Abstract
Objective
Our goal was to determine the mechanism by which mitochondrial oxidative stress impairs collateral growth in the heart.
Approach and Results
Rats were treated with rotenone (mitochondrial complex I inhibitor that increases reactive oxygen species production) or sham-treated with vehicle and subjected to repetitive ischemia protocol for 10 days to induce coronary collateral growth. In control rats, repetitive ischemia increased flow to the collateral-dependent zone; however, rotenone treatment prevented this increase suggesting that mitochondrial oxidative stress compromises coronary collateral growth. In addition, rotenone also attenuated mitochondrial complex I activity and led to excessive mitochondrial aggregation. To further understand the mechanistic pathway(s) involved, human coronary artery endothelial cells were treated with 50 ng/ mL vascular endothelial growth factor, 1 µmol/L rotenone, and rotenone/vascular endothelial growth factor for 48 hours. Vascular endothelial growth factor induced robust tube formation; however, rotenone completely inhibited this effect (P<0.05 rotenone versus vascular endothelial growth factor treatment). Inhibition of tube formation by rotenone was also associated with significant increase in mitochondrial superoxide generation. Immunoblot analyses of human coronary artery endothelial cells with rotenone treatment showed significant activation of adenosine monophosphate activated kinase (AMPK)-α and inhibition of mammalian target of rapamycin and p70 ribosomal S6 kinase. Activation of AMPK-α suggested impairments in energy production, which was reflected by decrease in O2 consumption and bioenergetic reserve capacity of cultured cells. Knockdown of AMPK-α (siRNA) also preserved tube formation during rotenone, suggesting the negative effects were mediated by the activation of AMPK-α. Conversely, expression of a constitutively active AMPK-α blocked tube formation.
Conclusions
We conclude that activation of AMPK-α during mitochondrial oxidative stress inhibits mammalian target of rapamycin signaling, which impairs phenotypic switching necessary for the growth of blood vessels.
Keywords: collateral circulation, coronary circulation, mitochondria, reactive oxygen species
The metabolic syndrome (MS) is a constellation of metabolic abnormalities that markedly increases the risk of developing ischemic heart disease, atherosclerotic cardiovascular diseases, and type II diabetes mellitus.1 Since the initial descriptions in the 1920s, both the terminology and the clinical criteria have undergone various revisions.2,3 Recent studies indicate that >40% of US citizens aged >60 years have MS when evaluated by Adult Treatment Panel III criteria.4 Importantly, angiographic study by Abaci et al5 showed that patients with diabetes mellitus developed a less extensive coronary collateral circulation with nondiabetic patients. Although the effect of the MS on coronary collateral development is somewhat controversial, there is consensus that a significant cohort of patients do not show evidence of collateral growth.6 Coronary collaterals carry insufficient flow to completely prevent infarction in most cases, their presence is known to limit the damage.7 Thus, patients with well-developed collateral have better prognosis in recovering from myocardium infarction. However, the complex mechanisms mediating the enlargement and development of new blood vessels in the heart are not well understood.
Zucker Obese Fatty rats are a model of human MS, exhibiting many of the same afflictions including obesity, insulin resistance, hyperlipidemia, hyperinsulinemia, and hyperphagia.8 The Zucker Obese Fatty rats also demonstrated endothelial dysfunction and oxidative stress.9 We previously observed that coronary collateral growth (CCG) was markedly compromised in response to episodic ischemia in the Zucker Obese Fatty rats10,11 and that mitochondrial oxidative stress and dysfunction may underlie this problem. However, the underlying signaling pathway(s) remain elusive. Accordingly, the goal of this study was to define the basis by which mitochondrial oxidative stress corrupts vascular growth. Our findings suggest that mitochondrial bioenergetics, which is adversely affected by mitochondrial oxidative stress, ultimately mediates the phenotypic switch in endothelial cells (ie, from quiescent to migratory and proliferative phenotypes) and adenosine monophosphate activated kinase (AMPK)-α is the effector of impaired mitochondrial bioenergetics and function. Together our data support the importance of mitochondrial bioenergetics and oxidative stress in regulating phenotypic switch in endothelial cells and suggest that mitochondrial treatment may hold therapeutic potential in coronary arteriogenesis/angiogenesis.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Mitochondrial Oxidative Stress and Dysfunction Compromise CCG in Lean Rats in Response to Repetitive Ischemia
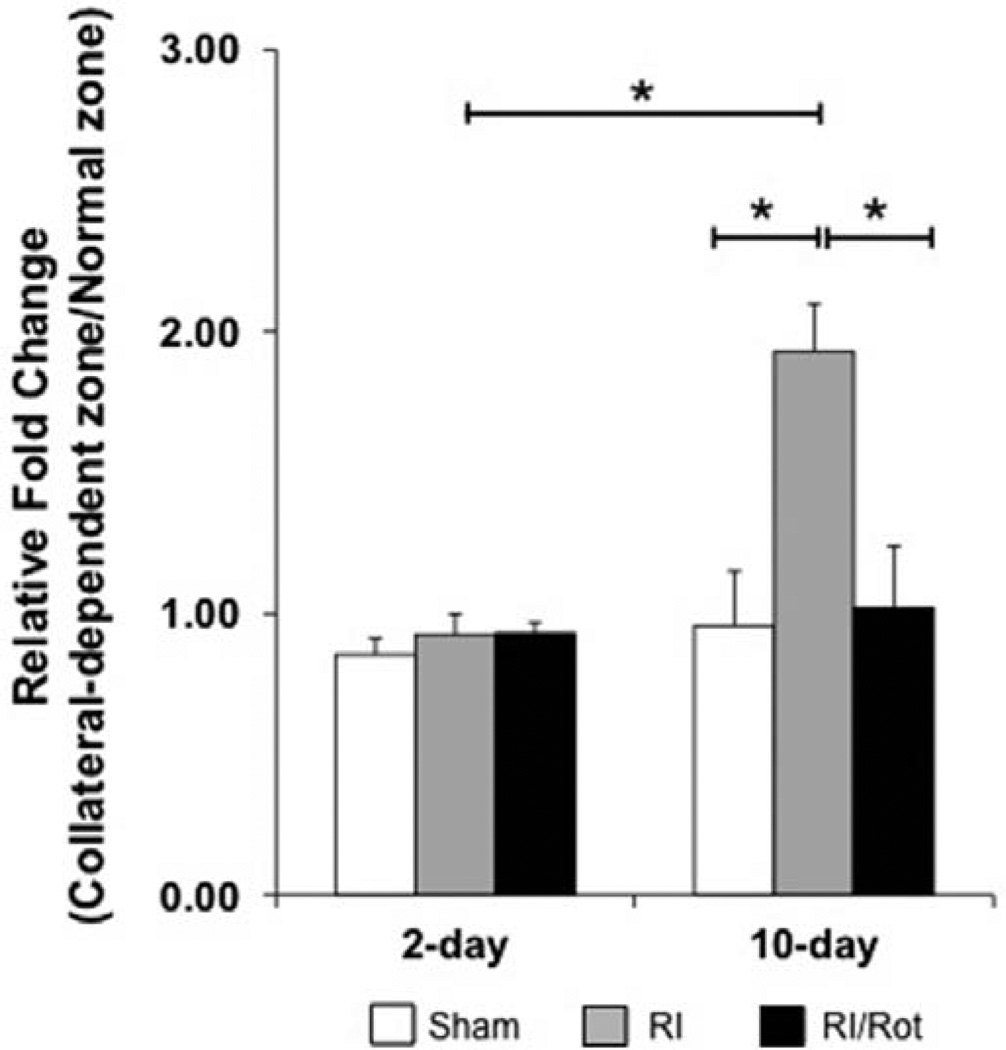
To assess the effect(s) of mitochondrial oxidative stress and dysfunction have on CCG, Wistar Kyoto rats were divided into sham, repetitive ischemia (RI), and RI/rotenone (Rot) groups (n=18–26 rats per group) and euthanized at days 2 and 10. Some rats were excluded from the study because of surgical failure, death resulted from severe myocardial infarction, and rotenone treatment. As shown in Figure 1A, the fold increase in the collateral-dependent zone (CZ)/normal zone (NZ) flow ratio is ≈1.0 (before/after RI) at day 2, indicating no obvious collateral growth to the CZ for the sham, RI, and RI/Rot groups (n=5, 4, and 2 rats per group). After 10 days of RI, the fold increase in ratio of CZ/NZ flow (before/after RI) was nearly doubled (1.93; n=8 rats; P<0.05) as compared with the sham-operated rats (n=6 rats) which showed no increase in the CZ/NZ flow ratio. Rotenone prevented this increase (P<0.05 versus RI).
Figure 1.
Mitochondrial oxidative stress and dysfunction compromise coronary collateral growth in lean rats in response to repetitive ischemia. Wistar Kyoto rats were euthanized at days 2 and 10 and further divided into 3 groups at each time point: sham, repetitive ischemia (RI), and RI/rotenone (Rot). At day 2, the fold change in the collateral-dependent zone (CZ)/normal zone (NZ) blood flow ratio (vs day 0) is unchanged from the basal time point before start of the RI protocol, implying no obvious growth of collaterals (n=5, 4, and 2 rats, respectively, for sham, RI, and RI/Rot groups). After 10 days of RI protocol, the fold increase in the ratio of blood flow compared with day 0 in the CZ/NZ was nearly doubled in the RI group as compared with the sham-operated rats (≈92% increase 10-day RI vs 10-day sham; *P<0.05; n=8 and 6 rats, respectively). Rot prevented this increase in the flow ratio in response to repetitive ischemia.
To assess the dosage efficacy of rotenone in the RI rat model, mitochondria were isolated from both the NZ and the CZ of the left ventricular tissues from the 10-day experimental groups and subjected to mitochondrial complex I (CI) activity assay (Figure I in the online-only Data Supplement). Ten-day RI increased CI activity in the CZ as compared with their NZ controls. It is also important to point out that CI activity was significantly blunted in both the NZ and the CZ tissues collected from 10-day RI/Rot compared with sham-operated and RI groups (reduction of ≈58% and 66%; n=3 rats per group; P<0.05). The striking suppression in CI activity in the rotenone-treated group might be the main reason for abrogated growth of coronary collaterals to CZ in response to RI.
Mitochondrial Oxidative Stress and Dysfunction Induce Mitochondrial Aggregation and Dyad Swelling in the Myocardium of Lean Rats in Response to RI
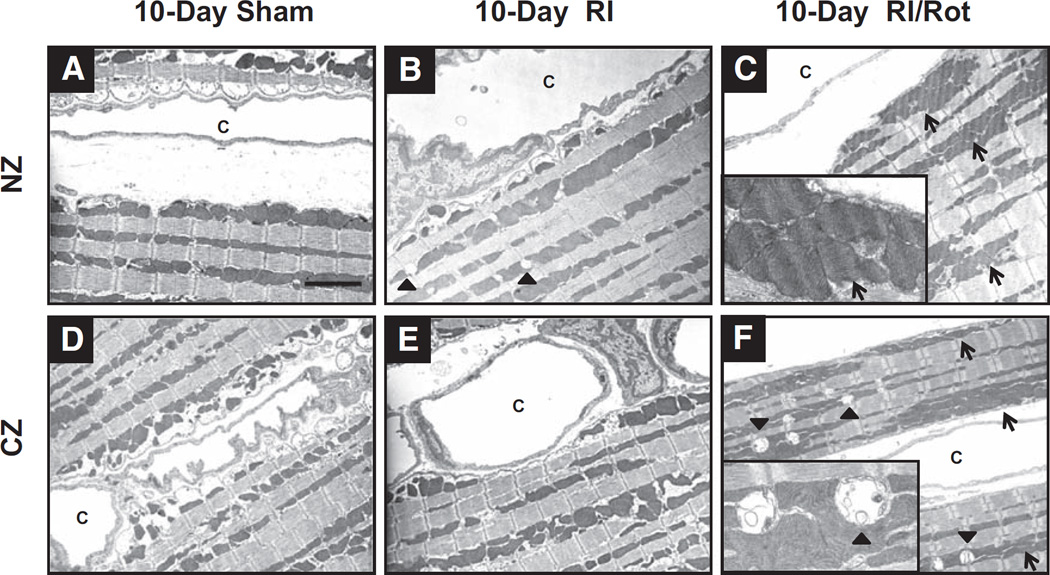
Representative electron micrographs showing ultrastructures of myocytes and vasculatures from left ventricular tissues of sham-operated, RI, and RI/Rot rats (n=3 rats per group). In general, intracellular architectures from both the NZ and the CZ of 10-day sham-operated rats were well preserved (eg, intact T-tubules, intercalated disks, sarcolemmal and basement membranes), as depicted in Figure 2A and 2D. In contrast, 10-day RI/Rot rats showed excessive mitochondrial aggregation, implying fusion of mitochondrial network (Figure 2C and 2F, opened arrow). In addition, consistent severe dyad swelling and rupture were also observed in the CZ of 10-day RI/Rot rats (Figure 2F, closed arrow). It is interesting to note that mild dyad swelling was also present in some NZ sections from 10-day RI rats (Figure 2B, closed arrow), suggesting perturbation of Ca2+ homeostasis in the myocardium.
Figure 2.
Mitochondrial oxidative stress and dysfunction induce mitochondrial aggregation and dyad swelling in the myocardium of lean rats in response to repetitive ischemia. Representative electron micrographs from 10-day sham (A, normal zone [NZ]; D, collateral-dependent zone [CZ]); 10-day RI (B, NZ; E, CZ); and 10-day repetitive ischemia (RI)/rotenone (Rot; C, NZ; F, CZ) rats, respectively (n=3 rats per group). Sham-operated rats showed well-preserved intracellular architectures (A and D). Rot induced mitochondrial aggregation (open arrow), indicating mitochondrial network fusion (C and F). This fusion process might be a mechanism for mitochondria to complement impair in function/bioenergetics for survival. It is also important to note that severe dyad swelling (solid arrow) was observed in Rot-treated rats, suggesting alteration in Ca2+ homeostasis in the myocardium (magnification, ×3000; insets, ×10 000). c indicates capillaries.
Mitochondrial Oxidative Stress and Dysfunction Inhibit Formation of Tubes on 2D Matrigel in Human Coronary Artery Endothelial Cells
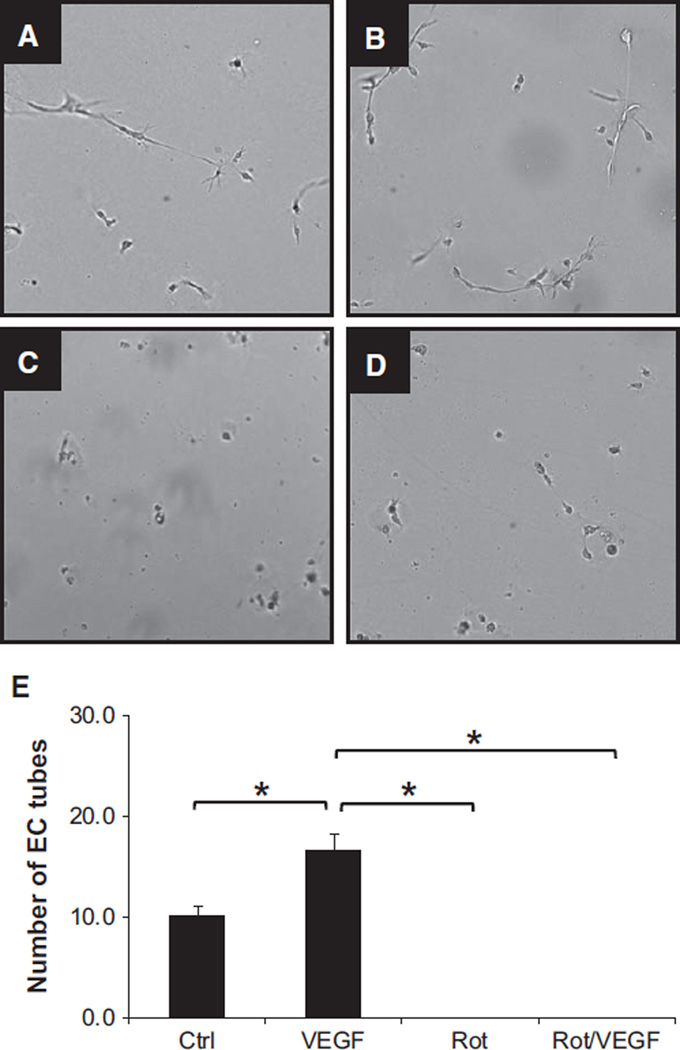
To further gain mechanistic insights into the effect of mitochondrial oxidative stress and dysfunction have on vascular growth, human coronary artery endothelial cells (HCAECs) were cultured on 2D Matrigel and subjected to rotenone and vascular endothelial growth factor (VEGF) treatment as described. Formation of tubes was significantly increased in response to VEGF (Figure 3A [control, 10.1±1.1] versus Figure 3B [VEGF, 16.5±1.7]; *P<0.05). In contrast, rotenone completely inhibited formation of tubes (Figure 3C [Rot, 0±0.0]; *P<0.05) and abolished the effects of VEGF (Figure 3B versus Figure 3D [Rot+VEGF, 0±0.0]; *P<0.05), suggesting that mitochondrial bioenergetics affects cellular signaling upstream of VEGF (n=4; 8–12 wells/condition).
Figure 3.
Mitochondrial oxidative stress and dysfunction inhibit tube formation on 2D Matrigel in human coronary artery endothelial cells (HCAECs). HCAECs were treated with vehicle (A), vascular endothelial growth factor (VEGF; 50 ng/mL; B), rotenone (Rot; 1 µmol/L; C), and Rot/VEGF (D). Bar graph summarizing the number of tubes formed under each condition is shown in E. Although VEGF induced robust formation of tubes on 2D Matrigel, Rot inhibited this process in the presence or absence of VEGF (*P<0.05; n=4; 8–12 wells/condition), implying that mitochondrial bioenergetics is the upstream regulator of growth factor–mediated signaling cascade.
Mitochondrial Oxidative Stress in HCAECs
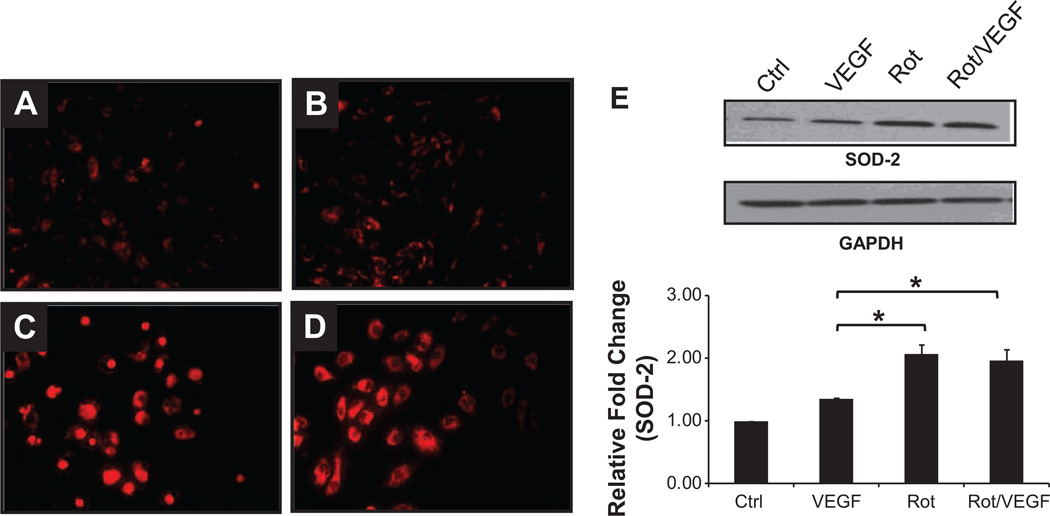
Production of reactive oxygen species (ROS) by mitochondria was examined using MitoSox Red. Similar fluorescent signals were observed for control and VEGF-treated HCAECs as illustrated in Figure 4A and 4B. However, there was substantially higher fluorescent intensity for rotenone and Rot/VEGF-treated cells (Figure 4C and 4D). Immunoblot analysis showed significant higher superoxide dismutase-2 protein expression for both the rotenone and the Rot/VEGF-treated cells (Figure 4E). Bright field images of HCAECs after VEGF, rotenone, and Rot/VEGF treatments are shown in Figure II in the online-only Data Supplement. Note that rotenone significantly reduces cell number and alters morphology of the cells (Figure IIA and IIB versus IIC and IID in the online-only Data Supplement).
Figure 4.
Mitochondrial oxidative stress in human coronary artery endothelial cells (HCAECs). HCAECs were treated with vehicle (A), vascular endothelial growth factor (VEGF; 50 ng/mL; B), rotenone (Rot; 1 µmol/L; C), and Rot/VEGF (D). Rot increases mitochondrial superoxide production as shown by the higher fluorescence intensity of MitoSox Red (A and B vs C and D; n=3). Superoxide dismutase (SOD)-2 expression, normalized against GAPDH, was significantly increased in Rot-treated cells (E; n=3; *P<0.05), suggesting upregulation of this antioxidant as compensation to the elevated mitochondrial oxidative stress.
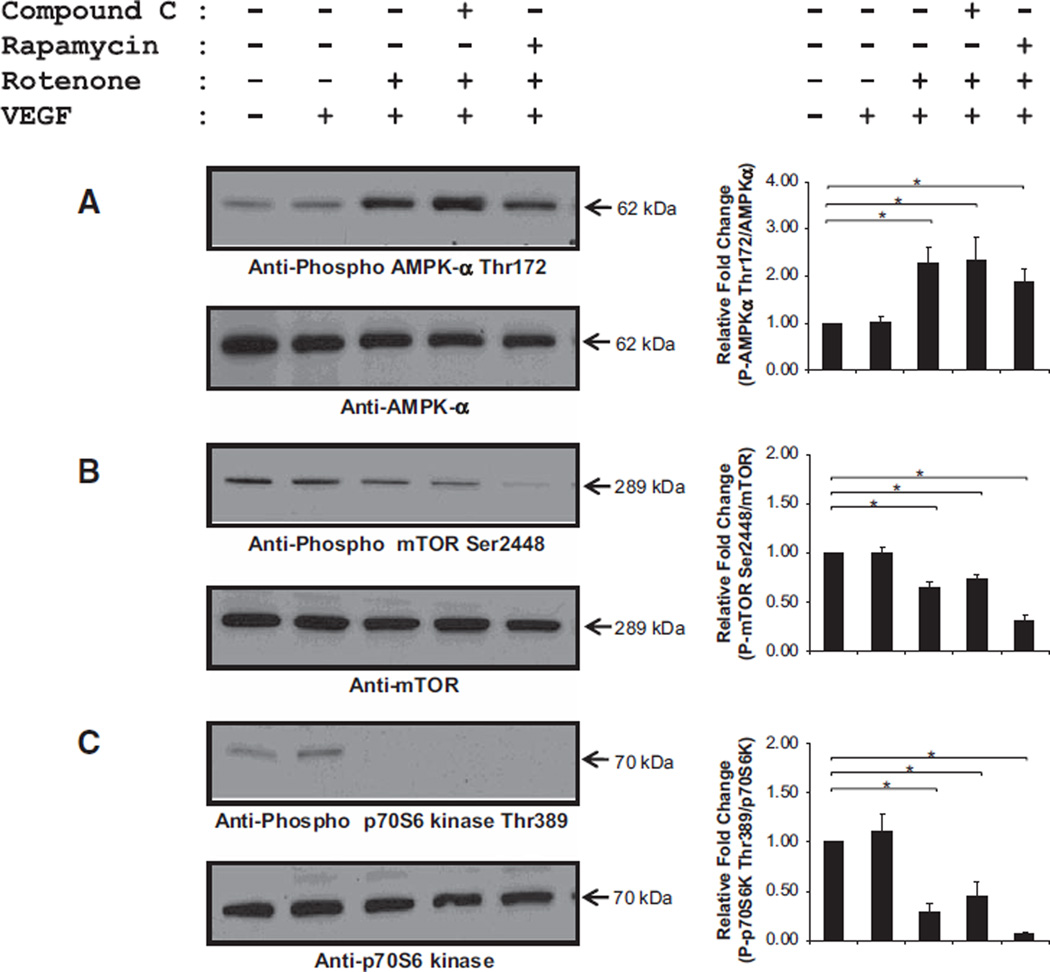
Corruption of Growth Factor Mediated–Signaling Cascade(s) via Altered AMPK-α/Mammalian Target of Rapamycin Signaling
Rotenone significantly increased phosphorylation of AMPK-α Thr172 (Figure 5A) and abolished the phosphorylation of mammalian target of rapamycin (mTOR) Ser2448 and p70 ribosomal S6 Thr389 as depicted in Figure 5B and 5C (n=5–9; *P<0.05). Neither compound C (AMPK-α inhibitor) nor rapamycin (mTOR inhibitor) blocked the activation of AMPK-α and the downregulation of mTOR and p70 ribosomal S6, suggesting that bioenergetic stress hinders the activity of mTOR which would prevent new protein synthesis and block phenotypic switching (ie, quiescent to proliferating cells). Dose response curves of compound C and rapamycin are shown in Figure III in the online-only Data Supplement.
Figure 5.
Impaired growth factor–mediated signaling cascade induced by mitochondrial oxidative stress and dysfunction. Human coronary artery endothelial cells were treated with vehicle, vascular endothelial growth factor (VEGF; 50 ng/mL), rotenone (Rot; 1 µmol/L), and Rot/VEGF, respectively. Rot significantly increased activation of adenosine monophosphate-activated kinase (AMPK)-α refecting energetic limitations (A). Activation of AMPK-α negatively regulates its downstream effectors, mammalian target of rapamycin (mTOR; B), and p70 ribosomal S6 (p70S6; C), which are essential for protein synthesis (n=5–9; *P<0.05).
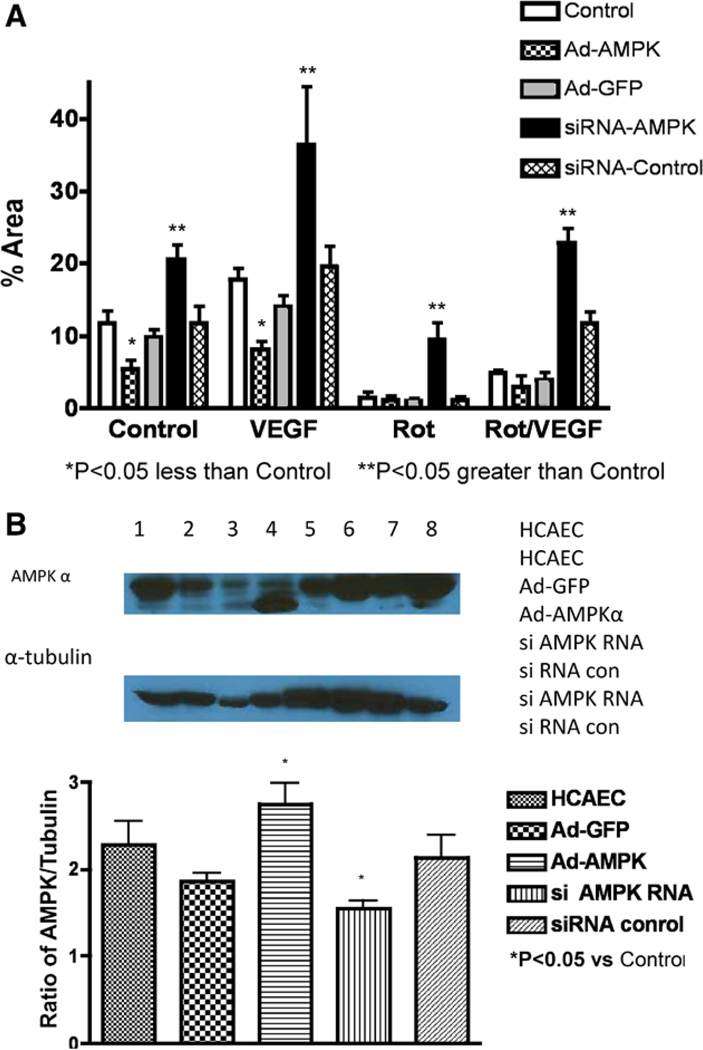
The effects of overexpression of an active AMPK-α or knockdown of AMPK-α on endothelial tube formation are shown in Figure 6. Overexpression of the active form of AMPK-α resulted in less tube formation during VEGF treatment compared with control VEGF treatment or to administration of the empty virus (expressing green fluorescent protein) with VEGF (Figure 6A). Knockdown of AMPK-α with the siRNA increased tube formation compared with the scrambled siRNA or controls (Figure 6A). Although the Western analyses revealed the expected changes in protein during knockdown or overexpression (Figure 6B), the physiological effects of these changes on tube formation were significant. Also, we would like to point out that the signal from the overexpression mutant that we report is only from the mutant, so the levels of total AMPK (wild type and mutant combined) would be even higher.
Figure 6.
Effects of AMPK overexpression and knockdown on endothelial tube formation. A, Tube formation by endothelial cells in Matrigel under control conditions, administration of vascular endothelial growth factor (VEGF), rotenone (Rot), and VEGF+Rot. Under these conditions cells were studied without treatment, after transduction of an adenovirus expressing constitutively active AMPK, the adenovirus-expressing green fluorescent protein (GFP), an siRNA to knockdown AMPK, and a scrambled control siRNA. Knockdown of AMPK increased tube formation during Rot, VEGF, and VEGF+Rot. In contrast, overexpression of the active AMPK decreased tube formation during VEGF. The scrambled siRNA and the adenovirus-expressing GFP did not have significant effects. B, AMPK and mutant AMPK protein levels. Knockdown by the siRNA decreased AMPK protein levels but the scrambled siRNA did not have an effect. Transduction with the active mutant resulted in protein levels slightly elevated compared with control levels. The adenovirus-expressing GFP did not have significant effects on AMPK protein levels. HCAECs indicates human coronary artery endothelial cells.
Mitochondrial Dysfunction in HCAECs
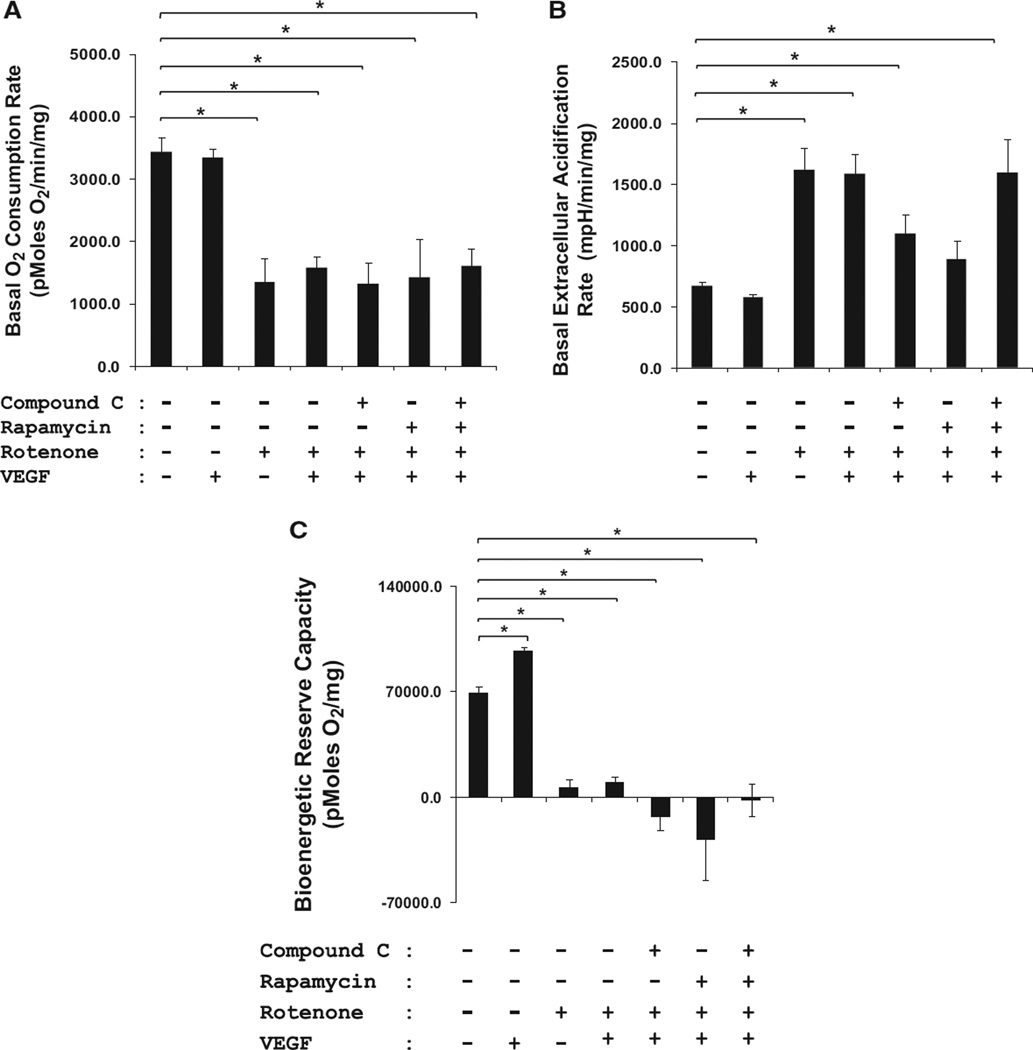
Mitochondrial functional indices of intact HCAECs were assessed using a Seahorse XF24-3 analyzer. On the basis of the oxygen consumption rate and the dose of carbonylcyanide-p-trifuoromethoxyphenylhydrazone (FCCP) to maximally stimulate oxygen consumption, density of 50 000 cells per well, and FCCP concentrations of 3 µmol/L were chosen for all experiments (Figure IVA and IVB in the online-only Data Supplement, respectively).
In our study, neither oxygen consumption rate nor extracellular acidification rate was significantly affected by VEGF compared with control HCAECs (Figure 7A). Rotenone, however, lowered the oxygen consumption rate and increased the extracellular acidification rate significantly as compared with controls and VEGF-treated cells (n=4; 2–4 wells/condition; *P<0.05), indicating severe mitochondrial dysfunction. Mitochondrial dysfunction induced by rotenone was not affected by compound C, rapamycin, or VEGF (n=4; 2–4 wells/condition; *P<0.05). Next, mitochondrial function was assessed using various bioenergetic stressors, namely oligomycin, FCCP, and antimycin A, in intact HCAECs (Figure 7B). To quantify the overall increase in the amount of oxygen consumed by the cells in the presence of FCCP, the oxygen consumption rate area under the curve for each group was calculated by multiplying the group average by the time interval for that rate. Exposure of either control or VEGF-treated HCAECs to 3 µmol/L FCCP resulted in up to ≈75 to 125 nmol of oxygen consumed per milligram proteins. However, Rot treatment significantly reduced oxygen consumption at baseline (compared with untreated controls), suggesting overt damage to mitochondrial respiratory complexes. We speculated that the negative values in oxygen consumption correlate with the production of O2·- leading to H2O2, and the metabolism of H2O2 yielding O2 and H2O. Compound C, rapamycin, and VEGF did not reverse the bioenergetic dysfunction caused by rotenone.
Figure 7.
A, Mitochondrial dysfunction in human coronary artery endothelial cells (HCAECs). Mitochondria are the major oxygen consumer, as they consume ≈90% of O2 in the cells. Therefore, oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) are direct representations of mitochondrial function. Basal OCR in pmol/min per milligram protein (A) and ECAR in millipH/min per milligram protein (B) were measured for 30 minutes (n=4; 2–4 wells/condition). Rotenone (Rot; 1 µmol/L) significantly blunted OCR and increased ECAR, as compared with control HCAECs (≈75%), indicating severe mitochondrial dysfunction (*P<0.05). C, Mitochondrial dysfunction and its effect on bioenergetic reserve capacity in HCAECs. Mitochondrial electron transport chain inhibitors, namely oligomycin (1 µg/mL), carbonylcyanide-p-trifuoromethoxyphenylhydrazone (3 µmol/L), and antimycin A (10 µmol/L), were injected sequentially. The reserve capacity was calculated by subtracting the maximal rate of oxygen consumption by the preoligomycin rate. Rot completely attenuated the bioenergetic reserve capacity (n=4; 2–4 wells/condition; *P<0.05). Neither compound C, rapamycin, nor vascular endothelial growth factor (VEGF) reversed the inhibitory effects of Rot.
Discussion
In this study, we reported the mechanism by which mitochondrial oxidative stress and dysfunction were transduced into inhibition of CCG in lean rats in response to RI. To further understand the mechanism(s) involved, HCAECs were used because of lack of established protocol to isolate coronary artery endothelial cells from rat. In HCAECs, VEGF treatment induced robust tube formation as compared with controls in 2D Matrigel; however, treatment with rotenone completely attenuated this effect. In addition, when AMPK-α was activated, even VEGF could not reverse the antiangiogenic effect induced by rotenone. Thus, this observation implies that mitochondrial bioenergetics and function are the important upstream regulators in mediating phenotypic switch in endothelial cells.
Rat Model of Mitochondrial Oxidative Stress and Dysfunction
Oxidative stress, either derived from excessive free radical generation and defective in the antioxidant defense, is often associated with the development of vascular disease in patients with MS risk factors. Enzymatic sources of ROS produced under pathological conditions via activation of vascular nicotinamide adenine dinucleotide phosphate oxidases, xanthine oxidase, and uncoupled of endothelial nitric oxide synthase are well characterized in cardiovascular diseases.7,12 Mitochondria are a major source of ROS in organs, especially in tissues requiring high energy demand, such as the myocardium. Instead of being transferred for energy production, some electrons leak from electron transport complexes and reduce oxygen, resulting in the production of superoxide anion (O2·-) followed by hydrogen peroxide (H2O2). Conversion of O2·- to H2O2 involves a series of enzymatic cascades by superoxide dismutases (ie, superoxide dismutase-1 and -2), followed by glutathione peroxidases, and catalase.12–14 The higher the energy demand, the greater the electron flux, and thus elevated ROS production. Mitochondrial production of ROS occurs primarily in complex I (nicotinamide adenine dinucleotide coenzyme Q reductase) and complex III (bcl complex).15
Mitochondrial oxidative stress and dysfunction have been implicated to play important role in the progression of Parkinson disease. In this regard, rotenone, which is a potent mitochondrial complex I inhibitor, has been used extensively to produce animal models with dysfunctional mitochondria that mimic this neurodegeneration disease.16 We, therefore, used rotenone to induce mitochondrial oxidative stress and dysfunction in our RI rat model. Surprisingly, subcutaneous bolus injections of rotenone, ranging from 0.5 to 2.0 mg/kg on alternate day, resulted in 100% mortality postmyocardial surgery in Wistar Kyoto rats. One possible explanation could be because of complete inhibition of complex I activity, which might have hindered the recovery process of the rats from the procedure. Osmotic mini pumps were then used to continuously infuse rotenone over the course of the experiment. With chronic rotenone infusion, we were able to obtain a survival rate of ≈90% postmyocardial surgery.
After rotenone treatment, mitochondrial complex I activity was inhibited significantly as compared with sham-operated and RI rats. Reduction in mitochondrial complex I activity was followed by excessive mitochondrial aggregation and dyad swelling. Dyad swelling and T-tubule network alterations have been described in various forms of human heart failure, including idiopathic dilated cardiomyopathy.17 Although it is unclear how faithfully rotenone-induced mitochondrial oxidative stress and dysfunction mimic the ischemic heart disease in human MS, findings from this study provide basis, indicating that bio-energetic deprivation in the myocardium is one of the reasons for abrogated growth of coronary collaterals in response to RI.
Mitochondrial Bioenergetics as Important Determinant of Vascular Cell Function
In the heart, mitochondria represent ≈40% to 50% of the myocyte volume and provide the unremitting energy via aerobic metabolism required for contraction.7,18–20 Aerobic respiration is, therefore, indispensable to sustain cardiac function and viability. At rest, the heart consumes up to ≈8 to 15 mL O2/ min per 100 g tissue.21 This is significantly more than that consumed by the brain (≈ 3 mL O2/min per 100 g tissue) and can increase to >70 mL of O2/min per 100 g of myocardial tissue during vigorous exercise by calling on the reserve capacity of the mitochondria and glycolysis.21 It should be noted that ≈90% oxygen is consumed by mitochondria and thus other components of oxygen consumption, for example, nicotinamide adenine dinucleotide phosphate oxidases, represent only a minor fraction of the total oxygen consumption.
Studies by others and from our laboratory have shown that the diseased/failing heart fails to supply the amount of oxygen required to meet energy demands, which has led to the hypothesis that a state of energy starvation may underlie myocardial pathology.22 In support of this concept, myocardial oxygen uptake is increased 2-fold in patients with left ventricular hypertrophy over normal subjects23; there is also an increase in the myocardial oxygen uptake in experimental hypertrophy, congestive heart failure, and diabetes mellitus.24 Furthermore, the postischemic heart uses more oxygen than the preischemic heart, and myocytes subjected to hypoxia/reoxygenation have an increased need for oxygen.25 As mitochondria are the major oxygen consumer in the cells, increased oxygen consumption would directly generate higher amount of mitochondrial O2·-. This vicious cycle of ROS-induced ROS-release may favor further elevated ROS production followed by more damages to mitochondrial electron transport chain complexes.26 However, the evidence for energy starvation would be signs of ischemia, such as lactate production.
Although it is well accepted that the myocardium requires significant amount of energy to support cardiac work,27 it is far less appreciated that any vascular growth, and the synthesis of proteins and nucleic acids, that are needed for division and proliferation of vascular cells also require energy. Furthermore, corruption in the balance between energy demand and supply because of mitochondrial oxidative stress and dysfunction might be the reason(s) contributing to impaired coronary vascular growth in response to RI.
Mitochondrial Bioenergetics and AMPK-α: The Controller of the Phenotypic Switch?
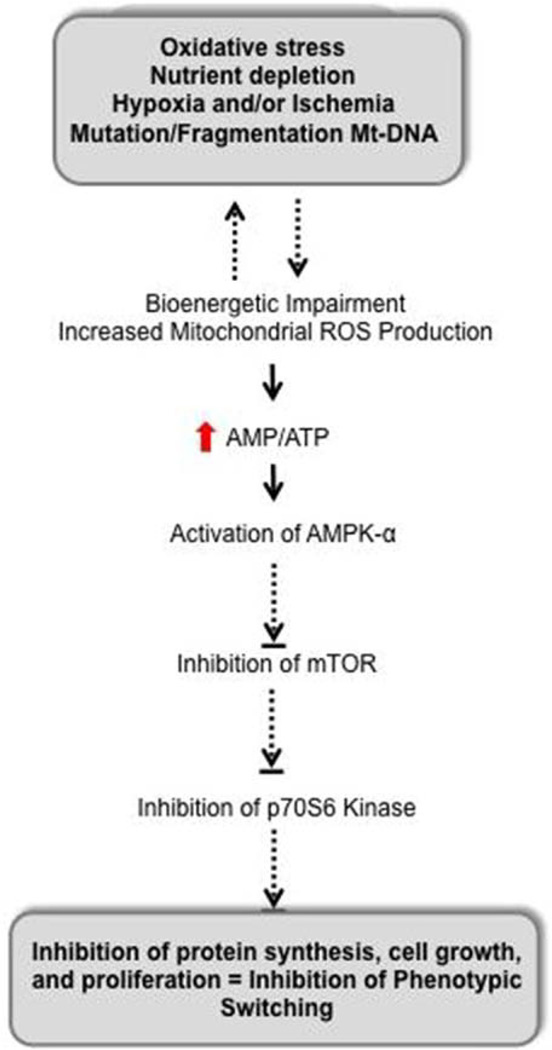
Almost all cellular activities require energy, usually in the form of ATP. Energy can also be stored in the form of other energy-rich compounds, such as the GTP and nicotinamide adenine dinucleotide, or as electrochemical gradient across the cellular membranes. We think that impairment in mitochondrial ATP-generating capacity will undoubtedly negatively impact cardiovascular functions, including phenotypic switching of cells from one phenotype (quiescence) to another (proliferation). An interesting point to note is that whether mitochondrial dysfunction in cardiovascular diseases is the cause or effect of signaling disorders or both remains debatable.28 On the basis of the results from our study, we hypothesize that mitochondrial dysfunction could be an initiating event resulting in compromised signal transduction as depicted in Figure 8 via deficiencies in the pool of free ATP, which is used not only for mechanical work but also for phenotypic switching in that kinase cascades that are activated when cells change phenotypes require ATP to phosphorylate their substrates. Subsequently, mitochondrial dysfunction causes direct impairment of mitochondrial role in downstream signaling events, or indirectly from the effects of bioenergetic deficiencies.
Figure 8.
Transduction of mitochondrial oxidative stress and dysfunction into impaired vascular growth. AMPK-α is a prototypical sensor of energy homeostasis, which is activated when cellular levels of AMP increase and ATP decreases. Activated AMPK then negatively regulates mammalian target of rapamycin (mTOR), which controls energy-demanding cellular processes, for example, protein synthesis, required for cell growth and proliferation. Thus, inhibition of mTOR signaling inhibits tube formation in human coronary artery endothelial cells, and we would also project that mitochondrial dysfunction in vivo would inhibit coronary collateral growth through similar mechanisms. Dephosphorylation/inactivation of p70S6 kinase, even in the presence of growth factor (ie, vascular endothelial growth factor), indicates that impaired mitochondrial bioenergetics would impair new protein synthesis. Thus, proper bioenergetics is critical to control interactions between phenotypic switching that requires new protein synthesis leading to vascular growth and cellular metabolism.
We speculate that AMPK-α is the downstream major molecular link between mitochondrial dysfunction and cellular signaling. This is in part because of low ATP conditions (eg, treatment with rotenone to induce mitochondrial oxidative stress and dysfunction will activate AMPK-α). AMPK-α then acts as a metabolic checkpoint in the cells, halting cell growth and suppressing ATP-consuming anabolic processes while stimulating ATP-generating catabolic processes to replenish the initial loss of ATP.29 Accordingly, knockdown of AMPK with siRNA prevented the inhibitory effect of rote-none on tube formation. The results with compound C are more difficult to interpret, but we state that this treatment did not inhibit the deleterious effect of rotenone as shown in both the oxidative metabolism profile and the immunoblot analysis, indicating mitochondrial bioenergetics is the upstream regulator of AMPK-α, although our central arguments for activation of AMPK-α center on reductions in ATP (ie, there is other evidence that other stimuli can activate this kinase). For example, H2O2 is reported to activate AMPK-α.30 Because rotenone increases mitochondrial ROS production from complex I, activation of AMPK after rotenone treatment could be related to its effects on ROS production. Regardless of the mechanism of AMPK-α activation, the data suggest that this kinase plays a key role in models of mitochondrial oxidative stress and impaired bioenergetics.
Acting in inverse fashion as compared with AMPK-α, activation of AMPK-α after bioenergetic stress can subsequently lead to dephosphorylation of the mTOR, which is the major regulator of cell growth and protein synthesis.31 mTOR is found in 2 different complexes within the cells, the mTOR complex 1 and mTOR complex 2.32 Studies have shown that only mTOR complex 1 is sensitive to inhibition by rapamycin, nutritional conditions, and energy status.32 mTOR complex 1 regulates protein synthesis by directly phosphorylating p70 ribosomal S6 kinase. p70 ribosomal S6 kinase activation positively impacts proteins synthesis that is needed for cell cycle progression, proliferation, angiogenesis, and survival pathways.29 This scheme is well documented in the literature and has been shown to modulate cardiac hypertrophy33–35; however, our results are the first to link this pathway with the mechanism underlying the negative effects of mitochondrial oxidative stress on vascular growth. Moreover, rapamycin did not block the effect of rotenone leading to the upregulation of AMPK-α, implying that mitochondrial bioenergetics is a key regulator in cardiovascular metabolism. Another implication of AMPK activation is the loss of Akt activity.36 AMP kinase activation leads to the dephosphorylation of Akt, which would negatively impact cell growth and survival. This action of AMP kinase on the Akt pathway could also be involved in the hampered growth of collaterals and in vitro tube formation after the induction of mitochondrial oxidative stress by rotenone.
It is also important to point out that compound C and rapamycin treatment alone significantly increased oxygen consumption rate in HCAECs (data not shown). This increase might be because of promotion of cell growth and survival pathways, as well as self-cannibalization process (ie, apoptosis and autophagy) after suppression of AMPK-α and mTOR signaling, respectively. Again, this emphasizes the importance of proper balance of pro- and antisurvival signaling to promote physiological vascular growth. We would be remiss to not mention an important caveat to the results with the pharmacological approach; namely, that compound C has a plethora of nonspecific effects. At the concentrations that effectively block AMP kinase, this antagonist also blocks many other kinases, including ERK8, Src, and Lck.37 For this reason, we incorporated genetic approaches to express a constitutively active AMPK-α and to knock down the expression of the kinase. These results supported the results obtained from the pharmacological studies, suggesting that AMP kinase plays a critical role in mediating the effects of mitochondrial oxidative stress.
Conclusions
The fundamental requirement of cellular growth (and vascular growth) depends on the ability to couple the availability of nutrients and oxygen (ie, optimal bioenergetic status) to signals from growth factors (eg, VEGF) to drive cell cycle progression and induce a phenotypic switch. Although AMPK-α/ mTOR signaling has been well implicated in tumorigenesis, its role(s) in angiogenesis has never been linked. The findings in this study reaffirm our hypothesis on the importance of mitochondrial bioenergetics in mediating a phenotypic switch in endothelial cells—from quiescence to migration/proliferation leading to CCG and tube formation in vitro—through AMPK-α/mTOR pathway, which is essential for vascular growth.
Supplementary Material
Significance.
Many vascular diseases are characterized by some type of mitochondrial dysfunction as shown by excessive production of reactive oxygen species and impairment in bioenergetics. Previously we have observed that scavenging mitochondrial free radicals will restore vascular growth, but to date, the precise mechanism by which mitochondrial dysfunction produces this impairment is unresolved. Our results point to activation of the cellular energy senor, AMP kinase, as a pivotal component that links mitochondrial dysfunction to impaired coronary collateral growth and reduced in vitro tube formation. Activation of AMP kinase inhibits mammalian target of rapamycin, which is critical for the activation of p70 ribosomal S6 kinase, a key kinase involved in protein synthesis. These results advance our understanding of how mitochondrial dysfunction can produce impairments in vascular growth and may then provide a platform for correcting the impairment via therapeutic interventions.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health grants HL32788, HL83366, RC1HL100828 (to W.M. Chilian), HL115540 (to L. Yin), by an American Heart Association Post-doctoral Fellowship 09POST2290021 (to Y.F. Pung) and from the Canadian Institute of Health Research to J.R.B. Dyck. J.R.B. Dyck is an Alberta Heritage Foundation for Medical Research Scholar.
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.301591/-/DC1.
Disclosures
None.
References
- 1.Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 2008;114:195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- 2.Magliano DJ, Shaw JE, Zimmet PZ. How to best define the metabolic syndrome. Ann Med. 2006;38:34–41. doi: 10.1080/07853890500300311. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Abaci A, Oğuzhan A, Kahraman S, Eryol NK, Unal S, Arinç H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz MB, Biyikoglu SF, Akin Y, Guray U, Kisacik HL, Korkmaz S. Obesity is associated with impaired coronary collateral vessel development. Int J Obes Relat Metab Disord. 2003;27:1541–1545. doi: 10.1038/sj.ijo.0802474. [DOI] [PubMed] [Google Scholar]
- 7.Pung YF, Chilian WM. Corruption of coronary collateral growth in metabolic syndrome: Role of oxidative stress. World J Cardiol. 2010;2:421–427. doi: 10.4330/wjc.v2.i12.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, Chilian WM. Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxid Redox Signal. 2009;11:1961–1974. doi: 10.1089/ars.2009.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the pre-diabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 10.Hattan N, Chilian WM, Park F, Rocic P. Restoration of coronary collateral growth in the Zucker obese rat: impact of VEGF and ecSOD. Basic Res Cardiol. 2007;102:217–223. doi: 10.1007/s00395-007-0646-3. [DOI] [PubMed] [Google Scholar]
- 11.Pung YF, Rocic P, Murphy MP, Smith RA, Hafemeister J, Ohanyan V, Guarini G, Yin L, Chilian WM. Resolution of mitochondrial oxidative stress rescues coronary collateral growth in Zucker obese fatty rats. Arterioscler Thromb Vasc Biol. 2012;32:325–334. doi: 10.1161/ATVBAHA.111.241802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 13.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 14.Fujita M, McKown DP, McKown MD, Franklin D. Changes in coronary flow following repeated brief coronary occlusion in the conscious dog. Heart Vessels. 1986;2:87–90. doi: 10.1007/BF02059961. [DOI] [PubMed] [Google Scholar]
- 15.Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 2010;88:993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 17.Crossman DJ, Ruygrok PN, Ruygrok PR, Soeller C, Cannell MB. Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS One. 2011;6:e17901. doi: 10.1371/journal.pone.0017901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David H, Meyer R, Marx I, Guski H, Wenzelides K. Morphometric characterization of left ventricular myocardial cells of male rats during postnatal development. J Mol Cell Cardiol. 1979;11:631–638. doi: 10.1016/0022-2828(79)90377-8. [DOI] [PubMed] [Google Scholar]
- 19.Guski H, Wassilev G, Meyer R, David H. Morphometric analysis of myocardial mitochondria in rats during adaptation to exercise. Cor Vasa. 1980;22:375–383. [PubMed] [Google Scholar]
- 20.Schaper J, Meiser E, Stämmler G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res. 1985;56:377–391. doi: 10.1161/01.res.56.3.377. [DOI] [PubMed] [Google Scholar]
- 21.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz AM. Changing strategies in the management of heart failure. J Am Coll Cardiol. 1989;13:513–523. doi: 10.1016/0735-1097(89)90586-x. [DOI] [PubMed] [Google Scholar]
- 23.Strauer BE. Cardiac energetics in clinical heart disease. Basic Res Cardiol. 1987;82(Suppl 2):389–402. doi: 10.1007/978-3-662-11289-2_38. [DOI] [PubMed] [Google Scholar]
- 24.Gunning JF, Cooper GIV, Harrison CE, Coleman HN., III Myocardial oxygen consumption in experimental hypertrophy and congestive heart failure due to pressure overload. Am J Cardiol. 1973;32:427–436. doi: 10.1016/s0002-9149(73)80033-5. [DOI] [PubMed] [Google Scholar]
- 25.Hill BG, Awe SO, Vladykovskaya E, Ahmed Y, Liu SQ, Bhatnagar A, Srivastava S. Myocardial ischaemia inhibits mitochondrial metabolism of 4-hydroxy-trans-2-nonenal. Biochem J. 2009;417:513–524. doi: 10.1042/BJ20081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 27.Finkel T, Hwang PM. The Krebs cycle meets the cell cycle: mitochondria and the G1-S transition. Proc Natl Acad Sci U S A. 2009;106:11825–11826. doi: 10.1073/pnas.0906430106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bokko PB, Francione L, Bandala-Sanchez E, Ahmed AU, Annesley SJ, Huang X, Khurana T, Kimmel AR, Fisher PR. Diverse cytopathologies in mitochondrial disease are caused by AMP-activated protein kinase signaling. Mol Biol Cell. 2007;18:1874–1886. doi: 10.1091/mbc.E06-09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin BY, Sartoretto JL, Gladyshev VN, Michel T. Endothelial nitric oxide synthase negatively regulates hydrogen peroxide-stimulated AMP-activated protein kinase in endothelial cells. Proc Natl Acad Sci U S A. 2009;106:17343–17348. doi: 10.1073/pnas.0907409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 33.Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 34.Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 36.King TD, Song L, Jope RS. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem Pharmacol. 2006;71:1637–1647. doi: 10.1016/j.bcp.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.