Abstract
Objectives: Pulmonary artery sling (PA sling) is frequently associated with tracheal and/or bronchial stenosis. Most PA sling patients receive left pulmonary artery (LPA) re-implantation and tracheoplasty under the cardiopulmonary bypass, but the postoperative complications of tracheoplasty remain a great challenge. In this study, we reviewed 14 PA sling children who received surgery in our hospital, and tried to find out whether tracheoplasty could be avoided or not. Methods: A total of 14 patients receiving surgery due to PA sling/tracheal stenosis were recruited. Complete tracheal ring was confirmed by fiberoptic bronchoscopy in all the patients preoperatively. The clinical outcome and the severity of trachea stenosis (tracheal diameter and length) were evaluated, and effectiveness of various managements was analyzed. Results: Fourteen PA sling/tracheal stenosis children underwent surgical treatment. Three patients needed intubation and mechanical ventilation for severe respiratory symptoms preoperatively. Eight patients received LPA re-implantation alone to relieve the trachea compression, and slide tracheoplasty was performed in one patient for extubation failure who finally died of air leakage. Six patients received LPA re-implantation and tracheal intervention simultaneously. Three patients received slide tracheoplasty, and one was discharged after recovery. The remaining 3 patients received tracheal stent implantation, but finally died. The diameter/length (%) in the survivors without tracheal intervention was significantly higher than that in patients with tracheal intervention. Conclusions: Patients with PA sling undergoing LPA re-implantation achieved a good outcome. Diameter/length (%) may be a more reliable indicator used for determination of tracheal intervention in surgical management of PA sling.
Keywords: Pulmonary artery sling, left pulmonary artery, implantation
Introduction
Pulmonary artery (PA) sling is a rare, congenital cardiovascular disease and usually associated with tracheal and/or bronchial stenosis. In PA sling patients, the anomalous left PA originates from the right PA, passes between trachea and esophagus, and finally arrives at the left lung. Complete tracheal rings are found in about 50%-65% of PA sling patients, and also referred to as “ring-sling” complex [1,2]. Because of the compression of anomaly LPA and tracheal stenosis, a number of patients present with respiratory symptoms after birth, such as cough, wheezing, stridor, and even severe respiratory distress resulting in sudden death [3]. Since Potts et al [4] for the first time reported the surgery for PA sling in 1954, the prognosis of PA sling in infants has not been significantly improved. It is generally believed that PA sling children with moderate respiratory symptoms need surgical treatment. Several techniques have been introduced for tracheoplasty and slide technique may be a best one. Although slide technique for tracheoplasty has been used in PA sling/tracheal stenosis patients, tracheal complications still remain a great challenge postoperatively. These complications include anastomotic leakage and tracheal granulation. In this study, we reviewed the outcome of PA patients in our hospital aiming to identify a type of PA sling with which patients have a good outcome after LPA reimplantation alone without tracheoplasty.
Materials and methods
This was a retrospective study, and 14 PA sling/tracheal stenosis children receiving surgical treatment in our hospital between April 2009 and July 2014 were recruited into present study. This study was approved by the Ethics Committee on human research. The characteristics of patients are shown in Table 1. The diagnosis of PA sling and tracheal stenosis or other cardiac abnormalities was based on routine echocardiography and computed tomography (CT). All CT scans were interpreted by the same radiologists in Department of Pediatric Radiology preoperatively, and the length and diameter of stenosis trachea were recorded.
Table 1.
Demographics and outcome of PA sling patients with tracheal stenosis
| Patients | Age (m) | Sex | Weight (kg) | Associated Anomalies | Tracheal stenosis (% of total length) | Preoperative ventilation | Surgery date | Tracheal Intervention | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 5 | M | 6 | 48 | 2009-4 | Slide | Survival | ||
| Case 2 | 2 | M | 5.3 | 67 | + | 2009-12 | Slide | Death | |
| Case 3 | 6 | F | 6.7 | 33 | 2010-2 | Slide | Death | ||
| Case 4 | 8 | M | 9 | 39 | 2011-11 | Stent | Death | ||
| Case 5 | 5 | F | 5.8 | 42 | + | 2011-11 | Stent | Death | |
| Case 6 | 7 | M | 9 | 20 | 2012-2 | Survival | |||
| Case 7 | 3 | F | 4.6 | PDA | 45 | + | 2012-2 | Survival | |
| Case 8 | 11 | M | 7.5 | PDA | 54 | 2012-6 | Stent | Death | |
| Case 9 | 6 | M | 6.3 | 45 | 2013-9 | Survival | |||
| Case 10 | 12 | F | 10 | 75 | 2013-11 | Slide | Death | ||
| Case 11 | 12 | F | 10 | ASD | 35 | 2013-5 | Survival | ||
| Case 12 | 23 | M | 13 | 25 | 2013-11 | Survival | |||
| Case 13 | 31 | F | 14 | ASD | 25 | 2014-4 | Survival | ||
| Case 14 | 6 | M | 6 | APW | 15 | 2014-7 | Survival |
Two surgical strategies were employed in this study. The first surgical strategy was LPA re-implantation and tracheal stenosis intervention performed simultaneously. Tracheal intervention was done by slide tracheoplasty and tracheal stent implantation. Median sternotomy and cardiopulmonary bypass were used. LPA was taken out between trachea and esophagus, and implanted into normal site of the main PA in front of the trachea. Slide tracheoplasty was performed by airway transection at the mid-point of stenotic segment. The airway tract was opened in the opposite direction of both ends, followed by re-anastomosis with a “slide” method. The newly formed trachea would be half of the length, double of the diameter, and 1/32 of the resistance [5].
The second surgical strategy was LPA re-implantation alone and performed to relieve the trachea compression without tracheal intervention. LPA re-implantation was performed off pump through median sternotomy. If there was difficulty or associated cardiac abnormalities were needed to repair, LPA re-implantation was performed on pump.
Statistical analysis
Descriptive statistics were used, and data were presented as mean ± standard deviation or median (range). The Mann-Whitney U-test was used for comparisons between groups (survivors without tracheal intervention and those with tracheal intervention).
Results
In this study, a total of 14 patients with PA sling and tracheal stenosis were recruited, and there were 8 males and 6 females. The median age at surgery was 7.5 months (range: 2 to 31 months). Before admission, the symptoms included repeating stridor, respiratory distress and bronchiolitis/pneumonia. Three patients (cases 2, 5 and 7) required intubation and mechanical ventilation for respiratory failure and carbon dioxide retention. All the patients have complete cartilage trachea rings (O-rings). Two patients had concomitant other lung anomalies. Bronchus (right upper lobe bronchus direct from trachea) was found in two patients (Cases 4 and 14) and pulmonary sequestration in another patient (Case 9). Associated cardiac anomalies were noted in 5 patients, 1 patient (Case 14) had aortopulmonary window (APW), 2 patients (Cases 11 and 13) had atrial septal defect (ASD) and 2 patients (Cases 7 and 8) had patent ductus arteriosus (PDA) (Table 1).
Surgical treatment
The managements of PA sling were as follows. Six patients received LPA re-implantation and tracheal interventions simultaneously. Median sternotomy and cardiopulmonary bypass were used in these patients. LPA was taken out between trachea and esophagus, and implanted into normal site of the main PA in front of the trachea. Among 6 patients, 3 underwent slide tracheoplasty and the remaining 3 received tracheal stent implantation after LPA reimplantation.
Eight patients received LPA re-implantation without tracheal intervention in first operation. Among them, LPA re-implantation was performed off pump in 6 patients. Two patients received surgery under CPB. Case 11 needed CPB for severe hypoxemia after left pulmonary artery clamping, and Case 14 required CPB for simultaneous APW repairing. Among 8 patients, only one (Case 10) needed a slide tracheoplasty for extubation failure and died at 28 days after surgery due to air leakage (Figure 1).
Figure 1.
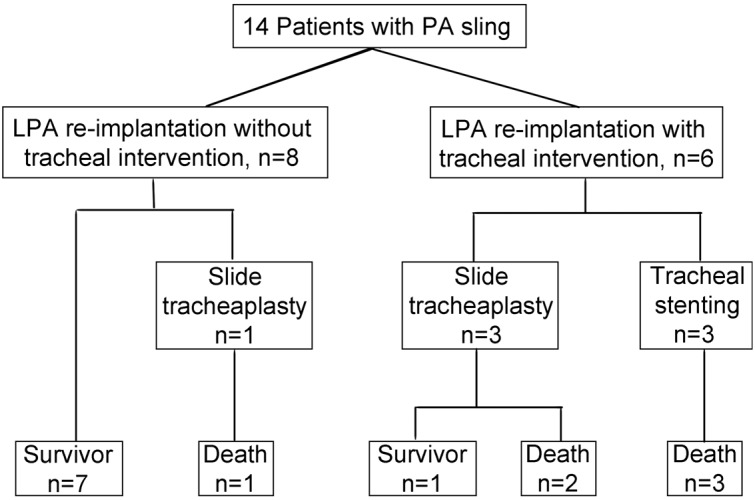
Management and outcome of 14 patients with pulmonary artery sling.
Therapeutic strategies in PCCU
In patients receiving LPA re-implantation without tracheal interventions, early extubation and non-invasive CPAP ventilation were employed in the Pediatric Critical Care Unit (PCCU) after anesthesia recovery. Among 8 patients, extubation was successfully performed in 7 patients at 0.5 h to 159 h postoperatively. One patient (Case 10) needed re-intubation, and received slide tracheoplasty on pump for severe carbon dioxide retention at 7 days after LPA re-implantation. This patient died of tracheal anastomotic leakage at 28 days after surgery. In this group, the remaining patients were followed up from 2 months to 23 months, and respiratory symptoms were relieved or disappeared.
Six patients underwent LPA re-implantation and tracheal intervention simultaneously. Among them, 3 received slide tracheoplasty under CPB. One patient survived, discharged after mechanical ventilation for 120 h, and followed up for 5 years without evident respiratory symptoms. The remaining 2 patients failed to extubate after tracheoplasty. Anastomotic granulation tissues could be seen by bronchoscopy. Balloon dilatation was performed but failed. They died of respiratory failure finally. The other 3 patients were admitted into PICU after LPA re-implantation on pump. Tracheal stent implantation was performed in PICU, but these 3 patients still failed to extubate after stent implantation and died of respiratory failure, necrotizing enterocolitis (NEC) and septicemia respectively.
Diameter and length of trachea in survivors without tracheal intervention
According to CT, in 14 patients, the narrowest diameter of the trachea was 2.62±0.61 mm. The stenosis length to the total tracheal length (from vocal cords to trachea carina) ratio was ≤ 25% in 4 patients, 25%-50% in 7 patients, 50%-75% in 2 patients and ≥75% in 1 patient. There were 7 patients who received LPA re-implantation alone survived to discharging. The narrowest tracheal diameter of these 7 patients was 2.89±0.56 mm. The stenosis length to total tracheal length ratio was ≤25% in 4 patients and 25%-50% in 3 patients. The duration of mechanical ventilation was 56.64±62.43 h and ICU stay was 6.43±4.07 d postoperatively.
In 7 patients receiving tracheal intervention, only 1 (Case 1) survived. In this group, the narrowest diameter was 2.36±0.58 mm. The stenosis length to total tracheal length ratio was 25%-50% in 4 patients, 50%-75% in 2 patients and ≥75% in 1 patient. The duration of mechanical ventilation was 562.29±432.19 h postoperatively and ICU stay was 25.1±16.47 d. There was no significant difference in the narrowest diameter of the trachea between survivors without tracheal interventions and those needing tracheal intervention (t=1.733, P=0.109). The stenosis length to total trachea length ratio in survivors without tracheal intervention was significantly smaller than that in patients needing tracheal intervention (Z=-2.56, P=0.010, 2-tailed). The duration of postoperative ventilation in survivors without tracheal intervention was significantly shorter than that in patients receiving tracheal intervention (t=-3.064, P=0.021) (Tables 2, 3).
Table 2.
Diameter of stenotic trachea, diameter/length ratio, duration of postoperative ventilation and ICU stay in two groups
| Survivors without tracheal intervention n=7 | Survivors with tracheal intervention n=7 | t-test | |
|---|---|---|---|
| Diameter of stenotic trachea (mm) | 2.89±0.56 | 2.36±0.58 | t=1.733, P=0.109 |
| Diameter/length (%) | 10.81±3.87 | 5.07±2.37 | t=3.351, P=0.007 |
| Duration of postoperative ventilation (min) | 56.64±62.43 | 562.29±432.19 | t=-3.064, P=0.021 |
| ICU stay (d) | 6.43±4.07 | 25.1±16.47 | t=-2.919, P=0.013 |
Table 3.
Length of stenotic trachea (% of total length) of survivors without and with tracheal intervention
| Length of stenotic trachea (% of total length) | Survivors without tracheal intervention (n=7) | Survivors with tracheal intervention (n=7) | Mann-Whitney U test |
|---|---|---|---|
| ≤25% | 4 | 0 | Z=-2.56, t=0.010 |
| >25%, ≤50% | 3 | 4 | |
| >50%, ≤75% | 0 | 2 | |
| ﹥75% | 0 | 1 | |
| Total | 7 | 7 |
There was no significant difference in the narrowest diameter of the trachea between survivors with and without tracheal intervention. Thus, the narrowest diameter of the trachea and the stenosis length to total tracheal length ratio (%) were used in this study. The ratio of narrowest diameter/stenosis length to total tracheal length (diameter/length [%]) was introduced and analyzed. The diameter/length (%) in the survivors without tracheal intervention was significantly higher than that in patients needing tracheal intervention (10.81±3.87 vs. 5.07 ± 2.37, t=3.351, P=0.007).
Follow-up of tracheal diameter in survivors without tracheal intervention
Among 7 survivors without tracheal intervention, 2 received CT postoperatively. The narrowest diameter of the trachea in CT increased from 3.2 mm to 5.2 mm (Case 7) and from 2.9 to 3.3 mm (Case 6). All of the survivors without tracheal intervention were followed up from 5 months to 2 years and had a good outcome, and the respiratory symptoms almost disappeared preoperatively.
Discussion
In this study, half of LPA sling children with tracheal stenosis received LPA re-implantation alone and survived to discharging. The diameter/length (%) of the trachea was 10.81±3.87 in this group and significantly higher than that in patients needing tracheal intervention (5.1±2.37). Instead of tracheal diameter, diameter/length (%) may be a better indicator for the evaluation of LPA re-implantaion alone in the first operation.
PA sling is a rare, congenital cardiovascular disease in children, and often associated with tracheal stenosis and complete tracheal cartilage ring [3]. A large survey in school-age children in Taiwan [6] showed that the natural incidence of PA sling was about 59 per million in school-age children. PA sling patients may survive to school-age without any clinical symptoms even in the presence of complete tracheal cartilage ring.
On the basis of clinical symptoms and CT scan, Anton-Pacheco et al [7] found PA sling could be divided into three types: mild (minimal symptoms and a tracheal diameter of about 4-6 mm in infants), moderate (continuous respiratory symptoms without respiratory distress) and severe (severe respiratory symptoms with respiratory distress needing mechanical ventilation). PA sling children with mild symptoms are frequently treated with conservative strategies in mostly medical centers due to the concern of risks of CPB and tracheal complications postoperative [7,8]. If PA sling/tracheal stenosis patients have associated with moderate and above clinical symptoms, LPA re-implantation and tracheoplasty are recommended simultaneously through median sternotomy under CPB [9-14]. Backer et al suggested that if PA sling patients exhibited complete tracheal rings, tracheoplasty was required [15]. Huang et al proposed that if PA sling patients had the diameter of the trachea of <3 mm, tracheoplasty and LPA re-implantation were needed [16].
Tracheoplasty can correct the abnormal LPA and tracheal stenosis in one surgery and ensure adequate ventilation early postoperatively. The surgical outcome of PA sling/tracheal stenosis children has been improved in recent decades [8,13,17], but the postoperative complications of tracheoplasty remain a great challenge, such anastomosis leakage, granulation tissue formation and tracheobronchomalacia. Neither tracheal stent implacement nor surgical treatment is an effective measure for the treatment of these complications [18-20]. Before June 2012, 3 PA sling/tracheal stenosis patients received slide tracheoplasty in our hospital, 2 patients died of granulation tissue growing at the tracheal anastomosis site (Case 2 and Case 3) and only 1 patient (Case 1) survived to discharging. All of three patients received tracheal stent implantation after LPA re-implantation, but died of respiratory failure. This is a major reason that we usually try to avoid tracheoplasty in the treatment of PA sling.
In this study, a majority of PA sling/tracheal stenosis patients with complete tracheal rings did show improved symptoms and survived to discharging after LPA re-implantation without tracheal intervention. Therefore, we speculate that most PA sling/tracheal stenosis children may survive by LPA re-implantation alone. There was no significant difference in the narrowest tracheal diameter between survivors with and without tracheal intervention. However, marked difference was noted in the narrowest diameter/length (%) of the trachea between them. It was 10.81±3.87 in survivors without tracheal interventions and 5.07±2.37 in survivors with tracheal intervention. Thus, diameter/length (%) may be a more reliable indicator used to avoid the tracheal intervention and predict a good outcome in PA sling patients. Since 2012, 8 PA sling children with complete tracheal rings have received LPA re-implantation alone in our hospital, and only 1 patient (Case 10) needed a second tracheoplasty after LPA re-implantation due to extubation failure. In Case 4, the stenosis length to total trachea ratio was 75%, and the narrowest tracheal diameter was 1.9 mm.
Intensive therapeutic strategies include early extubation and non-invasive CPAP in PCCU postoperatively. Even in the re-intubation patients after surgery, early re-extubation should be tried again. To achieve early extubation, CPB is avoided as far as possible during LPA re-implantation, which may reduce the tracheal secretions postoperatively and make extubation easier. Among 8 PA sling patients receiving LPA re-implantation alone, only 2 needed CPB during surgery (Cases 6 and 14). Case 6 needed CPB during LPA re-implantation due to hypoxemia after LPA clamping, and Case 14 required CPB for APW repairing.
Following up from 3 months to 2 years, respiratory symptoms of survivors without tracheal intervention were relieved after LPA re-implantation. No LPA obstruction was observed. This situation in this article was consistent with some other literature study [21,22].
Because PA sling is a rare, congenital vascular disease, only 14 PA sling/tracheal stenosis patients were included. The longest follow up period of survivors receiving LPA re-implantation alone was 2 years, and thus the long-term outcome of these patients remain unknown.
In conclusion, 8 PA sling/tracheal stenosis patients received LPA re-implantation alone. Early extubation and non-invasive CPAP were employed posteoratively, except 1 needing a slide tracheoplasty, and 7 patients survived to discharging and had a good outcome. The diameter/length (%) may be a more reliable indicator for the evaluation of tracheal intervention in the first operation of PA sling patients.
Disclosure of conflict of interest
None.
References
- 1.Berdon WE, Baker DH, Wung JT, Chrispin A, Kozlowski K, de Silva M, Bales P, Alford B. Complete cartilage-ring tracheal stenosis associated with anomalous left pulmonary artery: the ring-sling complex. Radiology. 1984;152:57–64. doi: 10.1148/radiology.152.1.6729137. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SR, Landing BH. Tracheostenosis and bronchial abnormalities associated with pulmonary artery sling. Ann Otol Rhinol Laryngol. 1976;85:582–590. doi: 10.1177/000348947608500504. [DOI] [PubMed] [Google Scholar]
- 3.Sade RM, Rosenthal A, Fellows K, Castaneda AR. Pulmonary artery sling. J Thorac Cardiovasc Surg. 1975;69:333–346. [PubMed] [Google Scholar]
- 4.Potts WJ, Holinger PH, Rosenblum AH. Anomalous left pulmonary artery causing obstruction to right main bronchus: report of a case. J Am Med Assoc. 1954;155:1409–1411. doi: 10.1001/jama.1954.73690340007008c. [DOI] [PubMed] [Google Scholar]
- 5.Tsang V, Murday A, Gillbe C, Goldstraw P. Slide tracheoplasty for congenital funnel-shaped tracheal stenosis. Ann Thorac Surg. 1989;48:632–635. doi: 10.1016/0003-4975(89)90777-7. [DOI] [PubMed] [Google Scholar]
- 6.Yu JM, Liao CP, Ge S, Weng ZC, Hsiung MC, Chang JK, Chen FL. The prevalence and clinical impact of pulmonary artery sling on school-aged children: a large-scale screening study. Pediatr Pulmonol. 2008;43:656–661. doi: 10.1002/ppul.20823. [DOI] [PubMed] [Google Scholar]
- 7.Anton-Pacheco JL, Cano I, Comas J, Galletti L, Polo L, Garcia A, Lopez M, Cabezali D. Management of congenital tracheal stenosis in infancy. Eur J Cardiothorac Surg. 2006;29:991–996. doi: 10.1016/j.ejcts.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 8.van Son JA, Hambsch J, Haas GS, Schneider P, Mohr FW. Pulmonary artery sling: reimplantation versus antetracheal translocation. Ann Thorac Surg. 1999;68:989–994. doi: 10.1016/s0003-4975(99)00677-3. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi M, Oshima Y, Hosokawa Y, Ohashi H, Tsugawa C, Nishijima E, Tsubota N. Concomitant repair of congenital tracheal stenosis and complex cardiac anomaly in small children. J Thorac Cardiovasc Surg. 1990;100:181–187. [PubMed] [Google Scholar]
- 10.Yamaguchi M, Ohashi H, Hosokawa Y, Oshima Y, Tsugawa C, Kimura K. Surgical treatment of airway obstruction associated with congenital heart disease in infants and small children. Eur J Cardiothorac Surg. 1991;5:479–485. doi: 10.1016/1010-7940(91)90144-9. [DOI] [PubMed] [Google Scholar]
- 11.Oshima Y, Yamaguchi M, Ohashi H, Yoshimura N, Tanaka T, Oka S, Ogawa K, Nishijima E, Tsugawa C. [Pulmonary artery sling with tracheal stenosis--primary repair in infancy] . Jpn J Thorac Cardiovasc Surg. 1998;46:347–353. doi: 10.1007/BF03217754. [DOI] [PubMed] [Google Scholar]
- 12.Oshima Y, Yamaguchi M, Yoshimura N, Ogawa K, Nishijima E, Tsugawa C. Primary repair of pulmonary artery sling with double outlet right ventricle and distal tracheal stenosis. J Cardiovasc Surg (Torino) 2002;43:849–851. [PubMed] [Google Scholar]
- 13.Backer CL, Mavroudis C, Gerber ME, Holinger LD. Tracheal surgery in children: an 18-year review of four techniques. Eur J Cardiothorac Surg. 2001;19:777–784. doi: 10.1016/s1010-7940(01)00736-9. [DOI] [PubMed] [Google Scholar]
- 14.Kimura K, Mukohara N, Tsugawa C, Matsumoto Y, Sugimura C, Murata H, Itoh H. Tracheoplasty for congenital stenosis of the entire trachea. J Pediatr Surg. 1982;17:869–871. doi: 10.1016/s0022-3468(82)80458-2. [DOI] [PubMed] [Google Scholar]
- 15.Backer CL, Russell HM, Kaushal S, Rastatter JC, Rigsby CK, Holinger LD. Pulmonary artery sling: current results with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012;143:144–151. doi: 10.1016/j.jtcvs.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Huang SC, Wu ET, Wang CC, Chen SJ, Chen YS, Chang CI, Chiu IS, Wang SS. Surgical management of pulmonary artery sling: trachea diameter and outcomes with or without tracheoplasty. Pediatr Pulmonol. 2012;47:903–908. doi: 10.1002/ppul.22516. [DOI] [PubMed] [Google Scholar]
- 17.Loukanov T, Sebening C, Springer W, Ulmer H, Hagl S. Simultaneous management of congenital tracheal stenosis and cardiac anomalies in infants. J Thorac Cardiovasc Surg. 2005;130:1537–1541. doi: 10.1016/j.jtcvs.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Konstantinov IE, d’Udekem Y, Saxena P. Interposition pericardial flap after slide tracheoplasty in pulmonary artery sling complex. Ann Thorac Surg. 2010;89:289–291. doi: 10.1016/j.athoracsur.2009.05.080. [DOI] [PubMed] [Google Scholar]
- 19.Wright CD, Graham BB, Grillo HC, Wain JC, Mathisen DJ. Pediatric tracheal surgery. Ann Thorac Surg. 2002;74:308–313. doi: 10.1016/s0003-4975(02)03613-5. discussion 314. [DOI] [PubMed] [Google Scholar]
- 20.Loeff DS, Filler RM, Vinograd I, Ein SH, Williams WG, Smith CR, Bahoric A. Congenital tracheal stenosis: a review of 22 patients from 1965 to 1987. J Pediatr Surg. 1988;23:744–748. doi: 10.1016/s0022-3468(88)80416-0. [DOI] [PubMed] [Google Scholar]
- 21.Oshima Y, Yamaguchi M, Yoshimura N, Sato S, Muraji T, Nishijima E, Tsugawa C. Management of pulmonary artery sling associated with tracheal stenosis. Ann Thorac Surg. 2008;86:1334–1338. doi: 10.1016/j.athoracsur.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Backer CL, Mavroudis C, Dunham ME, Holinger LD. Pulmonary artery sling: results with median sternotomy, cardiopulmonary bypass, and reimplantation. Ann Thorac Surg. 1999;67:1738–1744. doi: 10.1016/s0003-4975(99)00364-1. discussion 1744-1735. [DOI] [PubMed] [Google Scholar]


