Abstract
Objectives: The object of the study is to experimentally investigate the possible systemic side effects of Oxymetazoline including its nasal spray which has been in use for a long time both by the physicians and patients. There is no study in the literature to address the damages of oxymetazoline on the end organ. Materials and methods: The study conducted on 2 groups of rat. Group 1 (n = 8): Control; and Group 2 (n = 8): Oxymetazoline. During 4 week, the control group was applied with 2 drops of saline water on each nasal cavity 3 times a day and the other group was applied with 2 drops of oxymetazoline HCl 3 times a day. At the end of experiment, samples from mandible, parotid and tails of the rats were taken in 10% formalin for histopathological investigations. Results: In histopathological experiments, when compared with the control group, the oxymetazoline group showed significant increase in many of the histopathological parameters (ischemic changes: P = 0.0001; congestion: P = 0.0006; arterial thrombosis: P = Ns; PNL accumulations: P = 0.001; necrosis: P = 0.0001; and ulceration: P = 0.014). The results of histopathologic tests on the samples taken from mandible and parotid gland, in comparison with the control group, showed no significant increase (focal inflammation: P = Ns; and lymphocyte aggregation: P = Ns). Conclusion: Due to the damage that the long-term use of nasal spray including oxymetazoline, it may cause injury on the end organ, which we revealed in our histopathological experiments. We believe that it’s essential for the physicians to provide information on the side effects of the medicine to their patients who use for a long term.
Keywords: Nasal spray, oxymetazoline, systemic side effects, end-organ injury
Introduction
Considering the chemical properties of topical decongestants, they’re investigated in two groups [1]: Sympathomimetic agents (phenylephrine, ephedrine, and pseudoephedrine) and imidazoline derivatives (oxymetazoline, xylometazoline) [1]. Due to release of norepinephrine in the agents existing in both of the groups, they cause vasoconstriction by stimulating alpha-adrenergic receptors [1]. Generally, sympathomimetic agents selectively react to alpha 1 receptors and imidazoline derivatives react to alpha 2 receptors. Among the two groups of agents, only few medicines (pseudoephedrine and oxymetazoline) do not show selective reaction and are effective on both groups of receptors. Due to rapid elimination of nasal congestion, medicines containing nasal decongestants are frequently used both by the physicians and the patient [2]. Notwithstanding the fact that they eliminate the short-term nasal congestion rapidly, their effects on long term rebound congestion are going to be revealed [2]. Although the local decongestant drugs are mainly used due to their effects on peripheral alpha 2 receptors, they can affect the alpha 2 receptors in cardiovascular and nervous systems and consequently, can cause the respiratory depression [3,4]. When nasal decongestants as well as sympathomimetic agents, contained in local mydriatics used, they may cause systemic cardiovascular effects [5].
In this study, we experimentally investigated the possible systemic side effects of nasal sprays including oxymetazoline which have been in use for a long time both by the physicians and patients. Although the side effects of oxymetazoline on the circulatory system are known for us, there is no study in the literature to address the damages of oxymetazoline on the end organ.
Materials and methods
The ethics committee of Mustafa Kemal University approved the animal use protocol for this study in compliance with the guidelines of the Declaration of Helsinki (ID: 40595970/10). In this study we used 16 male Wistar Albino rats of 12-16 weeks (200-240 gr). The rats are classed as two groups. Group 1, control, was applied with 2 drops of serum physiologic (0.05 cc/drop) on both their nasal cavities 3 times a day for 4 weeks. Group 2, Oxymetazoline Group, was applied with 2 drops of 0.05% oxymetazoline (0.05 cc/drop) (Iliadin spray, Santa Farma) on both their nasal cavities 3 times a day for 4 weeks [6,7]. At the end of 4th week, samples were prepared and dissected from tails, sub-mandible and parotid gland. For the purpose of making the rats ready for the test, we used 10 mg/kg Ksilazin (Rompun, Bayer, Turkey) and 40 mg/kg Ketamin (Ketalar, Eczacibasi, Turkey) as intraperitoneal (i.p) anesthesia agent and they were taken to the test table. Blood samples were taken from the heart of the rats and euthanized. They were decapitated and the skin removed from head and put on formalin for light microscopy.
Pathological analysis
Tail, mandible, and parotid tissues from the rats were placed in 10% formalin solution for 24 hours for fixation and further pathological examination. Decalcification after fixation, the sections were subjected to routine histological tissue preparation, dehydrated and embedded in paraffin. Paraffin blocks were sliced in 5-µm thickness with microtome and the slices were subjected to routine hematoxyline and eosin (H&E) staining procedure and then were examined under a light microscope (Olympus BX50) by an expert pathologist. The tail tissues were scored for ischemic changes, congestion, arterial thrombosis, PNL (polymorph nuclear leukocytes) accumulation, necrosis, and ulceration parameters. The samples taken from mandible and parotid gland were histopathologically examined in terms of focal inflammation and lymphocyte aggregates.
Statistical analysis
The collected data were analyzed with SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). The normal distribution of continuous variables was assessed with the Kolmogorov-Smirnov test. Student t’ test was performed for comparison of the groups. A P value less than 0.05 was considered statistically significant for all the statistical data.
Results
The assessment of tail tissues
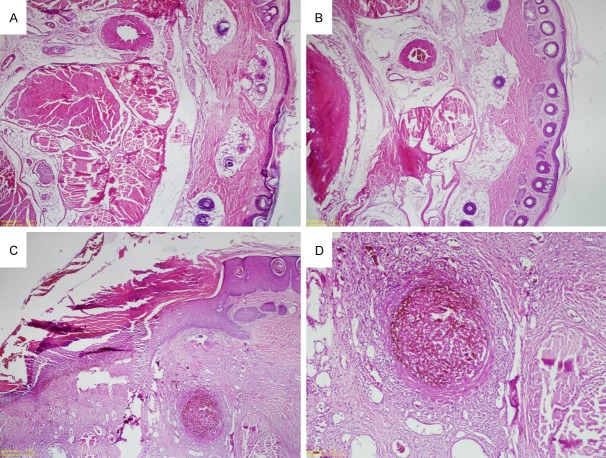
The pathological changes in the nasal mucosa of all the groups are summarized in Table 1 and Figure 1. In histopathological assessment, in terms of ischemic changes, congestion, arterial thrombosis, polymorph nuclear neutrophils (PNL) accumulations and necrosis, when compared with the control group, the oxymetazoline group showed significant increase in many of the histopathological parameters (ischemic changes: P = 0.0001; congestion: P = 0.0006; arterial thrombosis: P = non-significant (Ns); PNL accumulations: P = 0.001; necrosis: P = 0.0001; and ulceration: P = 0.014) (Table 1; Figure 1).
Table 1.
Histopathological scores of tail tissues
| Control | Oxymetazoline | P value | |
|---|---|---|---|
| Ischemic changes | 0.11 ± 0.33 | 2.25 ± 0.88 | 0.0001 |
| Congestion | 1.22 ± 0.44 | 2.50 ± 0.75 | 0.0006 |
| Arterial thrombosis | 0.00 ± 0.00 | 0.25 ± 0.46 | Ns |
| PNL accumulation | 0.00 ± 0.00 | 2.00 ± 1.41 | 0.001 |
| Necrosis | 0.00 ± 0.00 | 2.00 ± 1.06 | 0.0001 |
| Ulceration | 0.00 ± 0.00 | 0.50 ± 0.53 | 0.014 |
Ns: Non significant. Student t’ test was used.
Figure 1.
Tail histology of rats. A and B: Control group rats with normal tail histology (Hematoxylin-eosin (H&E), × 100). C and D: Ischemic changes, congestion, arterial thrombosis, neutrophil accumulation, necrosis and ulceration in the rats administered with oxymetazoline (H&E, × 100).
The assessment of mandible and parotid gland tissues
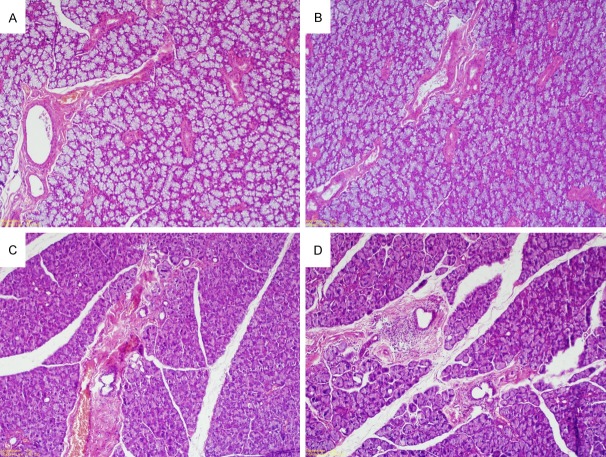
The results of histopathologic tests on the samples taken from mandible and parotid gland, in terms of focal inflammation and lymphocyte aggregation, in comparison with the control group, showed no significant increase (focal inflammation: P = Ns; and lymphocyte aggregation: P = Ns) (Figure 2).
Figure 2.
Mandibular and parotid gland histology of rats. A: Control group rats with normal mandibular histology (Hematoxylin-eosin (H&E), × 100). B: Oxymetazoline group rats with normal mandibular histology (H&E, × 100). C and D: Focal inflammation and minimal periductal inflammation in the rats’ parotid gland administered with oxymetazoline (H&E, × 100).
Discussion
In the study conducted by us, we noted the necrosis in the skin of tails of the rats applied with topical oxymetazoline and detached 90% of their tails. Such a finding does not exist in the control group. As a result of physiopathological tests we carried out at the end of our test, the damage of end organ has been proved due to necrosis.
The sympathomimetic amines affect both alpha and beta receptors. They are effective on alpha receptors but strong stimuli dependent vasodilatation effect of beta-receptors masked it [1]. Stimulation of beta-receptors in vasoconstriction followed by vasodilatation and the resulting rebound is important towards nasal congestion. The mechanism of negative feedback of derivatives of imidazoline, which is used since long ago, results in decrease of norepinephrine production in the presynaptic region [1]. As a result, the patients, who use the drug for long term, are forced to use higher doses of the drug. For the gaining the same result in the patients that use topical decongestants for long term, the increased doses of the drug is prescribed, as a result, more dozes of the material are entered into circulatory system via nasal mucus.
Among the side effects oxymetazoline including the local decongestants outlined in the prospective literature is: increase in runny or stuffy nose, blurred vision, fast, irregular, or pounding heartbeat, headache, dizziness, drowsiness, lightheadedness, high blood pressure, nervousness, trembling, trouble in sleeping and weakness. Furthermore, the systemic side-effects of use of topical oxymetazoline have been reported as event reports in the literature. But we have not found any matter relating to congestion, increase in PNL, ischemia and necrosis when reviewed the related literature. Abruzzo T. in course of a selective ophthalmic artery infusion chemotherapy on a 19-month patient suffering from retinoblastoma has reported the development of cerebral vasoconstriction following the use of topical nasal sympathomimetic agents [3]. So, if the indications show that the use topical sympathomimetic agents triggered local vasoconstriction in children, one should be careful when using such an agent [4]. In a study conducted by Loewan AH., it has been reported that the following the use of nasal spray of oxymetazoline reversible segmental cerebral vasoconstriction and severe headache has been observed in the adult women attended at the clinic. After use of sympathomimetic agents some mechanisms that are likely to be the cause of the problem in the central system (stroke) including acute hypertension, vasospasm, thrombosis, and angitis induced vasospasm have also been reported. In addition, the patients using sympathomimetic local and/or systemic medications should be asked about the sudden heavy headaches [4]. Soderman P. has reported the occurrence of reactions of cardiovascular system and central nervous system when the patients used oxymetazoline nasal drop of 0.01% to 0.05% doses [8]. Among the findings, they are some side effects like agitation, anxiety, insomnia, convulsions, tachycardia and vasoconstriction.
The cardiovascular side effects are also seen at the time of use of local decongestants. Fabi M. has reported the syncope development in the males following use of nasal oxymetazoline [9]. In general, sympathomimetics cause hypertension, tachycardia, peripheral vasoconstriction and common cause systemic toxicity. However, in some patients, as in these patients, due to toxicity central hypertensive alpha2 receptor-inducing causes bradycardic response is observed [9]. Like this, Glazner F. has reported bradycardia, hypotension, and syncope after application of oxymetazoline nasal spray on a male patient of 73 years old [10]. Thrush DN. has reported the case of development of cardiac arrest after use of local oxymetazoline on a child of 2 years old. The drugs are passed into the circulation system via nasal mucus, which is rich in vascular system. Thereafter, the vascular resistance, higher tension, the secondary effects like carotid and aortic baroreceptors stimulation and reflex bradycardia are occurred [11].
Since the lipophilic drug like oxymetazoline is systemically absorbed by the vessels, easily passes the blood-brain barrier and enter into vascular and central nervous system. When these drugs pass the brain barriers, like amphetamine caused noradrenergic strong stimulating effect on the central nervous system [12]. The reactive vasodilatation following the extended use of the drug, mucus reached of the vascular system is formed which the drug can easily pass through and enter into the circular system. Ticoll B and Shugar G. have reported that fully related these effects to a 41-year old patient suffering from paranoid psychosis as the result of use of nasal oxymetazoline spray [12]. Snow SS. has reported the addition to phenylephrine in a 26-year old patient which actually resulted in toxic psychosis [13].
In conclusion, the complications of central nervous system and also cardiovascular system have been reported after use of the local sympathomimetic agents. Such an information related to the effects of such agents on the end organs hasn’t been existent yet. Due to the damage that the long-term use of nasal spray including oxymetazoline, it may cause injury on the end organ, which we revealed in our histopathological experiments. We believe that it’s essential for the physicians to provide information on the side effects of the medicine to their patients who use for a long term.
Disclosure of conflict of interest
None.
References
- 1.Doshi J. Rhinitis medicamentosa: what an otolaryngologist needs to know. Eur Arch Otorhinolaryngol. 2009;266:623–625. doi: 10.1007/s00405-008-0896-1. [DOI] [PubMed] [Google Scholar]
- 2.Ramey JT, Bailen E, Lockey RF. Rhinitis medicamentosa. J Investig Allergol Clin Immunol. 2006;16:148–155. [PubMed] [Google Scholar]
- 3.Abruzzo T, Patino M, Leach J, Rahme R, Geller J. Cerebral vasoconstriction triggered by sympathomimetic drugs during intra-atrerial chemotherapy. Pediatr Neurol. 2013;48:139–142. doi: 10.1016/j.pediatrneurol.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Loewen AH, Hudon ME, Hill MD. Thunderclap headache and reversible segmental cerebral vasoconstriction associated with use of oxymetazoline nasal spray. CMAJ. 2004;171:593–594. doi: 10.1503/cmaj.1040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpay A, Ermis B, Ugurbas SC, Battal F, Sagdik HM. The local vasoconstriction of infant’s skin following instillation of mydriatic eye drops. Eur J Clin Pharmacol. 2010;66:1161–1164. doi: 10.1007/s00228-010-0890-6. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe H, Foo TH, Djazaeri B, Duncombe P, Mackay IS, Durham SR. Oxymetazoline nasal spray three times daily for four weeks in normal subjects is not associated with rebound congestion or tachyphylaxis. Rhinology. 2003;41:167–174. [PubMed] [Google Scholar]
- 7.Dokuyucu R, Cevik C, Ozler GS, Ozgur T, Arli C, Sefil F, Yonden Z. Determination of oxidative stress and effect of erdosteine on rhinitis medicamentosa in a rat model. Eur J Pharmacol. 2014;742:153–157. doi: 10.1016/j.ejphar.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Soderman P, Sahlberg D, Wiholm BE. CNS reactions to nose drops in small children. Lancet. 1984;1:573. doi: 10.1016/s0140-6736(84)90978-4. [DOI] [PubMed] [Google Scholar]
- 9.Fabi M, Formigari R, Picchio FM. Are nasal decongestants safer than rhinitis? A case of oxymetazoline-induced syncope. Cardiol Young. 2009;19:633–634. doi: 10.1017/S1047951109990722. [DOI] [PubMed] [Google Scholar]
- 10.Glazener F, Blake K, Gradman M. Bradycardia, hypotension, and near-syncope associated with Afrin (oxymetazoline) nasal spray. N Engl J Med. 1983;309:731. doi: 10.1056/NEJM198309223091213. [DOI] [PubMed] [Google Scholar]
- 11.Thrush DN. Cardiac arrest after oxymetazoline nasal spray. J Clin Anesth. 1995;7:512–514. doi: 10.1016/0952-8180(95)00060-u. [DOI] [PubMed] [Google Scholar]
- 12.Ticoll B, Shugar G. Paranoid psychosis induced by oxymetazoline nasal spray. CMAJ. 1994;150:375–376. [PMC free article] [PubMed] [Google Scholar]
- 13.Snow SS, Logan TP, Hollender MH. Nasal spray ‘addiction’ and psychosis: a case report. Br J Psychiatry. 1980;136:297–299. doi: 10.1192/bjp.136.3.297. [DOI] [PubMed] [Google Scholar]