Abstract
Aim: Cholangiocarcinoma is generally detected late in the course of disease, and current diagnostic techniques often fail to differentiate benign from malignant disease. Ongoing biomarker studies for early diagnosis of cholangiocarcinoma are still continues. By this study, we analyzed the roles of serum and biliary MMP-9 and TIMP-1 concentrations in the diagnosis of cholangiocarcinoma. Materials and methods: The 113 patients (55 males, 58 females) were included; 33 diagnosed with cholangiocarcinoma (malignant group) and 80 diagnosed with choledocholithiasis (benign group). MMP-9 and TIMP-1 concentrations were analyzed in serum and bile and compared in the malignant and benign groups. Results were evaluated statistically. Results: Biliary MMP-9 concentrations were significantly higher (576 ± 209 vs. 403 ± 140 ng/ml, p < 0.01) and biliary TIMP-1 concentrations were significantly lower (22.4 ± 4.9 vs. 29.4 ± 6.1 ng/ml, p < 0.01) in the malignant than in the benign group. In contrast, serum MMP-9 and TIMP-1 concentrations were similar in the two groups. Receiver operating curve analysis revealed that the areas under the curve of bile MMP-9 and TIMP-1 were significantly higher than 0.5 (p < 0.001). The sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios and accuracy were 0.94, 0.32, 0.36, 0.93, 1.40, 0.19 and 0.5 for biliary MMP-9, respectively, and 0.97, 0.36, 0.39, 0.97, 1.5, 0.08 and 0.54 for biliary TIMP-1, respectively. Conclusion: Serum and biliary MMP-9 and TIMP-1 tests do not appear to be useful in the diagnosis of cholangiocarcinoma.
Keywords: Cholangiocarcinoma, choledocholithiasis, TIMP-1, MMP-9
Introduction
Cholangiocarcinoma (CCA) is an aggressive malignant tumor arising from the epithelial lining of the biliary tract [1]. Unlike most human cancers, CCA is extremely difficult to diagnose because of its location and size [2,3]. Tumor masses often cannot be identified by radiological methods and endoscopy approaches are of limited usefulness in tissue diagnosis [4]. The prognosis of patients with CCA is generally poor with a 5-year survival rate below 5% [5]. Surgical resection has the potential to be curative, but most patients with CCA are diagnosed at advanced stages when surgery is no longer feasible [6].
Serum tumor markers are non-invasive diagnostic tools for identifying malignant tumors, and are commonly used to screen individuals for cancer, as prognostic factors and as indicators of treatment efficacy. Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) have been shown to be sensitive serum markers for identifying various cancers. These molecules are stored in granules of platelets and white cells and are released due to cellular disintegration during the coagulation process. Although serum levels of MMP-9 and TIMP-1 may be 3-6 times higher in serum than in plasma, these differences are not statistically significant.
Degradation of the extracellular matrix (ECM) is a prerequisite for cancer invasiveness and metastasis, with the latter processes strongly affecting the prognosis of cancer patients. The MMP family has been shown to play important roles in cancer cell invasion by digesting the ECM, and highly invasive tumor cells have been shown to secrete large quantities of these proteolytic enzymes [7]. Among the MMPs are the gelatinases, MMP-2 (gelatinase A) and MMP-9 (gelatinase B), which have been found to play key roles in tumor invasion. The most important endogenous suppressors of MMP activity are the TIMPs, with TIMP-1 and TIMP-2 inhibiting the activities of MMP-9 and MMP-2, respectively [8,9]. The levels of expression of the MMPs and TIMPs have been found to be altered in benign and malignant tumors, as well as during tumor invasion and metastasis, processes which require the breakdown and removal of ECM [10]. Previous studies have demonstrated that serum MMP-7 levels are elevated in patients with CCA and with colorectal, gastric, ovarian or renal cancers [11-14]. To our knowledge, no previous study has compared serum and bile concentrations of MMP-9 and TIMP-1 in patients with benign and malignant biliary strictures. We therefore measured these concentrations to assess the diagnostic role of these two markers in the prediction of CCA.
Materials and methods
Patients
Patients diagnosed with choledocholithiasis and cholangiocarcinoma were included in this study. A diagnosis of choledocholithiasis was based on patient history (right upper quadrant and epigastric pain, vomiting, fever) physical examination (Murphy sign), laboratory tests (elevated AST, ALT, alkaline phosphatase, GGT, and total and direct bilirubin concentrations), ultrasonography, MRCP, upper abdominal MRI, ERCP and/or EUS. A diagnosis of CCA was based on patient history (icterus, weight loss), physical examination (silent jaundice), imaging methods (CT, MRI, and PET) and laboratory tests (elevated AST, ALT, alkaline phosphatase, GGT, total and direct bilirubin, CEA, and CA 19-9 concentrations). Patients with sepsis or severe heart, renal or liver failure, as well as those unsuitable for ERCP due, for example, to bleeding tendency, were excluded from the study. All patients provided written informed consent. The study protocol was approved by the institutional review board (B.30.2.BAV.0.05.05/398-11.07.2012 and 71306642/050-01-04/105). All procedures were in accordance with the ethical standards of the committee on human experimentation of our institution and with the Declaration of Helsinki.
Sample collection
Blood samples were obtained before ERCP, and bile samples were obtained during ERCP using an ERCP catheter and EUS syringe. Serum and bile samples were stored at -80°C. AST, ALT, alkaline phosphatase, GGT, total protein, albumin, CRP, amylase, lipase, and total and direct bilirubin concentrations; as well as hematocrit, platelet counts, INR, and prothrombin time, were measured at Bezmialem Vakıf University. Serum and biliary MMP-9 and TIMP-1 concentrations were measured by ELISA assays (R & D Systems Europe Ltd, UK), performed in the Biochemistry Department of the Istanbul Cerrahpaşa Medicine Faculty.
Statistical analysis
All statistical analyses were performed using SPSS for Windows 15.0 software (Statistical Package for the Social Sciences). Quantitative data were reported as mean ± standard deviation; normally distributed parameters were compared using Student’s t-tests and nonnormally distributed parameters were compared using Mann Whitney U tests. Qualitative data were compared using the chi-square test. True and false positive rates [sensitivity and (1-specificity)] of the diagnostic accuracy of each test were evaluated using ROC curve analysis. The areas under the curve (AUCs) with 95% confidence intervals were calculated from the ROC curves for MMP-9 and TIMP-1 tests. ROC analysis was used to determine MMP-9 and TIMP-1 cutoff concentrations, as well as to determine their sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), FP, FN and accuracy. A p value < 0.05 was considered statistically significant.
Results
This prospective controlled cohort study involved 113 patients, including 55 men and 58 women, aged 31-87 years (mean age, 65.03 ± 12.21 years), who were evaluated between July 2012 and May 2013 in the gastroenterology clinic of Bezmialem Vakıf University. Of these 113 patients, 80 (70.8%) had choledocholithiasis and 33 (29.2%) had CCA (Table 1). The two groups were similar in mean age and gender distribution (p > 0.05).
Table 1.
Demographic and Laboratory Features
| Malignant | Benign | p | |
|
| |||
| Age, yr, mean ± SD | 66.06 ± 10.15 | 64.61 ± 13.00 | 0.569+ |
| Gender, n (%) | |||
| Female | 18 (54.5) | 40 (50.0) | 0.660++ |
| Male | 15 (45.5) | 40 (50.0) | |
|
| |||
| mean ± SD (Median) | mean ± SD (Median) | ||
|
| |||
| Serum MMP-9, ng/ml | 331.82 ± 16.53 | 330.37 ± 19.17 | 0.706 |
| Bile MMP-9, ng/ml | 575.85 ± 209.27 | 403.02 ± 140.12 | 0.001 |
| Serum TIMP-1, ng/ml | 220.91 ± 13.94 | 218.69 ± 16.93 | 0.507 |
| Bile TIMP-1, ng/ml | 22.42 ± 4.88 | 29.41 ± 6.09 | 0.001 |
| AST+++, U/L | 109.53 ± 107.14 (78.3) | 98.02 ± 113.69 (49.9) | 0.243 |
| ALT+++, U/L | 144.56 ± 189.95 (73) | 122.24 ± 123.99 (76) | 0.920 |
| Alkaline Phosphatase+++, U/L | 423.86 ± 423.86 (319) | 210.74 ± 224.96 (145) | 0.006 |
| GGT++, U/L | 628.97 ± 950.35 (232) | 347.96 ± 401.76 (211) | 0.352 |
| Total Bilirubin+++, U/L | 7.55 ± 8.66 (2.6) | 2.76 ± 4.16 (1.2) | 0.022 |
| Direct Bilirubin+++, U/L | 5.81 ± 6.58 (2.6) | 4.67 ± 26.74 (0.8) | 0.007 |
| INR+++ | 1.17 ± 0.24 (1.1) | 3.30 ± 13.66 (1.1) | 0.714 |
| CRP++, mg/L | 11.62 ± 25.57 (2.9) | 4.31 ± 6.29 (1.4) | 0.105 |
| Hct+++, % | 34.90 ± 5.82 (35.9) | 41.94 ± 23.02 (39.8) | 0.001 |
| Amylase+++, U/L | 267.82 ± 713.28 (70) | 137.70 ± 248.95 (69) | 0.589 |
| Lipase+++, U/L | 101.46 ± 126.32 (38) | 128.41 ± 293.99 (39.5) | 0.686 |
| Total Protein+, gr/dl | 6.08 ± 1.11 | 6.53 ± 0.81 | 0.019 |
| Albumin+, gr/dl | 3.45 ± 0.79 | 3.96 ± 0.57 | 0.002 |
| Prothrombin Time+, sec | 14.67 ± 2.74 | 13.90 ± 2.57 | 0.164 |
| Sedimentation+, mm/h | 89.15 ± 20.37 | 72.84 ± 33.59 | 0.002 |
| Platelets+, n/mm3 | 237.35 ± 80.94 | 238.35 ± 70.47 | 0.933 |
Student’s t test;
χ2 test;
Mann Whitney U Test.
Mean serum MMP-9 and TIMP-1 concentrations were similar in the two groups (p > 0.05). However, mean biliary MMP-9 concentrations were significantly higher (575.85 ± 209.27 vs. 403.02 ± 140.12 ng/ml, p < 0.01) and mean TIMP-1 concentrations significantly lower (22.42 ± 4.88 vs. 29.41 ± 6.09 ng/ml, p < 0.01) significant lower in the CCA than in the benign group (Table 1).
Tests of other laboratory parameters showed that mean alkaline phosphatase (p < 0.01), total bilirubin (p < 0.05) and direct bilirubin (p < 0.01) concentrations and sedimentation rate (p < 0.01) were significantly higher in the CCA group. In contrast, mean hematocrit (p < 0.01) and total protein (p < 0.05) and albumin (p < 0.01) concentrations were significantly lower in the CCA group. Similar concentrations of AST, ALT, GGT, CRP, amylase, and lipase, and similar INR, prothrombin time, and platelet counts, were observed in the two groups (p > 0.05 each; Table 1).
Using a serum MMP-9 cut-off of 325 ng/ml, we found that the sensitivity, specificity, PPV, NPV, accuracy, and positive and negative likelihood ratios were 0.7273, 0.3625, 0.32, 0.7631, 0.469, 1.1408 and 0.7523, respectively. A biliary MMP-9 cutoff of 350 ng/ml resulted in a sensitivity, specificity, PPV, NPV, accuracy, and positive and negative likelihood ratios of 0.9394, 0.325, 0.3647, 0.9286, 0.5044, 0.1864 and 1.3917, respectively (Table 2).
Table 2.
Concentrations of MMP-9 and TIMP-1 predictive of a diagnosis of cholangiocarcinoma
| Sensitivity | Specificity | PPV | NPV | PLR | NLR | Accuracy | |
|---|---|---|---|---|---|---|---|
| Serum MMP-9 (cut-off: 325 ng/ml) | 0.7273 | 0.3625 | 0.32 | 0.7631 | 1.1408 | 0.7523 | 0.4690 |
| Bile MMP-9 (cut-off: 350 ng/ml) | 0.9394 | 0.325 | 0.3647 | 0.9286 | 1.3917 | 0.1864 | 0.5044 |
| Serum TIMP-1 (cut-off: 205 ng/ml) | 0.9697 | 0.1375 | 0.3168 | 0.9167 | 1.1243 | 0.2204 | 0.3805 |
| Bile TIMP-1 (cut-off: 31 ng/ml) | 0.9697 | 0.3625 | 0.3855 | 0.9667 | 1.5211 | 0.0836 | 0.5398 |
PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
A serum TIMP-1 concentration of 205 ng/ml had a sensitivity, specificity, PPV, NPV, accuracy, and positive and negative likelihood ratios of 0.9697, 0.1375, 0.3168, 0.9167, 0.3805, 1.1243 and 0.2204, respectively; whereas a biliary TIMP-1 cut-off of 31 ng/ml had a sensitivity, specificity, PPV, NPV, accuracy, and positive and negative likelihood ratios of 0.9697, 0.3625, 0.3855, 0.9667, 0.5398, 1.5211 and 0.0836, respectively (Table 2).
ROC analyses showed that the AUCs for serum and biliary MMP-9 concentrations of 325 and 350 ng/ml, respectively, were 0.539 (95% CI 0.424-0.654) and 0.741 (95% CI 0.635-0.848), respectively. The AUCs for serum and biliary TIMP-1 concentrations of 205 and 31 ng/ml were 0.565 (95% CI 0.452-0.677) and 0.818 (95% CI 0.738-0.899), respectively. When the AUCs for these parameters were compared with a random value of 0.5 (the worst value under the curve), only serum MMP-9 (p = 0.001) and biliary TIMP-1 (p = 0.001) concentrations were significantly higher than 0.5 (p < 0.01) (Figure 1).
Figure 1.
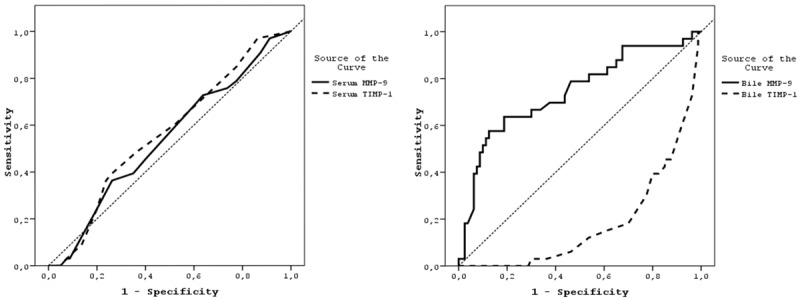
ROC Curves for the Diagnosis of Cholangiocarcinoma. The areas under the curves showed that serum MMP-9 (p = 0.001) and bile TIMP-1 (p = 0.001) concentrations were predictive of cholangiocarcinoma.
Discussion
As expected, we found that alkaline phosphatase and total and direct bilirubin concentrations, as well as sedimentation levels, were significantly higher in patients with CCA than in patients with choledocholithiasis. In addition, hematocrit, total protein and albumin concentrations were significantly lower in the CCA group. None of the other laboratory parameters differed significantly in these two patient groups.
Immunohistochemical assays have shown that MMP-9 and MMP-7 expression is higher in CCA specimens than in adjacent normal tissue [15,16], while another study found that serum MMP-9 concentrations did not differ significantly in patients with CCA and benign conditions [11]. Although MMP-9 and TIMP-1 levels are increased by other diseases, our study focused only on patients with CCA and choledochal stones. The AUC for serum MMP-9 was 0.539 (95% CI 0.424-0.654) in our study, similar to the AUC of 0.59 (95% CI 0.455-0.722) reported previously [11]. We found that the sensitivity and specificity of serum MMP-9 were 73% and 36%, respectively, similar to the 64% and 42%, respectively, reported earlier [11].
To our knowledge, no previous study has reported a relationship between serum TIMP-1 concentration and CCA. We found that the sensitivity, specificity and accuracy of serum TIMP-1 were 97%, 14% and 38%, respectively. A study assessing the prognostic efficacy of serum TIMP-1, MMP-9 and CA19-9 concentrations in patients with pancreatic ductal adenocarcinoma found that the sensitivity, specificity and AUC of TIMP-1 were 47.1%, 69.2% and 0.64, respectively; although that study found that circulating MMP-9 and TIMP-1 concentrations were inferior to CA19-9 as markers of malignancy. The combination of high sensitivity and low specificity of serum MMP-9 and TIMP-1 concentrations may be due to the lesions in the benign group acting like CCA. MMP-9 are involved in inflammation, tissue remodeling, wound healing, mobilization of matrix-bound growth factors and processing of cytokines [17-20], conditions not specific to CCA. This reduces the specificity of serum MMP-9 and TIMP-1 concentrations as markers of CCA, suggesting that the concentrations of these proteins were not helpful in the diagnosis of CCA.
We found that biliary MMP-9 concentrations were significantly higher and biliary TIMP-1 concentrations significantly lower in the malignant than in the benign group (P < 0.001 each). However, the concentrations of these proteins cannot be used as absolute evidence for the presence or absence of malignant disease. RT-PCR of samples from patients with intra-hepatic CCA showed a loss of balanced expression between MMPs and TIMPs, especially decreased expression of TIMPs, indicating the invasiveness of ICC [7]. The sensitivity, specificity and accuracy were 94%, 32.5%, and 50.4%, respectively, for biliary MMP-9 and 97%, 36%, and 54%, respectively, for TIMP-1. NGAL concentration has been reported to be a significant indicator for differentiating between benign and malignant biliary disorders, whereas MMP-9 concentration was not [21]. Another study found that the bile concentrations of MMP-2 and proMMP-9 were significantly higher in patients with liver metastasis of colorectal cancer than in patients with metastasis-free colorectal cancer [22]. Although we found that biliary MMP-9 and TIMP-1 concentrations differed significantly in our CCA and choledocholithiasis groups, these concentrations showed high sensitivity, low specificity and medium accuracy rates, precluding their use in the diagnosis of CCA.
In conclusion, we have shown that serum and biliary MMP-9 and TIMP-1 concentrations were ineffective in the differential diagnosis of CCA and benign conditions, due to their higher sensitivity, lower specificity and medium accuracy rates. These assays should be used only in conjunction with other investigations and procedures in the diagnosis of disease. Serum and biliary MMP-9 and TIMP-1 concentrations should not replace any established clinical methods in the diagnosis of CCA.
Acknowledgements
We thank biologist Nihan Eroğlu for her kind help with this study.
Disclosure of conflict of interest
None.
References
- 1.Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 1: epidemiology and cytology. J Gastroenterol Hepatol. 2002;17:1049–1055. doi: 10.1046/j.1440-1746.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 2.Saito M, Hige S, Takeda H, Tomaru U, Shibata M, Asaka M. Combined hepatocellular carcinoma and cholangiocarcinoma growing into the common bile duct. J Gastroenterol. 2001;36:842–847. doi: 10.1007/s005350170007. [DOI] [PubMed] [Google Scholar]
- 3.Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Miyazaki M. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525–1531. doi: 10.1046/j.1365-2168.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- 4.Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10:427–432. doi: 10.3748/wjg.v10.i3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 6.Tolek A, Wongkham C, Proungvitaya S, Silsirivanit A, Roytrakul S, Khuntikeo N, Wongkham S. Serum α1β-glycoprotein and afamin ratio as potential diagnostic and prognostic markers in cholangiocarcinoma. Exp Biol Med (Maywood) 2012;237:1142–1149. doi: 10.1258/ebm.2012.012215. [DOI] [PubMed] [Google Scholar]
- 7.Jo Chae K, Rha SY, Oh BK, Koo JS, Kim YJ, Choi J, Park C, Park YN. Expression of matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 and -2 in intraductal and nonintraductal growth type of cholangiocarcinoma. Am J Gastroenterol. 2004;99:68–75. doi: 10.1046/j.1572-0241.2003.04025.x. [DOI] [PubMed] [Google Scholar]
- 8.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: An imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s–5059s. [PubMed] [Google Scholar]
- 9.Blavier L, Henriet P, Imren S, Declerck YA. Tissue inhibitors of matrix matalloproteinases in cancer. Ann N Y Acad Sci. 1999;878:108–119. doi: 10.1111/j.1749-6632.1999.tb07677.x. [DOI] [PubMed] [Google Scholar]
- 10.Schröpfer A, Kammerer U, Kapp M, Dietl J, Feix S, Anacker J. Expression pattern of matrix metalloproteinases in human gynecological cancer cell lines. BMC Cancer. 2010;10:553. doi: 10.1186/1471-2407-10-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leelawat K, Sakchinabut S, Narong S, Wannaprasert J. Detection of serum MMP-7 and MMP-9 in cholangiocarcinoma patients: evaluation of diagnostic accuracy. BMC Gastroenterol. 2009;9:30. doi: 10.1186/1471-230X-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragutinovic V, Izrael-Zivkovic L, Radovanovic N. Relation of matrix metalloproteinase-9 to different stages of tumors in the serum of gastric cancer. Dig Dis Sci. 2009;54:1203–1207. doi: 10.1007/s10620-008-0472-y. [DOI] [PubMed] [Google Scholar]
- 13.Gao ZL, Zhang C, Du GY, Lu ZJ. Clinical significance of changes in tumor markers, extracellular matrix, MMP-9 and VEGF in patients with gastric carcinoma. Hepatogastroenterology. 2007;54:1591–1595. [PubMed] [Google Scholar]
- 14.Sarkissian G, Fergelot P, Lamy PJ, Patard JJ, Culine S, Jouin P, Rioux-Leclercq N, Darbouret B. Identification of pro-MMP-7 as a serum marker for renal cell carcinoma by use of proteomic analysis. Clin Chem. 2008;54:574–581. doi: 10.1373/clinchem.2007.090837. [DOI] [PubMed] [Google Scholar]
- 15.Itatsu K, Zen Y, Ohira S, Ishikawa A, Sato Y, Harada K, Ikeda H, Sasaki M, Nimura Y, Nakanuma Y. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007;27:1174–1184. doi: 10.1111/j.1478-3231.2007.01577.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Kim JS, Kang CD, Lee SJ, Kim JY, Yeon JE, Park JJ, Shim JJ, Byun KS, Bak YT, Lee CH. Expression of epidermal growth factor receptor, ErbB2 and matrix metalloproteinase-9 in hepatolithiasis and cholangiocarcinoma. Korean J Gastroenterol. 2005;45:52–59. [PubMed] [Google Scholar]
- 17.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 18.Lyons JG, Birkedal-Hansen B, Pierson MC, Whitelock JM, Birkedal-Hansen H. Interleukin-1 beta and transforming growth factor-alpha/epidermal growth factor induced expression of M(r) 95,000 type IV collagenase/gelatinase and interstitial fibroblast-type collagenase by rat mucosal keratinocytes. J Biol Chem. 1993;268:19143–19151. [PubMed] [Google Scholar]
- 19.Okada Y, Naka K, Kawamura K, Matsumoto T, Nakanishi I, Fujimoto N, Sato H, Seiki M. Localization of matrix metalloproteinase 9 (92-kilodalton gelatinase/type IV collagenase = gelatinase B) in osteoclasts: implications for bone resorption. Lab Invest. 1995;72:311–322. [PubMed] [Google Scholar]
- 20.Tamura T, Nakanishi T, Kimura Y, Hattori T, Sasaki K, Norimatsu H, Takahashi K, Takigawa M. Nitric oxide mediates interleukin-1-induced matrix degradation and basic fibroblast growth factor release in cultured rabbit articular chondrocytes: a possible mechanism of pathological neovascularization in arthritis. Endocrinology. 1996;137:3729–3737. doi: 10.1210/endo.137.9.8756539. [DOI] [PubMed] [Google Scholar]
- 21.Latif A, Zabron A, Horneffer-Van der Sluis V, Wadsworth C, Westaby D, Vlavianos P, Taylor-Robinson S, Edwards R, Khan S. PTU-068 Biliary matrix metalloproteinase 9 levels are independent of neutrophil gelatinase-associated lipocalin in patients with malignant biliary obstruction. Gut. 2012;61(Suppl):A211–A212. [Google Scholar]
- 22.Okada N, Ishida H, Murata N, Hashimoto D, Seyama Y, Kubota S. Matrix metalloproteinase-2 and -9 in bile as a marker of liver metastasis in colorectal cancer. Biochem Biophys Res Commun. 2001;288:212–216. doi: 10.1006/bbrc.2001.5741. [DOI] [PubMed] [Google Scholar]


