Abstract
Objective: to investigate the correlation between the parameters of prostate cancer (PCa) at contrast-enhanced ultrasound (CEUS) with PCa risk. Methods: 84 patients (68 ± 8 years; range, 33-79 years) who had undergone CEUS were included. All the images were offline analyzed. Parameters (maximum intensity (IMAX), rise time (RT), time to peak (TTP) and mean transit time (mTT)) were recorded and compared with PSA level, Gleason score, clinical stages and PCa risk. Results: Age was correlated significantly with PCa risk. RT and mTT of outer gland were associated with PCa risk. No significant correlation was found between PSA and CEUS enhancement parameters. Furthermore, with the exception of IMAX of inner gland and IMAX of outer gland, there were no significant differences of enhancement parameters in Gleason score groups and clinical stages groups. Conclusion: The enhancement parameters of PCa at CEUS may be used to predict PCa risk. And it is helpful for the choice of therapeutic options.
Keywords: Contrast-enhanced ultrasound, prostate cancer, prostate cancer risk
Introduction
A growing number of early-stage PCa are detected by widespread serum prostate-specific antigen levels (PSA) screening [1,2]. The majority of patients with PCa always undergo radical prostatectomy in clinical current trends [3,4]. However, besides of radical prostatectomy, the therapeutic options of PCa include active surveillance, low-dose-rate brachytherapy adjuvant and external-beam radiation therapy [3-6]. The choice of therapeutic options is advisable to make according to stage-specific of PCa [7].
As we know, the PCa is divided into three categories which fit the following standard definitions: low-risk PCa (clinical stages of T1c-T2a, biopsy Gleason grade < 7 or PSA < 10 ng/mL), intermediate-risk PCa (clinical stages of T2b-2c, biopsy Gleason score 7 or PSA 10.1-20 ng/ml) and high-risk PCa (clinical stages of ≥ T3a, biopsy Gleason score > 7 or PSA > 20 ng/ml) [7-9]. A detailed and accurate assessment of the PCa risk offers a decision basis for choosing an optimum therapeutic plan.
Folkman [10] first came up with the theory that tumor associated angiogenesis plays a pivotal role in the growth and metastasis of tumors. CEUS as a new imaging technique can reflect the tissue microvascular perfusion dynamically. To our knowledge, the correlation of parameters of PCa at CEUS with PCa risk has not been reported. Whether CEUS can noninvasively identify PCa risk remains unknown. The purpose of our study was to investigate the correlation between the parameters of CEUS with PCa risk.
Patients and methods
Patients
This retrospective study was approved by the Ethical Committee of our institution, and written informed consent was obtained from each patient.
The inclusion criterion were as follows: (1) From August 2012 to April 2014, The assessments of enhancement in the maximum transverse section of prostate tissue were obtained by CEUS in our institution ; (2) MRI was performed before prostate biopsy, and clinical stages of prostate cancer was classified; (3) Biopsy samples underwent histopathological examination with definitive diagnosis. According to the inclusion criterion, 88 patients were selected. Four patients were excluded because the section was changed during the CEUS examination. Therefore, 84 patients (68 ± 8 years; range, 33-79 years) were included in our study. The median PSA was 10.6 ng/ml (range, 4-100 ng/ml).
US examination
All patients keep left-lateral lie with bend knees. Conventional ultrasound imaging was performed with LOGIQ E9 machine (GE Healthcare, Milwaukee, Wl, USA) with a 5-9MHz transrectal endocavity probe (IC5-9-D). Gray-scale, color and power Doppler ultrasound were performed to measure the size. The maximum transverse section of prostate tissue was selected for CEUS examination. CEUS was performed with the same machine. The parameters were adjusted so that mechanical index was 0.08-0.1. Keep probe still and maintain parameter unchanged during the examination.
The contrast agent used in the study was SonoVue (Bracco, Milan, Italy). Being dissolved with 5-mL injection of 0.9% sodium chloride and 2.4 mL of the contrast agent was administrated via elbow vein in a bolus fashion. Continuous imaging was performed immediately after injection of the contrast agent and lasted for 6 minutes. CEUS images and video clips were stored on the hard disk as digital imaging and communications in medicine (DICOM) format for further offline analysis.
Offline analysis
The CEUS dynamic DICOM data were imported into SonoLiver software (TomTec Company, Germany). All the images were analyzed by an experienced operator who had the experiences in offline analysis with SonoLiver software at least 1 month. The CEUS video clips were dynamically observed. Firstly, the delimitation region of the interest (ROI) that including the entire section was depicted, and next a ROI with 9 mm2 in area was placed in the area of the most rapid and strongest enhancement with the Quality of Fit (QOF) > 75%. Three ROIs were placed selectively for each inner gland and the same for outer gland. Quantitative parameters as follows: maximum intensity (IMAX), rise time (RT), time to peak (TTP) and mean transit time (mTT) (Figure 1). The mean value of each parameter was recorded as the final value.
Figure 1.
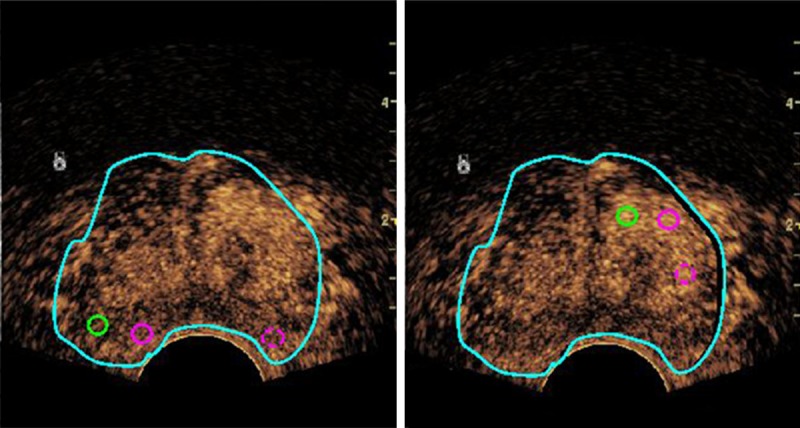
The offline analysis of CEUS video clips. The light blue circle represents the delimitation region of the interest (ROI) that including the entire section; Three analysis ROIs (one green and two circles) which with 9 mm2 in area were placed in the area of the most rapid and strongest enhancement selectively for each inner gland (right) and the same for outer gland (left).
Statistical analysis
Descriptive statistics were used in patient characteristics, including age, PSA, and Prostate volume. Levene analysis was performed to assess variance homogeneity and normal distribution was assessed by Kolmogorov-Smirnov analysis. The measurement data were expressed as mean ± SD. The differences in quantitative parameters in different groups (three categories of PCa risk) were evaluated by a one-way analysis of variance (ANOVA) test and least significant difference (LSD) test. All statistical analyses were performed using SPSS17.0 software (SPSS, Chicago, IL, USA). A P value < 0.05 was considered statistically significant.
Results
Comparison of age and prostate volume in different PCa risk groups
Age was correlated significantly with PCa risk (P = 0.008) (Figure 2). Age of low-risk PCa differed significantly with intermediate-risk and high-risk PCa (67 ± 7 years vs. 71 ± 8 years, P = 0.009 and 67 ± 7 years vs. 68 ± 10 years, P = 0.003). However, there were no significant differences between intermediate-risk and high-risk PCa group (P = 0.988). Furthermore, prostate volume did not correlate significantly with PCa risk (all P > 0.05) (Figure 2).
Figure 2.
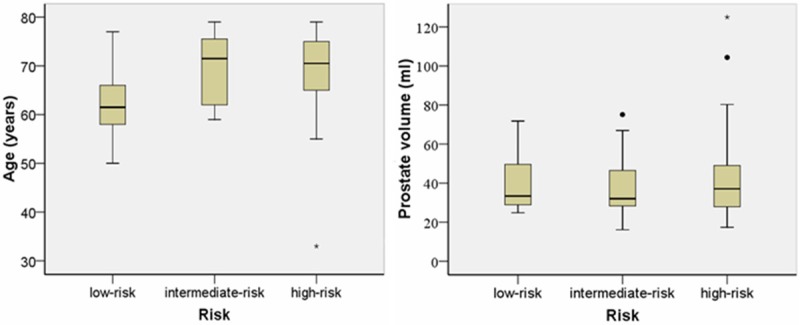
Box and whisker plots of age and prostate volume in different PCa risk group. Centre line = median, top of box = the first quartile, bottom of box = the third quartile, whiskers = data within 1.5 interquartile ranges, • and ★ = outliers.
Comparison of quantitative parameters in different PCa risk groups
There was significant differences in RT and mTT of outer gland in different PCa risk groups (P = 0.020 and P = 0.030) (Table 1). Pairwise comparison analyzed with the LSD showed that the RT was significantly longer to low-risk PCa compared with intermediate-risk PCa group and high-risk PCa group. (P = 0.007 and P = 0.019, respectively). But PCa risk did not correlate significantly with IMAX, TTP of outer gland and the quantitative parameters of inner gland (all P > 0.05).
Table 1.
Comparison of quantitative parameters in different PCa risk groups
| Low-risk | Intermediate-risk | High-risk | P value | ||
|---|---|---|---|---|---|
|
|
|
|
|||
| No. of patients | 14 | 20 | 50 | ||
| Inner gland | IMAX (%) | 234.1 ± 45.9 | 241.7 ± 41.8 | 244.3 ± 83.3 | 0.893 |
| RT (s) | 12.5 ± 4.7 | 12.5 ± 4.8 | 11.9 ± 4.4 | 0.897 | |
| TTP (s) | 18.9 ± 9.4 | 17.0 ± 9.7 | 16.3 ± 8.6 | 0.637 | |
| mTT (s) | 37.2 ± 12.7 | 36.2 ± 14.6 | 35.5 ± 18.7 | 0.944 | |
| Outer gland | IMAX (%) | 132.3 ± 66.0 | 110.7 ± 40.7 | 125.2 ± 45.4 | 0.390 |
| RT (s) | 15.5 ± 3.7 | 11.9 ± 3.0 | 12.8 ± 3.9 | 0.020 | |
| TTP (s) | 21.0 ± 7.5 | 16.6 ± 6.0 | 16.7 ± 6.1 | 0.074 | |
| mTT (s) | 36.3 ± 7.1 | 30.7 ± 7.3 | 30.3 ± 7.6 | 0.030 |
CEUS enhancement parameters analysis and its correlation with PSA, Gleason score and clinical stages
Descriptive statistics of IMAX, RT, TTP, and mTT of inner and outer prostate gland are summarized in Table 2. IMAX of inner gland was correlated significantly with Gleason score (P = 0.029). Pairwise comparison analyzed with the LSD showed that the IMAX of inner gland was significantly higher for Gleason score = 7 PCa compared with Gleason score < 7 PCa group. (P = 0.015). And compared with Gleason score > 7 PCa, the IMAX of > 7 group was significantly higher.
Table 2.
Relationship of CEUS enhancement parameters with PSA, Gleason score and clinical stages
| No. of patients | Inner gland | Outer gland | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| IMAX (%) | RT (s) | TTP (s) | mTT (s) | IMAX (%) | RT (s) | TTP (s) | mTT (s) | |||
| PSA | ≤ 10 | 26 | 256.4 ± 51.4 | 12.3 ± 5.0 | 18.3 ± 10.2 | 37.0 ± 11.6 | 120.7 ± 56.6 | 14.0 ± 4.5 | 18.8 ± 7.6 | 33.0 ± 7.1 |
| 10-20 | 16 | 218.1 ± 45.2 | 11.9 ± 4.0 | 14.9 ± 7.5 | 36.6 ± 14.2 | 118.1 ± 41.0 | 11.9 ± 2.4 | 15.5 ± 4.8 | 31.8 ± 7.8 | |
| ≥ 20 | 42 | 242.1 ± 84.2 | 12.2 ± 4.5 | 16.8 ± 8.9 | 35.0 ± 20.3 | 126.2 ± 46.1 | 13.0 ± 3.7 | 17.3 ± 6.2 | 31.4 ± 7.7 | |
| P | 0.224 | 0.950 | 0.482 | 0.883 | 0.821 | 0.217 | 0.287 | 0.323 | ||
| Gleason score | < 7 | 27 | 224 ± 39.9 | 12.3 ± 4.4 | 17.6 ± 8.7 | 34.9 ± 11.7 | 122.6 ± 55.9 | 14.2 ± 3.4 | 18.9 ± 6.7 | 34.1 ± 8.9 |
| 7 | 29 | 269.3 ± 93.3 | 11.9 ± 4.3 | 16.2 ± 9.4 | 40.0 ± 224.1 | 122.1 ± 52.2 | 12.3 ± 3.7 | 16.8 ± 6.0 | 29.9 ± 5.8 | |
| > 7 | 28 | 230.6 ± 55.5 | 12.4 ± 4.9 | 16.9 ± 9.0 | 322.7 ± 10.1 | 124.1 ± 36.5 | 12.7 ± 4.0 | 16.6 ± 6.7 | 30.3 ± 7.7 | |
| P | 0.029 | 0.918 | 0.855 | 0.246 | 0.988 | 0.156 | 0.361 | 0.080 | ||
| Clinical stages | T1c-T2a | 37 | 233.1 ± 46.1 | 12.5 ± 4.6 | 18.2 ± 9.4 | 39.3 ± 22.3 | 123.6 ± 55.2 | 14.1 ± 3.3 | 19.1 ± 6.5 | 33.2 ± 7.8 |
| T2b-2c | 25 | 260.1 ± 103.3 | 11.3 ± 5.0 | 15.1 ± 9.1 | 32.7 ± 12.6 | 103.6 ± 44.1 | 12.1 ± 4.0 | 16.4 ± 6.2 | 30.0 ± 8.6 | |
| ≥ T3a | 22 | 236.3 ± 52.6 | 12.6 ± 3.9 | 16.9 ± 8.1 | 34.0 ± 6.1 | 143.7 ± 29.6 | 12.4 ± 4.1 | 15.8 ± 6.4 | 29.8 ± 5.7 | |
| P | 0.298 | 0506 | 0.422 | 0.265 | 0.016 | 0.077 | 0.107 | 0.145 | ||
IMAX of outer gland was correlated significantly with clinical stages (P = 0.016). Pairwise comparison analyzed with the LSD showed that the IMAX of outer gland was significantly lower for T2b-2c PCa compared with clinical stages ≥T3a PCa group.
However, no significant correlation was found between PSA and CEUS enhancement parameters. Furthermore, With the exception of IMAX of inner gland and IMAX of outer gland, there were no significant difference of enhancement parametersbetween Gleason score groups and clinical stages groups (Table 2).
Discussion
The increasing incidence of low-risk PCa is due to widespread PSA screening [11]. Active surveillance was recently developed as an alternative treatment with fewer morbidities and complications than radical prostatectomy for some low-risk PCa [12-14]. PCa risk classification has implications for treatment. MRI has been reported to have value in predicting PCa risk [15,16]. The clinical stage of PCa mainly depends on MRI or other image features. In recent years, CEUS rapidly developed as a technology which is effective for improving the diagnostic accuracy of PCa [17-20]. SonoVue is blood pool agent that consists of a sulphur hexafluoride in an encapsulated shell [21], and it enhances the contrast of tissues and blood until it disintegrates. Several researches have showed this technology has a potential value in diagnosis of liver, prostate and other organs tumor [19,20,22]. But there are few studies on the relationship between parameters of CEUS and PCa risk.
Zullig et al. [23] reported that the median age of PCa in the United States was more than 65 years. In our study, Age of low-risk PCa was lower than intermediate-risk and high-risk PCa. It confirmed that age was associated with PCa aggressiveness. RT represented the time contrast agents arrived at the lesion, related to the blood supply of lesion. TTP represented the time of achieved maximum enhanced intensity was related to the speed of wash in. mTT showed the time that falled by half from maximum enhanced intensity was related to the speed of wash out. According to E. M. Jung’s study [24], by evaluating the mTT and RT, tumor detection was possible in 85.3% and 73.5% cases, quantitative parameters could be helpful for characterization of PCa. It is generally known, the main blood supplied artery for outer and inner prostate gland is prostate capsule artery and urethral artery, and they branched from the internal iliac artery [25]. In this study, the parameters of outer and inner prostate gland were evaluated separately. The RT and mTT of outer gland in low-risk PCa was greater than in intermediate-risk and high-risk PCa, which reflected in intermediate-risk and high-risk PCa the blood supply of outer gland was richer than that in low-risk PCa. But PCa risk did not correlate significantly with IMAX, TTP of outer gland and the quantitative parameters of inner gland.
The Gleason scoring system as a reference grading system for PCa related to the prognosis of PCa [26,27]. Halpern et al. [28] showed that CEUS could find higher Gleason score PCa. Bono et al. [29] found a significant difference in microvessel density (MVD) of PCa among different Gleason score groups, and the higher Gleason score PCa had higher MVD. CEUS can reflect the MVD of tissue dynamically, however, in our study, with the exception of IMAX of inner gland, there were no significant difference of enhancement parameters in Gleason score groups.
PSA is an important, non-invasive screening method for PCa, and has leaded to increased rates of PCa diagnoses. The precise clinical stage is important for the choice of therapeutic options [7]. In our study, PSA level did not correlate significantly with the quantitative parameters of inner and outer gland. And with the exception of IMAX of outer gland, there were no significant difference of enhancement parameters in clinical stages groups. Research had showed PSA level may be associated with PCa clinical stage, which has not come up with a unanimous conclusion until now. Gallee et al. [30] confirmed PCa cells cannot compound PSA, and the normal glandular epithelium were destroyed more seriously to bring lower PSA lever in higher clinical stage PCa. We thought that the unharmonious aspect between clinical stage and PSA affected the relationship of PCa risk and CEUS parameters. Beyond that, we selected the maximum transverse section of prostate tissue rather than suspicious area for CEUS examination also affected the results.
There were some limitations in our study. (1) It was retrospective design and (2) the clinical stages were only according to MRI results. Because not all cases underwent radical prostatectomy, the clinical stages couldn’t be compared with pathological stages. (3) Only the maximum transverse section of prostate tissue was selected for CEUS examination, which was not available to represent the whole gland. We will have a further research because of the inadequacy of the present study.
In conclusion, the enhancement parameters of PCa at CEUS may be used to predict PCa risk. And it is helpful for the choice of therapeutic options.
Acknowledgements
This study was supported by National Natural Science Foundation of China (81471673), the Opening Project of State Key Laboratory of High Performance Ceramics and Superfine Microstructure (SKL201412SIC) and the Research Project of Science and Technology Committee of Shanghai Municipality (124119a3201).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J. Clin. Oncol. 2004;22:2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson IM, Klotz L. Active surveillance for prostate cancer. JAMA. 2010;304:2411–2412. doi: 10.1001/jama.2010.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes JH, Ollendorf DA, Pearson SD, Barry MJ, Kantoff PW, Stewart ST, Bhatnagar V, Sweeney CJ, Stahl JE, McMahon PM. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304:2373–2380. doi: 10.1001/jama.2010.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupelian P, Kuban D, Thames H, Levy L, Horwitz E, Martinez A, Michalski J, Pisansky T, Sandler H, Shipley W, Zelefsky M, Zietman A. Improved biochemical relapse-free survival with increased external radiation doses in patients with localized prostate cancer: the combined experience of nine institutions in patients treated in 1994 and 1995. Int J Radiat Oncol Biol Phys. 2005;61:415–419. doi: 10.1016/j.ijrobp.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, Canby-Hagino E, Crawford ED. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 8.Roobol MJ, van Vugt HA, Loeb S, Zhu X, Bul M, Bangma CH, van Leenders AG, Steyerberg EW, Schroder FH. Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol. 2012;61:577–583. doi: 10.1016/j.eururo.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Roobol MJ, Zhu X, Schroder FH, van Leenders GJ, van Schaik RH, Bangma CH, Steyerberg EW. A Calculator for Prostate Cancer Risk 4 Years After an Initially Negative Screen: Findings from ERSPC Rotterdam. Eur Urol. 2013;63:627–633. doi: 10.1016/j.eururo.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 11.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14–19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, Walsh PC, Carter HB. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J. Clin. Oncol. 2011;29:2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 13.Whitson JM, Porten SP, Hilton JF, Cowan JE, Perez N, Cooperberg MR, Greene KL, Meng MV, Simko JP, Shinohara K, Carroll PR. The relationship between prostate specific antigen change and biopsy progression in patients on active surveillance for prostate cancer. J Urol. 2011;185:1656–1660. doi: 10.1016/j.juro.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Adamy A, Yee DS, Matsushita K, Maschino A, Cronin A, Vickers A, Guillonneau B, Scardino PT, Eastham JA. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 2011;185:477–482. doi: 10.1016/j.juro.2010.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oto A, Yang C, Kayhan A, Tretiakova M, Antic T, Schmid-Tannwald C, Eggener S, Karczmar GS, Stadler WM. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am J Roentgenol. 2011;197:1382–1390. doi: 10.2214/AJR.11.6861. [DOI] [PubMed] [Google Scholar]
- 16.Hambrock T, Hoeks C, de Kaa C H, Scheenen T, Futterer J, Bouwense S, van Oort I, Schroder F, Huisman H, Barentsz J. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012;61:177–184. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Uemura H, Sano F, Nomiya A, Yamamoto T, Nakamura M, Miyoshi Y, Miki K, Noguchi K, Egawa S, Homma Y, Kubota Y. Usefulness of perflubutane microbubble-enhanced ultrasound in imaging and detection of prostate cancer: phase II multicenter clinical trial. World J Urol. 2013;31:1123–1128. doi: 10.1007/s00345-012-0833-1. [DOI] [PubMed] [Google Scholar]
- 18.Tang J, Yang JC, Luo Y, Li J, Li Y, Shi H. Enhancement characteristics of benign and malignant focal peripheral nodules in the peripheral zone of the prostate gland studied using contrast-enhanced transrectal ultrasound. Clin Radiol. 2008;63:1086–1091. doi: 10.1016/j.crad.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Yi A, Kim JK, Park SH, Kim KW, Kim HS, Kim JH, Eun HW, Cho KS. Contrast-enhanced sonography for prostate cancer detection in patients with indeterminate clinical findings. AJR Am J Roentgenol. 2006;186:1431–1435. doi: 10.2214/AJR.04.1959. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto K, Moriyasu F, Shiraishi J, Saito K, Taira J, Saguchi T, Imai Y. Assessment of arterial hypervascularity of hepatocellular carcinoma: comparison of contrast-enhanced US and gadoxetate disodium-enhanced MR imaging. Eur Radiol. 2012;22:1205–1213. doi: 10.1007/s00330-011-2372-3. [DOI] [PubMed] [Google Scholar]
- 21.Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del MA, Summaria V, Maresca G, Pezzoli C, Llull JB. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol. 2002;41:200–206. doi: 10.1016/s0720-048x(01)00457-0. [DOI] [PubMed] [Google Scholar]
- 22.Gerst S, Hann LE, Li D, Gonen M, Tickoo S, Sohn MJ, Russo P. Evaluation of renal masses with contrast-enhanced ultrasound: initial experience. AJR Am J Roentgenol. 2011;197:897–906. doi: 10.2214/AJR.10.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zullig LL, Jackson GL, Dorn RA, Provenzale DT, McNeil R, Thomas CM, Kelley MJ. Cancer incidence among patients of the U. S. Veterans Affairs Health Care System. Mil Med. 2012;177:693–701. doi: 10.7205/milmed-d-11-00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung EM, Wiggermann P, Greis C, Eder F, Ehrich J, Jung W, Schreyer AG, Stroszczynski C, Ganzer R. First results of endocavity evaluation of the microvascularization of malignant prostate tumors using contrast enhanced ultrasound (CEUS) including perfusion analysis: first results. Clin Hemorheol Microcirc. 2012;52:167–177. doi: 10.3233/CH-2012-1594. [DOI] [PubMed] [Google Scholar]
- 25.Lee CH, Akin-Olugbade O, Kirschenbaum A. Overview of prostate anatomy, histology, and pathology. Endocrinol Metab Clin North Am. 2011;40:565–575. viii–ix. doi: 10.1016/j.ecl.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Mitterberger M, Pinggera GM, Horninger W, Bartsch G, Strasser H, Schafer G, Brunner A, Halpern EJ, Gradl J, Pallwein L, Frauscher F. Comparison of contrast enhanced color Doppler targeted biopsy to conventionalsystematic biopsy: impact on Gleason score. J Urol. 2007;178:464–468. doi: 10.1016/j.juro.2007.03.107. discussion 468. [DOI] [PubMed] [Google Scholar]
- 27.Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, Ma J, Fiorentino M, Kurth T, Loda M, Giovannucci EL, Rubin MA, Mucci LA. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3. J. Clin. Oncol. 2009;27:3459–3464. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpern EJ, Rosenberg M, Gomella LG. Prostate cancer: contrast-enhanced us for detection. Radiology. 2001;219:219–225. doi: 10.1148/radiology.219.1.r01ap21219. [DOI] [PubMed] [Google Scholar]
- 29.Bono AV, Celato N, Cova V, Salvadore M, Chinetti S, Novario R. Microvessel density in prostate carcinoma. Prostate Cancer Prostatic Dis. 2002;5:123–127. doi: 10.1038/sj.pcan.4500572. [DOI] [PubMed] [Google Scholar]
- 30.Gallee MP, Visser-de JE, van der Korput JA, van der Kwast TH, ten KF, Schroeder FH, Trapman J. Variation of prostate-specific antigen expression in different tumour growth patterns present in prostatectomy specimens. Urol Res. 1990;18:181–187. doi: 10.1007/BF00295844. [DOI] [PubMed] [Google Scholar]


