Abstract
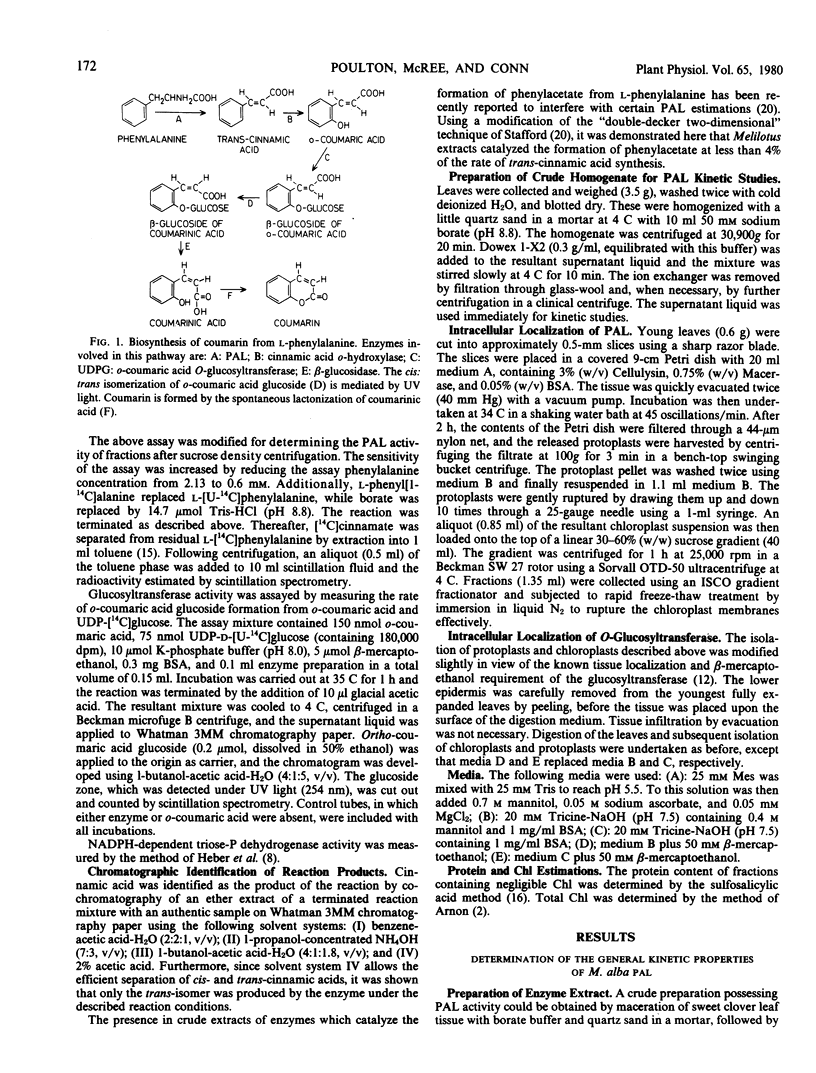
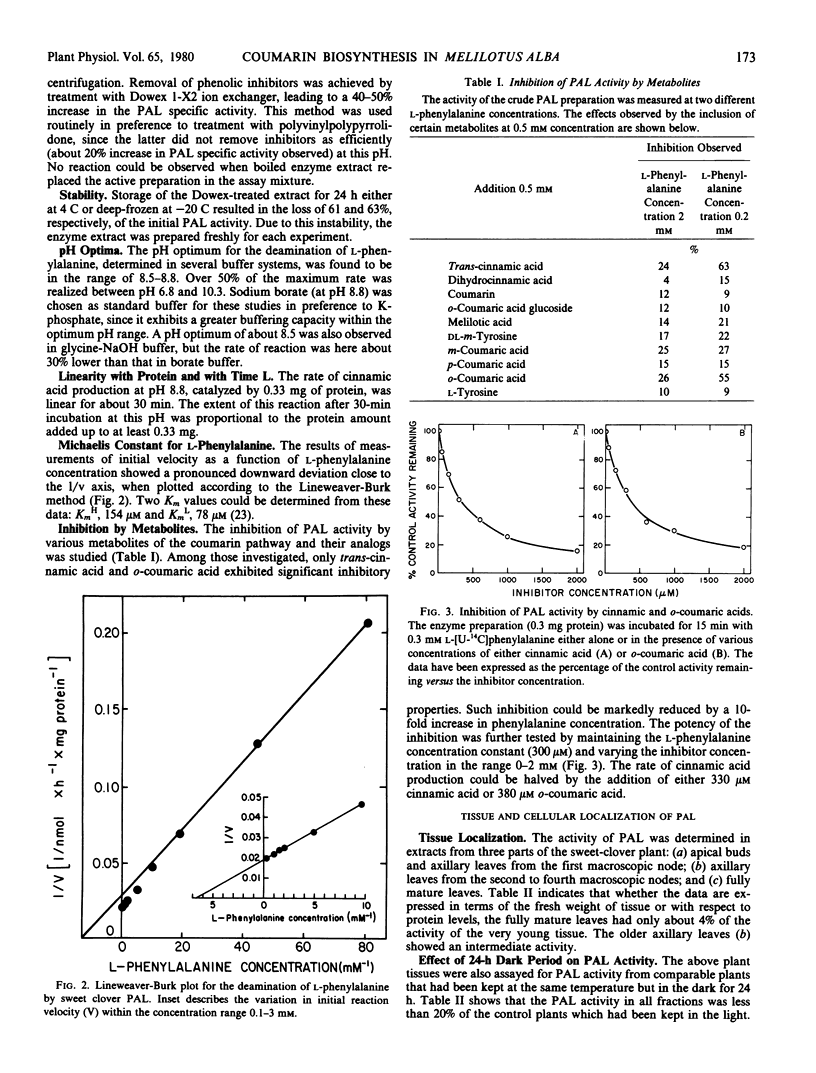
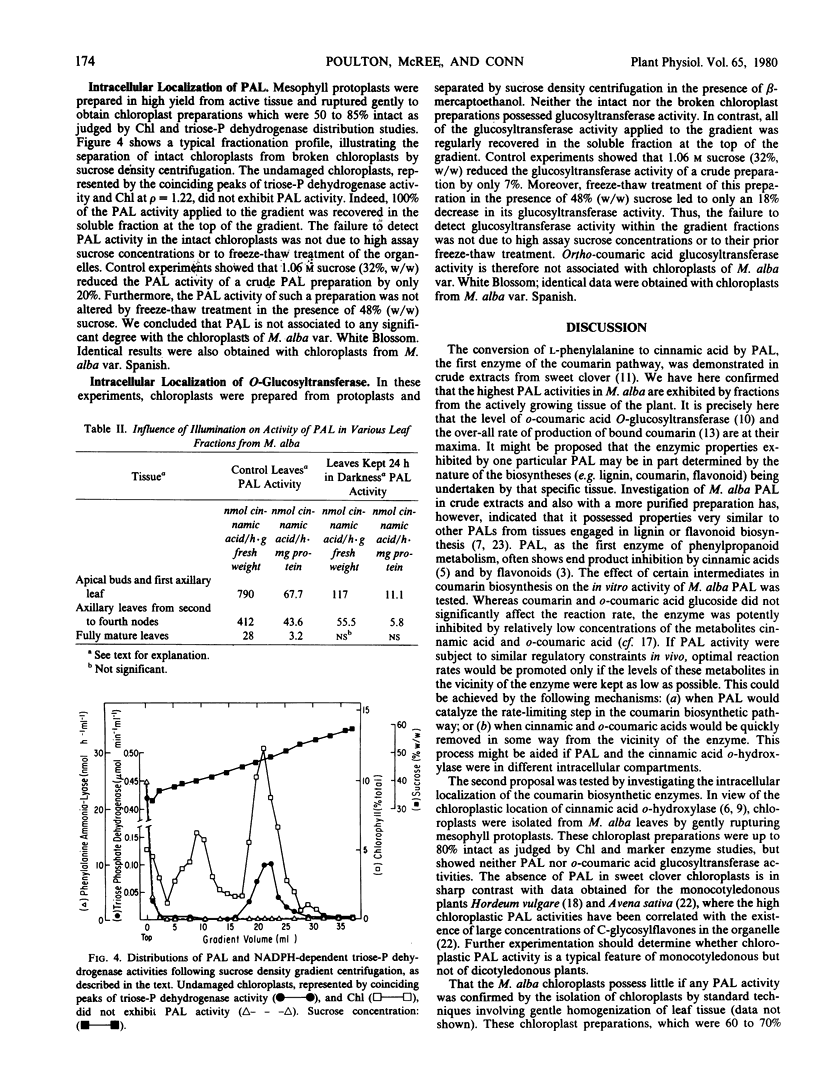
The localization of phenylalanine ammonia-lyase [EC 4.3.1.5] within sweet clover (Melilotus alba) leaves was investigated. Apical buds and axillary leaves contained 15 to 30 times more enzyme activity than did mature leaves. Mesophyll protoplasts were prepared by digesting young leaves with Cellulysin and Macerase and were gently ruptured yielding intact chloroplasts. These chloroplast preparations exhibited neither phenylalanine ammonia-lyase nor o-coumaric acid O-glucosyltransferase activities. The general enzymic properties of sweet clover leaf phenylalanine ammonia-lyase were similar to those described for this enzyme isolated from other plant species. The conversion of l-phenylalanine to trans-cinnamic acid, which occurred at an optimum pH of about 8.7, was strongly inhibited by the metabolites trans-cinnamic and o-coumaric acids. In contrast, o-coumaric acid glucoside, coumarin, p-coumaric acid, and melilotic acid had no significant effect on the reaction rate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attridge T. H., Stewart G. R., Smith Harry. End-product inhibition of Pisum phenylalanine ammonia-lyase by the Pisum flavonoids. FEBS Lett. 1971 Sep 15;17(1):84–86. doi: 10.1016/0014-5793(71)80569-0. [DOI] [PubMed] [Google Scholar]
- Gestetner B., Conn E. E. The 2-hydroxylation of trans-cinnamic acid by chloroplasts from Melilotus alba Desr. Arch Biochem Biophys. 1974 Aug;163(2):617–624. doi: 10.1016/0003-9861(74)90522-0. [DOI] [PubMed] [Google Scholar]
- Havir E. A. l-Phenylalanine Ammonia-Lyase (Maize): Evidence for a Common Catalytic Site for l-Phenylalanine and l-Tyrosine. Plant Physiol. 1971 Aug;48(2):130–136. doi: 10.1104/pp.48.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSUGE T., CONN E. E. The metabolism of aromatic compounds in higher plants. I. Coumarin and o-coumaric acid. J Biol Chem. 1959 Aug;234(8):2133–2137. [PubMed] [Google Scholar]
- KOUKOL J., CONN E. E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem. 1961 Oct;236:2692–2698. [PubMed] [Google Scholar]
- Kindl H. Der ortho-Hydroxylierung aromatischer Carbonsäuren in höheren pflanzen. Hoppe Seylers Z Physiol Chem. 1971 Jan;352(1):78–84. doi: 10.1515/bchm2.1971.352.1.78. [DOI] [PubMed] [Google Scholar]
- Kleinhofs A., Haskins F. A., Gorz H. J. Relationship of Phenylalanine Ammonia-lyase Activity to o-Hydroxycinnamic Acid Content in Melilotus alba. Plant Physiol. 1966 Oct;41(8):1276–1279. doi: 10.1104/pp.41.8.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A., Lewis L. L. Interference by a phenylacetate pathway in isotopic assays for phenylalanine ammonia-lyase in leaf extracts. Plant Physiol. 1977 Dec;60(6):830–834. doi: 10.1104/pp.60.6.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S., Hahlbrock K. Light-induced changes of enzyme activities in parsley cell suspension cultures. Purification and some properties of phenylalanine ammonia-lyase (E.C.4.3.1.5). Arch Biochem Biophys. 1975 Jan;166(1):54–62. doi: 10.1016/0003-9861(75)90364-1. [DOI] [PubMed] [Google Scholar]



