Abstract
Background: Treatment for metastatic breast cancer (MBC) in patients who have relapsed from anthracycline and taxane is difficult. S-1, an oral 5-FU derivative, has demonstrated a potential antitumor effect in patients with MBC. Thus, we evaluated the efficacy and safety of S-1 as second-line chemotherapy MBC patients in a phase II trial. Methods: The study was conducted at seven centers in China and enrolled MBC patients who had previously relapsed from one chemotherapy regimen. The median progression-free survival (PFS) was the primary end point. The treatment schedule involved the administration of S-1 at a standard dose based on the body surface area (BSA) in 28-day cycles with consecutive administration followed by a 14-day rest, as follows: 40 mg twice daily if BSA < 1.25 m2; 50 mg twice daily if 1.25 m2 ≤ BSA ≥ 1.5 m2; and 60 mg twice daily if BSA > 1.5 m2. Results: Thirty-three patients were included in the analysis. S-1 demonstrated moderate efficacy with a PFS of 3.3 months, a response rate of 33.3%, and a disease control rate of 72.7%. The treatment was well-tolerated with mild-to-moderate toxicity. Grade 3 adverse events (AEs) occurred in 4 patients (2 with hyperbilirubinemia, 1 with anorexia, and 1 with vomiting). Grade 4 AEs were not observed. Conclusion: S-1 demonstrated encouraging efficacy and safety in a prospective trial as second-line treatment in MBC patients. All AEs were manageable; however, bilirubin monitoring is recommended during treatment.
Keywords: Metastatic breast cancer, chemotherapy, S-1
Introduction
Advanced breast cancer is an incurable disease, whose priorities of treatment are anthracycline and taxane drugs. However, for patients who failed to be treated by these two drugs, there is no standard treatment protocol to be recommended. The treating principle for them is to extend the patients’ life time as long as possible and improve their life quality.
5-FU drugs have certain effects on breast cancer [1-3]. The conventional intravenous infusion of it can keep a stable plasma concentration for a long time, but it has a higher incidence of venous thrombosis, gastrointestinal reactions neutropenia, and oral mucositis [4,5]. In recent years, variety of oral-taken 5-FU precursor drugs has been developed, such as UFT, doxifluridine, capecitabine, etc., which can maintain 5-FU a stable plasma concentration within the body for a period with more convenient administration. However, its clinical application is limited by the high incidence of gastrointestinal reactions and hand-foot syndrome [6-9]. S-1 was further improved on the basis of these drugs and DPD enzyme inhibitors were added to the prescription to reduce the drug degradation and gimeracil is added to alleviate gastrointestinal side effects [10,11]. Although S-1 has a lot of evidence-based medical data of digestive tract tumors, which shows good therapeutic efficacy, there is not enough information of it applying in advanced breast cancer. The existing data are mainly collected by clinical studies in Japan, and the therapeutic efficacy of it is still in controversy [12-14]. In order to clarify the actual effects of S-1 monotherapy in patients with advanced breast cancer, meanwhile to observe the sensitivity and tolerance of Chinese patients with advanced breast cancer treated with S-1, a prospective, single-arm, II phase clinical study of using S-1 monotherapy in the treatment of advanced breast cancer after the failure of anthracycline and taxane drugs was designed to evaluate its efficacy and safety in the second-line therapy.
Patients and methods
The trial was approved by the Ethics Investigation Committees of the Cancer Institute & Hospital and the Chinese Academy of Medical Science (CAMS), and conducted in accordance with the Declaration of Helsinki and registered on clinicaltrial.gov (NCT01492543). Informed consent was obtained from each patient.
Patient eligibility
Female patients with MBC who had disease progression after treatment with one previous chemotherapy regimen for advanced disease were eligible. At least one measurable lesion according to RECIST 1.1 was required. Inclusion criteria were Eastern Cooperative Oncology Group Performance Status (ECOG-PS) 0 or 1, life expectancy > 12 weeks, and adequate hematologic, hepatic, and renal function. Patients with rapidly progressive disease, large-volume visceral disease, liver or renal dysfunction, or brain metastases were excluded.
Treatment and modification
Eligible patients were assigned to receive S-1 at a standard dose based on the body surface area (BSA) as follows: 40 mg/twice per day (if BSA < 1.25 m2); 50 mg/twice per day (if 1.25 m2 ≤ BSA ≥ 1.5 m2); and 60 mg / twice per day (if BSA > 1.5 m2) in cycles of 28-day consecutive administration followed by a 14-day rest. Adverse events (AEs) were graded according to the National Cancer Institute of Canada Common Toxicity Criteria (NCIC-CTC; version 4.0). For grade 3-4 AEs, the treatment was delayed until toxicity improved to grade 2 or better, or discontinued if the recovery time was > 3 weeks. Patients who experienced drug-related grade 4 non-hematologic toxicities were treated at two dose levels lower at first appearance and withdrawn at second appearance.
Response assessment
Patients were evaluated at baseline and thereafter every 6 weeks. Baseline assessments included a detailed history of previous treatments. A complete physical examination and laboratory evaluations were performed at baseline and at each follow-up visit. Tumor assessments at baseline comprised CT radiographs of chest, abdomen, and pelvis. Tumor evaluations utilize the same methods as at baseline. Tumor responses were classified using RECIST 1.1 criteria. Follow-up was continued until 8 weeks after the end of treatment.
Statistical considerations
The primary end point for the phase II trial was progression-free survival (PFS), defined as the length of time between the day when patients signed informed consent and the onset of disease progression or death. Secondary end points were objective response rate and safety. The major safety end point was the incidence and severity of AEs based on NCIC-CTC grades. All statistical analyses were based on the intention-to-treat (ITT) population. The Kaplan-Meier method was used to calculate PFS. Statistical testing was performed using a log-rank test. The 5% level was used as the cut-off for statistical significance throughout the study. Patients who did not experience an event (progression and/or death) by the time of analysis were censored at the last available follow-up date. Analyses of AEs were descriptive and were summarized as the worst grade of toxicity per patient.
Results
Baseline conditions of patients
36 patients were included and treated with S-1 from 2011 December to 2013 August, among which 3 patients were not included in the analysis. One was because of the withdrawal of informed consent, and the other two were excluded for the incomplete clinical data. Baseline characteristics of patients are shown in Table 1. The median age of the patients was 54 years old (31-71 years old). The median number of treatment cycles was 2 (1 cycle -10 cycles). 78.7% of patients (26/33) combined with visceral metastases and 21.3% of patients (7/33) were with bone and/or soft tissue metastases. 51.5% (17/33) were estrogen receptor positive and 10 cases were HER-2 positive, all of whom didn’t receive any trastuzumab treatment. Four patients received intravenous 5-FU treatment in the adjuvant chemotherapy and 2 were treated with Xeloda in the first-line treatment. After a median follow-up of 490 days (203 days -816 days), 31 patients were discontinued the treatment. 27 cases were out of the group due to disease progression and 4 cases were out for other reasons. There were no patients out of the group because of treatment intolerance. Currently, there were two patients still receiving treatment.
Table 1.
Baseline characteristics
| N | % | ||
|---|---|---|---|
| Age | ≥ 5 years old | 15 | 45.5 |
| < 5 years old | 18 | 54.5 | |
| Menopausal status | |||
| Pre-menopausal | 7 | 21.2 | |
| Post-menopausal | 26 | 78.8 | |
| ER/PR status | |||
| positive | 17 | 51.5 | |
| negative | 14 | 42.4 | |
| unknown | 2 | 6.1 | |
| HER-2 status | |||
| positive | 10 | 30.3 | |
| negative | 13 | 39.4 | |
| unknown | 10 | 30.3 | |
| Metastastic sites | |||
| Viseral metastasis | 26 | 78.8 | |
| Liver | 19 | 57.6 | |
| Lung | 15 | 45.5 | |
| Soft tissue or bone | 7 | 21.2 | |
| Previous treatment | |||
| Anthracyclin and Taxane | 33 | 100 | |
| platinum | 13 | 39.4 | |
| 5-FU | 6 | 18.2 |
Therapeutic efficacy evaluation
There were 1 case of CR, 10 cases of PR, 13 cases of SD and 9 cases of PD in the 33 patients. The objective response rate was 33.3% (11/33), and the disease control rate was 72.7% (24/33). The response rate of each sub-group is shown in Table 2. The response rate of patients with pulmonary metastasis was 13.3% (2/15), significantly lower than 31.6% of patients with liver metastases (6/19), and 57.1% of patients with bone/soft tissue metastases (4/7); the response rate of ER/PR receptor-negative patients was 42.9% (6/14), higher than that of ER/PR receptor positive patients (23.5%, 4/17), but the difference was not significant (P = 0.459). The response rate of HER-2 positive patients was higher than that of HER-2-negative patients, but the difference between them was not significant (40% vs. 30.8%, P = 0.866). Previous administration of 5-FU did not affect the therapeutic efficacy, for the response rate of the two groups were both 33.3%.
Table 2.
Analysis of response rate
| Response Rate (%, N) | P value | |
|---|---|---|
| Metastastic site | ||
| Liver | 31.6 (/19) | NA |
| lung | 13.3 (/15) | |
| Bone/Soft tissue | 26.9 (/26) | |
| ER/PR Status | 0.459 | |
| Negative | 42.9 (/14) | |
| Positive | 23.5 (4/17) | |
| HER-2 status | 0.866 | |
| Negative | 30.8 (4/13) | |
| Positive | 40 (4/10) | |
| Prior treatment | 1.000 | |
| 5-FU | 33.3 (2/6) | |
| Non-5-FU | 33.3 (9/27) | |
| Hyperbilirubinemia | ||
| Yes | 26.7 (4/15) | 0.458 |
| No | 38.9 (7/18) |
The response rate of patients with elevated bilirubin was 26.7% (4/15), which was slightly lower than that of patients with normal bilirubin (38.9%, 7/18). There was no significant difference between them (P = 0.458). After a median follow-up of 490 days (203 days -816 days), disease progression could be seen in 31 cases out of the 33 patients. The median PFS was 3.3 months (Figure 1), and the median OS had not been reached yet. Single factor analysis (Table 3) was made on factors of hormone receptor status, HER-2 status, visceral metastases, progression-free survival, first-line therapeutic efficacy, and previous 5-FU administration history. It could be concluded from the analysis that the above factors were of no statistical significance on the PFS images. No tumor progression had been seen in patients with the longest treatment continuing 17 months.
Figure 1.
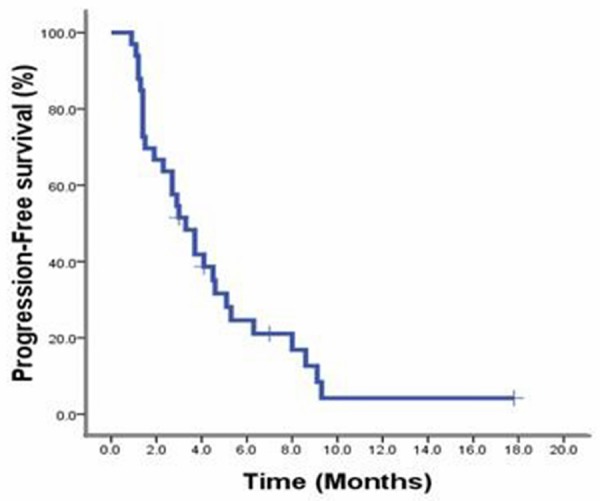
Progression-free survival of patients who receive S-1 monotherapy as second-line chemotherapy.
Table 3.
PFS analysis by prognostic factors
| Prognostic factors | N | PFS (months) | P value |
|---|---|---|---|
| Age | |||
| ≥ 60 y | 9 | 3.3 | 0.612 |
| < 60 y | 24 | 3.0 | |
| ER/PR status | |||
| positive | 17 | 2.9 | 0.984 |
| negative | 14 | 3.3 | |
| HER-2 status | |||
| positive | 10 | 2.7 | 0.572 |
| negative | 13 | 3.3 | |
| Viseral metastaisis | |||
| Yes | 26 | 2.9 | 0.489 |
| No | 7 | 3.7 | |
| Response of first-line therapy | |||
| Response (CR + PR) | 8 | 3.7 | 0.148 |
| No-response (SD + PD) | 20 | 1.5 | |
| DFS | |||
| > 3.5 years | 10 | 4.1 | 0.044 |
| ≤ 3.5 years | 20 | 3.0 | |
| TTP of first-line chemotherapy | |||
| ≥ 6 months | 6 | 6.0 | 0.262 |
| < 6 months | 27 | 3.0 | |
| Previous regimen | |||
| 5-FU | 6 | 3.7 | 0.151 |
| Non-5-FU | 27 | 3 | |
Safety
The incidence of adverse reactions is shown in Table 4. The most common adverse reactions were hyperbilirubinemia (45.5%), anorexia (33.3%) and fatigue (27.3%). Hematologic toxicities of them were all within 1-2 degrees. The 3-degree adverse reactions included hyperbilirubinemia (6.1%), anorexia (3.0%) and vomiting (3.0%). There were no 4-degree adverse reaction reports. The treatment of one patient was postponed for two weeks due to the III anorexia, and no patients required a dose reduction. Eight of all 15 cases of elevated bilirubin, were with liver metastases before treatment. The incidence of elevated bilirubin in patients with liver metastases was 42.1%; the one of patients without liver metastases was 50%. The median cycle of 15 cases of elevated bilirubin was 2 (1 cycle-10 cycles).
Table 4.
Adverse event profile by treatment (grades 1 to 4)
| AEs | RECIST 1.1 (n, %) | |||
|---|---|---|---|---|
| Non-hematologic AEs | 1 | 2 | 3 | 4 |
| hyperbilirubinemia | 9 (27.3%) | 4 (12.1%) | 2 (6.1%) | 0 |
| Anorexia | 8 (24.2%) | 2 (6.1%) | 1 (3.0%) | 0 |
| ALT/AST | 6 (18.2%) | 0 | 0 | 0 |
| Fatigue | 5 (15.2%) | 4 (12.1%) | 0 | 0 |
| nausea | 4 (12.1%) | 3 (9.1%) | 1 (3.0%) | 0 |
| diarrhea | 2 (6.1%) | 1 (3.0%) | 0 | 0 |
| Hand-foot syndrome | 1 (3.0%) | 0 | 0 | 0 |
| Oral mucositis | 1 (3.0%) | 2 (6.1%) | 0 | 0 |
| Skin rash | 0 | 1 (3.0%) | 0 | 0 |
| Hematologic AEs | ||||
| Leukopenia | 3 (9.1%) | 4 (12.1%) | 0 | 0 |
| Neutropenia | 1 (3.0%) | 5 (15.2%) | 0 | 0 |
| Thrombocytopenia | 2 (6.1%) | 1 (3.0%) | 0 | 0 |
| Anemia | 2 (6.1%) | 4 (12.1%) | 0 | 0 |
Discussion
This study is the first one using S-1 as the second-line therapy in the clinical study of patients with advanced breast cancer. All patients enrolled in this study were shown tumor progression after being treated with anthracycline and taxane in the adjuvant treatment or the first-line chemotherapy, and most of them were seen with visceral metastases at the same time. All factors mentioned above represented the main characteristics of patients with refractory breast cancer. The results showed that S-1 had good curative effect. The response rate was 33.3%, and the PFS was 3.3 months. The subgroup analysis showed that the response rate of patients with liver metastasis and bone metastasis was much higher and the previous administration of 5-FU in the adjuvant therapy or first-line therapy did not affect the therapeutic efficacy of S-1. The patients were of good overall tolerance and the adverse reactions were of controllable level of I to II. Elevated Bilirubin was the adverse reaction of highest incidence, whose occurrence had no connection with the existence of liver metastasis and was independent from the medication cycles.
The response rate of S-1 and PFS drawn by this study were consistent with the results of previous phase II clinical researches evaluating the therapeutic efficacy of using S-1 after taxane resistance in the first line or second line therapy [12], which were significantly better than those of the other retrospective analysis [13]. In this retrospective study, S-1 was used in the third line or above therapy of advanced breast cancer patients after anthracycline and taxane failing to take effects. The median starting time of S-1 was fifth line. The objective response rate of the results was only 3% and the disease control rate of it was only 8%. The results of this study proved that S-1 had certain therapeutic effects in the second-line treatment of anthracyclines and taxane resistant patients, which confirmed the prediction that the therapeutic efficacy could be improved by using S-1 in the first-line or second-line treatment of patients with advanced breast cancer. The reason that why S-1 did not perform well in the multi-courses treatment of patients with advanced breast cancer was that the general physical conditions and tolerance to treatment of patients would decline after treatment, and there would be possible occurrence of multiple-drug resistance to other chemotherapy drugs. This view had also been verified by a retrospective analysis in 2011, which included 33 patients with advanced breast cancer, who were failed to be treated by anthracycline and taxane. The response rate and disease control rate of patients treated by S-1 in the first-line and second-line treatment was better than the one of patients using S-1 in the third-line or above treatment [14].
It could be found by this study that the response rate of using S-1 in patients with liver metastases was higher than that of patients with lung metastases. The possible reason may be that tegafur contained in S-1 was decomposed to active ingredient 5-FU by liver microsomal P450 and cytochrome enzyme CYP2A6, while Jigme pyrimidine, mainly distributed in the liver, possessed selective antagonism action to DPD, the 5-FU metabolic enzyme synthesized by liver, which could increase the local concentration of 5-FU in liver, and enhanced the anti-tumor effects. Meanwhile, it could also be found that the previous administration of 5-FU did not affect the therapeutic efficacy of S-1 in the second-line treatment. The possible reason of it could be that the half-life of the drug was only 1.6-1.9 hours and the area under the curve of drug (AUC) of it was 870 ng.h/mL after intravenous injection of 5-FU, while the half-life of tegafur could be extended to 6.7-11.3 hours and the AUC of it could be increased to 19967 ng.h/mlL [15] after adding Jigme pyrimidine into S-1, which greatly extended the exposure time of tumor in effective drugs. The exposure time of tumor cells in the intravenous injection of 5-FU was short, so they were not completely resistant to the 5-FU drugs, which was still effective after the extension of the drug’s exposure time. There was no cross resistance between the two drugs.
The major adverse reactions in this study were elevated bilirubin, anorexia, fatigue, and nausea. The incidence of elevated bilirubin in this study was higher than that of other studies [12,14,16], such as in the phase II clinical study published by Toshiaki Saeki et al. [12], the elevated bilirubin ratio was 14.5%. In a study included 679 Japanese patients, the incidence of elevated bilirubin was 8.2% and the occurrence ratio of it in the third or above therapy was 0.4% [16]. No significant correlation between the liver metastases or medication cycles and the incidence or hyperbilirubinemia had been found in the exploration of possible reasons. One possible reason as speculated was that the DPD enzyme inhibitor contained in S-1 reduced the inactivation efficiency of 5-FU, resulting in an increase of liver accumulation and elevated liver toxicity; deficiency of DPD enzyme could be seen in 3% of Chinese patients, whose liver toxicity might be increased after being treated with S-1. Another possible reason leading to it was the single nucleotide polymorphism of CYP2A6. CYP2A6 was a key metabolism enzyme of S-1. Currently, single nucleotide polymorphisms of CYP2A6 that had been found were CYP2A6 × 1A, CYP2A6 × 1B and CYP2A6 × 4C, etc, all of which could influence efficiency of S-1 converting to 5-FU [17-19], and there were significant differences of distribution among ethnics [20], which might influence the therapeutic efficacy and toxicity of S-1. However, there were no statistics of single nucleotide polymorphisms of CYP2A6 collected from Chinese patients with breast cancer. Since there was no single nucleotide polymorphism of CYP2A6 or DPD enzyme activity being detected in this study, the speculations mentioned above could not be verified and needed further validation in future researches. It must be noted that although hyperbilirubinemia had been found by routine inspections in 45.5% of patients, no clinical symptoms associated with it could be seen. Therefore no reduction of drug dose or delay of treatment had been carried out and hyperbilirubinemia had been improved in 1 month after the discontinuance of drugs, indicating that the hyperbilirubinemia caused by S-1 was safe and controllable.
Compared with the researches of Xeloda monotherapy in treating patients with advanced breast cancer [21-24], the S-1 response rate and the progression-free survival period was similar to each other in this study. The single agent response rate of Xeloda monotherapy in the second-line treatment of patients with advanced breast cancer was 20%-30% and its PFS was 2.8-7.1 months. In terms of safety, III diarrhea occurred in 4%-14% and III hand-foot syndrome could be seen in 8%-26% of patients treated with Xeloda [21-24], which, to some extent, influenced the quality of life of patients. The activation of 5-FU in gastrointestinal tract and the gastrointestinal toxicity could be reduced by adding oteracil into S-1, which significantly decreased the incidence of diarrhea [12,13,16]. On the other hand, the conversion efficiency of tegafur was improved by adding the pyrimidine Jigme. The actual dosage of tegafur used in S-1 was lower than the one of it used independently, which reduced the production of its metabolites α-fluoro-β-propyl acid (FBAL), which was the main factor causing the hand-foot syndrome induced by 5-FU oral-taken drugs. Therefore, the incidence of hand-foot syndrome in patients treated with S-1 was significantly lower than that in patients treated with Xeloda [12,13,16].
This study had verified the safety and therapeutic efficacy of S-1 in the second-line therapy of Chinese patients with advanced breast cancer who were resistant to anthracycline and taxane and discovered that it might have better effects on patients with liver metastases. Currently, clinical researches of S-1 in combination with cisplatin and with Herceptin in treating advanced breast cancer are in progress [25-27]. There are also some studies exploring [28] to use S-1 in adjuvant chemotherapy. It should also be noted that the application of S-1 in the treatment of breast cancer is mainly based on the evidence collected from the phase II clinical study, therefore the III-phase clinical random control study with large volume of samples still needs to be carried out in the future to further confirm its efficacy and safety.
Disclosure of conflict of interest
None.
References
- 1.Blum JL, Barrios CH, Feldman N, Verma S, McKenna EF, Lee LF, Scotto N, Gralow J. Pooled analysis of individual patient data from capecitabine monotherapy clinical trials in locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2012;136:777–88. doi: 10.1007/s10549-012-2288-x. [DOI] [PubMed] [Google Scholar]
- 2.Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, Osterwalder B. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92:1759–68. doi: 10.1002/1097-0142(20011001)92:7<1759::aid-cncr1691>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J. Clin. Oncol. 1999;17:485–93. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 4.Scheithauer W, McKendrick J, Begbie S, Borner M, Burns WI, Burris HA, Cassidy J, Jodrell D, Koralewski P, Levine EL, Marschner N, Maroun J, Garcia-Alfonso P, Tujakowski J, Van Hazel G, Wong A, Zaluski J, Twelves C X-ACT Study Group. Oral capecitabine as an alternative to i. v. 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol. 2003;14:1735–43. doi: 10.1093/annonc/mdg500. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, McKendric J, Maroun J, Marshall J, Osterwalder B, Pérez-Manga G, Rosso R, Rougier P, Schilsky RL Capecitabine Colorectal Cancer Study Group. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:566–75. doi: 10.1093/annonc/mdf089. [DOI] [PubMed] [Google Scholar]
- 6.Ansfield FJ, Kallas GJ, Singson JP. Phase I-II studies of oral tegafur (ftorafur) J. Clin. Oncol. 1983;1:107–10. doi: 10.1200/JCO.1983.1.2.107. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura R, Tominaga T, Kimura M, Yanagita Y, Tamaki N, Asaishi K, Okamoto Y, Okuyama N, Takeuchi H, Inaba M, Doi T. Efficacy of doxifluridine combined with weekly paclitaxel therapy in the treatment of advanced or recurrent breast cancer: results of the JMTO BC01 phase II trial. Anticancer Drugs. 2008;19:911–5. doi: 10.1097/CAD.0b013e3283099e71. [DOI] [PubMed] [Google Scholar]
- 8.Crown JP, Dieras V, Staroslawska E, Yardley DA, Bachelot T, Davidson N, Wildiers H, Fasching PA, Capitain O, Ramos M, Greil R, Cognetti F, Fountzilas G, Blasinska-Morawiec M, Liedtke C, Kreienberg R, Miller WH Jr, Tassell V, Huang X, Paolini J, Kern KA, Romieu G. Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. J. Clin. Oncol. 2013;31:2870–8. doi: 10.1200/JCO.2012.43.3391. [DOI] [PubMed] [Google Scholar]
- 9.Sparano JA, Vrdoljak E, Rixe O, Xu B, Manikhas A, Medina C, Da Costa SC, Ro J, Rubio G, Rondinon M, Perez Manga G, Peck R, Poulart V, Conte P. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 2010;28:3256–63. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takechi T, Nakano K, Uchida J, Mita A, Toko K, Takeda S, Unemi N, Shirasaka T. Antitumor activity and low intestinal toxicity of S-1, a new formulation of oral tegafur, in experimental tumor models in rats. Cancer Chemother Pharmacol. 1997;39:205–11. doi: 10.1007/s002800050561. [DOI] [PubMed] [Google Scholar]
- 11.Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, Unemi N, Fukushima M. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602–6. [PubMed] [Google Scholar]
- 12.Saek T, Takashima S, Sano M, Horikoshi N, Miura S, Shimizu S, Morimoto K, Kimura M, Aoyama H, Ota J, Noguchi S, Taguchi T. A phase II study of S-1 in patients with metastatic breast cancer--a Japanese trial by the S-1 Cooperative Study Group, Breast Cancer Working Group. Breast Cancer. 2004;11:194–202. doi: 10.1007/BF02968301. [DOI] [PubMed] [Google Scholar]
- 13.Shien T, Shimizu C, Akashi-Tanaka S, Yonemori K, Kohno T, Hojo T, Ando M, Katsumata N, Kinoshita T, Fujiwara Y. Clinical efficacy of S-1 in pretreated metastatic breast cancer patients. Jpn J Clin Oncol. 2008;38:172–5. doi: 10.1093/jjco/hyn001. [DOI] [PubMed] [Google Scholar]
- 14.Hara F, Kiyoto S, Takahashi M, Takabatake D, Takashima S, Aogi K, Ohsumi S, Takashima S. Efficacy and safety of S-1 in patients with metastatic breast cancer: retrospective review in a single institution. Oncology. 2010;79:273–7. doi: 10.1159/000322371. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency. Teysuno 15 mg/4.35 mg/11.8 mg hard capsules: summary of product characteristics. http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001242/WC500104415.pdf. Accessed 11 April 2013. [Google Scholar]
- 16.Saito Y, Oshitanai R, Terao M, Tsuda B, Okamura T, Suzuki Y, Tokuda Y. Post-marketing safety evaluation of S-1 in patients with inoperable or recurrent breast cancer: especially in patients treated with S-1 + trastuzumab. Jpn J Clin Oncol. 2011;41:1051–8. doi: 10.1093/jjco/hyr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong SY, Lim HS, Nam BH, Kook MC, Kim YW, Ryu KW, Lee JH, Choi IJ, Lee JS, Park YI, Kim NK, Park SR. Association of CYP2A6 polymorphisms with S-1 plus docetaxel therapy outcomes in metastatic gastric cancer. Pharmacogenomics. 2009;10:1147–55. doi: 10.2217/pgs.09.48. [DOI] [PubMed] [Google Scholar]
- 18.Fang WJ, Mou HB, Jin DZ, Zheng YL, Zhao P, Mao CY, Peng L, Huang MZ, Xu N. Characteristic CYP2A6 genetic polymorphisms detected by TA cloning-based sequencing in Chinese digestive system cancer patients with S-1 based chemotherapy. Oncol Rep. 2012;27:1606–10. doi: 10.3892/or.2012.1678. [DOI] [PubMed] [Google Scholar]
- 19.Daigo S, Takahashi Y, Fujieda M, Ariyoshi N, Yamazaki H, Koizumi W, Tanabe S, Saigenji K, Nagayama S, Ikeda K, Nishioka Y, Kamataki T. A novel mutant allele of the CYP2A6 gene (CYP2A6*11) found in a cancer patient who showed poor metabolic phenotype towards tegafur. Pharmacogenetics. 2002;12:299–306. doi: 10.1097/00008571-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Soriano A, Vicente J, Carcas C, Gonzalez-Andrade F, Arenaz I, Martinez-Jarreta B, Fanlo A, Mayayo E, Sinués B. Differences between Spaniards and Ecuadorians in CYP2A6 allele frequencies: comparison with other populations. Fundam Clin Pharmacol. 2011;25:627–32. doi: 10.1111/j.1472-8206.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 21.Kamal AH, Camacho F, Anderson R, Wei W, Balkrishnan R, Kimmick G. Similar survival with single-agent capecitabine or taxane in first-line therapy for metastatic breast cancer. Breast Cancer Res Treat. 2012;134:371–8. doi: 10.1007/s10549-012-2037-1. [DOI] [PubMed] [Google Scholar]
- 22.Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O’Shaughnessy J. RIBBON-1: randomized, double-blind, placebo- controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J. Clin. Oncol. 2011;29:1252–60. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann M, Maass N, Costa SD, Schneeweiss A, Loibl S, Sütterlin MW, Schrader I, Gerber B, Bauer W, Wiest W, Tomé O, Distelrath A, Hagen V, Kleine-Tebbe A, Ruckhaeberle E, Mehta K, von Minckwitz G GBG-39 Trialists. First-line therapy with moderate dose capecitabine in metastatic breast cancer is safe and active: results of the MONICA trial. Eur J Cancer. 2010;46:3184–91. doi: 10.1016/j.ejca.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, Bell D, Rosso R, Mauriac L, Osterwalder B, Burger HU, Laws S. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–54. doi: 10.1023/a:1012281104865. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Ogiya R, Oshitanai R, Terao M, Terada M, Morioka T, Tsuda B, Niikura N, Okamura T, Saito Y, Tokuda Y. Feasibility and pharmacokinetics of combined therapy with S-1 and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic or recurrent breast cancer. Int J Clin Oncol. 2014;19:274–9. doi: 10.1007/s10147-013-0547-4. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama T, Morita S, Takashima T, Kamigaki S, Yoshidome K, Ito T, Taguchi T, Sakamoto J, Noguchi S. Phase I study of S-1 in combination with trastuzumab for HER2-positive metastatic breast cancer. Anticancer Res. 2011;31:3035–9. [PubMed] [Google Scholar]
- 27.Yunokawa M, Katsumata N, Yamamoto H, Kodaira M, Yonemori K, Shimizu C, Ando M, Tamura K, Fujiwara Y. A pilot feasibility study for cisplatin plus S-1 for the treatment for advanced or recurrent cervical cancer. Cancer Chemother Pharmacol. 2013;71:1369–74. doi: 10.1007/s00280-013-2137-6. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T. Evidence produced in Japan: tegafur-based preparations for postoperative chemotherapy in breast cancer. Breast Cancer. 2013;20:302–9. doi: 10.1007/s12282-013-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


