis, abdominal aneurysm, inflammation and malignant tumors [10-16]. Tenascin-C is also detected in the marginal zone between the infarct area and the intact area after myocardial infarct [17]. In the light of the recent researches, tenascin-C has been accepted as a predictor of left ventricular remodeling and prognosis after acute myocardial infarction [11,17,18]. Tenascin-C level has also been suggested to be related with the coronary lesion complexity after myocardial infarction [19]. The relationship between tenascin-C and myocardial reperfusion in patients undergoing coronary intervention after acute myocardial infarction has not been evaluated yet. Therefore, the aim of the study was to investigate the role of tenascin-C level in predicting the grade of myocardial reperfusion following the procedure in patients undergoing primary coronary revascularization after an acute myocardial infarction.
Materials and method
Fifty-eight patients with acute anterior myocardial infarction who underwent primary PCI to left anterior descending artery (LAD) were enrolled the study. The exclusion criteria included the following; patients who had received fibrinolytic therapy, angiographically any significant stenosis in the right or circumflex coronary artery together with an LAD artery lesion, history of coronary artery disease, cancer, chronic inflammatory disorders (rheumatoid arthritis, osteoarthritis, etc.), serum creatinine concentration higher than 2, 5 mg per deciliter.
Acute anterior myocardial infarction was diagnosed on the basis of clinical symptoms and the presence of ST elevation consistent with myocardial infarction in electrocardiograms (ECG). Patient history, physical examination, 12-lead ECGs, echocardiography, coronary angiography findings, accompanying systemic diseases and medications were recorded. The patients were assessed with respect to cardiovascular risk factors. After the coronary care unit (CCU) admission, the blood samples were drawn to measure complete blood count and basic biochemical parameters.
Hyperlipidemia was diagnosed as low-density cholesterol (LDL) was greater than 100 mg/dl or lipid-lowering medication was used. Blood pressure of all patients was measured. Systolic blood pressure (SBP) equal or greater than 140 mmHg and/or diastolic blood pressure equal or greater than 90 mmHg or under control by using blood pressure-lowering medications were defined as hypertension. Patients with a fasting glucose level equal or greater than 126 mg/dl on two separate occasions or using oral antidiabetic drugs or insulin were defined as diabetes mellitus.
Acute anterior MI was identified by having ST elevation ≥ 1 mm in V1-6 and reciprocal changes on the surface ECG and chest pain longer than 30 minutes. In all patients, the culprit lesion was shown by coronary angiography for confirming the diagnosis of acute anterior myocardial infarction.
Tenascin-C measurement
Peripheral blood samples for tenascin-C were obtained at the admission of coronary care unit. This samples were centrifuged and then stored at -70°C. Tenascin-C levels were analyzed after the blood samples of all study patients were obtained
Echocardiography
All patients underwent a complete transthoracic echocardiography (TTE) and Doppler study using multiple views in left lateral decubitus position. Echocardiographic examination was performed within 24 hours of admission. TTE study was performed using a 3, 5 MHz transducer on a GE Vivid 7 Pro device. Echocardiographic measurements were made according to the criteria recommended by American Society of Echocardiography [20]. Left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), and ejection fraction (LVEF) were measured from the apical 4-chamber view using the modified Simpson method.
Angiography
All patients underwent coronary angiography to determine the infarct-related artery (IRA) and revascularization. Before catheterization, all patients were administered aspirin (300 mg, sublingually) and clopidogrel (600 mg, PO). Following insertion of the femoral sheath, a heparin bolus of 100 IU/kg was given. Primary angioplasty and stenting procedures were completed according to standard techniques. Determination of infarct-related artery was accomplished by observing a thrombosed critical stenosis or complete obstruction of LAD in certain locations. A narrowing of ≥ 70% in other vessels were defined as additional vessel disease.
Recanalization of the infarct-related artery was evaluated by angiography after primary percutaneous coronary intervention. Quantitative coronary angiographic measurements were performed using ACOM PC Lite version 2.0 (Siemens, Munih, Germany) software package. Myocardial perfusion of the patients assessed using the MBG method described by Arnoud van’t Hof et al. in 1998 [6]. Glycoprotein IIb/IIIa inhibitor use was left to operator’s discretion. All patients were followed in coronary intensive care unit until stabilization was achieved. Surface ECGs were taken and ST segment resolution was assessed (resolution > 50% was considered significant) 90 minutes and 24 hours after the procedure.
Statistical analysis
The study data analyzed using the SPSS version 13.0 software package (SPSS Inc, Chicago, Illinois, USA). The continuous variables were expressed as mean ± standard deviation while the categorical variables were expressed as number and percentage (%). Normally distributed variables were compared across groups by means of student t-test. The non-parametric variables compared using the Mann-Whitney U test. A multivariable linear regression analysis was used to determine which parameters play an important role in the prediction of high tenascin-C levels. Categorical variables were compared via Chi-square test. A p value less than 0.05 was considered statistically significant for all the statistical assessments.
Results
The study population was classified according to their myocardial blush grades. Thirty-one patients with MBG 0, MBG 1 and MBG 2 were defined as Group I, twenty-seven patients with MBG 3 were defined as Group II. The demographic, clinical, echocardiographic, biochemical, electrocardiographic and angiographic data of the two groups were compared (Tables 1, 2).
Table 1.
Demographic and biochemical characteristics of study groups
| Parameters | Group I (n:31) | Group II (n:27) | p value |
|---|---|---|---|
| Age (years) | 55 ± 12.5 | 49.3 ± 11.1 | 0.07 |
| Men/women | 26/5 | 24/3 | 0.43 |
| Body Mass Index (kg/m2) | 27.3 ± 3.8 | 26.9 ± 3.2 | 0.69 |
| Smoking (n, %) | 15 (48%) | 19 (70%) | 0.07 |
| Hypertension (n, %) | 5 (16%) | 5 (18%) | 0.54 |
| Diabetes mellitus (n, %) | 3 (9%) | 1 (3%) | 0.36 |
| Heredity (n, %) | 2 (6%) | 2 (7%) | 0.64 |
| Heart rate (beats/min) | 70.7 ± 5.5 | 68.2 ± 8.8 | 0.22 |
| Tenascin-C (ng/ml) | 31.4 ± 17.6 | 15.9 ± 3.5 | < 0.001 |
| Glukose (mg/dl) | 127.8 ± 38.1 | 126.4 ± 32.8 | 0.88 |
| Urea (mg/dl) | 14.7 ± 3 | 15 ± 4.7 | 0.77 |
| Creatinin (mg/dl) | 0.81 ± 0.2 | 0.81 ± 0.3 | 0.97 |
| HDL-cholesterol (mg/dl) | 40.4 ± 11.3 | 36.6 ± 10.4 | 0.19 |
| LDL-cholesterol (mg/d) | 109 ± 29.9 | 122.4 ± 37.1 | 0.13 |
| Triglyserid (mg/dL) | 119.9 ± 85.7 | 128.2 ± 92.2 | 0.72 |
| WBC (× 103/mm3) | 12.36 ± 3.35 | 12.54 ± 3.06 | 0.83 |
| Platelet count (× 109/L) | 231.22 ± 50.94 | 238.88 ± 58.60 | 0.59 |
| CK-MB (U/L) | 267.6 ± 206.1 | 165.7 ± 157.6 | 0.04 |
| Troponin I (ng/ml) | 209.9 ± 126 | 80.1 ± 92.8 | < 0.001 |
LDL: Low density lipoprotein, HDL: High density lipoprotein, WBC: White Blood Cell, CK-MB: Creatine kinase myocardial band, Data are presented as means ± SD.
Table 2.
Angiographically, echocardiographic and electrocardiographic parameters in study groups
| Parameters | Group I (n:31) | Group II (n:27) | p value |
|---|---|---|---|
| LAD Lesion (Proximal/Middle-Distal (n, %) | 22/9 | 19/8 | 0.59 |
| LVEDV (ml) | 98.7 ± 24.9 | 86.3 ± 12.7 | 0.03 |
| LVESV (ml) | 53.5 ± 19.1 | 43.8 ± 10.7 | 0.03 |
| LVEF (%) | 43.4 ± 8.6 | 52.7 ± 7.1 | < 0.001 |
| ST segment resolution on ECG (< 50%/> 50%) | 14/17 | 3/24 | 0.003 |
LAD: Left anterior desending artery, LVEDV: Left ventricular end-diastolic volume, LVESV: Left ventricular end-systolic volume, LVEF: Left ventricular ejection fraction.
The two groups did not significantly differ with respect to the major cardiovascular risk factors such as hypertension, diabetes mellitus, smoking, and heredity. Both groups were similar with regard to mean age and gender distribution.
Comparison of the groups with respect to the laboratory data revealed that Group 1 had significantly higher cardiac injury markers; CK-MB and Troponin I (P < 0.05 and P < 0.001, respectively). Tenascin-C level was significantly higher in the Group I compared to Group II (P < 0.001). No significant differences were noted between the routine biochemical data of both groups.
The two groups were not significantly different with respect to lesion localization in the coronary artery. In group I, left ventricular ejection fraction was significantly lower (P < 0.001), left ventricular end-diastolic volume and left ventricular end-systolic volume were significantly higher (P = 0.03) as compared to group II. Group I had a worse ST segment resolution on surface ECG compared to Group II (P = 0.003).
In correlation analyzes, tenascin-C was not significantly correlated to age, LDL, CK-MB, and WBC. On the other hand, tenascin-C was a significantly positively correlated with troponin-I (r = 0,596, P < 0,001) and CRP (r = 0,615, P < 0,001). Also There was a significant negative correlation between tenascin-C and MBG, LVEF, and ST-segment resolution on ECG (r = -0,626, P < 0,001, r = -0,411, P = 0,002 and r = -0,631; P < 0,001, respectively) (Table 3; Figure 1).
Table 3.
Correlations between the tenascin-C and the patient variables
| Parameters | r value | p value |
|---|---|---|
| Age | 0.075 | 0.57 |
| LDL | -0.183 | 0.17 |
| CK-MB | 0.100 | 0.45 |
| Troponin I | 0.596 | < 0.001 |
| WBC | -0.112 | 0.40 |
| CRP | 0.615 | < 0.001 |
| ST segment resolution | -0.631 | < 0.001 |
| LVEF | -0.411 | 0.002 |
| MBG | -0.626 | < 0.001 |
LDL: Low density lipoprotein, CK-MB: Creatine kinase myocardial band, WBC: White blood Cell, CRP: C reactive protein, LVEF: Left ventricular ejection fraction, MBG: Myocardial blush grade.
Figure 1.
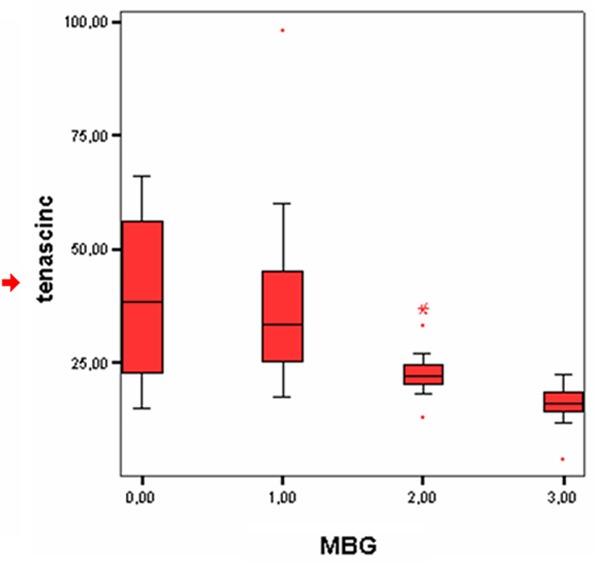
Distribution of tenascin-C levels according to myocardial blush grade.
MBG had no significant correlation with age, LDL, and WBC. On the other hand, there was a significant correlation with ST segment resolution and a significant positive correlation with LVEF (P < 0.001). MBG was a significantly negative correlation with CK-MB, CRP, and Troponin I (P = 0.03, P = 0.009, and P < 0.001, respectively) (Table 4).
Table 4.
Correlations between the myocardial blush grade and the patient variables
| Parameters | r value | p value |
|---|---|---|
| Age | -0.242 | 0.60 |
| LDL | 0.225 | 0.09 |
| CK-MB | -0.280 | 0.03 |
| Troponin I | -0.625 | < 0.001 |
| WBC | 0.141 | 0.29 |
| CRP | -0.347 | 0.009 |
| ST segment resolution | 0.472 | < 0.001 |
| LVEF | 0.532 | < 0.001 |
LDL: Low density lipoprotein, CK-MB: Creatine kinase myocardial band, WBC: White blood Cell, CRP: C reactive protein, LVEF: Left ventricular ejection fraction.
The multivariable linear regression analysis determined that MBG, ST segment resolution, CK-MB, and CRP levels were significant predictors of Tenascin-C level (Table 5).
Table 5.
Predictors of tenascin-C level in in multivariate linear regression analysis
| OR (CI 95%) | p value | |
|---|---|---|
| MBG | 2.04 (19.8-59.38) | < 0.001 |
| LVEF | 0.43 (0.42-0.27) | 0.66 |
| ST segment resolution | 3.49 (0.42-0.27) | 0.001 |
| Troponin I | 1.16 (0.01-0.05) | 0.25 |
| CK-MB | 2.75 (7.02-0.04) | 0.008 |
| CRP | 4.1 (0.1-0.28) | < 0.001 |
MBG: Myocardial blush grade, LVEF: Left ventricular ejection fraction, CK-MB: Creatine kinase myocardial band, WBC: White blood cell, CRP: C reactive protein.
Our study showed that, when a cut-off value of 19.95 ng/dl is selected for tenascin-C, MBG 3 was predicted with a sensitivity of 92.3%, and a specifity of 83.3%. The area under curve was calculated 0.911 (Figure 2).
Figure 2.
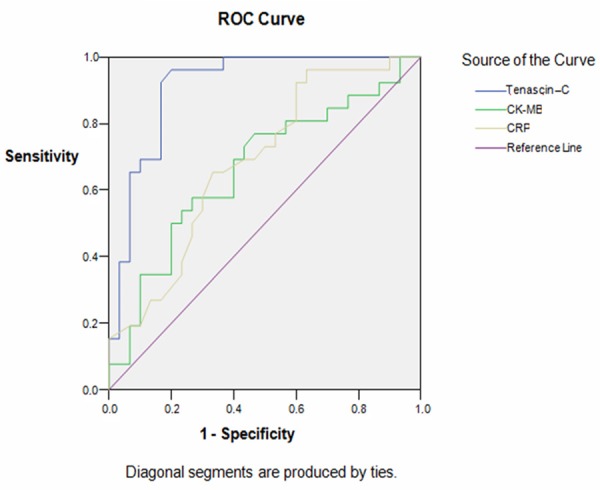
Receiver-operating characteristic (ROC) curve analysis for tenascin-C level in predicting myocardial blush grade (sen: 92.3%; spe: 83.3%; AUC: 0.911).
Discussion
This study showed that the tenascin-C levels were significantly higher in Group I compared to Group II in acute myocardial infarction patients undergoing primary PCI. This study also demonstrated that LVEF levels were significantly lower in patients with a higher tenascin-C level.
ST elevation myocardial infarction is characterized by an acute occlusion of blood flow to the myocardial territory supplied by the infarct-related artery (IRA). Myocardial ischemia results in life-threatening complications including electrical instability, heart failure, left ventricular remodeling, and valvular dysfunction. The primary goal in the treatment of STEMI is to open infarct-related artery as soon as possible. This protects patients against major adverse cardiac events [21]. Epicardial blood flow is provided by primary PCI in 90% of patients [2,3]. However, a significant proportion of patients may suffer impaired myocardial reperfusion and an associated poor prognosis despite providing of epicardial coronary blood flow [4,5].
Myocardial perfusion is affected by a multitude of parameters including blood pressure, inflammatory mediators, microvascular bed, and left ventricular diastolic pressure. Many invasive techniques and non-invasive tests have been used for assessment of myocardial perfusion. Among them, myocardial blush grade (MBG) is one of the most commonly employed angiographic parameters.
Henrique et al. examined 924 patients with TIMI 3 flow after primary angioplasty and found that the low MBG groups had a larger enzymatic infarct size and significantly lower LVEF. The authors demonstrated a significantly increased mortality and adverse cardiac events in the low MBG group over a period of 5 to 27 months. The same study also found a significantly higher prevalence of LAD as the IRA in the low MBG group [22]. Çelik et al. reported that tenascin-C level was related with the coronary lesion complexity after myocardial infarction, and tenascin-C level was higher in patients with total IRA occlusion [19]. In our study, the IRA was LAD in all subjects. In accordance with the literature, we found that the low MBG group had significantly higher levels of cardiac injury markers and a significantly lower LVEF, the latter being in relation with increased LVEDV and LVESV.
An analysis of the Horizons AMI study investigated the prognostic significance of MBG and ST segment resolution (STR) after primary angioplasty [23]. MBG and STR were evaluated in a total of 2367 patients. Overall, 77.7% of the patients had a MBG of 2 or 3 and 75.1% had a STR of > 50%. A discordance between MBG and STR was noted in 31% of the patients. At the end of the study, the group with a MBG of 2 or 3 had significantly lower 3-year mortality. In contrast, STR was not correlated to mortality. As a result of this study, there was a 70% concordance between MBG and STR, it was observed that MBG is an important parameter in predicting the survival. In agreement with the results of the Horizons AMI study, our results showed that the patient group with a low MBG also had a significantly worse STR.
In the previous studies, it has been reported that a negative correlation between MBG and LVEF. These results suggest that MBG is a significant independent predictive marker of mortality, microvascular circulation, and myocardial perfusion in patients undergoing primary PCI after AMI [24]. In accordance with the literature, in the present study a negative correlation was shown between MBG and LVEF.
Tenascin-C is an extracellular matrix glycoprotein considered to be an important marker for left ventricular remodeling and prognosis after acute myocardial infarction [11,18,25]. Sato et al. reported that Serum tenascin-C level on day 5 after admission is potentially useful for early risk stratification after AMI beyond established prognostic markers [11].
On the other hand, there is no study in the literature investigating the relationship between tenascin-C and myocardial reperfusion after primary PCI in AMI. We found higher tenascin-C levels in AMI patients with poor myocardial reperfusion after primary PCI to IRA compared to those who achieved normal reperfusion after that intervention. The present study gains importance as it is the first study in this field.
Wallner et al. showed all atherosclerotic plaques with an organized lipid core or ruptured intimal surface strongly expressed tenascin-C. Also tenascin-C expression correlated with the infiltration of macrophages [28]. In 2014 Sakamoto et al. reported that tenascin-C expression and accumulation in arterial mural injury contributed to both plaque inflammation and rupture [10]. Furthermore, Schaff et al. discovered that thrombocytes interact with tenascin-C through von Willebrand factor and the adhesion that occurs via that interaction subsequently triggers thrombocyte activation [27]. These data clearly reveal that tenascin-C is not only related to plaque instability but also to thrombogenicity after plaque rupture in acute coronary syndrome. This explains why patients with high level of tenascin-C level had a low MBG and low LVEF in our study and other studies. The local inflammatory response in coronary artery after an acute coronary event increased tenascin-C level in unstable plaque, systemic increase in tenascin-C level and inflammatory enzymes along with and tenascin-C mediated triggered thrombocyte activation and increased thrombogenicity all adversely affect coronary microcirculation. Supporting our hypothesis, the lowest tenascin-C levels were detected in MBG 3 patients and, in addition, CRP level was also lower in that patient population compared to the other group. On the other hand, higher tenascin-C and CRP levels and lower LVEF levels were found in the patient group with a lower MBG grade.
Patients with poor coronary reperfusion despite recanalization of IRA with mechanical revascularization eventually suffer myocardial stunning, leading to a lack of expected recovery in left ventricular pump function. We believe that an increased rate of poor reperfusion after PCI in patients with higher tenascin-C levels may be one of the reasons of poor prognosis in them. Based on the results of our study, we may suggest that, in patients with high tenascin-C levels, myocardial reperfusion may remain far from ideal even after successful PCI. In this context, high tenascin-C not only predicts long-term outcomes like left ventricular remodeling and prognosis, but it may also aid in the predicton of the effectiveness of primary PCI.
There are some limitations of our study. The basic limitations of our study are the small sample size and lack of long-term follow-up data. Other limitations may include the enrollment of acute coronary syndrome patients only with single-vessel disease and utilization of only one technique to assess myocardial reperfusion. Therefore, there is a need for future studies with larger sample size underwent primary PCI that will explore the relationship between tenascin-C levels and myocardial reperfusion assessed by multiple different methods and also evaluate the long-term outcomes of that relationship.
As a conclusion, our study demonstrated that, it can be estimated that in patients with high tenascin-C levels myocardial reperfusion was inadequate, even underwent succesfull PCI. In this context, increased tenascin-C may help predict not only left ventricular remodelling and prognosis but also the effectiveness of primary PCI.
Disclosure of conflict of interest
None.
References
- 1.Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van’t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 2.Haager PK, Christott P, Heussen N, Lepper W, Hanrath P, Hoffmann R. Prediction of clinical outcome after mechanical revascularization in acute myocardial infarction by markers of myocardial reperfusion. J Am Coll Cardiol. 2003;41:532–8. doi: 10.1016/s0735-1097(02)02870-x. [DOI] [PubMed] [Google Scholar]
- 3.Grines CL, Cox DA, Stone GW, Garcia E, Mattos LA, Giambartolomei A, Brodie BR, Madonna O, Eijgelshoven M, Lansky AJ, O’Neill WW, Morice MC. Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1999;341:1949–56. doi: 10.1056/NEJM199912233412601. [DOI] [PubMed] [Google Scholar]
- 4.Roe MT, Ohman EM, Maas AC, Christenson RH, Mahaffey KW, Granger CB, Harrington RA, Califf RM, Krucoff MW. Shifting the open-artery hypothesis downstream: the quest for optimal reperfusion. J Am Coll Cardiol. 2001;37:9–18. doi: 10.1016/s0735-1097(00)01101-3. [DOI] [PubMed] [Google Scholar]
- 5.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am CollCardiol. 2009;54:281–92. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Van’t Hof AW, Liem A, Suryapranata H, Hoorntje JC, Jan de Boer M, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction myocardial blush grade. Circulation. 1998;97:2302–6. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 7.Sato I, Shimada K. Quantitative analysis of tenascin in chordae tendineae of human left ventricular papillary muscle with aging. Ann Anat. 2001;183:443–8. doi: 10.1016/S0940-9602(01)80202-8. [DOI] [PubMed] [Google Scholar]
- 8.Imanaka-Yoshida K, Hiroe M, Yoshida T. Interaction between cell and extracellular matrix in heart disease: multiple roles of tenascin-C in tissue remodeling. Histol Histopathol. 2004;19:517–25. doi: 10.14670/HH-19.517. [DOI] [PubMed] [Google Scholar]
- 9.Willems IE, Arends JW, Daemen MJ. Tenascin and fibronectin expression in healing human myocardial scars. J Pathol. 1996;179:321–5. doi: 10.1002/(SICI)1096-9896(199607)179:3<321::AID-PATH555>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto N, Hoshino Y, Misaka T, Mizukami H, Suzuki S, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Serum tenascin-C level is associated with coronary plaque rupture in patients with acute coronary syndrome. Heart Vessels. 2014;29:165–70. doi: 10.1007/s00380-013-0341-2. [DOI] [PubMed] [Google Scholar]
- 11.Sato A, Hiroe M, Akiyama D, Hikita H, Nozato T, Hoshi T, Kimura T, Wang Z, Sakai S, Imanaka-Yoshida K, Yoshida T, Aonuma K. Prognostic value of serum tenascin-C levels on long-term outcome after acute myocardial ınfarction. J Cardiac Fail. 2012;18:480–6. doi: 10.1016/j.cardfail.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Imanaka-Yoshida K, Hiroe M, Yasutomi Y, Toyozaki T, Tsuchiya T, Noda N, Maki T, Nishikawa T, Sakakura T, Yoshida T. Tenascin-C is a useful marker for disease activity in myocarditis. J Pathol. 2002;197:388–94. doi: 10.1002/path.1131. [DOI] [PubMed] [Google Scholar]
- 13.Satta J, Soini Y, Pöllänen R, Pääkkö P, Juvonen T. Tenascin expression is associated with a chronic inflammatory process in abdominal aortic aneurysms. J Vasc Surg. 1997;26:670–5. doi: 10.1016/s0741-5214(97)70068-5. [DOI] [PubMed] [Google Scholar]
- 14.Goh FG, Piccinini AM, Krausgruber T, Udalova IA, Midwood KS. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J Immunol. 2010;184:2655–62. doi: 10.4049/jimmunol.0903359. [DOI] [PubMed] [Google Scholar]
- 15.Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett. 2006;244:143–63. doi: 10.1016/j.canlet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Ohno Y, Izumi M, Yoshioka K, Ohori M, Yonou H, Tachibana M. Prognostic significance of tenascin-C expression in clear cell renal cell carcinoma. Oncol Rep. 2008;20:511–16. [PubMed] [Google Scholar]
- 17.Imanaka-Yoshida K, Hiroe M, Nishikawa T, Ishiyama S, Shimojo T, Ohta Y, Sakakura T, Yoshida T. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab Invest. 2001;81:1015–24. doi: 10.1038/labinvest.3780313. [DOI] [PubMed] [Google Scholar]
- 18.Sato S, Aonuma K, Imanaka-Yoshida K, Yoshida T, Isobe M, Kawase D, Kinoshita N, Yazaki Y, Hiroe M. Serum tenascin-C might be a novel predictor of left ventricular remodeling and prognosis after acute myocardial infarction. J Am Coll Cardiol. 2006;47:2319–25. doi: 10.1016/j.jacc.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Celik A. The relationship between tenascin-C levels and the complexity of coronary lesion after myocardial infarction. J Atheroscler Thromb. 2011;18:693–7. doi: 10.5551/jat.6577. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiography. 2005;12:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Keeley EC, Boura JA, Grines CL. Primary angioplasty vs. intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 22.Henriques JP, Zijlstra F, van’t Hof AW, de Boer MJ, Dambrink JH, Gosselink M, Hoorntje JC, Suryapranata H. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation. 2003;107:2115–9. doi: 10.1161/01.CIR.0000065221.06430.ED. [DOI] [PubMed] [Google Scholar]
- 23.Brener SJ, Dizon JM, Mehran R, Guerchicoff A, Lansky AJ, Farkouh M, Brodie B, Guagliumi G, Witzenbichler B, Fahy M, Parise H, Stone GW. Complementary prognostic utility of myocardial blush grade and ST-segment resolution after primary percutaneous coronary intervention: Analysis from the HORIZONS-AMI trial. Am Heart J. 2013;166:676–83. doi: 10.1016/j.ahj.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Tsvetkov H, Mosseri M. Myocardial blush grade: an ınterventional method for assessing myocardial perfusion. Isr Med Assoc J. 2008;10:465–7. [PubMed] [Google Scholar]
- 25.Celik A, Kalay N, Sahin O, Duran M, Korkmaz H, Kobat MA, Kurtoglu E, Dogan A, Muhtaroglu S, Baran O, Inanc MT, Ozdogru I, Oguzhan A, Topsakal R. The importance of cardiac biomarkers on remodelling after myocardial infarction. J Clin Med Res. 2012;4:20–5. doi: 10.4021/jocmr759w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallner K, Li C, Shah PK, Fishbein MC, Forrester JS, Kaul S, Sharifi BG. Tenascin-C is expressed in macrophage-rich human coronary atherosclerotic plaque. Circulation. 1999;99:1284–9. doi: 10.1161/01.cir.99.10.1284. [DOI] [PubMed] [Google Scholar]
- 27.Schaff M, Receveur N, Bourdon C, Wurtz V, Denis CV, Orend G, Gachet C, Lanza F, Mangin PH. Novel function of tenascin-C, a matrix protein relevant to atherosclerosis, in platelet recruitment and activation under flow. Arterioscler Thromb Vasc Biol. 2011;31:117–24. doi: 10.1161/ATVBAHA.110.206375. [DOI] [PubMed] [Google Scholar]
- 28.Wallner K, Li C, Shah PK, Fishbein MC, Forrester JS, Kaul S, Sharifi BG. Tenascin-C is expressed in macrophage-rich human coronary atherosclerotic plaque. Circulation. 1999;99:1284–9. doi: 10.1161/01.cir.99.10.1284. [DOI] [PubMed] [Google Scholar]


