Abstract
Objective: Cancer is one of the most common risk factor of venous thromboembolism (VTE). Our previous studies have shown that integrin subunits β1, β2 and β3 were the core proteins of venous thrombi and potential useful biomarker of VTE. This study aimed to explore the expression status of core proteins (integrin subunits β1, β2 and β3) in cancer patients. Methods: This is a case-control study. A total of 144 inpatients (54 females) with clinically proven cancers were recruited into this study, meanwhile 200 inpatients without cancer matched in sex and age were recruited as control group. Flow cytometry was done to measure the expressions of blood integrin β1, β2, β3 and cellular immunity related variables (CD3, CD4, CD8, CD4/CD8, CD16CD56 and CD19). The association degree between increased core proteins and cancers was analyzed by calculating the relative risk (RR). Results: The expression of integrin β1 and β3 were markedly increased in patients with cancer (P=0.001 and 0.008). Integrin β2 was also mildly increased in patients with cancer (P=0.274). The relative risk ratio (RR) of increased integrin β1, β2 and β3 in cancer patients was 1.655 (95% CI: 1.321-2.074, P=0.000), 1.314 (95% CI: 1.052-1.642, P=0.021) and 1.852, (95% CI: 1.097-3.126, P=0.028), respectively. Combined analysis with integrin β1, β2 and β3 showed that the relative risk ratio (RR) of increased in cancer patients was 4.895 (95% CI: 1.645-14.563, P=0.002). CD3, CD4, CD4/CD8 and CD19 were significantly decreased (P=0.004, P=0.000, P=0.000, P=0.000, respectively) in patients with cancer, while CD8 and CD16CD56 were markedly increased in cancer patients (P=0.005, P=0.035). Conclusions: As the core proteins of venous thrombi, integrin β1 and β3 were markedly increased expression in patients with cancer, which maybe explain the increased risk of VTE in cancer patients. A weakened or disordered immune system might be the basis of VTE in condition.
Keywords: Core protein, Integrin β1, Integrin β2, Integrin β3, venous thromboembolism, cancer
Introduction
Venous thromboembolism (VTE) includes deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE), which is a serious and potentially fatal disorder [1]. Cancer is one of the most common risk factor of VTE. The incidence of VTE in patients with cancer is about 4%-20%, and it has been a leading cause of death in cancer patients [2-4]. There is evidence showing that about 20% Clinical first-episode patients with idiopathic VTE have been diagnosed malignant tumor in 6 months to 2 years. The prevalence of VTE in patients with malignancy is 4-7 times higher than that of patients without malignancy [5,6]. VTE has been an important contributor to morbidity and mortality among patients with cancer [7]. Why have malignancy patients had a high incidence of VTE? The molecular mechanisms were not clear.
Acute venous thrombosis is red thrombus, which is composed of red blood cells, platelets, white blood cells and plasma proteins. In 2011, we reported that the main component of red thrombus in acute PE patients was fibrinogen, rather than fibrin, with only a small quantity of cellular cytoskeletal and plasma proteins [8]. In our further studies, genomics analysis, proteomics analysis and bioinformatics analysis of acute venous thrombi of PE patients confirmed that integrin β1, β2 and β3 were the core proteins of acute venous thrombi [9,10]. Integrin β1 mainly localized on lymphocytes, integrin β2 mainly localized on neutrophils and integrin β3 mainly localized on platelets. Moreover, activated integrin β3 was involved in the accumulation of platelet, receptors of integrin β2 and β3 bound to fibrinogens to form the biofilter-like grid structure of thrombi in which red blood cells filled, forming red thrombi. We also found that the filamentous mesh-like structure was widespread in the veins of cancers, and a large amount of red blood cells and cancer cells were found in this biofilter-like grid structure [11].
Integrin β1, β2, β3 subunits are core proteins and potential biomarkers of VTE [12]. Is there any relevance between core proteins of acute venous thrombi-integrin β1, β2 and β3 and cancer? In this study we will explore the expression of Integrin β1, β2, β3 subunits in patients with cancer, and investigate their clinical importance.
Material and methods
Study population
A total of 144 cases of inpatients with cancer diagnosed from April 2011 to April 2012 in affiliated Tongji Hospital of Tongji University were recruited into this study, including 90 males and 54 females, aged 25-91 years, with a mean age of 67.36 years old. Cancers including: lung cancer, intestinal cancer, hepatic cancer, gastric cancer, prostate cancer, breast cancer, esophageal cancer, pancreatic cancer, cervical cancer, kidney cancer, ovarian cancer, bladder cancer, nasopharyngeal cancer and laryngeal cancer. All cancers were confirmed by imaging or pathology. Meanwhile, 200 cases of age and gender matched inpatients without cancer were recruited as control group, including 114 males and 86 females, aged 21-93 years (mean 68.17 years). Cancer was excluded in the control group by clinical symptoms, signs and imaging. Patients with acute infection, autoimmune disease or patients taking immunosuppressive drugs were excluded. Patients with clinical symptomatic venous thrombus were also excluded. This study was approved by the Ethics Committee of Affiliated Tongji Hospital of Tongji University, and informed consent was obtained before study.
Blood collection and measurements
Detailed clinical data were collected from each cancer patient and control patient on admission. Blood routine test, hsCRP and d-dimer were detected. HsCRP was detected by immune scatter turbidimetry, using Siemens BNII specific protein and auxiliary reagent. D-dimer was detected by Latex enhanced immune turbidimetric turbidity method, using SYSMEX CA1500 automatic blood coagulation analyzer. Fasting venous blood (2 ml) was collected from the cubital vein in the morning and anti-coagulated with EDTA. Two hours later, the anti-coagulated blood was processed as follows.
Monoclonal antibodies against integrin β1 (CD29), β2 (CD18) and β3 (CD61) (BD company) were used to detect the integrin β1, β2 and β3, respectively. Three tag monoclonal antibodies (BECKMAN-COULTER) were used for detection of CD3, CD4 and PC5, FITC and PE label were used for CD8, CD3, CD4 and CD8, respectively. CD16CD56 and CD19 also used PE label. In brief, 100 μL of EDTA treated blood was added to each tube and control tube was also included. Then, 20 μL of mouse IgG1-PC5, IgG1-FITC or IgG1-PE was added (20 μL of IgG2-PE was mixed with CD29), followed by addition of corresponding fluorescence antibodies (20 μL). Following vortexing, incubation was done in dark for 30 min at room temperature. Then, 500 μL of hemolysin (BECKMAN-COULTER) was added, followed by incubation at 37°C for 30 min. Following washing, 500 μL of sheath fluid was added to each tube, followed by flow cytometry (EPICS XL-4; BECKMAN- COULTER). The PMT voltage, fluorescence compensation and sensitivity of standard fluorescent microspheres (EPICS XL-4; BECKMAN-COULTER) were used to adjust the flow cytometer and a total of 10000 cells were counted for each tube. The corresponding cell population in the scatterplot of isotype controls was used to set the gate, and the proportion of positive cells was determined in each quadrant (%). SYSTEM-II was used to process the data obtained after flow cytometry.
Statistical analysis
SPSS18.0 statistical software was used for statistical analysis. Normality test was performed for all measurement data using the Kolmogorov-Smirnov test, with P>0.05 as normal distribution. Data of normal distribution were expressed as means ± SD and were compared with student’s t-test between groups. Corrected t-test was applied when heterogeneity of variance. Non-normal data were expressed as median P50 and interquartile range (P25-P75), and group comparison was analyzed using nonparametric test (Mann-Whitney U test). Categorical data were compared using chi-square test. The association degree between two categorical variables was analyzed by calculating the relative risk (Relative Risk, RR). P<0.05 was considered statistically significant for all tests.
Results
Patients’ characteristics
A total of 144 patients with cancer and 200 patients without cancer matched in age and sex were enrolled into this study. Among 144 patients with cancer, 43 (29.86%) were diagnosed with lung cancer, 25 (17.73%) were diagnosed with intestinal cancer, 17 (12.06) were diagnosed with hepatic cancer, 13 (9.22%) were diagnosed with gastric cancer, 11 (7.8%) were diagnosed with prostate cancer, 10 (7.09%) were diagnosed with breast cancer, 6 (4.26%) were diagnosed with esophageal cancer, 6 (4.26%) were diagnosed with pancreatic cancer, 3 (2.13%) were diagnosed with cervical cancer, 2 (1.42%) were diagnosed with kidney cancer, 2 (1.42%) were diagnosed with ovarian cancer, 2 (1.42%) were diagnosed with bladder cancer, 2 (1.42%) were diagnosed with nasopharyngeal cancer and 2 (1.42%) were diagnosed with laryngeal cancer. Patients’ demographics, type of cancer and comorbidities are shown in Table 1.
Table 1.
The baseline characteristics of 144 patients with cancer and controls
| Patients with cancer | Controls (%) | P value | |
|---|---|---|---|
|
| |||
| (%) N=144 | N=200 | ||
| Mean age (SD) | 67.36 (12.67) | 68.17 (12.09) | 0.549 |
| Gender, male | 90 (62.5) | 114 (57.0) | 0.319 |
| Cancer typing | |||
| Lung cancer | 43 (29.86) | ||
| Intestinal cancer | 25 (17.73) | ||
| Hepatic cancer | 17 (12.06) | ||
| Gastric cancer | 13 (9.22) | ||
| Prostate cancer | 11 (7.8) | ||
| Breast cancer | 10 (7.09) | ||
| Esophageal cancer | 6 (4.26) | ||
| Pancreatic cancer | 6 (4.26) | ||
| Cervical cancer | 3 (2.13) | ||
| Kidney cancer | 2 (1.42) | ||
| Ovarian cancer | 2 (1.42) | ||
| Bladder cancer | 2 (1.42) | ||
| Nasopharyngeal cancer | 2 (1.42) | ||
| Laryngeal cancer | 2 (1.42) | ||
| Comorbidities | |||
| CAD | 15 (10.42) | 30 (15) | 0.199 |
| Hypertension | 34 (23.61) | 51 (25.5) | 0.705 |
| CI | 18 (12.5) | 37 (18.5) | 0.140 |
| DM | 29 (20.14) | 36 (18) | 0.676 |
Ages are shown with mean (SD); categorical data are shown with the number and Percentage of the sample group. Ages were compared by Student’s t test. The frequency of categorical data was compared with the chi-square test. Abbreviations: CAD, coronary artery disease; CI, cerebrovascular infarction; DM, diabetes mellitus.
Plasma d-dimer and hsCRP levels
The median levels of d-dimer and hsCRP were all significantly higher in patients with cancer when compared with patients without cancer (P=0.000 and 0.000) (Table 2).
Table 2.
Expression of cellular immunity, HsCRP and d-dimer in patients with cancer and controls
| Patients with cancer (pg/ml) | controls (pg/ml) | P value | |
|---|---|---|---|
|
|
|||
| N=144 | N=200 | ||
| CD3 | 60.71 (14.64) | 64.91 (12.29) | 0.004 |
| CD4 | 32.31 (11.30) | 37.35 (11.26) | 0.000 |
| CD8 | 25.00 (9.77) | 22.16 (7.94) | 0.005 |
| CD4CD8 | 1.30 (0.87-2.08) | 1.80 (1.40-2.50) | 0.000 |
| CD16CD56 | 11.95 (9.92-16.18) | 9.75 (5.43-15.75) | 0.035 |
| CD19 | 6.64 (3.88-12.10) | 10.20 (6.35-15.28) | 0.000 |
| D-Dimer | 0.19 (0.05-0.39) | 0.08 (0.05-0.24) | 0.000 |
| HsCRP | 11.40 (4.70-44.05) | 3.00 (0.83-14.90) | 0.000 |
CD3, CD4, CD8 were shown with mean (SD) and compared by Student’s t test. CD4/CD8, CD16CD56, CD19, D-Dimer and HsCRP were expressed as median (p25th-p75th) and compared by Mann-Whitney U test.
Blood cellular immunity related variables
When comparing cellular immunity related variables (CD3, CD4, CD8, CD4/CD8, CD16CD56 and CD19), significant differences of all cellular immunity related variables were found between two groups. CD3, CD4, CD4/CD8 and CD19 were markedly decreased in patients with cancer (P=0.004, P=0.000, P=0.000 and P=0.000 respectively), while CD8 and CD16CD56 were increased (P=0.000 and P=0.035) (Table 2).
Blood integrin levels
When compared with the control group, the expression of integrin β1 and β3 were markedly increased in patients with cancer (P=0.000 and P=0.008), while integrin β2 was only mild increased in patients with cancer (P=0.274) (Table 3 and Figure 1). The relative risk ratio (RR) of increased integrin β1, β2 and β3 in patients with cancer were 1.655 (95% CI: 1.321-2.074, P=0.000), 1.314 (95% CI: 1.052-1.642, P=0.021) and 1.852 (95% CI: 1.097-3.126, P=0.028), respectively (Table 4). Combined integrin β1, β2 and β3 analysis showed (integrin β1, β2 and β3 increased at the same time means rise, otherwise normal) the relative risk ratio (RR) of increased in patients with cancer was 4.895 (95% CI: 1.645-14.563, P=0.002) (Table 4).
Table 3.
Expression of integrin β1, β2 and β3 in patients with cancer and controls
| Patients with cancer (pg/ml) | Controls (pg/ml) | P value | |
|---|---|---|---|
|
| |||
| N=144 | N=200 | ||
| Integrin β1 | 12.34 (5.40) | 9.63 (4.53) | 0.000 |
| Integrin β2 | 89.82 (6.63) | 88.99 (7.12) | 0.274 |
| Integrin β3 | 10.33 (3.55) | 9.39 (2.99) | 0.008 |
Integrin β1, β2, β3 were shown with mean (SD) and compared by Student’s t test.
Figure 1.
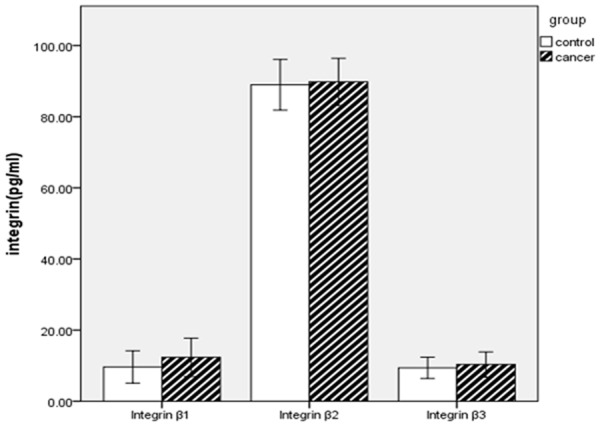
Integrin β1, integrin β2, integrin β3 levels in patients with cancer and controls.
Table 4.
Relative risk of increased expression of integrin β1, β2 and β3 in patients with cancer
| Patients with cancer | Controls | RR | 95% CI | P value | |
|---|---|---|---|---|---|
|
|
|||||
| above/normal | above/normal | ||||
| integrin β1 | 87/57 | 73/127 | 1.655 | 1.321-2.074 | 0.000 |
| integrin β2 | 78/65 | 83/117 | 1.314 | 1.052-1.642 | 0.021 |
| integrin β3 | 28/116 | 21/179 | 1.852 | 1.097-3.126 | 0.028 |
| Combination of integrin β1, β2 and β3 | 14/129 | 4/196 | 4.895 | 1.645-14.563 | 0.002 |
Discussion
Integrins are a kind of widespread cell surface receptors, which mediate interactions between cells and cells, cells and extracellular matrix (ECM). As signal receptor, integrins play an important role in the cell growth, migration, proliferation and differentiation of many aspects, and are one of the key members of the family of cell adhesion molecules [13]. Integrins are heterodimers consisting of noncovalently linked α and β transmembrane glycoprotein subunits. They consist of at least 18 α and 8 β subunits, producing 24 different heterodimers [14]. The β1 subunit is expressed mainly on cell surface of lymphocytes, and its ligands consist of laminins, collagens, thrombospondin, vascular cell adhesion molecule 1 and fibronectin [15]. The β2 subunit is distributed on cell surface of neutrophils and monocytes, and ligands for this subunit include fibrinogen, complement component iC3b, intracellular adhesion molecule-1, factor X and so on [16]. The β3 subunit is observed on platelets, and this subunit binds fibrinogen, fibronectin, vitronectin von Willebrand factor (vWF) and thrombospondin [17].
Cancer is a risk factor of VTE, and VTE is an important cause of death in cancer [18-20]. This study explored the expression of integrin β1, β2, β3 subunit in patients with cancer, the results showed that integrin β1, β2, β3 subunit were all increased in patients with cancer, among them integrin β1 and β3 were increased significantly. The relative risk ratio (RR) of increased integrin β1, β2 and β3 in patients with cancer were 1.655, 1.314 and 1.852 respectively. Combined integrin β1, β2 and β3 analysis showed that the relative risk ratio (RR) of increased in patients with cancer was 4.895. As core proteins of venous thrombosis, the increased expression of integrin β1, β2 and β3 in patients with cancer maybe explain the relative high risk of VTE in cancer patients.
The plasma levels of hsCRP and d-dimer were all significantly higher in patients with cancer in this study. As nonspecific inflammation markers, hsCRP was associated with venous thrombosis [21]. Elevated levels of serum hsCRP are a risk factor of VTE in cancer patients, which shows the role of nonspecific inflammation in the prone of VTE in patients with cancer [22]. Our study have shown that the incidence of VTE in patients with malignant tumor is the result of nonspecific inflammatory repair of small veins after destroyed by tumor cells invasion, as demonstrated by morphological examination and immunohistochemistry [11]. This is different from infective inflammation. D-dimer is a degradation product of cross-linked fibrin that is formed immediately after thrombin-generated fibrin clots are degraded by plasmin and reflects a global activation of blood coagulation and fibrinolysis. Being the best-recognized biomarker for the initial assessment of suspected VTE, d-dimer has a high sensitivity of 83%-96%, but a poor specificity (around 40%) [23-25], as core proteins of venous thrombosis, integrin β1, β2 and β3 had been proved a new useful biomarker of VTE both with a high sensitivity and an approving specificity in our previous study [12]. For those having increased integrin β1, β2 and β3 in patients with cancer, early treatment and prevention should be given, in order to reduce the incidence of VTE in high-risk groups.
In this study, cellular immune function was reduced or disordered in patients with cancer. Our previous studies had shown that VTE patients had association with compromised cellular immunity [26,27]. A weakened immune system could be the basic condition of VTE occurrence. These findings suggest malignant tumor patients with compromised cellular immunity possess the intrinsic basic conditions for VTE and thus have an increased risk of VTE.
Acknowledgements
The study was granted by “12 th Five-year” National Science & Technology Supporting Program (2011BAI11B16).
Disclosure of conflict of interest
None.
References
- 1.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611–7. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 2.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Liebman HA, White RH, Wun T, Lyman GH. The risk of venous thromboembolism in patients with cancer. American Society of Clinical Oncology. Cancer Thromb. 2008:240–8. [Google Scholar]
- 4.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J. Clin. Oncol. 2007;25:70–6. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 5.Wun T, White RH. Epidemiology of cancer-related venous throm-boembolism. Best Pract Res Clin Haematol. 2009;22:9–23. doi: 10.1016/j.beha.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, pro-thrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 7.Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, Jahanzeb M, Kakkar A, Kuderer NM, Levine MN, Liebman H, Mendelson D, Raskob G, Somerfield MR, Thodiyil P, Trent D, Francis CW American Society of Clinical Oncology. American Society of Clinical Oncology Guideline: recommendations for venous throm-boembolism prophylax is and treatment in patients with cancer. J. Clin. Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Gong Z, Jiang J, Xu W, Duan Q, Liu J, Qin C. Confusion of wide thrombolytic time window for acute pulmonary embolism: mass spectrographic analysis for thrombus proteins. Am J Respir Crit Care Med. 2011;184:145–146. doi: 10.1164/ajrccm.184.1.145. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y, Duan Q, Wang L, Gong Z, Wang Q, Song H, Wang H. Genomic characteristics of adhesion molecules in patients with symptomatic pulmonary embolism. Mol Med Rep. 2012;6:585–590. doi: 10.3892/mmr.2012.940. [DOI] [PubMed] [Google Scholar]
- 10.Wang LM, Duan QL, Yang F, Yi XH, Zeng Y, Tian HY, Lv W, Jin Y. Activation of circulated immune cells and inflammatory immune adherence are involved in the whole process of acute venous thrombosis. Int J Clin Exp Med. 2014;7:566–572. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LM, Duan QL, Yi XH, Zeng Y, Gong Z, Yang F. Venous thromboembolism is a product in proliferation of cancer cells. Int J Clin Exp Med. 2014;7:1319–1323. [PMC free article] [PubMed] [Google Scholar]
- 12.Song Y, Yang F, Wang L, Duan Q, Jin Y, Gong Z. Increased expressions of integrin subunit β1, β2 and β3 in patients with venous thromboembolism: new markers for venous thromboembolism. Int J Clin Exp Med. 2014;7:2578–2584. [PMC free article] [PubMed] [Google Scholar]
- 13.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavers M, Afzali B, Macey M, McCarthy DA, Irshad S, Brown KA. Differential expression of beta1 and beta2 integrins and L-selectin on CD4+ and CD8+ T lymphocytes in human blood: comparative analysis between isolated cells, whole blood samples and cryopreserved preparations. Clin Exp Immunol. 2002;127:60–65. doi: 10.1046/j.1365-2249.2002.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lityńska A, Przybylo M, Ksiazek D, Laidler P. Differences of alpha3beta1 integrin glycans from different human bladder cell lines. Acta Biochim Pol. 2000;47:427–34. [PubMed] [Google Scholar]
- 16.Solovjov DA, Pluskota E, Plow EF. Distinct roles for the alpha and beta subunits in the functions of integrin alphaMbeta2. J Biol Chem. 2005;280:1336–45. doi: 10.1074/jbc.M406968200. [DOI] [PubMed] [Google Scholar]
- 17.Gerber DJ, Pereira P, Huang SY, Pelletier C, Tonegawa S. Expression of alpha v and beta 3 16-integrin chains on murine lymphocytes. Proc Natl Acad Sci U S A. 1996;93:14698–703. doi: 10.1073/pnas.93.25.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 19.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–10. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 20.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–4. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 21.Vormittag R, Vukovich T, Schönauer V, Lehr S, Minar E, Bialonczyk C, Hirschl M, Pabinger I. Basal high-sensitivity-C-reactive protein levels in patients with spontaneous venous thromboembolism. Thrombosis and Haemostasis. 2005;93:488–93. doi: 10.1160/TH04-11-0745. [DOI] [PubMed] [Google Scholar]
- 22.Kröger K, Weiland D, Ose C, Neumann N, Weiss S, Hirsch C, Urbanski K, Seeber S, Scheulen ME. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol. 2006;17:297–303. doi: 10.1093/annonc/mdj068. [DOI] [PubMed] [Google Scholar]
- 23.Bounameaux H, Cirafici P, de Moerloose P, Schneider PA, Slosman D, Reber G, Unger PF. Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet. 1991;337:196–200. doi: 10.1016/0140-6736(91)92158-x. [DOI] [PubMed] [Google Scholar]
- 24.Bozic M, Blinc A, Stegnar M. D-dimer, other markers of haemostasis activation and soluble adhesion molecules in patients with different clinical probabilities of deep vein thrombosis. Thromb Res. 2002;108:107–114. doi: 10.1016/s0049-3848(03)00007-0. [DOI] [PubMed] [Google Scholar]
- 25.Di Nisio M, Squizzato A, Rutjes AW, Büller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5:296–304. doi: 10.1111/j.1538-7836.2007.02328.x. [DOI] [PubMed] [Google Scholar]
- 26.Haoming S, Lemin W, Zhu G, Aibin L, Yuan X, Wei L, Jinfa J, Wenjun X, Yuqin S. T cell-mediated immune deficiency or compromise in CTEPH patients. Am J Respir Crit Care Med. 2011;183:417–8. doi: 10.1164/ajrccm.183.3.417. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Song H, Gong Z, Duan Q, Liang A. Acute Pulmonary Embolism and Dysfunction of CD3+ CD8+ T Cell Immunity. Am J Respir Crit Care Med. 2011;184:1315. doi: 10.1164/ajrccm.184.11.1315. [DOI] [PubMed] [Google Scholar]


