Abstract
We report a case of high-grade invasive urothelial carcinoma with squamous differentiation of the urinary tract. A 72-year-old woman was referred to our hospital because of asymptomatic gross hematuria. A right-sided laparoscopic radical nephroureterectomy with bladder cuff removal and right-sided pelvic lymphadenectomy were performed at our institution. Postoperative pathological examination showed high-grade urothelial carcinoma with squamous differentiation. Five months later, CT scan of the neck diagnosed it as lymph nodes metastasis. Following the laparoscopic radical nephroureterectomy, chemotherapy with gemcitabine and cisplatin or nedaplatin was carried out. After several cycles’ chemotherapy, nearly all the enlarged lymph node disappeared. Seven years and five years passed, urothelial carcinoma has not recurred after the surgery and all the lymph node disappeared respectively.
Keywords: Chemotherapy, lymph nodes metastasis, ureteral urothelial carcinoma
Introduction
Ureteral cancer is rare. It represents about 25% of upper transitional urinary tract cell carcinomas [1-3]. Batata et al [2] found only 2.5% of 2566 primary urothelial carcinomas to be ureteral cancers. Of the 41 invasive ureteral cancers in Batata’s study, 92.7% were urothelial carcinomas [2]. Williams and Mitchell [3] found a 1:54 ratio of cases of ureteral carcinoma to cases of bladder carcinoma and reported that twice as many men had ureteral carcinoma as did women. Patients with upper urinary tract cancers usually present symptoms of gross hematuria, but fank pain, dysuria, and frequency of urination are other less common presentations [4]. The patient with high grade invasive ureteral carcinoma and systematic lymph node metastasis usually has a poor prognosis, the mean overall survival of the patients who had distant metastases was 19 months [5]. We present the first case of systematic lymph nodes metastasis of ureteral urothelial carcinoma in an elderly woman whose disease-free survival is more than five years, and she lived more than seven years after the surgery.
Case report
In February, 2007, a 72-year-old woman presented with asymptomatic gross hematuria was evaluated at an outside institution. A computed tomography (CT) scan of the abdomen and pelvis showed an obstructive mass in the right ureter and a tumor is suspected. Magnetic resonance urography showed the L3-4 of the right ureter is constructed and the upper of the right ureter is expanded. Urinalysis showed positive for red blood cells. Then the patient was referred from that hospital to our institution for follow-up on the right ureteral carcinoma. In July, 2007, a right-sided laparoscopic radical nephroureterectomy with bladder cuff removal and right-sided pelvic lymphadenectomy was performed at our institution. Postoperative pathological examination showed high-grade urothelial carcinoma with squamous differentiation, part of the mesenchyma is mucoid degeneration and ridgy, the level of invasion was deep muscle layer, the nerve around of the ureter was not infiltrated by tumor, angiolymphatic invasion was not identified, and the surgical margins of resection were negative, and no recurrences or complications were reported. Five months later, the both sides of neck lymph node are enlargement, like a walnut, no tenderness. Then she had a computed tomography (CT) scan which diagnosed it as systematic lymph nodes metastasis (Figure 1). Pathology examination showed malignant cells accompanied by necrosis, and then it can be diagnosed as ureteral carcinoma with multiple lymph nodes metastasis.
Figure 1.
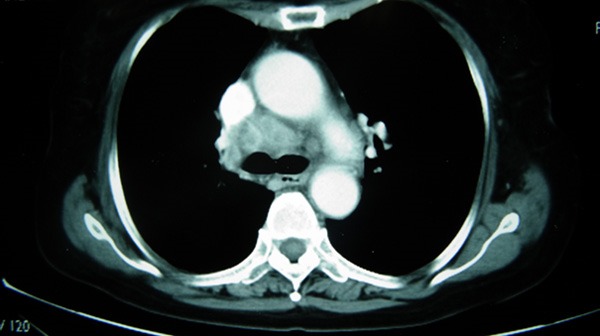
CT scan showed enlarged lymph nodes before chemotherapy.
Adjuvant chemotherapy was then performed with four cycles of cisplatin, a 100 mg/m2 on day1 and gemcitabine 1000 mg/m2 on days 1 and 8 combination, every 21 days from February, 2008 to April, 2008. A follow-up of the patient in May 2007, including a neck CT and thoracic CT, showed lymph nodes size had decreased significantly. From May 2008 to September 2008, six cycles of systemic chemotherapy with gemcitabine were administered. At the time of restaging, PET-CT showed that the lymph node size had decreased significantly and some of them diminished (Figure 2). In August 2009, she presented with pain in the right lower quadrant abdomen and vague pain in the left loin, especially when peeing, there is no urinary frequency, urgency, and urine pain , and she presented with edema of her right lower limb with no pain . Computed tomography scan of pelvis showed common iliac arteriovenous lymph node metastasis. Then two cycles of systemic chemotherapy with gemcitabine and nedaplatin were administered. The edema of her right lower limb and right lower quadrant abdominal pain were palliated. On September 2009, computed tomography scan of pelvis showed no obvious abnormalities. Then four cycles of systemic chemotherapy with gemcitabine and nedaplatin were administered. The patient is now followed-up at the outpatient clinic without evidence of recurrent disease 7 years after the surgery.
Figure 2.

PET-CT showed enlarged lymph nodes disappeared after chemotherapy.
Discussion
Urothelial carcinoma of the upper urinary tract is relatively rare, occurring in 5% of all urothelial tumors. Ureteral urothelial carcinoma is even less common than that of the renal pelvis, accounting for about 25% of all upper urinary tract tumors. Over the past 30 years, the incidence of disease in the ureter increased from 0.69 to 0.91 cases per 100 000 person-years, renal pelvic disease incidence decreased slightly from 1.19 to 1.15 cases per 100 000 person-years, and the total incidence of upper-tract tumors rose from 1.88 to 2.06 cases per 100 000 person-years [6].
Systematic lymph node metastases of ureteral cancer represent an important treatment challenge. The patient with high grade invasive ureteral carcinoma and systematic lymph node metastasis usually has a poor prognosis, the mean overall survival of the patients who had distant metastases was 19 months [5]. Tumor stage and grade are the only significant prognostic factors for both cancer-specific and overall survival [7]. The 5-year survival rate for each stage of the Heney et al [8] series was 100% for stage 0, 95% for stage A, 82% for stage B, 29% for stage C and 0% for stage D. The 5-year survival rate of pN0 patients was 69.6%, whereas that of N1 patients was 22% and that of N2 patients was 0% (P < 0.0001) [5]. Patients with distant metastases appeared to have an ominous prognosis, with survival rates under 10% some 3 years after diagnosis [6]. And increasing age at the time of diagnosis was significantly associated with poorer survival outcomes. For example, 5-year overall survival decreased from 75% for patients < 50 years of age to 50% for patients aged 70-79 years and < 20% for those aged ≥ 85 years [6].
We herein report a case of high-grade invasive urothelial carcinoma with squamous differentiation and systematic lymph node metastasis successfully treated by nephroureterectomy followed by chemotherapy and more than 5-year disease-free survival were obtained, and without evidence of recurrent disease 7 years after the surgery. To the best of our knowledge, this is the first case in which gemcitabine-based chemotherapy achieved a good response in an urothelial carcinoma patient with systematic lymph node metastasis.
Acknowledgements
This article is supported by the National Natural Science Foundation of China (NO 81372749).
Disclosure of conflict of interest
None.
References
- 1.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523–1525. [PubMed] [Google Scholar]
- 2.Batata MA, Whitmore WF, Hilaris BS, Tokita N, Grabstald H. Primary carcinoma of the ureter: a prognostic study. Cancer. 1975;35:1626–1632. doi: 10.1002/1097-0142(197506)35:6<1626::aid-cncr2820350623>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Williams CB, Mitchell JP. Carcinoma of the ureter--a review of 54 cases. Br J Urol. 1973;45:377–387. doi: 10.1111/j.1464-410x.1973.tb12175.x. [DOI] [PubMed] [Google Scholar]
- 4.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 5.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. 2011;107:1059–1064. doi: 10.1111/j.1464-410X.2010.09675.x. [DOI] [PubMed] [Google Scholar]
- 6.Simsir A, Sarsik B, Cureklibatir I, Sen S, Gunaydin G, Cal C. Prognostic factors for upper urinary tract urothelial carcinomas: stage, grade, and smoking status. Int Urol Nephrol. 2011;43:1039–1045. doi: 10.1007/s11255-011-9915-z. [DOI] [PubMed] [Google Scholar]
- 7.Chen WJ, Kuo JY, Chen KK, Lin AT, Chang YH, Chang LS. Primary urothelial carcinoma of the ureter: 11-year experience in Taipei Veterans General Hospital. J Chin Med Assoc. 2005;68:522–530. doi: 10.1016/S1726-4901(09)70087-5. [DOI] [PubMed] [Google Scholar]
- 8.Heney NM, Nocks BN, Daly JJ, Blitzer PH, Parkhurst EC. Prognostic factors in carcinoma of the ureter. J Urol. 1981;125:632–636. doi: 10.1016/s0022-5347(17)55143-5. [DOI] [PubMed] [Google Scholar]


