SUMMARY
Wnt signaling plays an essential role in developmental and regenerative myelination of the CNS; however, contributions of proximal regulators of the Wnt receptor complex to these processes remain undefined. To identify components of the Wnt pathway that regulate these processes, we applied a multifaceted discovery platform and found that Daam2-PIP5K comprise a novel pathway regulating Wnt signaling and myelination. Using dorsal patterning of the chick spinal cord we found that Daam2 promotes Wnt signaling and receptor complex formation through PIP5K-PIP2. Analysis of Daam2 function in oligodendrocytes (OLs) revealed that it suppresses OL differentiation during development, after white matter injury (WMI), and is expressed in human white matter lesions. These findings suggest a pharmacological strategy to inhibit Daam2-PIP5K function, application of which stimulates remyelination after WMI. Put together, our studies integrate information from multiple systems to identify a novel regulatory pathway for Wnt signaling and potential therapeutic target for WMI.
INTRODUCTION
Oligodendrocytes (OLs) produce and assemble the myelin sheath, which insulates axons in the CNS and facilitates saltatory conduction. Given their vital role in CNS physiology, loss of myelinating OLs can result in several neurological disorders, including multiple sclerosis (MS) in adults and cerebral palsy (CP) in children (Chang et al., 2002; Franklin, 2002; Khwaja and Volpe, 2008; Woodward et al., 2006). OLs have a robust capacity for regeneration (or remyelination), suggesting that endogenous cellular mechanisms can be employed for repair of white matter injury (WMI). However, areas of WMI in both adults and children are populated by “stalled” OL precursors (OLPs), suggesting that the molecular mechanisms governing OLP differentiation into OLs is suppressed by inhibitory signals within these lesions (Billiards et al., 2008; Buser et al., 2012; Hammond et al., 2014; Kuhlmann et al., 2008). Therefore, a comprehensive understanding of the molecular mechanisms regulating OLP differentiation into OLs may reveal therapeutic strategies that stimulate remyelination after WMI.
A central tenet of regenerative biology is that restorative processes parallel those found in development. Among the key molecular processes that suppress OLP differentiation during development and after WMI is Wnt signaling. Chronic activation of canonical Wnt signaling in OLPs results in delayed developmental and regenerative myelination (Emery, 2010; Fancy et al., 2009, 2014; Tawk et al., 2011). These functional studies, coupled with elevated expression of key pathway components in OLP populations in human WMI, suggest that Wnt signaling is a crucial regulatory node that is dysregulated after WMI and a potential target for therapeutic intervention (Fancy et al., 2011). Studies on Wnt signaling have focused on how key distal transcriptional effectors of this pathway regulate OLP differentiation; however, the contributions of the proximal membrane and intracellular components that transduce these signals have not been examined in this context. One critical aspect of Wnt signal transduction is the clustering of Wnt receptor complexes into signalosomes, which are required for the initiation, amplification, and maintenance of Wnt signaling (Bilic et al., 2007; He et al., 2004; MacDonald et al., 2009; Mao et al., 2001; Schwarz-Romond et al., 2007). Wnt receptor signalosomes represent a potential therapeutic target because they function at the cell membrane and are therefore more accessible for pharmacological manipulation. In spite of these potential therapeutic attributes, the mechanisms that regulate Wnt receptor clustering and subsequent signalosome formation remain uncharacterized during OLP development and remyelination.
A key step in signalosome formation is the aggregation and clustering of Frizzled/LRP/Dishevelled/Axin complexes, which facilitates amplification and maintenance of Wnt signaling (Bilic et al., 2007; Pan et al., 2008; Qin et al., 2009). Recently we found that Daam2 is required for canonical Wnt signaling during patterning in the dorsal spinal cord, functioning through the clustering and formation of Wnt receptor signalosomes (Lee and Deneen, 2012). At the biochemical level, Daam2 promotes the aggregation of Dishevelled (Dvl) complexes, which supports signalosome formation and potentiates Wnt activity. While these studies implicate Daam2 as a vital component of the Wnt receptor complex and provide a framework for understanding signal-osome formation, the underlying mechanisms of Daam2 function remain poorly characterized.
During gastrulation the PIP5K-Ptdlns(4,5)P2 (PIP2) pathway controls LRP5/6 phosphorylation and subsequent clustering of Wnt receptor complexes (Pan et al., 2008). Activation of PIP5K is regulated through its association with Dvl, in a Wnt3a-dependent manner (Qin et al., 2009). That Daam2 synergizes with Wnt3a in the dorsal spinal cord and also associates with Dvl suggests a possible functional link with the PIP5K-PIP2 pathway during Wnt signalosome formation in the CNS (Lee and Deneen, 2012). Importantly, a deeper mechanistic understanding of Wnt signalosome formation can potentially offer novel therapeutic insight into repair after WMI, as the role of Daam2 and its associated biology in OLP populations during development and after WMI remains completely undefined.
Using this premise as our starting point, we show that Daam2 regulates canonical Wnt signaling and signalosome formation through the PIP5K-PIP2 axis. Examination of Daam2 function during OLP development revealed that it suppresses their differentiation into OLs. These developmental findings were subsequently applied to WMI, where we found that Daam2 is similarly expressed in OLPs in human white matter lesions and functions to suppress their differentiation in mouse models of WMI. Our mechanistic studies suggested that pharmacological inhibition of PIP5K function might be an approach to suppress the Wnt pathway in OLPs. Translation of these findings to our WMI models revealed that inhibition of PIP5K promotes OLP differentiation in vitro and remyelination during in vivo regeneration. Together, these data identify a novel mechanism that regulates Wnt signaling in OLPs and point to a potentially new therapeutic approach for stimulating myelin repair after WMI.
RESULTS
Daam2 Regulates Canonical Wnt Signaling through PIP5K-PIP2
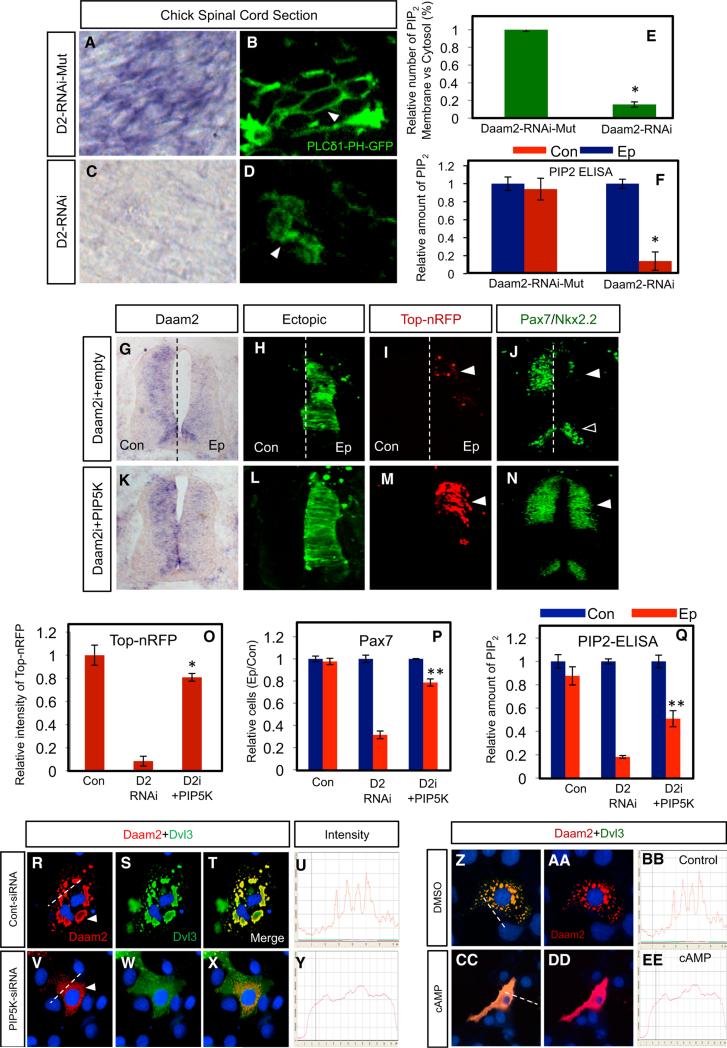
Previous studies have linked PIP2 production to Wnt signalo-some formation (Pan et al., 2008). Given that Daam2 regulates signalosome formation, we assessed whether it regulates PIP2 activity in chick spinal cord by co-electroporating Daam2-shRNAi (or mutant shRNAi control) with a PLCd-PH-GFP reporter, which binds PIP2 and reports its intracellular localization (Balla and Várnai, 2002). We found that knockdown of Daam2 results in decreased membrane localization of the reporter (Figures 1A and 1B versus 1C–1E). In parallel, we performed a PIP2 ELISA assay on these chick spinal cords and found a drastic decrease in PIP2 production in the presence of the Daam2-shRNAi (Gray et al., 2003; Pan et al., 2008) (Figure 1F); together these data indicate that knockdown of Daam2 results in decreased production of PIP2. Further biochemical analysis of these interrelationships using in vitro lipid binding assays (Heo et al., 2006) indicates that Daam2 does not directly associate with PIP2 (Figures S1A–S1F), suggesting an indirect regulatory relationship.
Figure 1. Daam2 Regulates Dorsal Patterning and Canonical Wnt Signaling through PIP5K-PIP2 Signaling.
(A–D) Chick spinal cord electroporated with Daam2-shRNAi (or mutant control), PLCg-PH-GFP reporter, and TOP-nRFP (red). Arrow in (B) denotes membrane localization of GFP reporter, indicating PIP2 activity.
(F) PIP2-ELISA on spinal cord electroporated with Daam2-shRNAi or mutant control. Quantification in (E) and (F) is from four independent experiments.
(K–N) Overexpression of PIP5K rescues dorsal patterning and canonical Wnt signaling in the absence of Daam2; (G–J) Daam2-shRNAi control. Arrowheads denote reduced dorsal marker expression (Pax7, TOP-nRFP), open arrowheads denote ventral markers with no change (Nkx2.2).
(O–Q) Quantification of dorsal patterning (Pax7), canonical Wnt activity (TOP-nRFP), and PIP2 production from chick experiments; values are derived from at least four independent embryos, six sections per embryo.
(R–U) Co-expression of Daam2/Dvl3 results in clustering; siRNA knockdown of PIP5K (V–Y) inhibits assembly of Daam2/Dvl3 complexes.
(Z–EE) Treatment with Sp-8-pCPT-cAMPs inhibits assembly of Dam2/Dvl3 complexes. Dashed lines in (R), (V), (Z), and (CC) are line intensity graphs shown in (U), (Y), (BB), and (EE), respectively, and are representative of 50 such measurements. Ep, electroporated side; Con, control side. All chick experiments were harvested at E4. Error bars in all graphs are SD (*p < 0.005; **p < 0.01; in P–R comparisons are between D2i and D2i/PIP5K conditions). Please also see Figure S1.
The production of PIP2 is regulated by PIP5K (van den Bout and Divecha, 2009), raising the possibility that Daam2 regulates PIP2 production, and Wnt signaling, through PIP5K. To test this hypothesis, we examined whether PIP5K overexpression rescues dorsal patterning and canonical Wnt signaling in the absence of Daam2. As indicated in Figure 1, co-electroporation of PIP5K with the Daam2-shRNAi restored canonical Wnt activity and dorsal patterning in the developing spinal cord (Figures 1G–1P). Moreover, ELISA assays analysis that PIP5K rescued PIP2 production, further correlating Daam2-dependent Wnt activity to PIP2 production (Figure 1Q). To confirm the role of PIP5K in Wnt signaling, we performed shRNAi knockdown in chick and found a loss of dorsal patterning and Wnt signaling, phenotypes that parallel knockdown of Daam2 (Figures S1G–S1U). To establish the functional relationship between Daam2 and PIP5K during Wnt signalosome formation we used in vitro clustering assays (Bilic et al., 2007; Lee and Deneen, 2012). siRNA knockdown of PIP5K completely blocked the clustering of Wnt receptor complexes mediated by Daam2, reinforcing their cooperative relationship during the construction of Wnt receptor complexes at the cellular level (Figures 1R–1Y; see Figures S1V and S1W).
Daam2 and PIP5K Biochemical Interaction Regulates Wnt Signaling
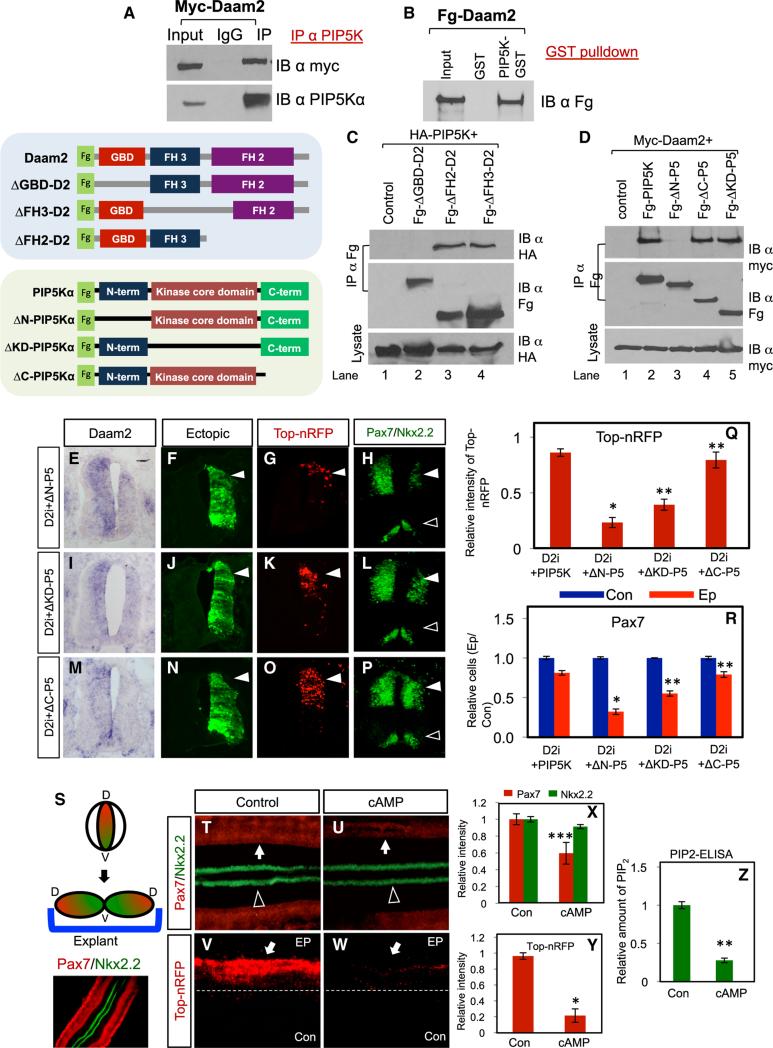
Further examination into their relationship revealed that Daam2 and PIP5K co-immunoprecipitation (coIP) and subsequent GST pull-down studies confirmed that they directly interact (Figures 2A and 2B). Investigation into the nature of the biochemical relationship between Daam2 and PIP5K revealed that the Daam2-GBD domain and the N-terminal region of PIP5K are required for their associations (Figure 2C, lane 2; Figure 2D, lane 3). These results suggest that the N-terminal region of PIP5K is required for its function toward Wnt signaling. To test this, we performed rescue analysis of Daam2-shRNAi with the PIP5K mutants in the chick spinal cord, finding that the PIP5K mutants lacking either the N terminus (ΔN) or the kinase domain (ΔKD) do not rescue Wnt signaling or dorsal patterning, while the C-terminal deletion (ΔC) does (Figures 2E–2R). These data indicate that the kinase and the N-terminal domains of PIP5K are required for its function in this context. Another key finding from our biochemical studies is that the Daam2-GBD domain is required for its interaction with PIP5K (Figure 2C, lane 2). Importantly, the GBD domain is essential for interactions with Rho-GTPases, which also associate with Daam2 (Figure S1Y). These observations, put together with previous studies demonstrating that Sp-8-pCPT-cAMPs (cAMP) inhibit PIP5K activity through Rho signaling (Yang et al., 2004), suggest an approach for inhibiting Daam2/PIP5K function. To examine whether this pharmacological approach similarly suppresses Wnt signaling in the spinal cord, we cultured chick spinal cord explants (see Figure 2S) with Sp-8-pCPT-cAMPs. Analysis revealed a selective loss of dorsal markers and canonical Wnt signaling, which is likely due to reduced PIP5K activity, as PIP2 production is also drastically reduced (Figures 2T–2Z). Finally, treatment with Sp-8-pCPT-cAMPs blocked Daam2-mediated Wnt receptor clustering (Figures 1Z–1EE and S1X). Collectively, these data indicate that Daam2 regulates Wnt signaling through PIP5K-PIP2 and suggest that inhibition of this pathway suppresses Wnt signaling in the developing CNS.
Figure 2. Structural Analysis of Daam2 and PIP5K Biochemical Interaction.
(A and B) Immunoblots of HEK293 cell extracts expressing Myc-Daam2 and co-immunoprecipitating endogenous PIP5K; GST pulldown studies with GST-tagged PIP5K and FLAG-tagged Daam2.
(C) CoIP experiments between HA-tagged PIP5K and various Daam2 mutants listed.
(D) CoIP experiments between Myc-tagged Daam2 and various PIP5K mutants listed.
(E–R) Rescue analysis of various PIP5K mutants in chicks co-electroporated with Daam2-shRNAi.
(S) Schematic of chick explant system.
(T–W) Chick spinal cord was electroporated with TOP-nRFP and GFP control and grown as an explant. Arrows in (T)–(W) indicate reduced expression of Pax7 and TOP-nRFP reporters in the presence of Sp-8-pCPT-cAMPs. Open arrowhead denotes Nkx2.2 expression.
(X and Y) Quantification of dorsal (Pax7) and ventral (Nkx2.2) patterning and canonical Wnt activity (TOP-nRFP) from these experiments; values are derived from at least four independent embryos, six sections per embryo.
(Z) PIP2 ELISA on chick explants treated with Sp-8-pCPT-cAMPs; values are derived from three independent experiments. Ep, electroporated side; Con, control side. All chick experiments were harvested at E4. Error bars are SD, and *p < 0.006; **p < 0.0004; ***p < 0.05. Please also see Figure S1.
Daam2 Suppresses OLP Differentiation during Development
To investigate a potential role for Daam2 in glial populations we characterized its expression patterns during gliogenesis in the developing and post-natal spinal cord. To determine the extent of its expression in the OL and astrocyte lineages, we assessed its co-expression with markers of these glial lineages in the developing spinal cord of a Daam2LacZ/+ mouse we recently generated (Figure S2; see Supplemental Information). Analysis of Daam2 expression at E18.5 revealed co-localization with Olig2 in OLPs and GLAST in astrocyte precursors (ASPs) (Figures 3A and 3B). Daam2 expression was also detected in OL and astrocyte populations (Figures 3C and 3D). These findings were corroborated by double in situ hybridization (Figures S3A–S3H). Analysis in the adult spinal cord revealed similar expression patterns (Figures 3E–3H). These data indicate that Daam2 is expressed in both OL and astrocyte lineages in the embryonic and adult spinal cord.
Figure 3. Daam2 Is Expressed in Glial Lineages and Suppresses OLP Differentiation in the Developing Spinal Cord.
(A–H) Double immunofluorescence on spinal cord from Daam2LacZ/+ mice at E18.5 and 2 months of age. Please see Figure S2 for double in situ hybridization images of Daam2 and the relevant glial lineage markers. Dashed lines in (A)–(D) demarcate white matter (WM).
(I–N) Overexpression of Daam2 in the chick spinal cord suppresses OLP differentiation at E11. (I) Ectopic Daam2 expression is detected with α-Myc. (J–K) analysis of OL markers, (L) OLP populations, and (M–N) astrocyte precursors. Arrows in (J)–(K) denote reduced OL marker expression on the electroporated side (Ep) of the spinal cord.
(O and P) Quantification of the number of MBP-, PLP-, and PDGFRα-expressing cells, derived from at least eight sections from three E11 chick embryos. Ep, electroporated side; Con, control side. Error bars are SD (*p < 0.005; **p < 0.01). Please also see Figures S2 and S3.
That Wnt signaling plays a vital role in suppressing OLP differentiation, and Daam2 is a key regulator of Wnt signaling, raises the possibility that Daam2 may regulate OLP differentiation. To test this, we overexpressed Daam2 in the embryonic chick spinal cord and found a reduction in the number of MBP- and PLP-expressing cells at E11 (Figures 3I, 3K, and 3O; see Figures S3Z–S3CC for high-magnification images). Importantly, this was coupled with no change in the number of OLPs or ASPs, indicating the suppression of OL differentiation by Daam2 is not due to a loss of OLP populations or interconversion of glial sub-lineages (Figures 3L–3N and 3P). Next, we assessed OL differentiation in the spinal cord of Daam2 knockout mice (see Figure S2), finding that loss of Daam2 results in the precocious differentiation of MBP- and PLP-expressing OLs at P0 (Figures 4A and 4B versus 4D, 4E, 4G, and 4H; see Figures S3DD–S3GG for high-magnification images). This effect is not secondary to changes in the constituency of other glial lineages, as there were no differences in the number of OLPs (Figure 4C versus 4F and 4I) or astrocytes between Daam2+/− and Daam2−/− mice (Figures S4A and S4B). Subsequent analysis at later post-natal time points revealed that the levels of MBP and PLP expression were comparable between the Daam2+/− and Daam2−/−, indicating that Daam2 is required for the proper timing of OLP differentiation (Figures S4C–S4J). To confirm that loss of Daam2 accelerates OLP differentiation, we generated OLP cultures from Daam2+/− and Daam2−/− embryos. A total of 3 days after induction of differentiation, we found a drastic increase in the number of MBP- and PLP-expressing cells in the Daam2-knockout cultures (Figures 4J and 4K versus 4M, 4N, 4P, and 4Q), with no effect on the expression of OLP markers (Figure 4L versus 4O and 4R). Consistent with a role in the timing of differentiation, a similar, albeit less drastic, enhancement of OLP differentiation was witnessed 7 days post-differentiation (Figures S4K–S4P). Together, these gain- and loss-of-function studies indicate that Daam2 suppresses OLP maturation by regulating the time course of their maturation into MBP- and PLP-expressing OLs.
Figure 4. Loss of Daam2 Stimulates OLP Differentiation during Development.
(A–F) Analysis of P0 spinal cord from Daam2+/− and Daam2−/− mice reveals increased expression of OL markers in the absence of Daam2 (arrows). There were no changes in the number of cells expressing the OLP marker PDGFRα (C, F) or markers of other glial lineages (see Figure S3).
(G–I) Quantification of OL and OLP markers is derived from at least six sections, from three animals of each genotype.
(J–R) In vitro analysis of OL differentiation using Daam2+/− and Daam2−/− OLP cultures at 3 days post-differentiation revealed increased expression of OL markers (arrowheads in M and N).
(S–W) Daam2+/− or Daam2−/− cultures were transfected with the PLCγ-PH-GFP reporter (PIP2-GFP) and TOP-nRFP (red), revealing reduced membrane production of PIP2 (arrows in S and U) and Wnt activity (arrows in T and V) in the absence of Daam2.
(X) Quantification of Wnt activity in Daam2−/− OLPs.
(Y–CC) In vitro analysis of OLP cultures treated with Sp-8-pCPT-cAMPs (cAMP), analyzed at 3 and 7 days after induction of differentiation. The quantification for all in vitro studies is derived from two independently derived sets of cultures and is representative of three experiments, performed in triplicate. Error bars are SD (*p < 0.005; **p < 0.01). Please also see Figures S2–S4.
Daam2 Suppresses OLP Differentiation through PIP5K
Having established that Daam2 regulates Wnt signaling through the PIP5K-PIP2 axis (see Figures 1 and 2), we next examined whether Daam2 functions through this mechanism to suppress OLP differentiation. Using double in situ hybridization, we found that Daam2 and PIP5K are co-expressed in the post-natal spinal cord, and that PIP5K is similarly expressed in OLP populations in the spinal cord (Figure S3). To determine whether PIP2 production is dysregulated in the absence of Daam2, we transfected OLPs generated from Daam2+/− and Daam2−/− embryos with the PLCδ-PH-GFP reporter, finding reduced membrane localization, coupled with diffuse, cytosolic distribution of the reporter in the absence of Daam2 (Figures 4S and 4T versus 4U and 4V). This effect is consistent with reduced membrane production of PIP2 and parallels our results in the chick spinal cord. Given the link between PIP2 production and Wnt signaling (Figure 1) (Pan et al., 2008; Qin et al., 2009), we sought to correlate these changes in OLP differentiation and membrane production of PIP2 to Wnt activity. To this end we measured Wnt activity in Daam2+/− and Daam2−/− OLPs by transfecting them with the TOP-nRFP Wnt reporter (Lee and Deneen, 2012). Subsequent analysis revealed a significant reduction in the number of cells reporting Wnt activity in Daam2−/− OLP populations (Figure 4T versus 4V and 4X). These results parallel those found during patterning of the embryonic spinal cord (see Figure 1) and suggest a direct mechanistic link between the Daam2-PIP2 axis and Wnt signaling in OLPs.
To extend this model and establish a direct link between Daam2 and PIP2 in OLPs we next determined whether Daam2 suppresses OLP differentiation through PIP5K by examining whether overexpression of PIP5K in Daam2−/− OLP populations is sufficient to block accelerated OLP differentiation. Toward this, Daam2−/− OLPs were infected with lentivirus containing Cherry or PIP5K and harvested at 3 and 7 days after the induction of differentiation. Analysis of OL differentiation revealed that PIP5K overexpression blocked the precocious and enhanced appearance of MBP- and PLP-expressing cells in Daam2−/− OLPs (Figures S4Q–S4U). These effects of PIP5K were not due to a loss of OLP populations, as the number of Olig2-expressing cells was unchanged, and indicates that Daam2 function during OLP differentiation is mediated in part through PIP5K and, again, parallels our observations from the chick spinal cord (Figure 1). That Daam2 signals through the PIP5K-PIP2 axis during OLP differentiation suggests that inhibition this signaling axis may similarly promote OLP maturation. To test this possibility, we generated OLPs from embryonic spinal cord and added Sp-8-pCPT-cAMPs after induction of differentiation. Analysis at 3 and 7 days post-differentiation revealed precocious and enhanced OL differentiation, marked by expression of MBP, coupled with no changes in the number of OLPs (Figures 4Y–4CC). These findings suggest that Daam2 functions through PIP5K and implicate PIP5K-PIP2 signaling in the suppression of OLP differentiation.
Daam2 Suppresses OLP Differentiation after WMI
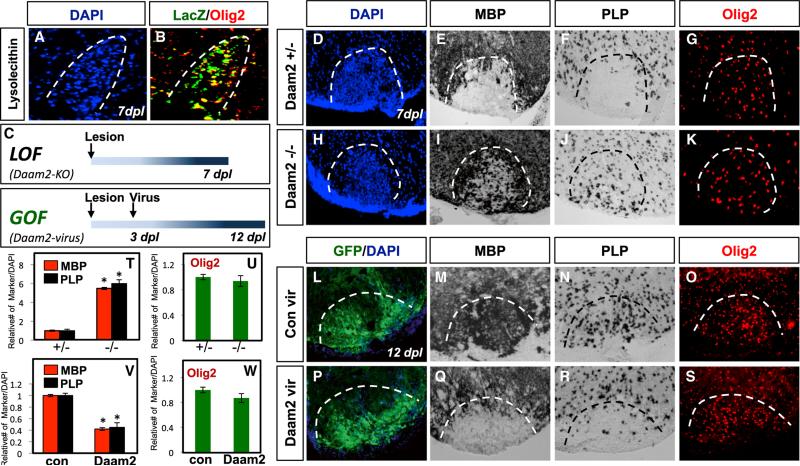
Our foregoing data describing the ability of Daam2 to suppress OLP differentiation in developmental systems led us to investigate whether it has a similar function during remyelination after WMI in the adult spinal cord. Toward this objective, we examined Daam2 expression and function in a lysolecithin model of WMI, a well-established gliotoxic injury model that allows for the precise evaluation of remyelination repair kinetics (Figure S5A) (Fancy et al., 2009). Using the Daam2-LacZ mouse, we performed lysolecithin lesioning on adult spinal cord, finding that Daam2 is indeed expressed in OLP populations at induced lesion sites (Figures 5A and 5B). To address whether Daam2 similarly suppresses OLP differentiation during remyelination, we next performed lysolecithin lesioning on Daam2+/− and Daam2−/− mice and analyzed the extent of OLP differentiation at 7 days post lesion (dpl), a time point that precedes normal remyelination and is devoid of mature OL populations (Figures 5C and S5). Analysis of OLP differentiation in Daam2 knockout mice revealed a 5-fold increase in the number of MBP- and PLP-expressing cells compared to heterozygote controls (Figures 5E and 5F versus 5I, 5J, and 5T), without any effect on the number of Olig2-expressing cells (Figures 5G, 5K, and 5U). Additionally, the reactive astrocyte and inflammatory responses are unaffected by the loss of Daam2 (Figures S5B–S5E). These data indicate that Daam2 suppresses OLP differentiation after WMI.
Figure 5. Daam2 Suppresses OLP Differentiation after WMI.
(A and B) Co-expression of Daam2-LacZ and Olig2 at 7 dpl in a mouse lysolecithin lesion.
(C) Schematic of the loss-of-function (LOF) and gain-of-function (GOF) paradigms.
(D–K) Loss of Daam2 results in increased expression of OL markers at 7 dpl, without affecting the number of OLPs.
(L–S) Viral overexpression of Daam2 suppresses the expression of OL markers at 12 dpl; overexpression of Daam2 in lesions did not affect the number of OLPs. Dashed lines in (D)–(S) indicate lesion borders.
(T–W) Quantification of OL and OLP marker expression in LOF and GOF experiments. For the LOF analysis, experiments were performed on at least three animals per genotype, and quantification involved at least eight sections per animal. For the viral GOF analysis, experiments were performed on at least six animals per condition, and quantification involved at least eight sections from three animals demonstrating the highest expression of the respective viral transgene. Error bars are SD (*p < 0.005; **p < 0.01). Please also see Figure S5.
Next we performed the complementary gain-of-function experiments in the lesioned spinal cord. For this, the spinal cords of wild-type mice were subjected to lysolecithin lesioning and followed by a secondary injection of lentivirus containing GFP or Daam2-IRES-GFP at 3 dpl, during the height of OLP recruitment to the lesion (Figure 5C, bottom). Because we anticipated that Daam2 overexpression would suppress OLP differentiation, we evaluated lesions at 12 dpl, a time point where a significant number of OLPs have differentiated into OLs. As expected, the GFP control showed extensive OL differentiation (Figures 5M and 5N), while the lesions injected with the Daam2-containig virus exhibited a drastic decrease in the number of MBP- and PLP-expressing OLs (Figures 5Q, 5R, and 5V). This decrease in OL differentiation was not the result of diminished OLPs or changes in the reactive astrocyte response, since the number of Olig2- and GFAP-expressing cells was not affected by Daam2 overexpression (Figures 5O, 5S, 5W, and S5F–S5I). Together these studies indicate that Daam2 suppresses OLP differentiation after WMI and, in conjunction with our previous studies, reveal that its function is conserved across development and repair.
Inhibition of PIP5K Stimulates Remyelination after WMI
Our discovery that Daam2 functions through the PIP5K-PIP2 axis to regulate Wnt signaling and OLP differentiation, coupled with the role of Daam2 in repair after WMI, suggest that this mechanism is similarly reutilized during repair after WMI. This notion is further supported by our observation that PIP5K is expressed in OLP populations in lysolecithin-induced white matter lesions (Figures 6A and 6B). Moreover, because PIP5K is expressed in these lesions, and there is an associated pharmacological agent that inhibits its activity, this pathway may represent a therapeutically actionable target for white matter disorders. Indeed, Sp-8-pCPT-cAMPs suppresses Wnt signaling (Figure 1W) and promotes OLP differentiation (Figures 4Y–4CC), suggesting that it may similarly stimulate remyelination after WMI. Application of this biology toward repair after WMI is critical, as there is a dearth of pharmacological strategies that promote OLP differentiation after injury (see Discussion).
Figure 6. Inhibition of PIP5K Activity Promotes Remyelination after WMI.
(A and B) In situ hybridization demonstrating co-expression of PIP5K and PDGFRα at 7 dpl in a mouse lysolecithin lesion.
(C) Schematic of experimental paradigm.
(D–K) Treatment with Sp-8-pCPT-cAMPs results in increased expression of OL markers at 7 dpl, compared to the vehicle control, without affecting the number of OLPs.
(L and M) Quantification of OL and OLP marker expression was performed on at least eight sections from six mice per condition (Sp-8-pCPT-cAMPs or control). Error bars in graphs are SD.
(N–P) Myelin formation was assessed using electron microscopy (EM) on 70-nm sections from lesions at 10 dpl. This analysis revealed significant increases in the thickness of the restored myelin sheaths (t test, p = 0.0043); G-ratios were significantly different between the control (G-ratio = 0.904, SEM = 0.004) and the Sp-8-pCPT-cAMPs groups (G-ratio = 0.860, SEM = 0.003). EM and G-ratio analysis was performed on three animals per condition. Scale bar in (N), 2 μm. *p < 0.005; **p < 0.01. Please also see Figure S6.
To determine the effects of inhibiting PIP5K activity on post-injury remyelination, we co-injected lysolecithin with Sp-8-pCPT-cAMPs (or vehicle control) into the spinal cord of wild-type mice, harvesting lesioned spinal cords at 3 dpl and 7 dpl (Figure 6C). Analysis at 3 dpl revealed the presence of a demyelinated lesion, populated with Olig2-expressing OLPs in both Sp-8-pCPT-cAMPs and control conditions, indicating that treatment with Sp-8-pCPT-cAMPs does not interfere with lesioning or recruitment of OLPs to the lesion site (Figures 6I–6P). Moreover, the reactive astrocyte and inflammatory responses were unaffected (Figures S6A–S6D). Next, we analyzed lesioned spinal cord at 7 dpl, a time point that is normally devoid of differentiated OLs, and observed a drastic increase in PLP- and MBP-positive cells at the lesion site in the presence of Sp-8-pCPT-cAMPs compared to vehicle (Figures 6E and 6F versus 6I, 6J, and 6L). The OLP, reactive astrocyte, and inflammatory responses were unaffected (Figures 6G, 6K, 6M, and S6E–S6H). These data indicate that inhibition of PIP5K promotes OL differentiation after WMI. To further substantiate these findings, we next performed transmission electron microscopy (EM) analysis on the vehicle and Sp-8-pCPT-cAMPs-treated lesions harvested at 10 dpl to assess the formation of compacted myelin. Our EM analysis revealed a substantial increase in myelin thickness in lesions treated with the Sp-8-pCPT-cAMPs compared to vehicle control (Figures 6N–6P), indicating that the precocious OL differentiation manifests in enhanced myelin formation (i.e., smaller G-ratios, see Figure 6 legend). Together these data indicate that inhibition of PIP5K stimulates remyelination after WMI and identifies this pathway as a potential therapeutic target for WMI.
Daam2-PIP5K Signaling Axis Suppresses Remyelination after Hypoxic Injury
In the lysolecithin injury model, our pharmacological and viral manipulations are administered to the lesion site raising the possibility that they indirectly stimulate OL differentiation and remyelination through non-CNS-derived inflammatory cells or reactive astrocytes. To examine this possibility we performed both viral and pharmacological manipulations in ex vivo cerebellar slice cultures after lysolecithin demyelination, a well-established system for modeling remyelination ex vivo (Birgbauer et al., 2004; Huang et al., 2011). Here, we made cerebellar slices, and treated them with lysolecithin to induce demyelination; 2 days later Daam2-virus (or control) or Sp-8-pCPT-cAMPs (or control) was added (Figures 7A and S6U–S6X). Analysis of OL differentiation after viral or drug addition revealed that overexpression of Daam2 inhibited OL differentiation, while treatment with the Sp-8-pCPT-cAMPs stimulated OL differentiation (Figures 7B–7E). These findings parallel our in vivo results and indicate that the effects of our viral and pharmacological manipulations are not mediated through non-CNS inflammatory or reactive astrocyte populations.
Figure 7. Daam2-PIP5K Signaling Suppresses Myelination after Hypoxia.
(A) Schematic of ex vivo remyelination experimental paradigms.
(B–E) Ex vivo cerebellar slices were prepared and treated with lysolecithin and subjected to Daam2 virus or Sp-8-pCPT-cAMPs.
(F–I) Ex vivo slices were prepared, subjected to acute hypoxia, and then treated with Daam2 virus or Sp-8-pCPT-cAMPs. Subsequently, the slices were harvested and stained for MBP and Cspr. These experiments were performed three independent times, in triplicate; the figures are representative. Please also see Figure S6.
In addition to lysolecithin-induced remyelination, the ex vivo cerebellar system can also be used to model WMI following hypoxia (An et al., 2011; Fancy et al., 2011). Toward this, cerebellar slices were established and maintained at 1% oxygen for 2 days, after which there was a complete loss of mature OL populations (Figures S6Q and S6R). After this acute hypoxia, the cultures were returned to normoxia and treated with either the Daam2-virus or Sp-8-pCPT-cAMPs (Figures 7F–7I, S6Y, and S6Z). Subsequent analysis of OL regeneration revealed that Daam2 overexpression blocked OL differentiation, while Sp-8-pCPT-cAMPs stimulated both OL differentiation (MBP) and myelination (Cspr) in these cultures.
That Daam2 is sufficient to suppress myelination after acute hypoxia ex vivo led us to determine whether it has a similar function in a neonatal model of hypoxic injury (HI). Established models of neonatal HI involve placing pups and nursing mothers in 10.5% oxygen from P3 to P11, which results in the loss of OLs, followed by a period of regeneration during perinatal development (Figure 8A) (Scafidi et al., 2014). We generated litters containing Daam2+/− and Daam2−/− pups and subjected them to HI from P3 to P11. Analysis of OL differentiation in P18 brains revealed significant increases in OL differentiation (MBP) and myelination (CASPR) in the Daam2−/− mice (Figures 8B–8E), without changes in the number of Olig2-expressing cells (Figures S7F and S7G). Next we performed the complementary gain-of-function studies by combining in utero electroporation (IUE) of the cortex with PiggyBac-mediated gene expression. Standard IUE approaches do not allow for long-term expression because the plasmids do not integrate; here we used a dual PiggyBac system that allows for plasmid integration, facilitating long-term expression (Figures 8A and S7A) (Chen and LoTurco, 2012; Glasgow et al., 2014). As indicated in Figures 8F–8I, over-expression of Daam2 resulted in decreased expression of MBP and CASPR on the electroporated side of the cortex, coupled with no changes in the number of Olig2-expressing cells (Figures S7H and S7I). These findings, combined with our ex vivo studies, provide evidence that Daam2-PIP5K signaling suppresses myelination after HI and further consolidates the key contributions of this signaling axis in the suppression of remyelination after WMI.
Figure 8. Daam2 Suppresses Myelination after Neonatal Hypoxic Injury.
(A) Schematic of neonatal mouse hypoxic injury (HI) model and our combined in utero electroporation (IUE) and dual PiggyBac (PB) overexpression system.
(B–E) Analysis of oligodendrocyte differentiation (MBP) and myelination (CASPR) in subcortical white matter regions from P18 Daam2+/− and Daam2−/− mice.
(F and G) Complementary overexpression studies using the combined PB/IUE approach to overexpress Daam2. Inset in (G) is lower-magnification image showing the region of the white matter demonstrating high ectopic expression of Daam2-t2a-GFP; see Figure S8. Analysis of oligodendrocyte differentiation at P18 revealed decreased MBP and CASPR expression on the side of the cortex electroporated with the PB containing Daam2.
(J and K) PIP5K co-localizes with Olig2-expressing cells within human HIE white matter lesions.
(L and M) Daam2 co-localizes with Olig2-expressing cells within a human white matter lesion. Arrows denote cells co-expressing PIP5K or Daam2 and Olig2. Ep, electroporated side; Con, control side. The knockout studies are representative of six animals from each genotype, derived from three independent litters. For the PB/IUE experiments, the images are representative of five animals from two independent experiments. Please also see Figure S7.
Daam2 and PIP5K Are Expressed in OLPs in Human White Matter Disorders
Collectively, our findings implicate the Daam2-PIP5K pathway as playing a key role in OLP differentiation during regenerative myelination after gliotoxic injury in the adult and HI in neonates. To determine whether this pathway is present in human WMI associated with neonatal HI we investigated whether Daam2 and PIP5K are expressed in OLP populations within the subcortical white matter of human neonatal brain damaged by hypoxic-ischemic encephalopathy (HIE). HIE was diagnosed as previously described based on both clinical and neuropathological correlations (Fancy et al., 2011). Using in situ hybridization for human PIP5K (Figure 8J) or Daam2 (Figure 8L), in combination with immunostaining for Olig2 (Figures 8K and 8M), we found that both PIP5K and Daam2 are expressed in OLP populations in areas of gliotic subcortical white matter. These findings, together with our functional studies, suggest that the Daam2-PIP5K pathway contributes to the suppression of remyelination after human WMI.
DISCUSSION
In this study we used dorsal patterning in the embryonic chick spinal cord as a discovery platform to delineate a novel mechanism between Daam2 and PIP5K that regulates canonical Wnt signaling during dorsal patterning of the spinal cord. Given that Wnt signaling also plays a key role in suppressing OLP differentiation, we applied these findings to OL development and discovered that Daam2 similarly suppresses OLP differentiation during developmental and regenerative myelination. Examination of human HIE lesions revealed that Daam2 and PIP5K are expressed in OLP populations, implicating its function in the suppression of remyelination in human WMI. Furthermore, pharmacological inhibition of PIP5K promotes OLP differentiation and stimulates remyelination after WMI. These studies reveal a novel mechanism regulating Wnt receptor complex formation that dictates OLP development and regeneration, and in the process identified a potential therapeutic target for stimulating remyelination after WMI.
New Mechanisms of Wnt Signalosome Formation in the CNS
Mechanisms that regulate clustering of Wnt receptor complex components and subsequent signalosome formation have remained enigmatic. Here we found that Daam2 regulates the clustering of Wnt receptor signalosomes through its association with PIP5K. Wnt signalsome formation by this complex is mediated through PIP2, revealing a novel role for Daam2 in the regulation of PtdIns lipids and at the same time providing critical new insight into the regulation of PIP2 in canonical Wnt signaling (Figure 9). Together these studies identify a novel pathway regulating the construction of Wnt receptor signalsomes that directly influences two key Wnt-dependent processes in the CNS: (i) embryonic patterning of the dorsal spinal cord and (ii) differentiation of OLP populations during developmental and regenerative myelination.
Figure 9. Model for Daam2-PIP5K Function in Wnt Signaling and Remyelination.
Daam2 associates with PIP5K and promotes the clustering of Wnt receptor complexes into signalosomes through PIP2. The net result of signalosome formation is the amplification and maintenance of Wnt signal transduction, which in OLP populations serves to suppress their differentiation into myelinating OLs during development and after WMI. Our pharmacological studies point to the Daam2-PIP5K relationship as a potential therapeutic node that could be used to screen for inhibitors that disrupt Daam2-PIP5K interactions, culminating in decreased levels of Wnt signaling and increased OL generation after WMI. ** PIP2 phosphorylation of LRP6 was demonstrated previously in Xenopus (Pan et al., 2008).
In the course of our studies we found that PIP5K directly interacts with Daam2 and rescues Wnt signaling and PIP2 production in its absence. Deletion mapping of these proteins found that the Daam2-GBD domain is required for association with PIP5K. The nature of the Daam2/PIP5K interaction implicates Rho-GTPases, as GBD domain is essential for these interactions, which also associate with Daam2 (Figure S2). This biochemical relationship, combined with previous studies implicating Rho signaling in the inhibition of PIP5K function, paved the way for our use of Sp-8-pCPT-cAMPs (see below). Besides the potential use of these biochemical relationships as a potential target for WMI, these observations raise several key mechanistic questions that will warrant further investigation, namely: how does the Daam2/PIP5K complex regulate PIP2 production? The trans-location of PIP5K to the membrane is a key regulatory step in the production of PIP2, therefore it is possible that Daam2 facilitates the membrane localization of PIP5K. Interestingly, the Daam2 associates with Rho-GTPases and Dishevelled, both of which have been implicated in the translocation of PIP5K (Halstead et al., 2010; Lee and Deneen, 2012; Pan et al., 2008). Together these observations suggest that Daam2 serves as a trafficking hub for PIP5K that ultimately regulates the levels of PIP2.
Daam2: A Novel Regulator of OLP Differentiation
Our studies on dorsal patterning in the embryonic chick spinal cord directly implicate Daam2 and its associated biology in the canonical Wnt pathway. Given that Wnt signaling plays a central role in the suppression of OLP differentiation during development, we extended our studies on Daam2 to the OL lineage. Our overexpression and mouse knockout studies indicate that Daam2, similar to other positive regulators of the Wnt pathway, suppresses OLP differentiation during development and after WMI. Together, these studies represent the first characterization of Daam2 function in glial lineages during development and after human WMI injury. Furthermore, these studies indicate that proximal regulators of the Wnt receptor complex regulate OLP development, suggesting that modulating levels of Wnt signaling at the receptor level may be a therapeutically tractable approach for WMI (Figure 9) (also see below).
Characterization of Daam2 expression in the OL lineage revealed that it is expressed in both OLPs and mature OL populations. This diverges from the expression patterns of other Wnt pathway components, which are expressed in OLP populations, and downregulated during differentiation into OLs. Many of these other Wnt components are distal effectors of the pathway and subject to strict feedback regulation (Fancy et al., 2011, 2014; Shimizu et al., 2005). Since Daam2 acts proximally, and is not a distal Wnt pathway effector, it is unlikely to be subject to the same strict levels of regulation. Indeed, overexpression of activated β-catenin or Wnt3a does not affect expression of Daam2, indicating that it is regulated in a Wnt-independent manner (data not shown). That Daam2 is expressed in mature OLs raises the question of what, if any, role it might have in these populations. Given that its function is reliant on Wnt ligands and receptor complex formation, it is possible that it functions in a non-canonical Wnt pathway in these cell populations. Indeed, Daam2 regulates cytoskeletal dynamics through Wnt5a and non-canonical Wnt signaling in the developing gut (Welsh et al., 2013). Moreover, Daam1 was originally identified as a regulator of Wnt-dependent planar cell polarity (PCP) during gastrulation and has been linked to actin remodeling (Habas et al., 2001). While the non-canonical Wnt pathway has not been implicated in OL development or function, actin-cytoskeletal dynamics have been implicated in CNS myelination (Kim et al., 2006; Waggener et al., 2013; Wang et al., 2008). Another possibility is that Daam2 functions in OLs through the Akt pathway. Activation of Akt requires the conversion of PIP2 to PIP3, and given the role of Daam2 in PIP2 production, it's possible that it indirectly contributes to Akt-dependent myelination in OL populations (Flores et al., 2008; Narayanan et al., 2009; Norrmén and Suter, 2013).
Analysis of the Daam2 knockout mouse revealed precocious OLP differentiation, which is consistent with reduced Wnt activity. However, examination at later post-natal time points revealed normalized OL numbers between knockout and control animals, indicating that Daam2 is critical for the timing of OLP differentiation. It is likely these effects are due to redundant mechanisms in place to safeguard Wnt receptor complex integrity during this sensitive period of lineage development. For example, excess levels of Dvl can promote clustering and restore canonical Wnt signaling in the absence of Daam2 in the chick (Lee and Deneen, 2012). In the context of an OLP, it is likely that loss of Daam2 either influences the kinetics of Wnt receptor complex formation, or its stability, the net result being a change in the level of Wnt signals that are transduced. Such changes in signaling levels culminate in the accelerated differentiation of OLPs early in development. These observations indicate that Daam2 function is required for the initiation of Wnt signaling and hence is an “early/activating” event in the transduction of the signal and ultimate activation of the pathway in OLPs.
Given these developmental dynamics and the functionally redundant nature of the Wnt receptor complex, it is surprising that manipulation of Daam2 expression has an effect on OLP differentiation after WMI. While it is possible that Daam2 functions in other cell populations present in lesions, our ex vivo studies rule out non-CNS-derived cells, and manipulation of Daam2 did not affect the constituency of reactive astrocytes, suggesting its effects on OLP differentiation after WMI are cell autonomous. Put together, these data suggest that remyelinating OLPs are sensitive to changes in the levels of Wnt signaling and point to components of the Wnt receptor complex as potential therapeutic targets for WMI. This possibility is reinforced by the expression of Daam2 and PIP5K in OLP populations in human HIE. Targeting members of this complex may be advantageous because they function at the cell membrane and are more accessible to pharmacological agents than transcriptional or cytosolic effectors. Therefore a comprehensive understanding of which components of the Wnt receptor complex are expressed in remyelinating OLPs and the nature of their interactions is essential in developing these therapeutic strategies. Furthermore, it is likely that lesions contain a different constituency of Wnt ligands than during development, which will influence signalosome formation. Given the central role of Daam2 in this process, decoding how Wnt ligands present in lesions regulate its signalosome organizing function will further guide the development of these strategies.
New Therapeutic Pathways for WMI
Leveraging insight gained from our studies on dorsal patterning in the embryonic chick spinal cord, we found that pharmacological inhibition of PIP5K activity stimulates OLP differentiation in vitro and provokes accelerated remyelination in animal and ex vivo models of WMI in the adult spinal cord and neonatal brain. These findings point to the Daam2-PIP5K-PIP2 signaling axis as a potential therapeutic target for stimulating myelin regeneration after WMI.
Here we used Sp-8-pCPT-cAMPs as a means to indirectly inhibit PIP5K activity. This compound is a modified analog of cAMP that contains a p-chlorophenylthio moiety, which significantly enhances its lipophilicity (Schwede et al., 2000). This chemical property likely facilitates its entry into cells and contributes, in part, to its ability to stimulate remyelination after injury (Figure 5). That a one-time “pulse” injection of Sp-8-pCPT-cAMPs coincident with lesioning can promote longer-term remyelination is striking. One explanation for these results is that resident OLP populations within the white matter at the time of lesioning are exposed to Sp-8-pCPT-cAMPs. Exposure of these OLPs to the compound (facilitated by its lipophilicity) provokes developmental changes (i.e., inhibition of Daam2/PIP5K and Wnt) that stimulate their differentiation. Therefore, it is possible that the acceleration of remyelination we witness after lysoleci-thin lesioning is the result of exposing these resident OLP populations to this compound at the time of lesioning.
Prior studies on Sp-8-pCPT-cAMPs show that it functions through Rho to inhibit translocation of PIP5K, which culminates in impaired PIP2 production (Yang et al., 2004). These studies, and the nature of the Daam2/PIP5K biochemical interaction and function toward Wnt signaling, provided the rationale for testing Sp-8-pCPT-cAMPs in this context. However, because Sp-8-pCPT-cAMPs is a cAMP analog there are a few caveats that must be considered. First, prior in vitro studies implicated CREB activation in OLP differentiation, though whether these effects are due to direct cAMP-PKA signaling remains poorly defined (Raible and McMorris, 1989; Pende et al., 1997). Nevertheless, it is possible that the effects on OLP differentiation we observe are due in part to direct activation by cAMP signaling. Second, cAMP signaling can influence a wide range of pathways that are also implicated in OLP differentiation (FGF/PDGF, IGF, integrins) suggesting that the effects of Sp-8-pCPT-cAMPs could also be mediated in part through these other pathways. Despite these caveats, we show that treatment of both chick explants and in vitro OLPs with Sp-8-pCPT-cAMPs resulted in decreased PIP2 production and Wnt signaling, indicating that it is functioning in part through these mechanisms in CNS and OLP populations. Moreover, we also show that Sp-8-pCPT-cAMPs blocks Wnt receptor clustering in vitro. Together, our studies, across multiple systems, in conjunction with the observations from Yang et al. support the use of Sp-8-pCPT-cAMPs as an indirect inhibitor of PIP5K and Wnt signaling. These observations in fact highlight potential cross-talk between cAMP-Rho and PIP5K-Daam2 signaling in the regulation of Wnt receptor clustering and are key areas of future basic studies that have critical translational implications for WMI and a host of other Wnt-related diseases.
Similar to other pharmacological agents, this compound likely has other “off-target” effects that will limit its efficacy in a clinical setting. Moreover, because PIP5K-PIP2 and cAMP signaling have wide-ranging effects on cell physiology (van den Bout and Divecha, 2009), both inside and outside the CNS, it may be therapeutically advantageous to develop agents that antagonize Daam2-PIP5K interactions, as this relationship may confer specificity to the Wnt pathway in the CNS and/or OLP populations (Figure 9). Another potential regulatory node to target in this context is the relationship between Rho-GTPases and Daam2-GBD (Figure S1), as this interaction may also specifically link PIP5K translocation and Wnt signaling. Finally, there is the possibility that Sp-8-pCPT-cAMPs functions through astrocytes or neurons to promote remyelination. This possibility seems unlikely, because the effects we witness on myelination after injury are very robust and occur immediately. Moreover, to our knowledge, there is no prior evidence that promoting cAMP signaling in neurons or astrocytes provokes a cell non-autonomous effect after injury that drives robust remyelination in OLs after injury. Therefore, the most parsimonious explanation for our injury results is that the compound is acting on OLP populations.
To our knowledge, there is only one other pharmacological agent that stimulates OLP differentiation after WMI, XAV939, which functions by inhibiting tankyrase activity, resulting in stabilized Axin protein levels and suppression of Wnt signaling (Fancy et al., 2011). Similar to Sp-8-pCPT-cAMPs, XAV939 acts indirectly on the Wnt pathway, and is likely to also influence other signaling mechanisms. One therapeutic strategy to limit potential “off-target” effects of both compounds might be to combine them at sub-threshold levels and determine if they synergistically suppress Wnt activity and stimulate OLP differentiation. Future studies will be directed toward combinatorial approaches in vitro and in animal models to ascertain whether these compounds synergistically stimulate OLP differentiation and remyelination.
In spite of these limitations, our genetic and pharmacological studies, spanning early embryonic development and adult injury, indicate that the Daam2-PIP5K-PIP2 axis represents a key regulatory node for Wnt signaling and an actionable pathway for remyelination after WMI. The development of WMI therapeutics based on this biology will require a deeper understanding of how Daam2 regulates PIP5K activity and PIP2 production and their associations in this context. Combining tractable developmental models to dissect these mechanistic relationships in vivo, with high-throughput drug screening approaches that interfere with these relationships, may be an efficacious, multi-disciplinary approach toward identifying new clinical agents.
EXPERIMENTAL PROCEDURES
Mice
To generate the Daam2 knockout mice, we obtained the Daam2 tm1a (KOMP) targeted ES cells, produced by the KOMP Repository (http://www.komp.org). The Daam2 tm1a (KOMP) mouse line was generated by ES cell microinjection in the ES Cell Core Facility at the Baylor College of Medicine (see Supplemental Experimental Procedures). All procedures were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine and conform to the US Public Health Service Policy on Human Care and Use of Laboratory Animals.
Constructs, siRNAs, and Pharmacological Treatments
Daam2 constructs used in this study have been described previously (Lee and Deneen, 2012). Tagged cDNAs for human PIP5K were cloned into mammalian expression vectors (3×HA-pCS2+, 3×FLAG−pcDNA). The human and mouse PIP5K siRNAs were designed using the siRNA Design Program (Dharmacon) and synthesized by Thermo Scientific. The sequences of all of the siRNAs are listed in Table S1. For pharmacological inhibition of PIP5K we used Sp-8-pCPT-cAMPs (Sigma) (Yang et al., 2004).
In Ovo Electroporation and Chick Spinal Cord Explants Culture
Chick Daam2 and PIP5K shRNAi were cloned into avian retrovirus RCAS vectors. The constructs were electroporated into the neural tube of stage 11 to stage 13 chick embryos as described previously (Lee and Deneen, 2012). For chick spinal cord explant culture, the electroporated chick embryos were harvested and dissected using a micro tungsten needle to trim the spinal cord in ice-cold PBS. Dissected spinal cord was moved into transwell plates and maintained in DMEM/F12 with 0.1% fetal bovine serum (FBS) and N2 supplement for 48 hr.
In Situ Hybridization and Immunohistochemistry
Spinal cords were fixed in 4% paraformaldehyde and then cry-protected in 20% sucrose. To detect effective knockdown of Daam2 and PIP5K in the chick, we generated mRNA probes and performed in situ hybridization (Lee and Deneen, 2012). For chick spinal cord immunohistochemistry, the following antibodies were used: mouse anti-Pax7 (DSHB), anti-Nkx2.2 (DSHB), anti-Myc (Sigma), and rat anti-HA (Sigma). Mouse spinal cord was analyzed using in situ hybridization: MBP, PLP, PDGFRα; and immunohistochemistry: chick anti-beta galactosidase (Abcam 1:1,000), mouse anti-MBP (Covance 1:500), mouse anti-PLP (1:500), rabbit anti-Olig2 (Abcam 1:1,000), rabbit anti-GFAP (Dako 1:1,000), rabbit anti-Iba-1 (Wako 1:1,000).
OLP Culture
Oligosphere cultures were performed as previously described (Fancy et al., 2012; Harrington et al., 2010). For viral infection of OLPs, cells were dissociated and plated on poly-D-lysine-coated coverslips at a density of 1.5 × 104 cells/cm2 in oligodendrocyte precursor media (OPM) subsequently infected with either PIP5K-lenti-cherry or control cherry fluorescent protein virus for 14 hr. For Sp-8-pCPT-cAMPs studies, 100 μM of drug or vehicle control was added immediately after the induction of differentiation.
Induction of Demyelination with Lysolecithin in Mouse Spinal Cord and Viral Misexpression or Inhibitor Treatment in Remyelinating Lesions
Lysolecithin-induced demyelinated lesions were generated in the white matter of the ventrolateral spinal cord of 8- to 10-week-old mice. Mice were harvested 3 days post lesion (dpl), 7 dpl, and 12 dpl, and analyzed. For viral misexpression studies, the animal was injected with lysolecithin in white matter lesion of spinal cord, followed by secondary focal injection of the lesioned region with either a Daam2-containing lentivirus or control lentivirus at 3 dpl. For these viral experiments, mice were perfused at 12 dpl. For pharmacological treatments, 500 mM of Sp-8-pCPT-cAMPs and lysolecithin were injected day 0, and harvested at 3 dpl and 7 dpl to assess the effect on OLP differentiation. EM analysis was performed at 10 dpl as described in Chang et al. (2010). Vehicle control experiments included co-injection of lysolecithin with PBS.
Ex Vivo Cerebellar Slice Culture
Slice culture was performed as described previously (Birgbauer et al., 2004; Fancy et al., 2011). Briefly, the sagittal slices of postnatal day 0–1 mouse cerebellum were cut at 350 μM using a McIlwain tissue chopper, and the slices were plated on Millicell-CM culture inserts (Millipore, 0.4 μm) in slice culture medium (50% MEM with Earle's salts, 35% Earle's balanced salt solution, 25% heat-inactivated horse serum, glutamax, fungizone, penicillin-streptomycin, and glucose) for up to 14–28 days at 37°C.
HI and PB/IUE
HI was performed as described in Scafidi et al. (2014). P3 pups and nursing mothers were placed in 10.5% oxygen for 8 days then returned to normoxia until harvested at P18. IUE was performed as previously described in Chen and LoTurco (2012); Dual PiggyBac system is also described in Chen and LoTurco (2012) and Glasgow et al. (2014). Electroporation was performed at embryonic day 15.
Human HIE Tissue
All human tissue was collected in accordance with guidelines established by the University of California, San Francisco Committee on Human Research (H11170-19113-07). The procurement of tissue and its pathological diagnosis as HIE tissue has been described in detail previously (Fancy et al., 2011).
Supplementary Material
Highlights.
Daam2 regulates Wnt signaling through a direct interaction with PIP5K
Daam2 inhibits OL differentiation during development and after white matter injury
Pharmacological inhibition of PIP5K stimulates remyelination after injury
Daam2 and PIP5K are expressed in OLPs in human white matter injury
ACKNOWLEDGMENTS
We thank Alana Hoffmann, Burak Tepe, and Chih-Chun Hsu for technical assistance; Jason Yustein for the use of his hypoxia culture chamber; Mauro Costa-Mattioli for assistance with the slice culture; and Vittorio Gallo and Ben Arenkiel for manuscript consultation. This work was supported by grants from the National Multiple Sclerosis Society (RG 4623A1/2 to B.D., TA 3054-A-1 to H.K.L.), the Sontag Foundation (B.D.), and the NIH (R01 NS071153 to B.D., R01 NS044916 to M.N.R.). This work was also supported in part by the Baylor College of Medicine IDDRC (HD024064) and by the Integrated Microscopy Core at Baylor College of Medicine, with funding from the NIH (HD007495, DK56338, and CA125123), the Dan L. Duncan Cancer Center, and the John S. Dunn Gulf Coast Consortium for Chemical Genomics.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, one table, and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2015.02.024.
REFERENCES
- An SS, Pennant WA, Ha Y, Oh JS, Kim HJ, Gwak SJ, Yoon DH, Kim KN. Hypoxia-induced expression of VEGF in the organotypic spinal cord slice culture. Neuroreport. 2011;22:55–60. doi: 10.1097/WNR.0b013e3283418b00. [DOI] [PubMed] [Google Scholar]
- Balla T, Várnai P. Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci. STKE. 2002:pl3. doi: 10.1126/stke.2002.125.pl3. 2002. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH, Ligon KL, Volpe JJ, Kinney HC. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008;18:153–163. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgbauer E, Rao TS, Webb M. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J. Neurosci. Res. 2004;78:157–166. doi: 10.1002/jnr.20248. [DOI] [PubMed] [Google Scholar]
- Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Chang KJ, Susuki K, Dours-Zimmermann MT, Zimmermann DR, Rasband MN. Oligodendrocyte myelin glycoprotein does not influence node of ranvier structure or assembly. J. Neurosci. 2010;30:14476–14481. doi: 10.1523/JNEUROSCI.1698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, LoTurco J. A method for stable transgenesis of radial glia lineage in rat neocortex by piggyBac mediated transposition. J. Neurosci. Methods. 2012;207:172–180. doi: 10.1016/j.jneumeth.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 2011;14:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Glasgow SM, Finley M, Rowitch DH, Deneen B. Evidence that nuclear factor IA inhibits repair after white matter injury. Ann. Neurol. 2012;72:224–233. doi: 10.1002/ana.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Baranzini SE, Silbereis JC, Shiow LR, Yuen TJ, Huang EJ, Lomvardas S, Rowitch DH. Parallel states of pathological Wnt signaling in neonatal brain injury and colon cancer. Nat. Neurosci. 2014;17:506–512. doi: 10.1038/nn.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Glasgow SM, Zhu W, Stolt CC, Huang TW, Chen F, LoTurco JJ, Neul JL, Wegner M, Mohila C, Deneen B. Mutual antagonism between Sox10 and NFIA regulates diversification of glial lineages and glioma subtypes. Nat. Neurosci. 2014;17:1322–1329. doi: 10.1038/nn.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A, Olsson H, Batty IH, Priganica L, Peter Downes C. Nonradioactive methods for the assay of phosphoinositide 3-kinases and phosphoinositide phosphatases and selective detection of signaling lipids in cell and tissue extracts. Anal. Biochem. 2003;313:234–245. doi: 10.1016/s0003-2697(02)00607-3. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Halstead JR, Savaskan NE, van den Bout I, Van Horck F, Hajdo-Milasinovic A, Snell M, Keune WJ, Ten Klooster JP, Hordijk PL, Divecha N. Rac controls PIP5K localisation and PtdIns(4,5)P2 synthesis, which modulates vinculin localisation and neurite dynamics. J. Cell Sci. 2010;123:3535–3546. doi: 10.1242/jcs.062679. [DOI] [PubMed] [Google Scholar]
- Hammond TR, Gadea A, Dupree J, Kerninon C, Nait-Oumesmar B, Aguirre A, Gallo V. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron. 2014;81:588–602. doi: 10.1016/j.neuron.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EP, Zhao C, Fancy SP, Kaing S, Franklin RJ, Rowitch DH. Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Ann. Neurol. 2010;68:703–716. doi: 10.1002/ana.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JK, Jarjour AA, Ffrench-Constant C, Franklin RJ. Retinoid X receptors as a potential avenue for regenerative medicine in multiple sclerosis. Expert Rev. Neurother. 2011;11:467–468. doi: 10.1586/ern.11.34. [DOI] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J. Neurosci. 2006;26:5849–5859. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Lee HK, Deneen B. Daam2 is required for dorsal patterning via modulation of canonical Wnt signaling in the developing spinal cord. Dev. Cell. 2012;22:183–196. doi: 10.1016/j.devcel.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J. Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmén C, Suter U. Akt/mTOR signalling in myelination. Biochem. Soc. Trans. 2013;41:944–950. doi: 10.1042/BST20130046. [DOI] [PubMed] [Google Scholar]
- Pan W, Choi SC, Wang H, Qin Y, Volpicelli-Daley L, Swan L, Lucast L, Khoo C, Zhang X, Li L, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008;321:1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Fisher TL, Simpson PB, Russell JT, Blenis J, Gallo V. Neurotransmitter- and growth factor-induced cAMP response element binding protein phosphorylation in glial cell progenitors: role of calcium ions, protein kinase C, and mitogen-activated protein kinase/ribosomal S6 kinase pathway. J. Neurosci. 1997;17:1291–1301. doi: 10.1523/JNEUROSCI.17-04-01291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Li L, Pan W, Wu D. Regulation of phosphatidylinositol kinases and metabolism by Wnt3a and Dvl. J. Biol. Chem. 2009;284:22544–22548. doi: 10.1074/jbc.M109.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DW, McMorris FA. Cyclic AMP regulates the rate of differentiation of oligodendrocytes without changing the lineage commitment of their progenitors. Dev. Biol. 1989;133:437–446. doi: 10.1016/0012-1606(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Scafidi J, Hammond TR, Scafidi S, Ritter J, Jablonska B, Roncal M, Szigeti-Buck K, Coman D, Huang Y, McCarter RJ, Jr., et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014;506:230–234. doi: 10.1038/nature12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- Schwede F, Maronde E, Genieser H, Jastorff B. Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacol. Ther. 2000;87:199–226. doi: 10.1016/s0163-7258(00)00051-6. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kagawa T, Wada T, Muroyama Y, Takada S, Ikenaka K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev. Biol. 2005;282:397–410. doi: 10.1016/j.ydbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Tawk M, Makoukji J, Belle M, Fonte C, Trousson A, Hawkins T, Li H, Ghandour S, Schumacher M, Massaad C. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J. Neurosci. 2011;31:3729–3742. doi: 10.1523/JNEUROSCI.4270-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bout I, Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J. Cell Sci. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- Waggener CT, Dupree JL, Elgersma Y, Fuss B. CaMKIIbeta regulates oligodendrocyte maturation and CNS myelination. J. Neurosci. 2013;33:10453–10458. doi: 10.1523/JNEUROSCI.5875-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tewari A, Einheber S, Salzer JL, Melendez-Vasquez CV. Myosin II has distinct functions in PNS and CNS myelin sheath formation. J. Cell Biol. 2008;182:1171–1184. doi: 10.1083/jcb.200802091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh IC, Thomsen M, Gludish DW, Alfonso-Parra C, Bai Y, Martin JF, Kurpios NA. Integration of left-right Pitx2 transcription and Wnt signaling drives asymmetric gut morphogenesis via Daam2. Dev. Cell. 2013;26:629–644. doi: 10.1016/j.devcel.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- Yang SA, Carpenter CL, Abrams CS. Rho and Rho-kinase mediate thrombin-induced phosphatidylinositol 4-phosphate 5-kinase trafficking in platelets. J. Biol. Chem. 2004;279:42331–42336. doi: 10.1074/jbc.M404335200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.