Abstract
This study investigated whether goji berry extract (GBE), a known antioxidant, reduces ischemic reperfusion injury when administered to rats exposed to experimental testis torsion. A total of 32 Sprague-Dawley male rats were randomized into 4 groups, including the control (sham), goji, torsion, and torsion-goji groups. The treatment groups received intraperitoneal GBE prior to torsion. The left testes of the animals were subjected to torsion via 5 hours of ischemia and 6 hours of reperfusion. TAC (total antioxidant capacity), TOS (total oxidant status) and OSI (oxidative stress index) levels were calculated. Approximately 5-μm-thick sections were stained with hematoxylin-eosin (H&E) and examined under a light microscope. Statistical analyses were performed with the SPSS 15 software package. The mean serum TAC level was significantly increased in Groups 2 and 4 compared with Groups 1 and 3 in biochemical analyses (for both P < 0.001). The mean serum TOS level was significantly increased in Group 3 compared with Groups 1, 2, and 4 (P < 0.001, P < 0.001, and P = 0.003, respectively). Comparison of the groups with regard to histopathological examination revealed that Group 4 exhibited a significantly higher rate of hemorrhage and congestion compared with Groups 1 and 2 (P = 0.038). The groups did not differ significantly with respect to degeneration. Ischemic reperfusion injury associated with testis torsion was reduced by the antioxidant effect of GBE. Further experimental and clinical studies are needed to confirm the agent’s efficacy for this indication.
Keywords: Antioxidant, lycium, goji berry extract, testis torsion
Introduction
Testis torsion is a serious urological condition that occurs when the spermatic cord and associated structures twist around themselves, and it requires urgent surgery [1,2]. It is one of the most common causes of testicular loss in childhood and adolescence. If testicular torsion is not treated within 4 to 6 hours (h), spermatogenic cell loss occurs [3]. However, even in men who have undergone surgical detorsion within this time period, the ipsilateral testis often becomes permanently dysfunctional [3,4]. Ischemic/reperfusion (I/R) injury following torsion/detorsion of the testes results in the development of significant pathology. One of the main reasons for (I/R) injury in testes involves increased reactive oxygen species (ROS) and reactive nitrogen species (RNS) levels in testis tissue with ischemia [5]. These radicals both increase the area of injury and impair sperm quality in the testis tissue via enhanced blood flow with reperfusion [6]. Recently, antioxidant agents have been used to prevent the I/R injury that occurs with unilateral testis torsion and numerous studies have experimentally confirmed that these agents reduce I/R injury [7-12].
Among traditional Chinese herbal products, goji berry (GB) is an herb from the Solanaceae family that has drawn attention due to its antioxidant effects. GB is also colloquially known as wolfberry (WB) or Lycium barbarum (LB). Its widespread use and its beneficial effects that have been reported anecdotally make GB an attractive agent for scientists. Experimental studies have demonstrated that GB reduces blood sugar and lipid levels and exhibits anti-aging, immuno-modulating, antitumor, anti-fatigue, and male fertility-enhancing effects. In various studies, GB was used as a liver-protecting agent in alcohol-induced liver necrosis, a hepatoprotective agent in toxic hepatitis associated with carbon tetrachloride in rats, a cytoprotective agent in the endoplasmic reticulum, and an antioxidant biomarker-increasing agent in healthy adults [13-28]. GB mainly exerts its effects via ion exchange chromatography of a polysaccharide complex composed of six monosaccharides (galactose, glucose, rhamnose, arabinose, mannose, and xylose) that exhibits bioactivity. GB extract (GBE) is obtained when this polysaccharide-rich region is separated [20]. Given that GBE exhibits an antioxidant effect in various organs, we examined its effects in a testis torsion model. In this regard, we determined whether GBE administration reduces I/R injury in rats subject to experimental testis torsion.
Materials and methods
Animals and experimental protocol
This study employed 32 male Sprague-Dawley rats weighing 200 to 250 grams (g). The rats were housed and received follow-up at Dicle University Health Sciences and Research Center (Diyarbakır, Turkey). Rats were randomly selected for the experimental study. The rats were housed in 14 × 9 × 8-centimeter (cm) wooden cages. Prior to the experiment, all rats were fed a standard rat chow and water ad libitum. All rats were maintained in an air-conditioned room at 21°C with a 12 h:12 h light:dark cycle. All rats were handled humanely. The animals were fasted prior to the experiment. The Dicle University Animal Experiments Local Ethics Committee (Diyarbakır, Turkey) approved this study.
A total of 32 male Sprague-Dawley rats were randomized into 4 groups (n = 8).
Group 1: Control (sham operation) Group.
Group 2: Goji Group.
Group 3: Torsion Group.
Group 4: Torsion + Goji Group.
In the control group (Group 1), the left testis was explored and re-closed without performing any torsion procedure (sham operation); saline (2 milliliter (ml)) was intraperitoneally injected for 7 days. The second group received 100 milligrams/kilogram (mg/kg) GBE (2 ml) via intraperitoneal injection for 7 days. The third group underwent detorsion and reperfusion after being subjected to torsion-induced ischemia for 5 hours. This group was injected with intraperitoneal saline (2 ml) for 7 days. The fourth group was administered intraperitoneal GBE at a dose of 100 mg/kg [21] for 7 days. On the last day, torsion-induced ischemia was induced for 5 hours followed by detorsion and reperfusion.
Surgical procedure
After an overnight fasting period, the rats were anesthetized with intraperitoneal (IP) ketamine HCl (Ketalar® 50 milligrams/deciliter (mg/dl), Eczacıbaşı) at 40 mg/kg and xylazine hydrochloride (Rompun® 2%, Bayer). The operation was performed under sterile conditions. The anesthetic duration was the same for all groups Testes were localized via a left scrotal incision, and testis torsion was achieved by twisting the testis 720 degree in a clockwise direction and suturing it to the scrotal skin with 5.0 silk.
The testes were detorsioned and sutured to the scrotal skin with 5.0 silk upon the completion of the 5-hour torsion period. The scrotal skin was closed with 3.0 silk. In the torsion groups, intracardiac blood and testis tissue samples were obtained from the rats under sedation after a 6-hour observation period following detorsion and reperfusion. The rats were sacrificed via cervical dislocation, and their left testes were removed at the end of the experiment.
Extract preparation
To prepare fruit extracts for the antioxidant activity assay, 50 g of shade-dried and powdered roots were soaked in 500 ml of ethanol for 7 days at room temperature. The filtrate was collected and evaporated to dryness to yield 2 g of the extract [26,27]. A GB extract rich in antioxidants was obtained after processing GB with ethanol [27,28]. After being dissolved in 2 ml of normal saline, the GB extract obtained was administered to the rats at single dose of 100 mg/kg [21] intraperitoneally for 7 days.
Biochemical analysis and oxidant-antioxidant parameters
Blood samples obtained at maximal amounts via the intracardiac route were centrifuged, and the sera were kept at -70°C until the biochemical analysis. TAC (total antioxidant capacity), TOS (total oxidant status), and OSI (oxidative stress index) levels were calculated. TAC and TOS levels of supernatant fractions were calculated using a new automated measurement technique described by Erel [29,30].
In this method, a hydroxyl radical is produced, which is the most potent biological radical. In the assay, a ferrous ion solution (Reagent 1) is admixed with the hydrogen peroxide present in Reagent 2. The subsequently emerging radicals, including the brown-colored dianisidinyl radical cation produced by the hydroxyl radical, are also potent radicals. This method allows measurements of the antioxidative effect of the sample against the potent free radical reactions, which is initiated by the hydroxyl radical product. The assay exhibits excellent precision values that are typically less than 3%. The results are expressed as nmol Trolox Equiv./mg protein.
The sample contains oxidants that oxidize the ferrous ion-o-dianisidine complex to a ferric ion. Glycerol molecules that are abundant in the reaction medium augment the oxidation reaction. In an acidic medium, the combination of ferric ion and xylenol orange produces a colored complex. The color intensity is related to the total amount of oxidant molecules in the sample. The assay is calibrated with hydrogen peroxide, and the results are expressed in terms of nmol H2O2 Equiv./mg protein.
The percent ratio of TOS and TAC levels represents the OSI. The following formula is used to calculate the OSI value: OSI (Arbitrary Unit) = TOS (nmol H2O2 Equiv./mg protein)/TAC (nmol Trolox Equiv./mg protein) [31]. The results were expressed as arbitrary units.
Histopathological evaluation
The tissue samples were fixed in 10% formol solution and embedded in paraffin blocks. Approximately 5-μm-thick sections were stained with hematoxylin-eosin (H&E) and examined under light microscopy. A single pathologist who was blind to the study protocol examined the specimens. As described by Mikuz [32], interstitial injury was graded on a scale from 0 to 3 as follows: Grade 0, normal interstitium; Grade 1, interstitial capillary edema and congestion; Grade 2, interstitial hemorrhage; Grade 3, hemorrhagic infarct and degeneration. Vascular congestion, testis parenchyma hemorrhage, and seminiferous tubule degeneration were graded 0 to 3 in ascending order. Hemorrhage levels were classified as follows: Grade 0, no hemorrhage; Grade 1: slight hemorrhage; Grade 2: moderate hemorrhage; Grade 3: diffuse hemorrhage.
Statistical analysis
SPSS for Windows 15.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The results are presented as the mean + SD. Chi-square tests for categorical variables were used for univariate statistical analyses. The Mann-Whitey U test was used for multiple comparisons. Kruskal Wallis one-way variance analysis was used when sample sizes were not equal and groups did not exhibit homogenous distributions in in-group comparisons. Statistical significance was set as P < 0.01 for comparisons between groups and P < 0.05 for other comparisons.
Results
Statistically significant differences were noted between the groups with respect to TAC, TOS, and OSI levels (Table 1).
Table 1.
Biochemical analysis results
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | P-valuea |
|---|---|---|---|---|---|
| TOS (µmol/L) | 32.88 ± 14.70 | 47.60 ± 24.16 | 120.94 ± 44.26 | 65.99 ± 16.55 | < 0.001 |
| TAC (mmol/L) | 1.30 ± 1.02 | 3.09±0.71 | 1.32 ± 0.32 | 3.02 ± 0.58 | < 0.001 |
| OSI (%) | 3.48 ± 2.34 | 1.63 ± 0.97 | 9.41 ± 3.75 | 2.20 ± 0.45 | < 0.001 |
Kruskal Wallis one-way variance analysis.
Comparison of the groups according to serum TAC, TOS and OSI levels (Table 2)
Table 2.
Comparison of the groups according to sera TAC, TOS and OSI levels
| Parameter | Groups | P-valuea | |
|---|---|---|---|
| Serum TAC | I (1.30 ± 1.02) | II (3.09 ± 0.71) | < 0.008 |
| III (1.32 ± 0.32) | 1.000 | ||
| IV (3.02 ± 0.58) | < 0.008 | ||
| II (3.09 ± 0.71) | III (1.32 ± 0.32) | < 0.001 | |
| IV (3.02 ± 0.58) | 1.000 | ||
| III (1.32 ± 0.32) | IV (3.02 ± 0.58) | < 0.001 | |
| Serum TOS | I (32.88 ± 14.70) | II (47.60 ± 24.16) | 1.000 |
| III (120.94 ± 44.26) | < 0.001 | ||
| IV (65.99 ± 16.55) | 0.138 | ||
| II (47.60 ± 24.16) | III (120.94 ± 44.26) | < 0.001 | |
| IV (65.99 ± 16.55) | 1.000 | ||
| III (120.94 ± 44.26) | IV (65.99 ± 16.55) | 0.003 | |
| Serum OSI | I (3.48 ± 2.34) | II (1.63 ± 0.97) | 0.690 |
| III (9.41 ± 3.75) | < 0.001 | ||
| IV (2.20 ± 0.45) | 1.000 | ||
| II (1.63 ± 0.97) | III (9.41 ± 3.75) | < 0.001 | |
| IV (2.20 ± 0.45) | 1.000 | ||
| III (9.41 ± 3.75) | IV (2.20 ± 0.45) | < 0.001 | |
The Mann-Whitey U test was used for multiple comparisons.
Groups 2 and 4 exhibited significantly increased mean serum TAC levels compared with Groups 1 and 3 (P < 0.001).
Group 3 exhibited significantly increased mean serum TOS levels compared with Groups 1, 2, and 4 (P < 0.001, P < 0.001, and P = 0.003, respectively).
Group 3 exhibited a significantly higher mean serum OSI level compared with Groups 1, 2, and 4 (P < 0.001 for each).
Regarding the results of the histopathological grades (Table 3), Groups 1 and 2 exhibited no histopathological change and were assigned a grade of 0, whereas Groups 3 and 4 exhibited different levels of histopathological changes.
Table 3.
Histopathological examination results
| Parameter | Grade | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|---|
| Hemorrhage | (0) | 8 | 8 | 0 | 0 |
| (I) | 0 | 0 | 0 | 3 | |
| (II) | 0 | 0 | 2 | 4 | |
| (III) | 0 | 0 | 6 | 1 | |
| Congestion | (0) | 8 | 8 | 0 | 0 |
| (I) | 0 | 0 | 0 | 2 | |
| (II) | 0 | 0 | 2 | 4 | |
| (III) | 0 | 0 | 6 | 1 | |
| Degeneration | (0) | 8 | 8 | 0 | 0 |
| (I) | 0 | 0 | 8 | 8 | |
| (II) | 0 | 0 | 0 | 0 | |
| (III) | 0 | 0 | 0 | 0 |
Comparison among the groups revealed that Group 4 exhibited more hemorrhaging and congestion than Groups 1 and 2 (P = 0.038). Group 3 exhibited greater hemorrhaging and congestion than Group 4 (P = 0.020). No significant differences were noted among the groups with regard to degeneration.
Group 3 exhibited increased hemorrhaging and congestion compared with Groups 1 and 2 (P = 0.003). Although Group 4 exhibited a greater extent of hemorrhaging and congestion compared with Groups 1 and 2, this difference was not statistically significant.
According to the results of the histopathological examination; Group 1 normal testis parenchyma, group 2 display mild vascular congestion (Figure 1A, 1B). Group 3 also contained a lot of damage rate in the rat testis parenchyma, while group 4 also shows that this damage is reduced (Figure 1C, 1D).
Figure 1.
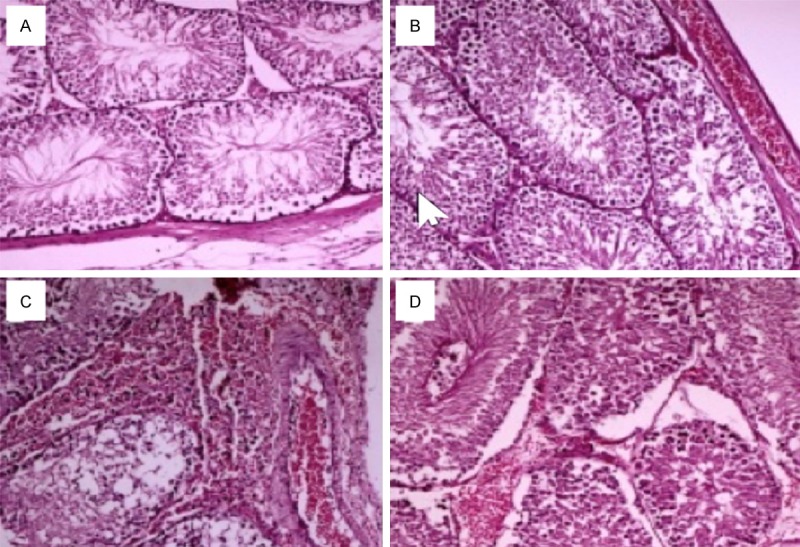
Histologic findings in rat testis. A. Normal testis parenchyma in a rat from the control group (H&E, 100 ×). B. Testis parenchyma displaying mild vascular congestion in a rat from the goji group (H&E, 100 ×). C. Severe congestion, hemorrhaging and injury to the seminiferous tubule in a rat from the torsion group (H&E, 100 ×). D. Testis parenchyma exhibiting mild vascular congestion and mild hemorrhaging in a rat from the torsion + goji group (H&E, 100 ×).
Discussion
Testis torsion is not a fatal condition, but it may result in organ loss if not recognized in a timely manner. Therefore, an early diagnosis both prevents organ loss and reduces the risk of infertility. Various studies have reported that fertility is impaired after unilateral testis torsion [1,2,33]. The mechanism of contralateral testis injury after unilateral testis torsion is not entirely clear.
In testis torsion, a minimum of 1 to 5 hours of ischemia is required to sustain injury [9]. In our study, one testis of the rats was twisted 720 degrees clockwise; this torsion position was maintained for 5 hours and detorsioned thereafter. The injury caused by I/R insult after detorsion may be more severe than that caused by the ischemia itself. Timely diagnosis and treatment of testis torsion is an important issue; the diagnosis is often delayed, necessitating surgical therapy (orchiectomy). Both early detorsion and ischemic reperfusion following orchiectomy are known to cause injury to ipsilateral and contralateral testes. In the pathogenesis of this injury, oxidant molecules have been thought to play a role; thus, agents with antioxidant properties, such as allopurinol, caffeic acid phenethyl ester, melatonin, selenium, resveratrol, and N-acetylcysteine [7-12], have been used experimentally. These agents reduce ischemic reperfusion injury. We were the first to study the effect of GBE, which possesses known antioxidant properties, on testis torsion.
Mammalian testes are very sensitive to oxidants, and oxidants form the mechanistic basis for the injury. Conversely, testicular injury is relieved by the administration of antioxidant molecules. Buchelli et al reported an increased total antioxidant capacity (TAC) in GBE-administered rats [34]. We similarly found that TAC increased and TOS and OSI levels were statistically significant. Another experimental study revealed that oral administration of GBE to rats provides in vivo protection against heat-induced testis injury, in vitro protection against cellular oxidative injury caused by H2O2, and beneficial effects for fertilization, thus confirming the efficacy of GBE in testicular tissues [23]. Similarly, the results of the present study biochemically demonstrated that rats administered GBE and subject to the experimental testis torsion procedure were exposed to less ischemic reperfusion injury than rats not administered GBE. Histopathological examination confirmed that Group 4 exhibited reduced hemorrhaging and congestion compared with Group 3 but did not display any differences in degeneration. These results suggest that GBE was more beneficial in lower grades given its ability to reduce hemorrhaging and congestion; GBE was not as successful in the higher grades given that it did not affect degeneration.
Conclusion
We determined that GBE reduced ischemic reperfusion injury via its antioxidant effects in testis torsion. However, further experimental and clinical studies are needed to confirm its efficacy for this indication.
Acknowledgements
This study was conducted at the Dicle University Medical Faculty Health Research Center with the approval of the ethics committee for animal studies. This study was not supported by any financial sources; it was financed by the authors’ own budgets.
Disclosure of conflict of interest
None.
References
- 1.Yıldız H, Durmus AS, Simsek H, Yaman M. Protective effect of sildenafil citrate on Contralateral testis injury after unilateral testicular torsion/detorsion. Clinics (Sao Paulo) 2011;66:137–142. doi: 10.1590/S1807-59322011000100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vısser AJ, Heyns CF. Testicular function after torsion of the spermatic cord. BJU Int. 2003;92:200–203. doi: 10.1046/j.1464-410x.2003.04307.x. [DOI] [PubMed] [Google Scholar]
- 3.Wei SM, Yan ZZ, Zhou J. Beneficial effect of taurine on testicular ischemia reperfusion injury in rats. Urology. 2007;70:1237–1242. doi: 10.1016/j.urology.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Lysiak JJ, Turner SD, Nguyen QA, Singbartl K, Ley K, Turner TT. Essential role of neutrophils in germ cell-specific apoptosis following ischemia/reperfusion injury on the mouse testis. Biol Reprod. 2001;65:718–725. doi: 10.1095/biolreprod65.3.718. [DOI] [PubMed] [Google Scholar]
- 5.Power RE, Scanlon R, Kay EW, Creagh TA, Bouchier-Hayes DJ. Long-term protective effects of hypothermia on reperfusion injury post-testicular torsion. Scand J Urol Nephrol. 2003;37:456–460. doi: 10.1080/00365590310014508. [DOI] [PubMed] [Google Scholar]
- 6.Turner TT, Tung KS, Tomomasa H, Wilson LW. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod. 1997;57:1267–1274. doi: 10.1095/biolreprod57.6.1267. [DOI] [PubMed] [Google Scholar]
- 7.Prillaman HM, Turner TT. Rescue of testicular function after acute experimental torsion. J Urol. 1997;157:340–345. [PubMed] [Google Scholar]
- 8.Koltuksuz U, Irmak MK, Karaman A, Uz E, Var A, Ozyurt H, Akyol O. Testicular nitric oxide levels after unilateral testicular torsion/detorsion in rats pretreated with caffeic acid phenethyl ester. Urol Res. 2000;28:360–363. doi: 10.1007/s002400000145. [DOI] [PubMed] [Google Scholar]
- 9.Abasiyanik A, Dagdonderen L. Beneficial effects of melatonin compared with allopurinol in experimental testicular torsion. J Pediatr Surg. 2004;39:1238–1241. doi: 10.1016/j.jpedsurg.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Uguralp S, Mizrak B, Bay Karabulut A. Resveratrol reduces ischemia reperfusion injury after experimental testicular torsion. Eur J Pediatr Surg. 2005;15:114–119. doi: 10.1055/s-2004-830359. [DOI] [PubMed] [Google Scholar]
- 11.Cay A, Alver A, Kucuk M, Işik O, Eminağaoğlu MS, Karahan SC, Değer O. The effects of N-acetylcysteine on antioxidant enzyme activities in experimental testicular torsion. J Surg Res. 2006;131:199–203. doi: 10.1016/j.jss.2005.11.572. [DOI] [PubMed] [Google Scholar]
- 12.Avlan D, Erdougan K, Cimen B, Dusmez Apa D, Cinel I, Aksoyek S. The protective effect of selenium on ipsilateral and contralateral testes in testicular reperfusion injury. Pediatr Surg Int. 2005;21:274–278. doi: 10.1007/s00383-005-1365-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang HX, Ng TB. Natural products with hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities. Life Sci. 1999;65:2663–2677. doi: 10.1016/s0024-3205(99)00253-2. [DOI] [PubMed] [Google Scholar]
- 14.Amagase H, Sun B, Borek C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr Res. 2009;29:19–25. doi: 10.1016/j.nutres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Lin CL, Wang CC, Chang SC, Inbaraj BS, Chen BH. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int J Biol Macromol. 2009;45:146–151. doi: 10.1016/j.ijbiomac.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Hu Y, Wang D, Zhang F, Zhao X, Abula S, Fan Y, Guo L. Lycium barbarum polysaccharide inhibits the infectivity of Newcastle disease virus to chicken embryo fibroblast. Int J Biol Macromol. 2010;46:212–216. doi: 10.1016/j.ijbiomac.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Li XM, Ma YL, Liu XJ. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J Ethnopharmacol. 2007;111:504–511. doi: 10.1016/j.jep.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Bryan JK, Costa D, Giese N, Nummy K, Rapp C, Seamon E. Goji (Lycium spp) in Natural Standard Monograph. Natural Standard Inc; Available: http://www.naturalstandard.com/ (accessed 6 May 2008) [DOI] [PubMed] [Google Scholar]
- 19.Xiao J, Liong EC, Ching YP, Chang RC, So KF, Fung ML, Tipoe GL. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress andnecroinflammation. J Ethnopharmacol. 2012;139:462–470. doi: 10.1016/j.jep.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Chang D, Kong H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules. 2011;16:2542–2550. doi: 10.3390/molecules16032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin SM, Kil HR, Park K, Noh C. Gene expression in rat hearts following oral administration of a single hepatotoxic dose of acetaminophen. Yonsei Med J. 2012;53:172–180. doi: 10.3349/ymj.2012.53.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian JY, Liu D, Huang AG. The efficiency of flavonoids in polar extracts of Lycium chinense Mill. fruits as free radical scavenger. Food Chem. 2004;87:283–288. [Google Scholar]
- 23.Luo Q, Li Z, Huang X, Yan J, Zhang S, Cai YZ. Lycium barbarum polysaccharides: protective effects against heat-induced damage of rat testes and H2O2-induced DNA damage in mouse testicular cells and beneficial effect on sexual behavior and reproductive function of hemicastrated rats. Life Sci. 2006;79:613–621. doi: 10.1016/j.lfs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Luo Q, Cai YZ, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 25.Cai YZ, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ananthi R, Chandra N, Santhiya ST, Ramesh A. Genotoxic and antigenotoxic effects of Hemidesmus indicus R. Br. root extract in cultured lymphocytes. J Ethnopharmacol. 2010;127:558–560. doi: 10.1016/j.jep.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Mu M. Studies on extraction of polysaccharides from Lycium barbarum. J Anhui Agric Sci. 2007;35:3736–3737. [Google Scholar]
- 28.Meng LY, Qiu AS, Lan TF, Jiang CC. Optimization of ultrasound extraction technology of Lycium barbarum polysaccharide and study on its antioxidation ability. J Anhui Agric Sci. 2009;37:12168–12170. [Google Scholar]
- 29.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95. doi: 10.1186/1471-2334-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikuz G. Testicular torsion: simple grading for histological evaluation of tissue damage. Appl Pathol. 1985;3:134–139. [PubMed] [Google Scholar]
- 33.Cankorkmaz L, Köylüoğlu G, Özer H, Yıldız E, Sümer Z, Özdemir Ö. The role of apoptosis and protective effect of carnitine in contralateral testicular injury in experimental unilateral testicular torsion. Ulus Travma Acil Cerrahi Derg. 2009;15:529–534. [PubMed] [Google Scholar]
- 34.Bucheli P, Vidal K, Shen L, Gu Z, Zhang C, Miller LE, Wang J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom Vıs Sci. 2011;88:257–262. doi: 10.1097/OPX.0b013e318205a18f. [DOI] [PubMed] [Google Scholar]


