Abstract
Objective: To observe the impact on maxillary growth of the use of buccal fat pads to pack palate relaxing incisions. Methods: We recruited 30 patients aged 3-4 years with a complete cleft palate. Half (15) of the patients underwent reparative surgery that entailed packing buccal fat pads into the bilateral raw bony surfaces and served as the experimental group. The remaining 15 patients underwent reparative surgery that entailed packing and fixing relaxing incisions with iodoform gauze and served as the control group. After a 5-year follow-up, the differences in data from X-ray cephalometry and upper dental models in the two groups were analyzed using the T test. Results: A significant difference (P < 0.05) in maxillary growth was observed between the two groups of children with cleft palate. Conclusion: The results supported the use of buccal fat pads to pack relaxing incisions which led to improved maxillary development in patients with cleft palate.
Keywords: Buccal fat pad, relaxing incision, cephalometric analysis, arch model, maxillary development
Introduction
Conventional palatoplasty generates post-operative scar tissue contraction due to the widely separated release and exposure of bone and mucosal tissues by bilateral relaxing incisions, which affects maxillary development [1]. The packing of iodoform gauze into relaxing incisions can also cause pain, fever, inconvenience during eating and infection [2].
Several studies have shown that the maxillary development of children who have undergone complete cleft palate surgery always differs considerably from that of unaffected normal children, and post-operative scars are considered to be an important reason for these differences [3,4]. This study was carried out on 30 patients with complete cleft palate who were randomized to receive buccal fat pads (experimental group) or iodoform gauze strips (control group) to repair cleft palate relaxing incisions. Through X-ray cephalometric and arch model analysis, the influence on maxillary growth of the different relaxing incision packing methods used in the cleft palate surgery was recorded.
Materials and methods
Participant recruitment
Thirty patients aged 3-4 years with complete cleft palate were recruited in the department of Stomatology and The Second People’s Hospital of Shenzhen between January 2006 and December 2007. Among the 30 cases (18 boys, 12 girls), 17 had a unilateral complete cleft palate and 13 had a bilateral complete cleft palate. They were divided randomly into two groups, with 15 patients undergoing reparative surgery that entailed filling the bilateral raw bony surfaces with buccal fat pads (experimental group) and the remaining 15 undergoing reparative surgery that entailed packing and fixing relaxing incisions with iodoform gauze (control group). All of the operations were performed by the same doctor to ensure that the same surgical methods were used.
None of the children received orthodontic, alveolar or orthognathic treatment before or after the operation. The 5-year follow-up data were obtained during subsequent visits.
Statistical analysis
During the 5-year follow-up visits, lateral skull X-ray slices and upper dental models were measured under standard conditions. On the lateral skull X-ray slice, fixed-pointed measures of the value of S-N, Ba-N, ∠SNA, N-A, ∠SNB, N-B, ∠ANB, N-ANS, ANS-PNS, and N-ANS/N-Me were taken (Figure 1). In the dental model, the upper dental arch length (a-b), the tooth bow front width (c-d) and the dental arch at the widest point (e-f) were measured (Figure 2).
Figure 1.
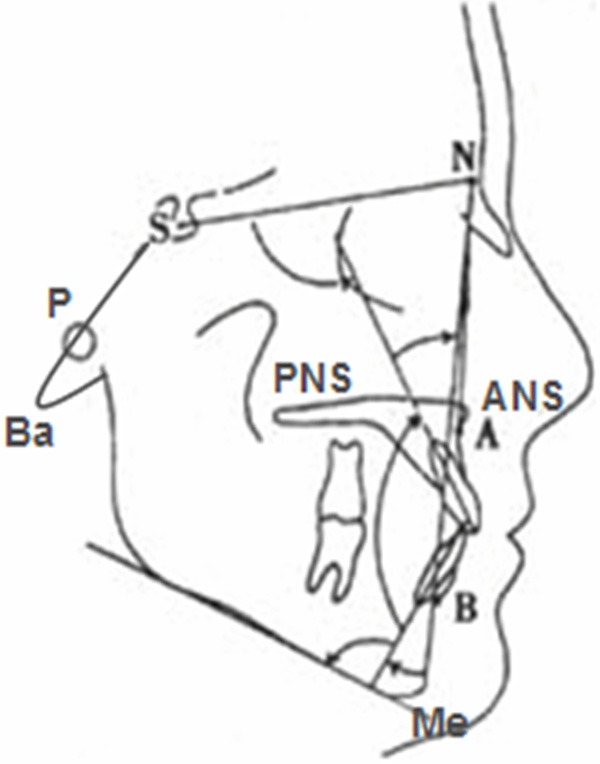
Landmarks of X-ray cephalometry.
Figure 2.
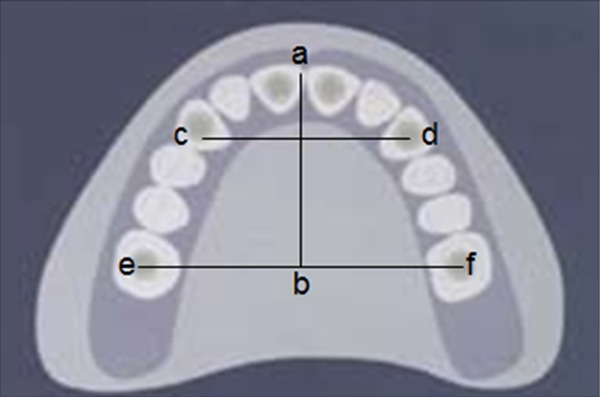
Measured landmarks in the upper arch model.
Results
The various measurement indices for two groups are shown in Table 1. The parameter values are the average of three measurements taken by the same doctor. We performed the T test using the means of the two groups. The P-value (Table 1) showed a statistically significant difference (P < 0.05) between the experimental and control groups. The results demonstrated that the maxillary growth in the experimental group was considerably improved compared with that in the control group.
Table 1.
Various measurement indices of the two groups (X̅ ± s)
| Measurement | Experimental group | Control group | P-value |
|---|---|---|---|
| S-N (mm) | 60.01 ± 2.58 | 57.66 ± 2.59 | < 0.01 |
| Ba-N (mm) | 95.55 ± 3.55 | 92.75 ± 4.15 | < 0.05 |
| SNA (°) | 80.53 ± 3.04 | 78.19 ± 2.63 | < 0.01 |
| N-A (mm) | -4.11 ± 3.30 | -8.85 ± 4.00 | < 0.01 |
| SNB (°) | 77.02 ± 2.64 | 76.38 ± 2.56 | < 0.05 |
| N-B (mm) | -10.75 ± 5.05 | -11.65 ± 5.9 | < 0.05 |
| ANB (°) | 1.28 ± 2.9 | -1.13 ± 3.12 | < 0.01 |
| N-ANS (mm) | 48.13 ± 3.27 | 45.56 ± 3.71 | < 0.01 |
| ANS-PNS (mm) | 40.01 ± 3.51 | 38.56 ± 3.67 | < 0.05 |
| N-ANS/N-Me | 0.41 ± 0.03 | 0.39 ± 0.04 | < 0.05 |
| a-b (mm) | 25.45 ± 3.05 | 23.68 ± 2.95 | < 0.01 |
| c-d (mm) | 31.34 ± 2.82 | 29.95 ± 3.17 | < 0.01 |
| e-f (mm) | 46.62 ± 3.16 | 47.02 ± 4.03 | < 0.05 |
*A (subspinale), the innermost point on the contour of the premaxilla between anterior nasal spine and the incisor tooth. ANS (anterior nasal spine), the tip of the anterior nasal spine. B (supramental), the innermost point on the contour of the mandible between the incisor tooth and the bony chin. S (sella), the midpoint of the cavity of sella turcica. N (nasion), the anterior point of the intersection between the nasal and formal bones. Ba (basion), the lowest point on the anterior margin of foramen magnum, at the base of the clivus. PNS (posterior nasal spine), the tip of the posterior spine of the palatine bone,at the junction of the hard and soft palates. Me (menton), the most inferior point on the mandibular symphysis. *N-A/N-B is the distance that each landmark projected to the Frankfort horizontal (FH) plane.
Discussion
The post-operative development of maxillofacial deformities in cleft palate mainly involves central surface dysplasia and alveolar crest collapse that result in severe underbite, midfacial retrusion and lower facial protrusion. Some studies have suggested that the appearance of facial deformities is related to the post-operative formation of large scars on the surface of the bare bone [5,6] that has been recognized to be repaired by granulation tissue, which corresponds to the scar tissue. The healing process on the surface of the bare bone is dependent on wound systolic pressure and scar tissue formation, and its influence on maxillary growth after surgery has been attributed to wound systolic pressure and the stiffness of the scar [3]. Some studies have shown that the scar tissue of the palate is stiffer than normal tissue, and is an important factor in the restriction of maxillary growth [7].
In conventional cleft palate surgery, intra-operative separation by an adhesive periosteal suture in the midline bares the bone on both sides of the relaxing incision, and the use of iodoform gauze to stimulate the formation of scar tissue leads to the restriction of maxillary growth, resulting in the inward incline of tooth and alveolar structures and facial deformities [8]. Thus, many researchers worldwide are trying to discover and use alternative methods to reduce scar formation and the occurrence of complications after cleft palate surgery [9,10].
The buccal fat pad is an ideal matrix for the repair of soft tissue defects in the oral cavity [11]. Because its blood supply is constant and rich and contains many anastomoses in the donor, the buccal fat pad has strong anti-infection and tissue repair properties, and rarely undergoes necrosis or absorption after transplantation [12]. With a complete capsular layer, the buccal fat pad can be used as a filling in various fissures. It is very soft and pliable, and is easily separated from the surrounding tissue. Buccal fat pads can repair defects of about 5 cm in diameter. When a bilateral pedicled buccal fat pad flap is applied simultaneously, this can repair more intra-oral defects [13,14].
The buccal fat pad is a non-essential tissue in the human body. Its removal has no effect on facial appearance or function and it adheres to the recipient site. Moreover, it can be removed and its transfer to the repair site completed in the same operating area [15]. The buccal fat pad can be packed to cover both sides of the cleft palate relaxing incision, where it forms epithelia, prevents the formation of scar tissue, promotes wound healing and reduces the force formed by tension generated during the period of wound healing, providing satisfactory conditions for the normal growth of the maxilla [16].
This study used buccal fat pads to fill the cleft palate relaxing incision as an experimental intervention, and used traditional iodoform gauze as a comparison reference. The results showed obvious differences in the maxillary growth of the two groups, with that of the experimental group being considerably improved compared with that of the control group, which was associated with a large scar area that formed after cleft palate surgery. The experimental group had a decreased area of palate scar tissue and therefore a reduced effect of the post-operative scar on maxillary growth. However, maxillary growth continues into adulthood, and the study should be expanded to focus on the patients who underwent cleft palate surgery when they are 8-9 years of age. The ultimate impact of this method therefore needs to be followed up further.
Acknowledgements
This work was partially supported by grants from the innovation of science and technology project of Shenzhen City (No. 201002048).
Disclosure of conflict of interest
None.
References
- 1.Rafael RR, Juan CLN. Secondary management of cleft lip/cleft palate deformities in both soft and hard tissues. J Oral Maxill Surgery. 2009;67:127. [Google Scholar]
- 2.Gosain AK. Discussion: two-stage palate repair with delayed hard palate closure is related to favorable maxillary growth in unilateral cleft lip and palate. Plast Reconstr Surg. 2010;125:1511–1513. doi: 10.1097/PRS.0b013e3181d512d0. [DOI] [PubMed] [Google Scholar]
- 3.Song QG, Shi B, Huang X, Li S, Lu Y. The influence to maxillary growth by exposed bone wound in hard palate of rat. West China J Stomatol. 2004;22:13–15. [PubMed] [Google Scholar]
- 4.Holland S, Gabbay JS, Heller JB, O’Hara C, Hurwitz D, Ford D, Sauder AS, Bradley JP. Delayed closure of the hard palate leads to speech problems and deleterious maxillary growth. Plast Reconstr Surg. 2007;119:1302–1310. doi: 10.1097/01.prs.0000258518.81309.70. [DOI] [PubMed] [Google Scholar]
- 5.Mohan S, Kankariya H, Harjani B. The use of the buccal fat pad for reconstruction of oral defects: review of the literature and report of cases. J Maxillofac Oral Surg. 2012;11:128–131. doi: 10.1007/s12663-011-0217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robiony M. The use of silicone sheet to improve buccal fat pad healing in palatal reconstruction. J Plast Reconstr Aesthet Surg. 2010;63:e729–e732. doi: 10.1016/j.bjps.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Liu ZH, Lei RC. Effect of two kinds of sutures on patients with palate cleft. China J Mod Med. 2004;14:64–65. [Google Scholar]
- 8.Rapidis AD, Alexandridis CA, Elefteriadis E, Angelopoulos AP. The use of the buccal fat pad for reconstruction of oral defects: review of the literature and report 15 cases. J Oral Maxillofac Surg. 2000;58:158–63. doi: 10.1016/s0278-2391(00)90330-6. [DOI] [PubMed] [Google Scholar]
- 9.MILLARD DR Jr. The island flap in cleft palate surgery. Surg Gynecol Obstet. 1993;116:297–300. [PubMed] [Google Scholar]
- 10.Liao YF, Lee YH, Wang R, Huang CS, Chen PK, Lo LJ, Chen YR. Vomer flap for hard palate repair is related to favorable maxillary growth in unilateral cleft lip and palate. Clin Oral Investig. 2014;18:1269–76. doi: 10.1007/s00784-013-1084-2. [DOI] [PubMed] [Google Scholar]
- 11.Silvano F, Andrea F, Bemardo B, Chiara C, Alice SM, Enrico S. A novel technique for cheek mucosa defect reconstruction using a pedicled buccal fat pad and buccinator myomucosal island flap. Oral Oncol. 2008;45:59–62. doi: 10.1016/j.oraloncology.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Yousuf S, Tubbs RS, Wartmann CT, Kapos T, Cohen-Gadol AA, Loukas M. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg Radiol Anat. 2010;32:427–436. doi: 10.1007/s00276-009-0596-6. [DOI] [PubMed] [Google Scholar]
- 13.Prashanth R, Nandini GD, Balakrishna R. Evaluation of versatility and effectiveness of pedicled buccal fat pad used in the reconstruction of intra oral defects. J Maxillofac Oral Surg. 2013;12:152–159. doi: 10.1007/s12663-012-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loukas M, Kapos T, Louis RG Jr, Wartman C, Jones A, Hallner B. Gross anatomical, CT and MRI analyses of the buccal fat pad with special emphasis on volumetric variations. Surg Radiol Anat. 2006;28:254–260. doi: 10.1007/s00276-006-0092-1. [DOI] [PubMed] [Google Scholar]
- 15.Fan L, Chen G, Zhao S, Hu J. Clinical application and histological observation of pedicled buccal fat pad grafting. Chin Med J (Engl) 2002;115:1556–9. [PubMed] [Google Scholar]
- 16.Diah E, Lo LJ, Huang CS, Sudjatmiko G, Susanto I, Chen YR. Maxillary growth of adult patients with unoperated cleft: answers to the debates. J Plast Reconstr Aesthet Surg. 2007;60:407. doi: 10.1016/j.bjps.2006.10.004. [DOI] [PubMed] [Google Scholar]


