Abstract
Background
The Ebola outbreak that is sweeping across West Africa is the largest, most volatile, and deadliest Ebola epidemic ever recorded. Liberia is the most profoundly affected country, with more than 3500 infections and 2000 deaths recorded in the past 3 months.
Objective
To evaluate the contribution of disease progression and case fatality on transmission and to examine the potential for targeted interventions to eliminate the disease.
Design
Stochastic transmission model that integrates epidemiologic and clinical data on incidence and case fatality, daily viral load among survivors and nonsurvivors evaluated on the basis of the 2000–2001 outbreak in Uganda, and primary data on contacts of patients with Ebola in Liberia.
Setting
Montserrado County, Liberia, July to September 2014.
Measurements
Ebola incidence and case-fatality records from 2014 Liberian Ministry of Health and Social Welfare.
Results
The average number of secondary infections generated throughout the entire infectious period of a single infected case, R, was estimated as 1.73 (95% CI, 1.66 to 1.83). There was substantial stratification between survivors (RSurvivors), for whom the estimate was 0.66 (CI, 0.10 to 1.69), and nonsurvivors (RNonsurvivors), for whom the estimate was 2.36 (CI, 1.72 to 2.80). The nonsurvivors had the highest risk for transmitting the virus later in the course of disease progression. Consequently, the isolation of 75% of infected individuals in critical condition within 4 days from symptom onset has a high chance of eliminating the disease.
Limitation
Projections are based on the initial dynamics of the epidemic, which may change as the outbreak and interventions evolve.
Conclusion
These results underscore the importance of isolating the most severely ill patients with Ebola within the first few days of their symptomatic phase.
Primary Funding Source
National Institutes of Health.
West Africa is overwhelmed by the most devastating Ebola epidemic known to date. It continues to increase exponentially, with the fastest rate of spread in Liberia (1). By August 2014, the number of cases in Liberia exceeded the capacity of all Ebola treatment units (2, 3). Because this public health crisis shows no signs of improvement and the risk for Ebola spreading beyond West Africa continues to mount, it is an international imperative to determine effective approaches to stem transmission of this virus.
Early Ebola symptoms include sudden high fever, muscle pain, and severe headache followed by pharyngitis, abdominal pain, and maculopapular rash (4), whereas the late phase is marked by vomiting, diarrhea, hemorrhagic diathesis, and multiorgan dysfunction (4). Ebola is primarily transmitted through direct contact with infected bodily fluids and contaminated materials. Therefore, close contacts of patients with Ebola, such as family members, health care workers, and those preparing bodies for burial, are at high risk for infection (5). In the current absence of pharmaceutical prophylaxis and treatment (6), control strategies rely on 1) active case ascertainment and isolation, 2) identification of patients’ contacts with monitoring of them for 21 days, and 3) identification of Ebola deaths for hygienic burial (7). However, the implementation of these approaches is falling short because of the rapid spread of the outbreak combined with the limited resources available.
The risk for Ebola transmission increases with the viral load of infected individuals, which for nonsurvivors is greatest in the later stages of illness and immediately after death (8). In combination with their viral load, the number of close contacts of patients determines transmissibility of the virus (9, 10). Both the viral load and number of contacts of a patient with Ebola may change during the infectious period (for example, ante- and postmortem contacts); thus, the contribution of these factors to disease transmission to other patients may vary with disease progression (also known as age-of-infection), defined as the number of days since exposure (11). This distinction is clinically relevant because the viral load of survivors peaks 4 days after symptom onset and then rapidly declines, whereas viral load of nonsurvivors continues to rise. In addition, among nonsurvivors, the mean viral load throughout the infection period is 100-fold higher than that among survivors (8, 12).
To our knowledge, previous Ebola transmission models have not considered the effect of disease progression and case fatality on transmission. We present the first Ebola transmission model that distinguishes between survivors and nonsurvivors and incorporates disease progression to evaluate Ebola transmission and the effectiveness of targeted control measures.
Our model integrates epidemiologic and clinical data on Ebola viral load, daily infection incidence, and case fatality together with primary data on contact mixing patterns collected from patients with Ebola in Montserrado County, Liberia. We used this model to evaluate the distribution of secondary cases resulting from infected individuals as disease progresses, differentiating between survivors and nonsurvivors. We then evaluated the potential effect of case isolation and social behavior change through contact reduction for controlling Ebola transmission in Liberia.
Methods
We analyzed incidence and case fatality of Ebola reported by the Liberian Ministry of Health and Social Welfare (MoHSW) for Montserrado County from 7 July 2014 to 22 September 2014 (13). In addition, we considered in our model individual-level contact-tracing data collected between 7 August and 26 August by MoHSW (Supplement 1, available at www.annals.org). Index cases in our data set were isolated, while their contacts were traced and monitored daily for symptoms during a period of 21 days. For each index case, date of symptom onset, clinical status (alive or deceased; suspected, probable, or confirmed case), and contact history were recorded. Contact history variables were the number of contacts and the date of last interaction between the contact and patient. If the contact became symptomatic, the date of symptom onset was recorded.
With minimal assumptions, we calculated the reproductive ratio, defined as the average number of secondary cases generated throughout the infectious period of a single case (14), and evaluated feasible intervention programs that could facilitate disease control.
The study was exempt from institutional review board approval because only deidentified data were used.
Model Framework
We divided each Ebola infection into 3 sequential phases: incubation, early symptomatic, and late symptomatic (4). We denoted the duration between exposure and time t as the day of infection. During the incubation phase, denoted η, individuals are not infectious (15). After this phase, individuals become infectious. The probability of transmitting Ebola depends on the magnitude of viral load in an infected individual at time t (16, 17) and the number of contacts with which the infected individuals interacted, C (t). We distinguished between viral load among survivors and nonsurvivors Vs(t), S ∈ {survivors, nonsurvivors} on the basis of clinical data demonstrating that the magnitude and pattern of viral load among survivors and nonsurvivors substantially differ throughout disease progression (8, 18, 19). Concomitantly, as symptoms become more severe as disease progresses, patterns of social interactions will probably be altered (20). Thus, the number of contacts that an infected individual has also depends on the phase of disease progression.
Taken together, we defined the probability of an infected individual i infecting a contact j at time t as follows:
| (1) |
where Ij(t) is an indicator variable that is equal to 1 if contact j has been exposed at time t and is 0 otherwise, n is the number of infected persons in Montserrado County, and is the relative infectiousness of individual i with survivorship indicator S at time t. Thus, is defined as follows:
| (2) |
where τi is the day of symptom onset for individual i, and νi is the duration of the infectious period. Within the symptom interval, the relative infectiousness depends on the contribution of the viral load to transmission, , and the number of contacts, Ci (t − τi). Thus, the relative infectivity of an individual on a particular day is based on the viral load and number of contacts on that day of infection.
R is calculated by summing the number of infections that occur on each day of the infectious period, averaging for the entire study population.
| (3) |
Model Parameterization
For each iteration of our simulations, we sampled the duration of the incubation period, the early phase of symptoms, and the late phase of symptoms from distributions based on clinical and epidemiologic data (Table). We assumed an incubation period ranging between 5 and 15 days, which is consistent with recent empirical estimates for the current Ebola outbreak (15, 21).
Table.
Parameters Used in the Stochastic Model
| Parameter | Symbol | Distribution Used for Uncertainty Analysis |
Source (Reference) |
|---|---|---|---|
| Number of contacts during early phase | CEarly | Sampled from data | 2014 Liberia; Supplement 1† |
| Number of contacts during late phase for nonsurvivors | CLate | Sampled from data, between 1 and 5 | Based on household size (22) |
| Incubation phase duration (d) | η | Triangular (mode 8, range [5, 15]) | 3, 9, 15, 30 |
| Late symptoms phase duration (d) | ψ | Uniform (range [1, 5]) | 18, 21, 31, 32 |
| Overall symptom duration (d) | ν | Triangular (mode 8, range [5, 14]) | 18, 21, 26 |
| Rate ratio of transmission risk | r | Evaluated | 1995 Zaire, 2000 Uganda; Supplement 1† (8, 9) |
| Daily viral load stratified by survivorship | Vs(t) | Log normal* | 2000 Uganda (8) |
Viral load was measured based on the mean and SD counts of daily RNA copy levels over 14 d after symptom onset and are stratified by survivorship.
Available at www.annals.org.
To evaluate the number of contacts of an infectious individual at time t, we generated a contact distribution from primary contact-tracing data collected between 3 August and 28 August in Montserrado County by the MoHSW. These data show the contacts of 245 infected individuals and the timing of symptom onset. Thus, these data provide information about the contact patterns of infected individuals through the infectious period, which we assume different from the contact patterns of those who are not ill. We found that the mean number of contacts for an infected individual is 5.47 (95% CI, 4.80 to 6.15) but can be as high as 33 (Supplement 2, available at www.annals.org). With the exception of nonsurvivors during their late phase of infection, we assumed that the number of contacts of an infected individual would be drawn from this distribution. Conservatively with respect to our findings, we also assumed that for nonsurvivors during the late phase of infection, the number of contacts would be drawn from the same distribution, truncated between 1 and 5, because the average household size in Montserrado County is 4.7 (22). This is because individuals in the late phase of disease are more likely to stay home and would therefore probably be in contact only with household members (Table).
To evaluate the daily infectiousness of an infected individual given contact assumptions, we also considered viral load over disease progression (8). The viral load estimates were based on Ebola RNA copy levels among survivors and nonsurvivors on each day of their infection, measured from a 2000–2001 Ebola outbreak in Uganda (8). Consistent with clinical studies on other viruses, we assumed that for any given contact, each 10-fold increase in viral load will lead to an r-fold increase in infectiousness, that is g(Vs(t)) = rLog(Vs(t)) 16, 17). We also took into account that the rate ratio of transmission risk, r, depends on the nature of the contact. We considered 3 types of common contacts parameterized from data on previous Ebola outbreaks: 1) conversation, 2) sharing a meal, and 3) sharing a bed (Supplement 3, available at www.annals.org). These types of contacts probably correspond to transmission routes of aerosol contact, body fluid contact, and sexual interactions, respectively (23). We evaluated the distribution of r (Supplement 4, available at www.annals.org) for each type of contact by using data from the 1995 Ebola outbreak in Zaire on relative risk for infection from contacts of patients with Ebola during their early and late phases of infection (9).
Numeric Simulations
We performed 1000 stochastic iterations of our model, each predicting transmission trajectories over the longitudinal period of data collection (Supplement 5, available at www.annals.org). For every infected individual in each iteration, we independently sampled from data-driven distributions, the incubation duration, the early-phase duration, the late-phase duration, the daily viral load, and the aggregate number of contacts for each case reported in our epidemiologic data set, stratified by early or late disease (Table; Supplement 3). Using equations 1 and 2, we evaluated for the survivors and nonsurvivors the distribution of the 1) number of secondary cases resulting from a single infection; 2) number of secondary cases over disease progression; and 3) mean number of secondary cases, R. Specifically, to generate the distribution of the number of secondary cases resulting from a single infection, we randomly drew an infected individual for each iteration and calculated the number of secondary cases that arose. This approach ensured independence between the sampling. We conducted the same procedure to evaluate the number of individuals infected by every individual daily through disease progression.
We also evaluated the potential effectiveness of case isolation in Ebola treatment units based on the disease progression by day of infection and survivorship. Specifically, we evaluated the probability of Ebola elimination through the isolation of cases starting t days after symptom onset. Because of logistic challenges in case detection (24) and shortage of isolation units, we assessed the effectiveness of case-isolating 50% to 100% of infected individuals. In addition, we assessed a more pragmatic strategy that included self-quarantine among 50% to 100% of the infected patients, considering contact reduction from symptom onset that varied between 0% and 100%. The effectiveness of the hospital isolation and self-quarantine interventions was measured in terms of the reduction in R that each intervention can achieve, respectively. Disease elimination can be attained when R is suppressed below 1. All analyses were conducted by using Mathematica, version 9.0 (Wolfram). The code is presented in Supplement 6 (available at www.annals.org).
Role of the Funding Source
The study was funded by the National Institutes of Health. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the manuscript for publication.
Results
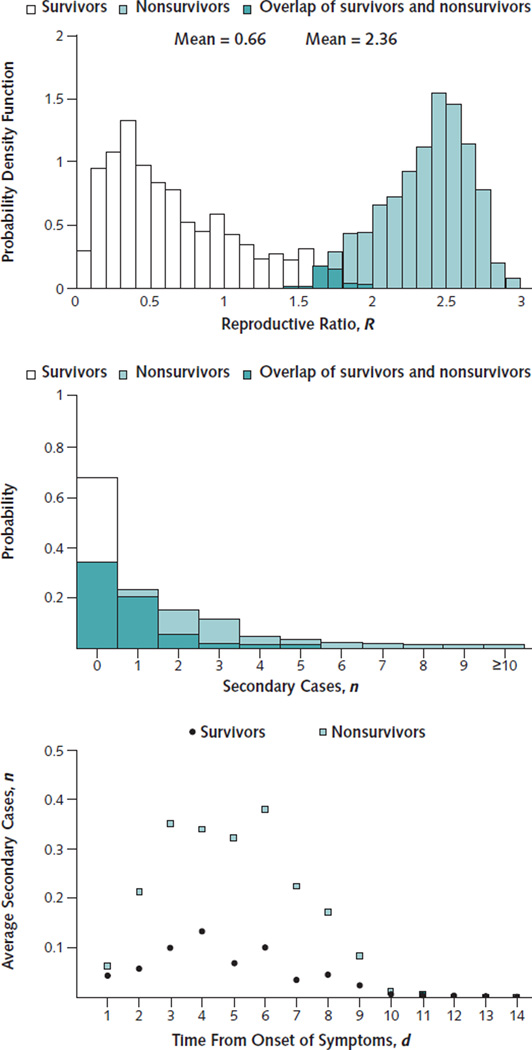
We calculated an R for Ebola in Liberia of 1.73 (95% CI, 1.66 to 1.83). Given this value of R, and assuming an overall infection duration of 16 days (Table), the incidence will double every 20 days. The reproductive ratio among the nonsurvivors, RNonsurvivors, is 2.36 (CI, 1.72 to 2.80), whereas that for the survivors, RSurvivors, is 0.66 (CI, 0.10 to 1.69) (Figure 1, top). The survivors had a 32% probability of infecting at least 1 individual during their infectious period compared with a 67% probability in nonsurvivors (Figure 1, middle). Consequently, nonsurvivors, who make up 63% (CI, 60% to 64%) of the population, are responsible for 86% (CI, 63% to 98%) of transmissions. From our calculation of the daily average number of secondary infections for survivors and nonsurvivors, we found that nonsurvivors have the highest risk for transmitting beyond 4 days from symptom onset (Figure 1, bottom). These results are robust regardless of our assumption that individuals have substantially fewer contacts during the late phase.
Figure 1.
Infectivity according to day of infection and survivorship.
Top. Distribution of the reproductive number among survivors, RSurvivors, and among nonsurvivors, RNonsurvivors. Middle. Distribution of secondary cases per infected individual among survivors and nonsurvivors. Bottom. Average number of secondary cases per day of symptomatic disease.
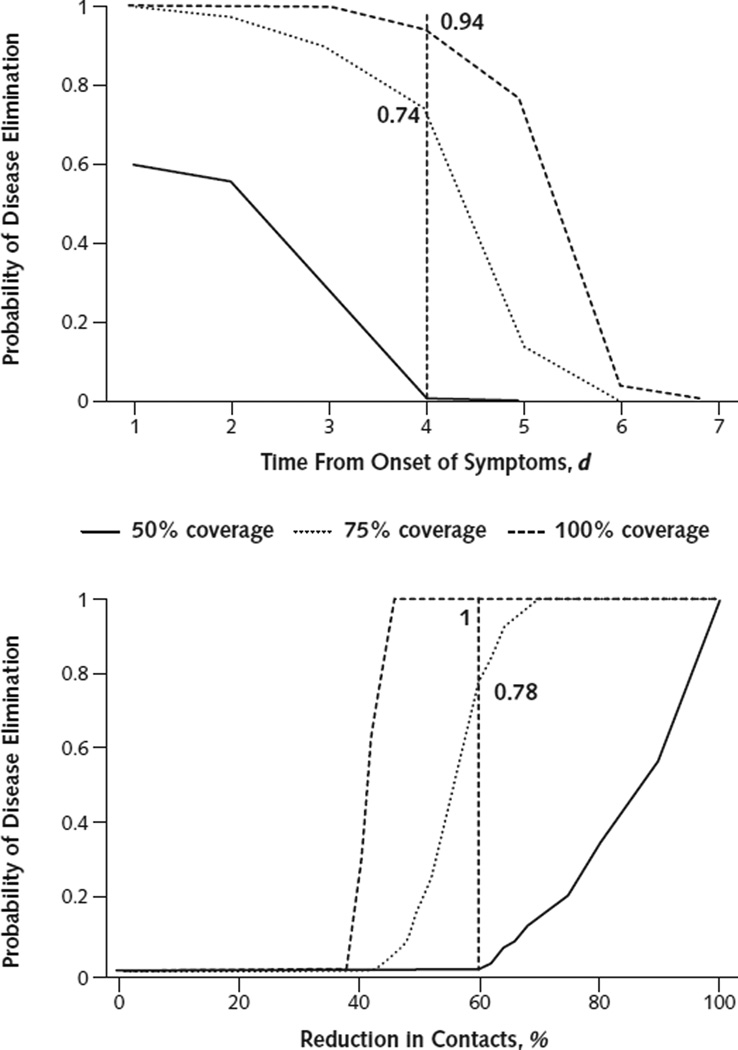
Nonsurvivors may be identified by their severe, Ebola-specific symptoms at the late phase of infection (21). Hence, we tested the effectiveness of targeting case isolation of the nonsurvivors following t days from symptom onset (Figure 2, top). Our results indicate that effective isolation of these nonsurvivors may achieve disease elimination if isolation occurs within 4 days from symptom onset. For example, isolating 75% of the nonsurvivors within 4 days from symptom onset has at least 74% probability of disease elimination (Figure 2, top; Supplement 7, available at www.annals.org). Adding the isolation of infected cases who would go on to survive marginally further reduced transmission (Supplement 7).
Figure 2.
Probability of disease elimination for different intervention strategies and coverages.
Top. Case isolation of nonsurvivors after symptom onset. Vertical dashed line indicates probability of disease elimination by isolating nonsurvivors within 4 days of symptom onset. Bottom. Percentage self-quarantine on first day of symptom onset. Vertical dashed line indicates probability of disease elimination achieved by a 60% reduction in contacts.
In addition, we evaluated a projected effectiveness of self-quarantine intervention (Figure 2, bottom), a pragmatic strategy in the absence of sufficient case isolation units. Self-quarantine of 75% of all infected individuals (both survivors and nonsurvivors), which reduced at least 60% of their contacts starting on the first day of symptoms, was projected to achieve elimination with at least 78% probability (Figure 2, bottom; Supplement 8, available at www.annals.org).
Discussion
We developed a data-driven stochastic model that included empirical contact information combined with viral load data to evaluate the differential contribution of disease progression and case fatality on disease transmission and to examine the effectiveness of interventions targeted to those who are most infectious. Our findings indicate that the number of secondary cases resulting from an infected individual varies with the phase of disease progression (early vs. late) and outcome (survival vs. nonsurvival) of infection, such that nonsurvivors and, in particular, nonsurvivors 4 days after symptom onset are most responsible for perpetuating the epidemic.
From a clinical perspective, survival cannot be predicted at the outset of symptoms. Our results indicate that case isolation of most infected individuals who are in critical condition within 4 days from symptom onset could facilitate disease elimination. Our analyses also indicate that a strategy of promoting self-quarantine that reduces by 60% the contacts of most infected individuals throughout their infectious period could facilitate disease elimination. However, this 60% reduction in contacts is beyond the reduction that occurs when people become symptomatic and would thus be challenging to implement. Instead, our results emphasize the importance of sufficient resources to provide case isolation for infected individuals, particularly for those most gravely ill. As the international community commits considerable assistance to address the Ebola outbreak (25), our findings indicate that such efforts should be directed toward expanding the capacity of hospitalized case isolation. The average period from symptom onset to hospitalization in Liberia has been estimated to be 5 days (21), compared with our finding that disease elimination would require case isolation of the most severely ill patients within 4 days. Consequently, the degree of improvement in contact tracing and case isolation necessary to achieve this improvement is substantial yet likely feasible, provided that enough hospital beds are available. The success of these strategies requires strong community engagement through effective communication and health education, as behavior change in affected and at-risk communities is paramount to the success of any Ebola control strategy.
This study has several limitations. The number of contacts was evaluated by using contact-tracing data from Liberia. However, the actual number of contacts may be higher as a result of underreporting. This would make the implementation of the self-quarantine strategy even more challenging because additional effort would be required for the intervention to facilitate disease elimination.
The actual case-fatality rate of the ongoing Ebola outbreak also remains unclear. Using our data-driven model, we estimated that the case-fatality rate of Ebola in Montserrado County is 63%. Previous estimates of case fatality for the current outbreak of Ebola in West Africa have been calculated as a ratio of the cumulative fatalities to the cumulative cases, leading to estimates of around 50% in Liberia (3). For an ongoing epidemic, particularly during the initial exponential phase, such calculations underestimate case fatality among the reported cases because there may be a substantial number of infected individuals who will still die. In contrast, the World Health Organization Ebola Response Team reports a Liberian case-fatality rate of around 75% (21). Given that the World Health Organization report is based only on cases identified through clinical care settings, which may disproportionately include more severe cases, it probably overestimates case fatality.
Our estimates of R for the current Ebola outbreak in Liberia are within the range of other recent estimates (3, 21). Recent models that have used simple mathematical models to evaluate R of the current outbreak (2, 3, 26, 27) have not incorporated temporal variation in infectiousness and contact behavior over the course of disease progression. To account for the evolution of infectivity with disease progression, we integrated clinical data on temporal variation of viral load for fatal and nonfatal Ebola cases, the relative risk for disease transmission from close contacts with an infected individual at different stages of disease progression, and contacts as reported through primary tracing data in Liberia.
We obtained our estimates of viral load data from the 2000–2001 Uganda outbreak (8), which demonstrated that viral load among nonsurvivors was substantially higher that that among survivors. Because this is consistent with additional previous Ebola outbreaks (18, 28, 29), we do not expect this assumption to qualitatively affect our results. However, if viral load data from the current outbreak become available, future studies could improve quantification of the relationship among viral load, type of contact, and Ebola infectiousness.
The higher transmissibility of nonsurvivors may be exacerbated by specific clinical symptoms associated with the bleeding diathesis that occurs in the late phase of infection, such as hematemesis, hematochezia, or even bleeding from mucous membranes and puncture sites. It is clinically plausible that these clinical features would be associated with the higher viral load among nonsurvivors. Nonsurvivors may be identified by these severe, Ebola-specific symptoms (20). A recent study demonstrated that specific symptoms of Ebola, including bleeding from the nose and gums, are associated with a higher risk for death, as is age older than 45 years. Further studies are needed to design a clinical algorithm to identify likely survivors from those likely to die in order to better inform clinical practice.
Case isolation and hygienic burial of the dead have been cornerstone strategies in public health efforts to contain the Ebola outbreak (30, 31). The effect of these strategies on disease transmission depends on the efficiency of disease surveillance for identification of active cases in affected communities and on contact tracing, as well as on hospital capacity for case isolation. Our results show that isolating infected individuals before they progress into their late phase of illness, which is also their most infectious period, may facilitate the reversal of the volatile Ebola outbreak in West Africa (32).
Supplementary Material
EDITORS' NOTES.
Context
The Ebola outbreak in West Africa is spiraling out of control. The need to determine how to deploy scarce resources to end this crisis is urgent.
Contribution
A stochastic model of disease transmission that incorporated both clinical and epidemiologic data from Liberia, including incidence, case-fatality rate, and previous estimates of viral load, among both survivors and nonsurvivors was developed. By using these data, the model predicted that isolating the most severely ill patients during the first days of symptomatic illness would have the greatest effect on reducing viral transmission.
Implication
Targeted isolation may offer the best hope of ending the Ebola epidemic in West Africa.
Acknowledgments
Grant Support: By the National Institutes of Health (U01 GM087719, U01 GM105627 and K24 DA017072).
Footnotes
Web-Only
Supplements
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M14-2255.
Reproducible Research Statement: Study protocol: Not applicable. Statistical code and data set: Available in Supplement 6.
Current author addresses and author contributions are available at www.annals.org.
Author Contributions: Conception and design: D. Yamin, S. Gertler, M.L. Ndeffo-Mbah.
Analysis and interpretation of the data: D. Yamin, S. Gertler, M.L. Ndeffo-Mbah.
Drafting of the article: D. Yamin, S. Gertler, M.L. Ndeffo-Mbah. Critical revision of the article for important intellectual content: D. Yamin, S. Gertler, M.L. Ndeffo-Mbah, L.A. Skrip, M. Fallah.
Final approval of the article: D. Yamin, S. Gertler, M.L. Ndeffo-Mbah, M. Fallah.
Statistical expertise: D. Yamin, S. Gertler, M.L. Ndeffo-Mbah, L.A. Skrip.
Obtaining of funding: D. Yamin.
Administrative, technical, or logistic support: D. Yamin.
Collection and assembly of data: D. Yamin, M. Fallah.
References
- 1.Ministry of Health and Social Welfare, Republic of Liberia. [on 30 September 2014];Liberia Ebola SitRep. 2014 123 Accessed at www.mohsw.gov.lr/documents/Liberia%20Ebola%20SitRep%20123Sept%2015,%202014.pdf. [Google Scholar]
- 2.Althaus C. Estimating the reproduction number of Ebola virus (EBOV) during the 2014 outbreak in West Africa. [on 2 September 2014];PLOS Curr Outbreaks. 2014 doi: 10.1371/currents.outbreaks.91afb5e0f279e7f29e7056095255b288. Accessed at http://currents.plos.org/outbreaks/article/estimating-the-reproduction-number-of-zaire-ebolavirus-ebov-during-the-2014-outbreak-in-west-africa/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiura H, Chowell G. Early transmission dynamics of Ebola virus disease (EVD), West Africa, March to August 2014. European Centre for Disease Prevention and Control (ECDC)–Health Comunication Unit. [on 30 September 2014];Eurosurveillance. 2014 19:20894. doi: 10.2807/1560-7917.es2014.19.36.20894. Accessed at www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20894. [DOI] [PubMed] [Google Scholar]
- 4.Formenty P. Ebola-Marburg viral diseases. In: Heymann DL, editor. Control of Communicable Diseases Manual. Washington, DC: American Public Health Assoc; 2008. [Google Scholar]
- 5.Khan AS, Tshioko FK, Heymann DL, Le Guenno B, Nabeth P, Kerstiëns B, et al. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999;179(Suppl 1):S76–S86. doi: 10.1086/514306. [PMID: 9988168] [DOI] [PubMed] [Google Scholar]
- 6.Galvani AP, Ndeffo-Mbah ML, Wenzel N, Childs JE. Ebola vaccination: if not now, when? Ann Intern Med. 2014;161:749–750. doi: 10.7326/M14-1904. [PMID: 25141813] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon MG, Schafer IJ. Centers for Disease Control and Prevention (CDC). Ebola viral disease outbreak—West Africa, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:548–551. [PMID: 24964881] [PMC free article] [PubMed] [Google Scholar]
- 8.Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent M, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–4341. doi: 10.1128/JVI.78.8.4330-4341.2004. [PMID: 15047846] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999;179(Suppl 1):S87–S91. doi: 10.1086/514284. [PMID: 9988169] [DOI] [PubMed] [Google Scholar]
- 10.Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [PMID: 18366252] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brauer F. Age of infection in epidemiology models. Electronic Journal of Differential Equations. 2005;12:29–37. [Google Scholar]
- 12.Basler CF, Wang X, Mühlberger E, Volchkov V, Paragas J, Klenk HD, et al. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci U S A. 2000;97:12289–12294. doi: 10.1073/pnas.220398297. [PMID: 11027311] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health and Social Welfare, Republic of Liberia. [on 30 September 2014];Situation report on the EBOLA virus disease epidemic in Liberia. 2014 Accessed at www.mohsw.gov.lr/documents/SITRep%20146%20Oct%208th,%202014.pdf.
- 14.Anderson RM, May RM, Anderson B. Infectious Diseases of Humans: Dynamics and Control. New York: Oxford Univ Pr; 1992. [Google Scholar]
- 15.Eichner M, Dowell SF, Firese N. Incubation period of ebola hemorrhagic virus subtype zaire. Osong Public Health Res Perspect. 2011;2:3–7. doi: 10.1016/j.phrp.2011.04.001. [PMID: 24159443] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2009;6(Suppl 6):S783–S790. doi: 10.1098/rsif.2009.0302.focus. [PMID: 19773292] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [PMID: 10738050] [DOI] [PubMed] [Google Scholar]
- 18.Baize S, Leroy EM, Georges AJ, Georges-Courbot MC, Capron M, Bedjabaga I, et al. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–168. doi: 10.1046/j.1365-2249.2002.01800.x. [PMID: 11982604] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElroy AK, Erickson BR, Flietstra TD, Rollin PE, Nichol ST, Towner JS, et al. Ebola hemorrhagic Fever: novel biomarker correlates of clinical outcome. J Infect Dis. 2014;210:558–566. doi: 10.1093/infdis/jiu088. [PMID: 24526742] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauch CT, Galvani AP. Epidemiology. Social factors in epidemiology. Science. 2013;342:47–49. doi: 10.1126/science.1244492. [PMID: 24092718] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Ebola Response Team. Ebola virus disease in West Africa—The first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [PMID: 25244186] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberia Institute of Statistics and Geo-Information Services. 2008 national population and housing census: preliminary results. [on 6 June 2008];2008 Accessed at http://unstats.un.org/unsd/dnss/docViewer.aspx?docID=2075. [Google Scholar]
- 23.Bausch DG, Towner JS, Dowell SF, Kaducu F, Lukwiya M, Sanchez A, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl 2):S142–S147. doi: 10.1086/520545. [PMID: 17940942] [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Why the Ebola outbreak has been underestimated. [on 1 September 2014];2014 Aug 22; Accessed at www.who.int/mediacentre/news/ebola/22-august-2014/en/
- 25.Cooper H, Fink S. Obama presses leaders to speed Ebola response. [on 17 September 2014];New York Times. 2014 Sep 16; Accessed at www.nytimes.com/2014/09/17/world/africa/obama-urges-world-powers-to-bolster-ebola-response.html?_r=0. [Google Scholar]
- 26.Legrand J, Grais RF, Boelle PY, Valleron AJ, Flahault A. Understanding the dynamics of Ebola epidemics. Epidemiol Infect. 2007;135:610–621. doi: 10.1017/S0950268806007217. [PMID: 16999875] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owers S, Patterson-Lomba O, Castillo-Chavez C. Temporal variations in the effective reproduction number of the 2014 West Africa Ebola outbreak. [on 18 September 2014];PLOS Curr Outbreaks. doi: 10.1371/currents.outbreaks.9e4c4294ec8ce1adad283172b16bc908. Accessed at http://currents.plos.org/outbreaks/article/temporal-variations-in-the-effective-reproduction-number-of-the-2014-west-africa-ebola-outbreak/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez A, Lukwiya M, Bausch D, Mahanty S, Sanchez AJ, Wagoner KD, et al. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78:10370–10377. doi: 10.1128/JVI.78.19.10370-10377.2004. [PMID: 15367603] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debré P, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. [PMID: 10202932] [DOI] [PubMed] [Google Scholar]
- 30.Frieden TR, Damon I, Bell BP, Kenyon T, Nichol S. Ebola 2014—new challenges, new global response and responsibility. N Engl J Med. 2014;371:1177–1180. doi: 10.1056/NEJMp1409903. [PMID: 25140858] [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. Ebola response roadmap. [on 30 September 2014];2014 Aug; Accessed at www.who.int/csr/resources/publications/ebola/response-roadmap/en/
- 32.Centers for Disease Control and Prevention. 2014 Ebola outbreak in West Africa. [on 30 September 2014]; Accessed at www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.