SUMMARY
Recurrent outbreaks of muscular sarcocystosis among tourists visiting islands in Malaysia have focused international attention on sarcocystosis, a disease once considered rare in humans. Sarcocystis species require two hosts, definitive and intermediate, to complete their life cycle. Humans can serve as definitive hosts, with intestinal sarcocystosis for two species acquired from eating undercooked meat: Sarcocystis hominis, from beef, and Sarcocystis suihominis, from pork. Symptoms such as nausea, stomachache, and diarrhea vary widely depending on the number of cysts ingested but appear more severe with pork than with beef. Humans serve as intermediate hosts for Sarcocystis nesbitti, a species with a reptilian definitive host, and possibly other unidentified species, acquired by ingesting sporocysts from feces-contaminated food or water and the environment; infections have an early phase of development in vascular endothelium, with illness that is difficult to diagnose; clinical signs include fever, headache, and myalgia. Subsequent development of intramuscular cysts is characterized by myositis. Presumptive diagnosis based on travel history to tropical regions, elevated serum enzyme levels, and eosinophilia is confirmed by finding sarcocysts in muscle biopsy specimens. There is no vaccine or confirmed effective antiparasitic drug for muscular sarcocystosis, but anti-inflammatory drugs may reduce symptoms. Prevention strategies are also discussed.
INTRODUCTION
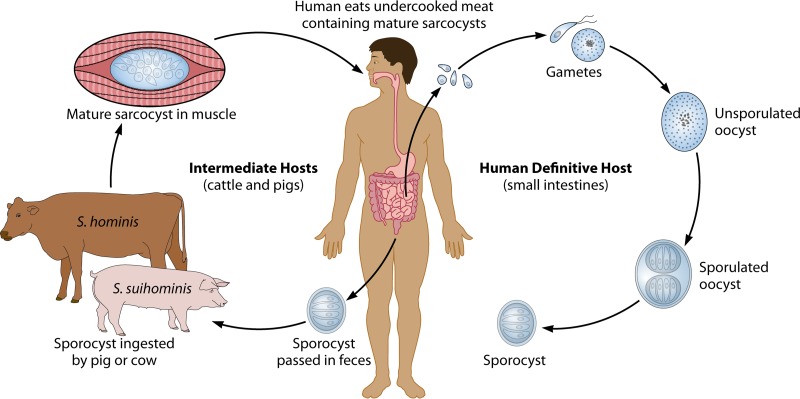
Sarcocystis is classified in the phylum Apicomplexa, along with species of Eimeria that cause coccidiosis in poultry and livestock; Toxoplasma gondii, which infects virtually all warm-blooded vertebrates; and species of Cystoisospora that infect humans and a variety of animals. Sarcocystis species are ubiquitous in nature and are found worldwide. Two hosts are required to maintain the life cycle: an intermediate or prey host, in which cysts (sarcocysts) containing infectious zoites infect the muscles, and a definitive, final, or predator host that ingests the cysts, becomes infected with intestinal-stage parasites, and excretes oocysts or sporocysts into the environment. For the >150 species of Sarcocystis, most intermediate hosts include herbivorous mammals and humans and other primates but also some birds, reptiles, and possibly fish. Definitive hosts include carnivores or omnivores, including humans and some reptiles and raptorial birds (1). Although others may exist, only Sarcocystis nesbitti has been identified in humans and nonhuman primates serving as intermediate hosts, with a snake possibly serving as the definitive host. However, this identification is based on a comparison of available congeners that most closely matched those of species in which snakes were the final hosts and has yet to be confirmed. Two species, Sarcocystis hominis and S. suihominis, have been identified in humans and nonhuman primates serving as definitive hosts (Fig. 1).
FIG 1.
Humans as definitive (final) hosts for Sarcocystis species.
Early knowledge of infections in humans is scant because the relationship between infection and symptoms was not understood, tissue specimens were rare, light microscopy (LM) had limitations, the life cycle was unknown, and other diagnostic tools were not developed. The first record of what would become recognized as Sarcocystis was in 1843 by Meischer, who observed long, thin, white cysts in muscles of a deer mouse in Switzerland (1). For the following 2 decades, this parasite with no scientific name was called Meischer's tubules. In 1865, a parasite with a similar appearance in muscles of a pig was then described by Kuhn, who proposed the name Synchytrium miescherianum (1). However, the name Synchytrium was already in use for another organism. Thus, in 1899, Labbe changed the name to Sarcocystis meischeriana (1), and it became the type species of the genus. In the following decades, many species of Sarcocystis were named based on finding intramuscular cysts in various animals, and these were referred to as sarcocysts. In some references to Sarcocystis, the term sarcosporidium was used. This possibly resulted from studies in which sarcocysts in culture media appeared to develop hyphae and mycelia resulting from contamination, and Sarcocystis therefore was thought to be a type of fungal organism. In 1967, electron micrographs clearly demonstrated that the organisms contained within the sarcocysts were not fungal spores but were zoites morphologically similar to those of other apicomplexan protozoa (2), removing any consideration that Sarcocystis was taxonomically related to fungi. Other life cycle stages remained unknown until the 1970s, when zoites freed from sarcocysts in the muscles of grackles (Quiscalus quiscula) developed into sexual-stage parasites and oocysts in cultured mammalian cells (3, 4). Additionally, sarcocysts from cattle were fed to cats, dogs, and humans, establishing three distinct species, with the proposed names Sarcocystis bovifelis, S. bovicanis, and S. bovihominis, combinations representing two hosts (5–7). Although these species names were later changed, the cumulative findings from this period established the requisite relationship of the two-host, prey-predator (intermediate host-definitive host) life cycle for all species of Sarcocystis.
Life Cycle Stages
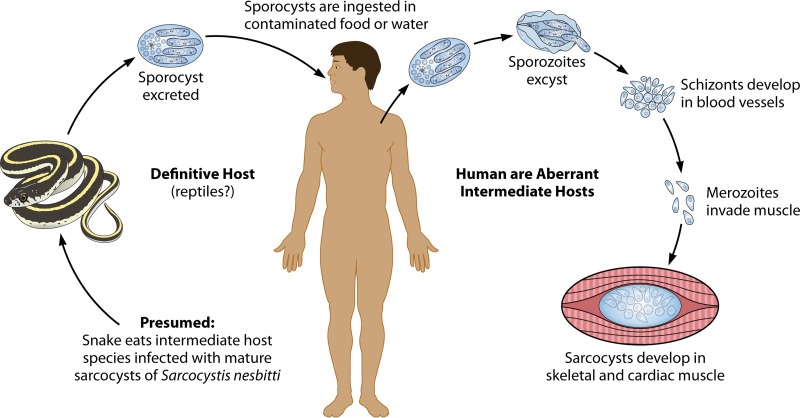
In intermediate hosts, including humans, only asexual-stage parasites are found (Fig. 2). The initial stages of asexual development are known from animal studies but have not been seen in human tissues. The following descriptions are based on Sarcocystis cruzi development in cattle. Infection begins when oocysts or sporocysts in feces from a final host become ingested by a susceptible intermediate host. Exposure to trypsin and bile causes the plates that form the sporocyst wall to disunite, liberating four motile sporozoites contained within. The sporozoites pass into or through the gut wall and are first found within endothelial cells that line small arteries in all parts of the body. This is the first of approximately four cycles of asexual development, called merogony or schizogony, the number and timing of which may vary with the species. During each of the first three cycles, nuclear division eventually gives rise to merozoites, which are motile, crescent-shaped organisms with a structure similar to that of sporozoites. Subsequent generations are found downstream, in arterioles, and then in capillaries and in veins in all parts of the body until the last generation develops in skeletal, smooth, and cardiac muscles, and sometimes in neural tissue, where sarcocysts are formed.
FIG 2.
Humans as aberrant intermediate hosts for Sarcocystis species.
For S. cruzi, the first generation is found ∼2 weeks after ingestion of sporocysts (Fig. 3A), and the second generation is found as singles or pairs of merozoites within mononuclear cells in peripheral blood nearly 4 weeks after ingestion of sporocysts (Fig. 3C). A few days to a week later, the third generation is seen as immature multinucleate schizonts or mature schizonts containing merozoites in endothelial cells of capillaries throughout the body, but they are especially prominent in renal glomeruli (Fig. 3B). Merozoites from these schizonts enter muscle cells, where they begin sarcocyst formation, first differentiating into a single round cell, called a metrocyte or mother cell. A series of divisions gives rise to numerous metrocytes as the sarcocyst grows while, concurrently, a wall develops, isolating the sarcocyst from the surrounding muscle (Fig. 3D). At the time when metrocytes develop into infectious bradyzoites, also referred to as cystozoites or just zoites, the sarcocyst is considered mature (Fig. 3E and F). The maturation time appears to differ among species and can take 2 months or more to complete, but the sarcocyst can then persist for months or years. Depending on the species, sarcocysts differ in size and shape from microscopic to macroscopic. They range from a few micrometers to several millimeters in length, range from narrow to wide in circumference, and have a great variety of wall structures that differ in thickness and in patterns of peripheral protrusions called cytophaneres. Seven morphologically unique wall structures were described in early reports of sarcocysts found in human muscles (8) (Table 1), but most subsequent reports of intramuscular sarcocystosis in humans did not identify wall morphology (9–31) (Table 2). Some sarcocysts have internal septa that form compartments, while in others, no septa are apparent. The septa and cytophaneres may be difficult to distinguish by light microscopy and are best seen by electron microscopy. Sarcocysts can be found in the muscles of limbs, tongue, esophagus, diaphragm, and heart but also in neural tissue in the brain, spinal cord, and Purkinje fibers.
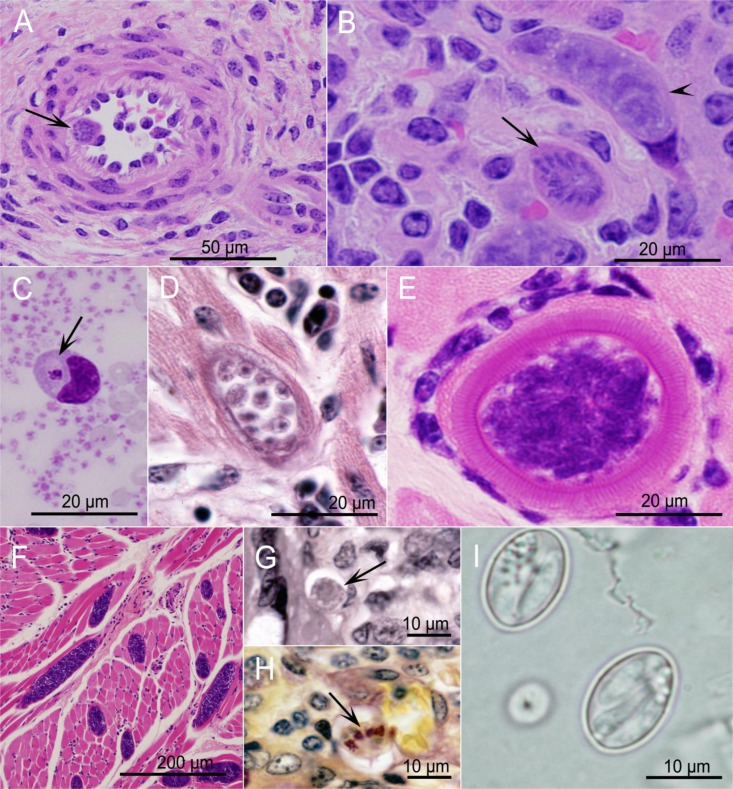
FIG 3.
Sarcocystis stages in tissues of intermediate hosts (A to F) and definitive hosts (G to I). Panels A to G show hematoxylin-stained images. All images are of S. cruzi, except panel E, which is an image of S. hominis. (A) Artery with a first-generation multinucleate schizont (arrow) in an endothelial cell. (B) Kidney glomerulus with immature (arrowhead) and mature (arrow) second-generation schizonts. (C) Blood smear with a merozoite in a mononuclear cell. (D) Heart with an immature sarcocyst containing globular metrocytes. (E) Skeletal muscle with a cross section of a mature sarcocyst with a thick striated wall surrounded by a mononuclear cell infiltrate. (F) Skeletal muscle with longitudinal and cross sections of sarcocysts. The was no inflammatory response. (G) Lamina propria of small intestine with a macrogametocyte (arrow). (H) Small intestine with sporulated sporocysts (arrow) (Whipf's polychrome stain). (I) Phase-contrast microscopy of two sporocysts in a fecal float.
TABLE 1.
Summarization of early reports of humans with sarcocystsc
| Country or region | Patient age (yr), gender | Sarcocyst location(s) | Biopsy or autopsy | Sarcocyst typeb | Original author(s), yra |
|---|---|---|---|---|---|
| Sudan | 36, M | Abdomen | A | 2 | Kartuli, 1893 |
| France | Adult, M | Larynx | A | 1 | Barbaran and St. Remy, 1894 |
| France | Adult, unknown | Skeletal muscle | A | 1 | Vuillemin, 1902 |
| Malaysia | 30, M | Tongue | A | 4 | Darling, 1919 |
| UK | Unknown | Heart | A | 6 | Manifold, 1924 |
| India | 55, M | Chest | B | 2 | Vasudevan, 1927 |
| USA via West Indies | 32, F | Heart | A | 7 | Lambert, 1927 |
| Indonesia | 20, M | Cheek | B | 4 | Bonne and Soewandi, 1929 |
| China | Adult, M | Leg | B | 1 | Feng, 1932 |
| UK | Adult, F | Heart | A | 5 | Hewett, 1933 |
| Panama | 11, F | Heart | A | 7 | Gilmore et al., 1942 |
| Panama | 48, F | Heart | A | 7 | Kean and Grocott, 1943 |
| Brazil | 32, F | Heart | A | 7 | DeFreitas, 1946 |
| USA via Germany | 31, M | Heart | A | 7 | Arai, 1949 |
| USA via Puerto Rico | 34, M | Heart | A | 7 | Arai, 1949 |
| India | 37, M | Leg | B | 2 | Dastur and Iyer, 1955 |
| India | 20, M | Leg | B | Dastur and Iyer, 1955 | |
| Sudan | 45, M | Foot | B | 3 | McKinnon and Abbott, 1955 |
| UK via India | 60, M | Pectoral | B | 2 | McGill and Goodbody, 1957 |
| Brazil | 40, F | Heart | A | 6 | Koberle, 1958 |
| Italy | 17, F | Heart | A | 5 | D'Arrigo and Squillaci, 1962 |
| Indonesia | 51, M | Pectoral | B | 4 | Van Thiel and Van den Berg, 1964 |
| UK via Southeast Asia | 51, M | Leg | B | 4 | Mandour, 1965 |
| Angola | 30, F | Chest | B | 2 | Liu and Roberts, 1965 |
| India | 22, F | Leg, arm | B | 2 | Gupta et al., 1973 |
| Southeast Asia | 21, M | Leg | B | 3 | Jeffrey, 1974 |
| UK via Malaysia | 34, M | Pectoral | B | 4 | Jeffrey, 1974 |
| Malaysia | 34, M | Larynx | B | 4 | Kutty and Dissanaike, 1975 |
| Malaysia | 12, F | Pharynx | A | 4 | Kutty et al., 1975 |
| India | 56, M | Arm | B | 3 | Agarwal et al., 1976 |
| India | 54, F | Leg | B | 3 | Thomas, 1976 |
| Malaysia | 20, M | Foot | B | 4 | Prathap and Dissanaike, 1976 |
| Malaysia | 23, M | Neck | A | 4 | Prathap and Dissanaike, 1978 |
| Unknown | Unknown | Skeletal muscle | A | 4 | Frenkel, 1976 |
| USA via Asia | 40, M | Arm | B | 4 | McLeod et al., 1979 |
| Uganda | 50, M | Leg | B | 1 | Beaver, 1979 |
| India | 50, M | Arm | B | 3 | Beaver, 1979 |
| Singapore | Unknown | Skeletal muscle | B | 4 | Beaver, 1979 |
| Singapore | Unknown | Skeletal muscle | B | 4 | Beaver, 1979 |
| Costa Rica | 9, M | Heart | A | 6 | Beaver, 1979 |
Original authors and years can be found in reference 8, and not all of these are included in the list of references in this study.
These types, described by Beaver et al. (8), differ from those described by Dubey et al. (1) and are defined as follows: type 1, thick, radially striated wall, large zoites often sparse in the center, and metrocytes; type 2, generally large sarcocysts, thin and smooth wall, medium zoites, and septa; type 3, small and medium sarcocysts, thin wall, medium zoites, and septa; type 4, long and medium sarcocysts, thin wall, small zoites, and septa evident in center; type 5, small sarcocysts, thin wall, medium zoites, and in myocardium; type 6, small to medium sarcocysts, thin wall, large zoites, and in myocardium; type 7, small sarcocysts, thin wall, small zoites, and in myocardium.
Data were compiled from reference 8. Abbreviations: A, autopsy; B, biopsy; F, female; M, male.
TABLE 2.
Summary of reports of muscular sarcocystosis in humans not included in the review by Beaver et al. and presented in chronological order of publication
| Country or region | Description of human subject(s) or specimen(s) | Sarcocyst location | Biopsy or autopsyc | Reference, yr of publication |
|---|---|---|---|---|
| Malaysia | 58-yr-old Ma | Tongue | B | 9, 1981 |
| India | 2 persons | Leg | B | 10, 1983 |
| Gluteus | B | |||
| Denmark? | 4 of 112 specimensb | Muscle | A? | 11, 1985 |
| Malaysia | 45-yr-old Ma | Nasopharynx | B | 12, 1987 |
| 53-yr-old Ma | Nasopharynx | B | ||
| Malaysia | 1 person | Skeletal muscle | B | 13, 1988 |
| Australia via Thailand | 31-yr-old M | Skeletal muscle | B | 14, 1990 |
| Egypt | 1 person | Skeletal muscle | B | 15, 1990 |
| Malaysia | 45-yr-old Ma | Tongue | B | 16, 1992 |
| 67-yr-old Fa | Tongue | B | ||
| 32-yr-old Fa | Pectoral muscle | B | ||
| Malaysia | 21 persons aged 16 to 57 yr | Tongue | A | 17, 1992 |
| Belgium via Brazil, Kenya, and Tanzania | 31-yr-old M | Thigh | B | 18, 1995 |
| India | 40-yr-old M | Thigh | B | 19, 1996 |
| 14-yr-old F | Arm | B | ||
| 52-yr-old F | Thigh | B | ||
| 23-yr-old F | Arm | B | ||
| Malaysia | 44-yr-old Fa | Thigh | B | 20, 1998 |
| 19-yr-old F | Calf | B | ||
| Malaysia | 7 adults, M | 1 muscle | B | 21, 1999 |
| Thailand | 66-yr-old Ma | Larynx | B | 22, 2011 |
| India | 20-yr-old M | Arm | B | 23, 2012 |
| India | 50-yr-old M | Neck | B | |
| India | 47-yr-old M | Leg | B | 24, 2013 |
| Pangkor Island, Malaysia | 89 people | Leg | B | 25, 2013 |
| 26, 2014 | ||||
| 27, 2014 | ||||
| Tioman Island, Malaysia | 39 F and 29 M subjects, aged 4 to 72 yr | Skeletal muscle from 15 patients; 1 PCR positive | B | 28, 2014 |
| 29, 2014 | ||||
| Tioman Island, Malaysia | 3 F and 3 M subjects | Febrile myositis syndrome | NTE | 30, 2014 |
| Tioman Island, Malaysia | 26 cases in Germany reported previously | Symptomatic changes; skeletal muscle | B | 31, 2014 |
Cancer patients.
Tissues examined by trichinoscopy.
NTE, no tissue examined.
Sexual stages occur in definitive hosts. After a susceptible host has eaten meat containing mature sarcocysts, the wall of the sarcocyst becomes digested or broken. Bradyzoites within the sarcocysts are released and can soon be found intracellularly in villi of the small intestine. Each bradyzoite transforms into either a microgamont (male) or a macrogamont (female) stage (Fig. 3G). Microgametocytes become multinucleate, and a sperm-like microgamete forms around each nucleus. A single flagellated microgamete finds and fuses with a macrogamont. Their nuclei combine, and the fertilized macrogamont develops into an oocyst that sporulates in situ, forming two sporocysts that each contain four sporozoites (Fig. 3H). Although oocysts are immobile, they reach the lumen of the intestine and are excreted with feces, sometimes intact with a barely visible wall, appearing as a pair of sporocysts, or more often, the fragile wall breaks, and individual sporocysts are released. Sporocysts of virtually all species are indistinguishable from one another, measuring ∼10 by 15 μm and containing four sporozoites and a cluster of residual granules (Fig. 3I). Sporocysts of S. hominis and S. suihominis have average sizes of 9.3 by 14.7 and 10.5 by 13.5 μm, respectively, and are immediately infectious when excreted (1).
Species Infecting Animals
Some species of Sarcocystis that infect agricultural and companion animals, such as cattle, sheep, and horses, are of economic importance because they cause illness that results in fever, lethargy, poor growth, poor feed use, reduced milk production, lameness, wool and hair loss, abortion, carcass condemnation at meat inspection, as well as death. Information obtained from such infections has been helpful in understanding aspects of clinical disease in humans. For example, data on hematology, serum enzyme level changes, the inflammatory response, histopathology, the location and timing of developmental stages, the febrile response, the negative impact on growth, abortion, and other factors have been well documented from experimental and outbreak studies of sarcocystosis in livestock and have been reviewed (1, 32).
Species Infecting Humans
Humans can be either final or intermediate hosts (Fig. 1 and 2). Humans can become final hosts after eating undercooked pork and beef harboring mature sarcocysts of S. suihominis and S. hominis, respectively. Tissues of many species of domesticated animals, wild mammals, birds, and reptiles that are eaten for meat throughout the world contain sarcocysts capable of infecting unidentified final hosts, possibly including humans and with unknown health consequences. Therefore, there may be additional undocumented species for which humans can serve as a definitive host. Although sporocysts in the feces are diagnostic for Sarcocystis infections of definitive hosts, they are so morphologically similar that species cannot be differentiated simply by the size and shape of sporocysts.
Within the Sarcocystis life cycle, humans who are infected with muscular sarcocystosis are considered aberrant intermediate hosts because they accidently substitute for the natural hosts that routinely serve as prey for a definitive predator host. The number of species for which humans can serve as an intermediate host is unknown, but there may be seven or more species based on differences in sarcocyst wall morphology observed in human tissue specimens (8). Sarcocyst wall morphology has also been used to differentiate species in animals (1). The use of sarcocyst wall morphology to distinguish species has been controversial because morphology can be difficult to discern by light microscopy, can be affected during the processing of tissues, and can change with the age of the sarcocysts. Molecular methods have been used but have been extremely limited. Greater use will extend and confirm species identity and improve diagnosis of infections.
Humans have been identified as an intermediate host for Sarcocystis nesbitti (26–28) based on 18S ribosomal DNA (rDNA) sequence data. Sarcocystis nesbitti, described by Mandour in 1969, was originally detected in muscles from a rhesus monkey based on LM, but its taxonomic validity is questionable, and there are neither transmission electron microscopy (TEM) morphological data nor LM photographic support for the original description. Nevertheless, the first report of Sarcocystis in nonhuman primates (Macaca fascicularis) in China, which was supported by LM and TEM studies (33), was based on the perceived resemblance to S. nesbitti, so those authors named the organisms that they found in M. fascicularis muscles S. nesbitti. Morphological similarities suggested that one species of Sarcocystis might infect Macaca fascicularis, Macaca mulatta, Papio papionis, Cercocebus atys, as well as humans (34). Sarcocysts in muscles from M. fascicularis identified as S. nesbitti by Yang et al. (33) were later examined by molecular methods (34), and 18S rDNA sequence data from two sarcocysts placed this species in a cluster with S. atheridis and S. singaporensis, species with rodent intermediate hosts and snake definitive hosts. This suggested that a final host for S. nesbitti might be a snake. PCR evidence of Sarcocystis was found in feces from a cobra (26); the 18S rDNA sequence clustered with Sarcocystis sequences from other snakes and with the two S. nesbitti sequences obtained from M. fascicularis by Tian et al. (34). Three reports from the Pangkor Island outbreak in Malaysia reported similar findings. Muscle biopsy specimens from the swollen jaw of one patient and the leg of another showed intramuscular sarcocysts identified by LM and TEM (26). The 18S rDNA sequences of Sarcocystis from these patients varied 1% from each other, and a BLAST analysis found that they shared 99% homology with S. nesbitti from the muscle of M. fascicularis (26). Another report also identified a patient with sarcocysts in the temporalis muscle and another with sarcocysts in a leg muscle, for which DNA sequences matched 100% of those for S. nesbitti in the clade with S. atheridis (25). Still another study (27) reported the same 18S rDNA findings as those reported by AbuBakar et al. (25) and Tian et al. (34). The 18S rDNA sequence from the muscle biopsy specimen of a patient who had visited Tioman Island in Malaysia also showed 100% homology with the S. nesbitti gene sequence reported under GenBank accession number HF544323 (28).
Sources of Intestinal and Muscular Sarcocystosis
The only source of intestinal sarcocystosis is ingestion of meat containing mature sarcocysts. The range of meats and meat products consumed by humans worldwide includes domestic and wild animals of many species. Although only two species are known to infect humans as definitive hosts, S. hominis from beef and S. suihominis from pork, many others may exist throughout the world but are as yet undefined.
The source of muscular sarcocystosis is ingestion of sporocysts, most likely through contaminated water or fresh produce or possibly through exposure to a contaminated environment. It is difficult to predict for unnamed species of Sarcocystis how specific either the intermediate or the final host species must be, but there are some generalized patterns. Dogs, coyotes, and foxes, but not humans or cats, are final hosts for S. cruzi, with intermediate hosts being restricted to cattle and bison, but not sheep, monkeys, pigs, or rats. Intermediate hosts of S. odocoileocanis include white-tailed deer, sheep, and cattle. There are other examples in which clusters of related host species serve as either intermediate or final hosts. Based on the likelihood that one species of Sarcocystis can infect multiple closely related host species, some species of Sarcocystis that infect nonhuman primates might also be expected to be able to infect humans. This is true for S. nesbitti, a species found in muscles of macaques and recently in humans; snakes and possibly other reptiles may serve as definitive hosts (33, 34). Because most cases of human muscular sarcocystosis have been found in tropical areas inhabited by many species of nonhuman primates and reptiles, and because some locations could be subject to contamination from reptilian feces, the combination of these factors appears conducive to human infection.
PREVALENCE, SYMPTOMS, AND DIAGNOSIS OF INFECTION IN HUMANS
Intestinal Sarcocystosis Species and Symptoms
Humans with intestinal sarcocystosis (Fig. 1) have been identified worldwide, with the exception of Africa and the Middle East, although such infections likely occur there given customs of eating raw or undercooked meat in those areas. Locations where cases of intestinal sarcocystosis have been reported are listed in Table 3, including infections that resulted after volunteers ingested naturally infected or experimentally infected meat (6, 7, 35–68). Early investigators, with no knowledge of the Sarcocystis life cycle, observed two parasites with characteristics of apicomplexan oocysts in stool samples of infected persons and named one Isospora belli, which was excreted as a distinctive unsporulated (internally undifferentiated) oocyst, unique in size and shape and unlike any other apicomplexan parasite. The other coccidian parasite, excreted as a sporulated oocyst (containing sporocysts with sporozoites) or as individual sporocysts, was named Isospora hominis (35, 37, 39, 44) and represented what is now recognized as multiple species of Sarcocystis, two of which are known to be S. hominis (6, 40, 41, 46–48, 51, 53, 54, 58, 59) and S. suihominis (6, 40, 50, 53, 62). However, other species may have been present because the oocysts and sporocysts of Sarcocystis species are morphologically indistinguishable. Most reports cite infections in persons in European countries, including The Netherlands, Germany, Poland, Slovakia, France, and Spain (Table 3). In Asia, infections have been reported in China, Tibet, Laos, and Thailand (Table 3). Other cases have been reported in Australia, Argentina, and Brazil (Table 3).
TABLE 3.
Reports of naturally acquired intestinal sarcocystosis in humans and attempted experimental infectionsb
| Country | Description of human subject(s) and/or samples | Natural or experimental infection | Species | Source of sarcocysts | Clinical sign(s) | Reference |
|---|---|---|---|---|---|---|
| Netherlands | 12 of 72 persons | N | I. hominisa | NI | NI | 35 |
| 5 of 17 autopsy specimens | ||||||
| Germany | 2 volunteers | E | S. hominis | Beef | 1 of 6 persons had severe influenza-like symptoms and mild diarrhea | 6 |
| 4 volunteers | S. suihominis | Pork | ||||
| Poland | 29-yr-old F | N | Sarcocystis sp. | NI | Symptoms very scant | 36 |
| 7 subjects (assumed to be children) | Sarcocystis sp. | |||||
| 92 unidentified subjects | Sarcocystis sp. | |||||
| Poland | 200 persons | N | I. hominisa | NI | NI | 37 |
| Germany | 1 volunteer | E | S. tenella | Sheep | None; no infection resulted from ingestion of macroscopic cysts from sheep | 7 |
| Germany | 12 of 150 persons | N | Sarcocystis sp. | Beef and pork | 6 persons had gastric and intestinal problems | 38 |
| Germany | 22 of 300 persons | N | I. hominisa | NI | 10 of 22 persons had gastric and intestinal problems; 12 persons had no gastrointestinal signs | 39 |
| Germany | 1 volunteer | E | S. hominis | Beef | Diarrhea and stomachache | 40 |
| 3 volunteers | S. suihominis | Pork | Bloating, inappetence, nausea, vomiting, diarrhea | |||
| Netherlands | 1 child | N | S. hominis | NI | Natural infection transmitted to calf | 41 |
| 3 volunteers | E | S. hominis | Beef | NI | ||
| Germany | 8 of 506 persons | N | NI | NI | Intermittent enteritis with diarrhea or rheumatic phenomena or asymptomatic | 42 |
| Poland | 13 of 125 children 7 to 18 yr old | N | NI | NI | NI | 43 |
| Slovakia | 12-yr-old F | N | I. hominisa | NI | Asymptomatic | 44 |
| United States | 2 persons | S. cruzi | Beef | Not infectious, asymptomatic | 45 | |
| Netherlands | 5 persons | N | S. hominis | Beef | Natural infection | 46 |
| 1 person | E | S. hominis | Beef | Experimentally infected with 15,000 sarcocysts | ||
| France via tropical areas | 2% of 3,500 samples | N | S. hominis | NI | 47 | |
| Thailand | 30-yr-old M | N | S. hominis | Beef | All 6 subjects had acute fever and acute abdominal pain, and 5 had leukocytosis; bacterial infection may have contributed to severity of illnesses | 48 |
| 3-yr-old F | N | S. hominis | Beef | |||
| 60-yr-old F | N | S. hominis | Beef | |||
| 9-yr-old M | N | S. hominis | Beef | |||
| 19-yr-old M | N | S. hominis | Beef | |||
| 70-yr-old M | N | S. hominis | Beef | |||
| China | 48-yr-old M | N | Sarcocystis sp. | Pork | Abdominal distension, pain, diarrhea, constipation, stomachache, dyspnea | 49 |
| Adult M | N | Sarcocystis sp. | Unknown | No information | ||
| China (Yunnan Province) | Adult M (naturally infected) | N | S. suihominis-like sporocysts | Pork | Asymptomatic human who ate pork excreted sporocysts infectious for pigs; sarcocysts in pig muscle did not infect monkeys | 50 |
| 4 monkeys | E | |||||
| China | 1 volunteer | E | S. hominis | Beef | NI | 51 |
| 2 monkeys | E | |||||
| Slovakia via North Vietnam | 14 of 1,228 workers | N | Sarcocystis sp. | Unknown | None | 52 |
| Tibet | 20.5%–22.9% of 926 persons | N | S. hominis | Beef | Asymptomatic | 53 |
| 0.6%–7% of 926 persons | S. suihominis | Pork | Asymptomatic | |||
| Laos | >10% of 1,008 persons | N | S. hominis | NI | NI | 54 |
| Thailand | 23.2% of 362 persons | N | Sarcocystis sp. | Possibly beef or pork | Asymptomatic | 55 |
| Australia | 2 of 385 persons | N | Sarcocystis sp. | NI | NI | 56 |
| China | 3 volunteers | E | Sarcocystis sp. | Cattle | All had clinical symptoms including abdominal pain, distension, watery diarrhea, and eosinophilia | 57 |
| 2 volunteers | E | Sarcocystis sp. | Water buffalo | |||
| Brazil | 6 of 7 volunteers | E | S. hominis | Beef kibbe | Diarrhea | 58 |
| Spain | 35-yr-old M | N | Sarcocystis sp. | Beef | Abdominal discomfort, loose stools | 59 |
| China (Guangxi) | 27 of 501 persons | N | S. suihominis suspected | Pork (raw) | 8 had diarrhea and abdominal pain; 1 had only abdominal pain | 60 |
| China (Guangxi) | 32 of 489 persons | N | S. suihominis suspected | Pork (raw) | NI | 61 |
| China | 1 volunteer | E | S. suihominis | Pork | Distension, diarrhea, fever, pain | 62 |
| Thailand | ||||||
| Ubon Ratchathani | 4.6% of 479 specimens | N | Sarcocystis sp. | NI | NI | 63 |
| Khon Kaen | 8% of 1,124 specimens | N | Sarcocystis sp. | NI | NI | |
| Argentina | 31-yr-old HIV patient | N | Sarcocystis sp. | NI | Diarrhea | 64 |
| China | 2 volunteers | E | S. sinensis | Buffalo | Became ill but did not excrete sporocysts | 65 |
| Northeast Thailand (Khon Kaen) | 1 of 253 persons | N | Sarcocystis sp. | NI | NI | 66 |
| Jordan | 19-yr-old M | N | S. hominis | Beef shawarma | Abdominal pain, diarrhea, nausea, vomiting, intermittent fever | 67 |
| Malaysia | 1 of 269 persons | N | Sarcocystis sp. | NI | NI | 68 |
| 20-yr-old F |
Isospora hominis was an early name used to identify sporocysts in feces and stages in lamina propria before Sarcocystis species were known.
NI, not indicated; F, female; M, male; N, natural; E, experimental.
As definitive hosts, humans can experience nausea, vomiting, acute and severe enteritis, or chronic enteritis, but many infections appear to be mild or asymptomatic. Differences depend on the number, and perhaps the species, of sarcocysts ingested. Few accurate data are available on the duration of infection or the numbers of oocysts and sporocysts excreted. Most case studies suffer from not knowing the time of onset of infection, the type or quantity of meat consumed, the species and number of sarcocysts consumed, and whether a patient ingested raw meat once or multiple times. The longest period of continuous sporocyst excretion (I. hominis) was 21 months or more for a patient in The Netherlands, while other patients excreted sporocysts for at least 6 months (35). A patient in Poland excreted sporocysts for at least 12 months (36). However, the most reliable information on the prepatent and patent periods is from human volunteer studies. In Germany, diaphragms from cattle and pigs were obtained from an abattoir, ground in a meat grinder, and found to contain zoites of Sarcocystis. This ground meat was then fed to volunteers who were not excreting oocysts or sporocysts in their stools (6). In the first experiment, two volunteers ate 500 g of raw beef diaphragm with onions and spices and began excreting sporocysts 9 days later, continuing for 40 days or longer. In the second experiment, four volunteers ate seasoned raw pork diaphragm and began excreting sporocysts 9, 13, and 17 days later; the fourth person remained uninfected. The patent period for the three volunteers was at least 30 days. For a volunteer in China who ingested S. suihominis-infected pork, the prepatent period was12 days, and the patent period was >120 days (50). Another volunteer excreted sporocysts from days 8 to 40 after eating beef (51). Two other volunteers in China who ate beef and three who ate water buffalo had prepatent periods of 10 to 12 days and patent periods of 11 to 29 days (57). Another volunteer in China excreted sporocysts of S. suihominis for an estimated 91 days beginning 10 days after eating pork (62). Of seven volunteers in Brazil who ate raw kibbe (beef), six began to excrete S. hominis oocysts/sporocysts 10 to 14 days later and excreted them for 5 to 12 days (58).
Intestinal Sarcocystosis in Europe
In The Netherlands, oocysts and sporocysts were identified as I. hominis in stool samples of 17 of 72 persons, some without illness and others suspected of suffering from chronic amoebiasis; additionally, in 5 of 17 subjects, intestinal mucosa scrapings taken at autopsy contained sporocysts of I. hominis (35). Also, in The Netherlands and in various other countries between 1960 and 1970, several surveys were conducted, in which stool samples from humans were examined (69). Ten percent to >50% of persons excreting sporocysts were in countries where raw meat was usually consumed. In a subsequent survey in The Netherlands, babies born to mothers who excreted sporocysts began excreting sporocysts at 9 to 10 months of age, correlating with them being fed raw or partially cooked, minced beef (69). In Poland, a 29-year-old woman working in an orphanage had single and double sporocysts in her feces for 12 months (36), which could have resulted from repeated reinfection. Neither her family nor her coworkers were found to be positive, but later, 7 persons at the orphanage were found to be infected, and at other locations in Szczecin Province, 92 additional persons were found to be infected.
In Germany, of 150 stool samples examined, I. hominis was detected in 12 persons who had eaten raw beef or pork (38). Six of the 12 persons had different gastric or intestinal symptoms, and 6 were asymptomatic. Also in Germany, a volunteer consumed 100 to 200 g of raw beef naturally infected with S. hominis or meat from cattle experimentally infected with S. hominis for 1 to 3 days on four separate occasions at intervals of several months (40). The volunteer had low-grade clinical symptoms of stomachache, nausea, and diarrhea beginning 3 to 6 h after meat consumption; symptoms lasted 24 to 36 h. Diarrhea and stomachache were again present when most sporocysts were excreted 14 to 18 days after the meat was consumed. Again in Germany, three volunteers who consumed 100 to 400 g of raw minced pork from pigs heavily infected with S. suihominis became symptomatic beginning 6 to 8 h later, with diarrhea, dyspnea, vomiting, bloat, nausea, stomachache, inappetence, and rapid pulse (40). Symptoms continued up to 48 h. Well-cooked pork from the same pigs caused no clinical symptoms in nine other volunteers who ate the meat. Based on these observations, S. suihominis was considered either pathogenic or toxic for humans. Others concluded that such pathological effects were due to toxicity (69).
In eastern Slovakia, a 12-year-old girl hospitalized for tuberculosis was incidentally found to be excreting oocysts and sporocysts of I. hominis in her stool (44). In central Slovakia, of 1,228 Vietnamese workers who immigrated over a period of 18 months, 14 excreted sporocysts of Sarcocystis for a mean of 49 days, with no gastrointestinal symptoms (52).
Intestinal Sarcocystosis in Asia
In Thailand, six patients 3 to 70 years of age with acute enteritis and leukocytosis underwent resection surgery of the ileum (48). The histopathological diagnosis indicated segmental eosinophilic enteritis or segmental necrotizing enteritis. Sexual stages and developing oocysts resembling those of Sarcocystis were observed in resected tissues from one patient, and sporocysts and numerous Gram-positive bacilli were observed in tissue samples from five others. Because sarcocysts were present in local market beef from Bos indicus cattle, and the patients ate raw beef in chili dishes, the authors of that report concluded that the cattle-human parasite S. hominis was responsible for the infections. In northern Thailand, Sarcocystis was found in 23.2% of stool samples from 362 asymptomatic laborers, 83.3% of whom were males (55). Of 253 stool samples from 140 female and 102 male villagers 2 to 80 years of age in Kaen Province, northeastern Thailand, 0.4% were positive for S. hominis (66). Of 479 stool samples collected from rural Ubon Ratchathani Province and 1,124 stool specimens from Khon Kaen in Thailand, 4.6% and 8% were positive for Sarcocystis (63). These findings and others (55, 66) suggest that northern Thailand is an area where enteric sarcocystosis is endemic. In neighboring Laos, stool samples from 1,008 persons were examined, and S. hominis was present in >10% of samples from persons >20 years of age (54).
In Tibet, stool specimens from 926 persons from Linzhi, Milin, and Duilongdeqing counties were examined by the zinc sulfate flotation method, and S. hominis was detected in 20.5 to 22.9% of the specimens in the three counties (53). S. suihominis was detected in 7.0, 0.6, and 0% of the specimens, respectively (53). Cases were usually asymptomatic, and most became negative after an undisclosed treatment.
In China, of stool specimens examined from 12 persons, single and double sporocysts were found in specimens from two men (49). No information was provided for one man, but the other, 48 years of age, had abdominal distention and pain, with alternating diarrhea and constipation and with stomachache and dyspnea. He had eaten raw pork between 13 and 65 days before his examination, and because he also had ova of Ascaris in his feces, the sporocysts were assumed to be those of S. suihominis. However, the presence of Ascaris cannot be accepted as a valid reason for assuming that sporocysts were S. suihominis without additional information. In Yunnan Province, China, a volunteer developed dizziness, abdominal pain, anemia, and diarrhea 3 days after consuming 60 g of raw beef from a calf experimentally infected with S. hominis (51). Also in China, three volunteers consumed >1,500 sarcocysts in skeletal muscles from naturally infected cattle, and two other volunteers ingested >14,000 sarcocysts in water buffalo meat (57). Beginning a week after ingestion and ending spontaneously 3 weeks later, symptoms included abdominal pain and swelling, diarrhea, and eosinophilia. In Guangxi, China, a volunteer who ate fresh pork containing sarcocysts of S. suihominis had abdominal distension beginning 5 h later, and from 8 to 36 h, he had watery diarrhea followed by fever; chills; dizziness; headache; muscle, joint, and upper abdominal pain; as well as loss of appetite (62). Another volunteer in China who ingested raw beef developed abdominal distention on the day that he consumed it; on the following day, he had stomach pain and diarrhea that lasted for 28 days (65). Sarcocystis hominis sporocysts were found in stool samples 11 to 29 days after consumption of beef (65). In two countryside villages in Guangxi, 22 men and 5 women out of 501 persons examined were excreting oocysts identified as Sarcocystis suihominis based on the finding that all persons had a history of eating raw pork (60). Of 247 men and 254 women in that study, 26 persons with positive specimens were >30 years of age, 8 had diarrhea and abdominal pain, 1 had only abdominal pain, and the others had no symptoms. It is not clear if those authors revisited one of the same villages, but similar results were reported, in which 32 of 489 fecal specimens from the Zhuang ethnic population were found to be positive for S. suihominis (61).
In India, the prevalence of S. suihominis in pigs and humans was high in an economically deprived sect (70, 71), probably linked to slaughter practices. A selected group of 20 children between 3 and 12 years of age belonged to families who reared pigs and slaughtered them in their backyard for selling pork for human consumption (71). The children of these families consumed the offal, including parts of the tail, which they ate raw with salt. The stools of these children were examined daily for 2 weeks, and 14 children, all of whom complained of abdominal pain and diarrhea, excreted sporocysts of Sarcocystis (71).
Intestinal Sarcocystosis in Australia
At a local hospital servicing five aboriginal communities in Western Australia, a 4-year parasitological survey was conducted, in which fecal specimens from 385 children and adults were examined (56). Sarcocystis detected in 2 specimens was attributed to adverse living conditions and inadequate hygiene.
Intestinal Sarcocystosis in North and South America
In each of two studies, a human volunteer and two dogs ate beef products from a retail grocery store in Maryland, and feces were examined daily for ∼3 weeks thereafter (45). In the first study, 227 g of undercooked roast beef was eaten daily for 5 days, and in the second study, 227 g of raw ground beef was eaten daily for 3 days. Because both dogs in both studies became infected, whereas the human volunteer did not, the findings suggested that S. cruzi was present and infectious in both types of beef. Neither the dogs nor the volunteer exhibited any clinical symptoms of infection.
Sarcocystosis was detected in stool and in duodenal and liver biopsy specimens from a 31-year-old AIDS patient in Argentina who had chronic diarrhea, hepatitis, and muscle pain (64). Sexual stages were seen in the lamina propria, sporulated oocysts were found in the stools, and schizont-stage parasites were seen in the liver. However, the illustrated objects do not appear to be schizonts, and other findings are inconsistent with what is known of the life cycle. Because those authors provide no explanation for these differences, the conclusion that these stages represent Sarcocystis is considered doubtful. A second, unrelated infection with another protist might explain this inconsistency. In 25 Arabian restaurants in Brazil, Sarcocystis was found in 50 samplings of kibbe (58). During a second period of collection, of four men and three women volunteers who ate between 128 and 260 g of kibbe, six excreted S. hominis oocysts/sporocysts in stools. Two volunteers had clinical symptoms: one had abdominal pain 1 day later and diarrhea for the first 3 days after eating the kibbe, and the other had diarrhea 11 days after eating the kibbe.
Diagnosis of Intestinal Sarcocystosis
The basis for a presumptive diagnosis of intestinal sarcocystosis includes enteritis and a history of having consumed undercooked meat, although infected persons can be asymptomatic. Confirmation requires identification of oocysts and or sporocysts in the stool. Sporocysts with sizes of ∼10 by 15 μm are easily seen by LM in a wet preparation just below the coverslip in a droplet of aspirated fluid from the surface of a fecal float and will autofluoresce when viewed by fluorescence microscopy. Flotation is performed by mixing feces with concentrated solutions of zinc sulfate, sucrose, sodium or cesium chloride, Percoll, or similar high-density solutions, followed by centrifugation at 500 × g to sediment fecal debris while concentrating the parasites at the surface (1). Species cannot be distinguished from one another by this method because they are so similar morphologically. The presence of asexual stages and sporulated oocysts in the intestine is more applicable for postmortem diagnosis, but biopsy or postsurgical specimens can reveal the presence of infection when stool specimens appear negative.
Extraintestinal Sarcocystosis
Humans can become infected with unknown numbers of Sarcocystis species acquired by ingesting contaminated food or water containing sporocysts excreted by infected carnivores (Fig. 2). In such cases, humans serve as an accidental and aberrant intermediate host, replacing the intermediate host found in nature. Based on studies of animal intermediate hosts, multiple generations of asexual reproduction develop in the vascular endothelium and in circulating monocytes, followed by the development of sarcocysts in myocytes of skeletal, cardiac, and smooth muscle. Based on animal studies, sarcocysts are the end stage in intermediate hosts. Sarcocysts may rupture from time to time, but the released bradyzoites die and are not known to initiate new infection.
Until recently, <100 humans had been diagnosed with muscular sarcocystosis (32). Most cases were diagnosed by use of incidental biopsy specimens, with no associated clinical symptoms, or at autopsies in tropical countries, of which nearly 50% worldwide were in Malaysia (8, 16, 17, 22, 24, 72). Of the 40 infections reviewed by Beaver et al. (8), 13, 8, 5, 4, 4, 3, and 1 were probably acquired in Southeast Asia, India, Central or South America, Africa, Europe, the United States, and China, respectively. Reports from Africa, the Middle East, and Central and South America continue to be rare or lacking. Examination of tongue muscle obtained at autopsy was extrapolated to suggest a sarcocystosis prevalence rate of ≤21% among Malaysians (17), although none of the >1,500 muscle biopsy specimens from limbs of patients with various muscle diseases, acquired over a 20-year period at the Medical Centre of the University of Malaya, are reported to have yielded sarcocyst-positive tissues (25). Until the 21st century, only ∼10 cases were reported to be symptomatic with acute muscular sarcocystosis (18, 19, 21, 73). All infections in humans until 2013 were reported as intramuscular sarcocysts of unknown species. Since then, studies of two separate outbreaks in Malaysia have identified S. nesbitti as the causative species and one capable of infecting humans (25–28).
Diagnosis during the early period leading to muscular infection is difficult because symptoms are nonspecific. Based on observations from outbreaks and experimental animal studies, muscular sarcocystosis might be suspected when a patient presents with a history of travel or residence in a tropical country, especially in Southeast Asia, and with various combinations of fever, myalgia, headache, cough, episodic weakness or fatigue, and arthralgia (21, 27, 28). This early phase of disease also might not present with objective laboratory abnormalities. Nonspecific and slightly elevated levels of hepatic enzymes (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), inflammatory markers (C-reactive protein and erythrocyte sedimentation rate [ESR]), or markers of general cell damage (lactic dehydrogenase [LDH]) might be encountered. Later, with the onset of myositis, muscle tenderness upon physical examination and possibly elevated serum creatinine phosphokinase (CK) levels and blood eosinophilia might be found. With negative test results for Toxoplasma and Trichinella, a presumptive diagnosis of sarcocystosis should be entertained. The detection of sarcocysts in a muscle biopsy specimen would confirm the diagnosis, although parasites might be diffusely distributed and difficult to find. Magnetic resonance imaging (MRI) has been suggested to be of benefit in guiding the choice of muscle biopsy site (27), although there is no evidence showing this method to be superior to guidance using physical examination findings, such as palpation to locate a point of maximal tenderness, visual observation to identify regions with swelling, or touch to identify areas of increased warmth. LM examination of stained histological sections and those with a positive periodic acid-Schiff reaction of the sarcocyst wall facilitate identification. Although eosinophilic myositis, muscle inflammation and necrosis, and interstitial and perivascular inflammation can be seen in some cases, inflammatory cells are not often observed in close proximity to sarcocysts (21, 27, 28, 74).
An accurate and sensitive diagnostic test is needed to improve diagnosis. Enzyme-linked immunosorbent assays (ELISAs), immunofluorescence assays (IFAs), and other serologic tests for antibody to Sarcocystis using bradyzoite antigen have been limited to specialized laboratories, have not been standardized, are not widely available, and have other inherent problems (75). Most of these tests no longer exist because there has been little or no continued demand for their use. In addition, the specificity of serologic assays for muscular versus intestinal sarcocystosis has not been proven, and cross-reactivity with other apicomplexans could be problematic. Development of a molecular method to detect Sarcocystis DNA in human blood or muscles could facilitate early diagnosis by expeditiously confirming the cause of the infection and identifying the species involved, all in one test. PCR has been applied for diagnostic blood testing in animals and might have the capability of detecting low numbers of circulating parasites at an early stage of infection, during merogony, at a point before entry of the organism into muscles (76).
Outbreaks and Case Descriptions
Much can be learned of the clinical signs of intramuscular sarcocystosis, the potential routes of infection, and the sources of infection from outbreaks. A series of outbreaks in Malaysia have been recorded (21, 25–30).
During a civic project in 1993 in Malaysia, seven members of a U.S. Air Force team were deployed for 1 week ∼80 km northeast of Kuala Lumpur in or near a jungle village (21). The seven persons had extensive opportunity for potential exposure to sporocysts in the environment from working shirtless and shoeless during heavy monsoon rains, by exposing themselves to possibly contaminated swimming and drinking water and soil, and by eating fresh vegetables that might not have been well cooked. Within 3 weeks after returning from deployment, two members of the jungle cohort presented with acute fever, myositis, and bronchospasm, with elevated liver enzyme levels and eosinophilia. Seven members of the jungle cohort, one of whom was asymptomatic, were tested for eosinophilia, sedimentation rate, and serum enzyme levels, including ALT, CK, LDH, and AST levels, and virtually all subjects had at least some levels elevated above normal. The timing of these laboratory determinations with respect to symptom onset or time beyond departure from the jungle area was not reported. Four subjects developed bronchospasm, myalgia, skin lesions, and swollen lymph nodes within 2 months after leaving Malaysia, which waxed and waned over the following 6 months, but two were much more symptomatic, with intermittent fevers, myalgias, arthralgia, muscle wasting, weight loss of 6 to 9 kg, fatigue, headache, and rashes. One of these two subjects remained symptomatic for several years, with a waxing-waning course; this patient underwent a muscle biopsy 3 months after illness onset, and sarcocysts were observed histologically. About a year later, possible Sarcocystis-related myocarditis was diagnosed based on borderline left atrial enlargement with electrocardiogram (ECG) abnormalities. These cardiac abnormalities subsided over the following year, but 5 years from the onset of illness, he still reported occasional episodes of pruritus, abdominal tenderness, and subcutaneous nodularity.
Another outbreak of muscular sarcocystosis included college students and teachers who visited Pangkor Island in the Malacca Strait of Malaysia (25–27). Of 92 persons who attended a retreat on the island, 89 became symptomatic within 26 days after leaving the island. Symptoms included fever (94%; 57% relapsing), myalgia (91%), headache (87%), and cough (40%). Eight persons with facial swelling for 4 to 6 weeks showed changes in their muscles of mastication by whole-body MRI consistent with inflammatory edema (27). Similar findings were observed for the back muscles of four persons and the calf muscles of two others. Four symptomatic patients underwent muscle biopsy. Of these patients, at least one sarcocyst was observed in the biopsy sections of three patients; a fourth specimen was definitively determined to be positive by PCR. Of the four patients with confirmed muscular sarcocystosis, two had elevated serum CK levels, and three had eosinophilia; however, the timing of these laboratory investigations was not well described (27). Sequences of 18S rRNA extracted from ground temporalis muscle tissue excised from one patient and from leg muscle of another matched 100% with sequences reported for Sarcocystis nesbitti (34), suggesting that sporocysts in snake feces were the probable source of infection. Although snake feces were collected from several sites in Malaysia, including Pangkor Island, Sarcocystis sporocysts were not recovered, but PCR evidence of S. nesbitti was found in feces of a cobra (Naja naja) collected in peninsular Malaysia (26).
Two waves of infection in 2011 and 2012 involved 99 tourists who vacationed on Tioman Island in the South China Sea off the Malaysian coast; all subjects were Europeans, except for two residents of Canada and a British resident of Singapore (28). Sixty-eight patients met the case definition, with myositis, eosinophilia, and negative trichinellosis serology. Diagnosis of myositis required at least one of the following signs: a complaint of muscle pain with a serum CK level of >200 IU per liter, muscle tenderness documented upon physical examination, or histological evidence of myositis in a muscle confirmed by biopsy. Sixty-two patients were considered probable cases, and six cases were confirmed by histological observation of intramuscular cysts compatible with sarcocysts. Symptoms that clustered during the second week and the sixth week after returning from the island included fever (82%), myalgia (100%), and headache (59%), similar to the symptoms of visitors to Pangkor Island, as well as fatigue (91%) and arthralgia (29%). Blood eosinophilia and elevated serum CK levels were first observed during the fifth week postdeparture. DNA recovered from sarcocysts in one biopsy sample identified the infecting species as Sarcocystis nesbitti. These first two waves of infection in 2011 and 2012 were associated with travel during the summer. No patients meeting the case definition were reported in 2013, but a series of infections in travelers returning to Germany from the same island was diagnosed beginning in the early spring of 2014 (30).
Additional data collected from 39 German patients with a history of travel to Tioman Island during 2011 to 2014, some of whom were included in the first report (28), were examined longitudinally (31). These patients had periods of illness from ranging from 0 to 23 months (median duration, 2.2 months), with 17% having symptoms lasting >6 months. Two patients had unresolved but diminished symptoms at 13 and 23 months. The median severity of pain on a scale of 0 to 10 was 6 (range, 0 to 10). This was the first study of the duration of muscular sarcocystosis in multiple patients.
Sarcocystosis and Cardiomyopathy
In 11 of the 40 cases cited by Beaver et al. (8), sarcocysts were found in heart muscle at autopsy. Sarcocysts appeared to be of three morphological types, suggesting three possible species. Seven persons were from the Caribbean and Central and South America. In one case, an 8-year-old boy in a hospital in Costa Rica was diagnosed with cardiac insufficiency and died 13 days later (8). One year earlier, he had been diagnosed with rheumatic fever. The autopsy revealed bilateral pulmonary thrombosis with infarction and chronic cardiopathology. Histopathology revealed numerous sarcocysts in cardiac muscle, with no associated inflammatory reaction. The morphology of the sarcocysts resembled that of sarcocysts found in myocardium in two other autopsy cases, one of whom had sarcocysts in Purkinje fibers.
Of the 68 confirmed cases associated with the outbreak on Tioman Island, Malaysia, 10 patients had a mildly elevated CK MB fraction (i.e., the bound combination of isoenzymes creatinine phosphokinase M and creatinine phosphokinase B), and 8 patients had a normal electrocardiogram, echocardiogram, or troponin level, but none were considered to have cardiac pathology (28). One patient had an echocardiogram showing mild dilatation of right ventricular outflow and was thought to have mild myocarditis, which ultimately resolved.
Sarcocystosis and Glomerulonephritis
Although glomerulonephritis associated with acute sarcocystosis with schizonts in the glomeruli is characteristic of S. cruzi infection in cattle, there is only one human case, in which a 47-year-old Indian man with intramuscular sarcocysts and glomerulonephritis was described (24). Whether the two were related or coincidental could not be established.
Sarcocystosis and Malignancy
Several cases of sarcocystosis have been detected in patients with various types of cancer.
At this time, insufficient data are available to clearly link sarcocystosis as an underlying cause or consequence of malignancy.
In Malaysia, when a patient with a malignant brain melanoma was examined to find the primary tumor site, sarcocysts were discovered in the nasopharynx and oropharynx (77). Subsequently, in Malaysia, 8 of 11 muscular sarcocystosis cases were persons with malignancies, mostly those of the nasopharynx and tongue (16). In Malaysia and other parts of Southeast Asia, many residents chew betel leaves in combination with other ingredients such as tobacco. In Sri Lanka, for example, a high prevalence of potential oral malignancies was associated with betel-quid chewing (78), so factors other than sarcocystosis that might contribute to malignancy cannot be ignored. Another case of malignancy with intramuscular sarcocysts that was reported in Malaysia involved a 44-year-old woman with progressive swelling, pain, and weakness of the midthigh; this was diagnosed as a malignant histiocytoma with sarcocysts in muscle fibers deep within the lesion (20). Of 1,063 laryngeal biopsy specimens examined in Thailand, only a single case of sarcocystosis concomitant with squamous cell carcinoma of the larynx was found (23). These contrasting findings provoke several hypotheses: (i) immunosuppression associated with malignancy might facilitate opportunistic parasitism with Sarcocystis, (ii) sarcocystosis might cause malignancy, or (iii) sarcocystosis in cancer patients might be coincidental in locations where there is a high prevalence of sarcocystosis. Ultimately, Shekhar et al. (20) concluded that there was no evidence of tissue reaction at the site of the parasite to induce neoplastic changes. Those authors did not consider the possible effect of earlier developmental stages or of other environmental or cultural factors. For example, areca nut, sometimes mixed with tobacco, slaked lime, and various spices, is chewed by millions of Indo-Asians and is strongly associated with cancer risk (79).
IMMUNITY, PROPHYLAXIS, AND TREATMENT
Intestinal Sarcocystosis
Although intestinal infections do not involve multiplication, development in the intestine consists of either a male or female stage from each bradyzoite within a sarcocyst. Therefore, such infections are self-limiting. Although some infections appear to persist for long periods, others might actually be the result of reinfection and the lack of protective immunity. A report of a volunteer who repeatedly infected himself by consuming sarcocysts in pork and beef suggests that there is little or no protective immunity to repeated intestinal infection (40). Neither prophylaxis nor therapeutic treatment for intestinal sarcocystosis of either animals or humans has been developed.
A patient with enteritis was diagnosed with Strongyloides stercoralis and I. hominis (Sarcocystis) infection and was treated with dithiazanine, but Sarcocystis persisted (35). Pyrimethamine, prescribed at 37 mg daily for 5 days and then at 25 mg daily for 10 days in combination with sulfisoxazole at 3 g per day for 14 days, was also not successful (44). A volunteer who ingested S. suihominis sarcocysts was treated with acetylspiramycin for 15 days at a dose of 0.2 g four times a day, but excretion did not stop until 30 days later (62).
Muscular Sarcocystosis
There are no vaccines to provide protection against muscular sarcocystosis, but protective immunity following an initial infection has been demonstrated in animals. Prophylaxis for muscular sarcocystosis was successful when the anticoccidial drugs amprolium, salinomycin, and halofuginone, designed to prevent Eimeria infections in poultry and livestock, were administered at the time of experimental infection in animal studies (80–83). Activity against the early development of asexual stages has been found, but efficacy for therapeutic treatment once intramuscular cyst formation has begun has not been investigated under experimentally controlled conditions. When salinomycin was administered to lambs for 29 days beginning 1 day before 100,000 or 1 million S. tenella sporocysts were fed, clinical sarcocystosis was reduced, but completion of the life cycle by some of the parasites was not prevented. At 63 days after the initial infection, lambs given salinomycin were challenged with 1 million S. tenella sporocysts and were found to have developed protective immunity (82). This might explain, at least in part, why foreign tourists to Tioman Island and not the local indigenous population became ill, if it is assumed that the local indigenous population was infected some time earlier (28, 84), and why native Malaysian students did not appear to become as ill as the foreign students during the Pangkor Island outbreak (27).
Severe Sarcocystis myositis in two dogs with fever, lymphopenia, thrombocytopenia, and elevated ALT and CK levels was confirmed by muscle biopsy (85). Treatment with anti-inflammatory drugs and clindamycin was unsuccessful. One dog recovered after treatment with decoquinate (an anticoccidial drug). The other dog died. Consequently, it is not clear if any antiparasitic drugs used for treatment after sarcocysts form are effective or if waning symptoms are the natural course of the infection.
Treatment of a patient involved in the outbreak among U.S. Air Force personnel in rural peninsular Malaysia began with 400 mg albendazole twice daily for 15 days, 18 months after symptoms began (21). Treatment elicited intense pruritus, but chronic symptoms gradually waned, and therapy was then discontinued. After several weeks, discomfort returned; therapy was initiated at 600 mg twice daily for 20 days, and symptoms abated. Those authors note that it was unclear if improvement was related to the use of albendazole or reflected the natural history of the infection. Patients returning from Tioman Island, Malaysia, received treatment with various antiparasitic agents, including albendazole, and some received treatment with oral steroids (28). Some clinicians reported relatively rapid symptom improvement resulting from oral steroids, including a patient with mild myocarditis who responded favorably to such treatment.
The antiprotozoal drug co-trimoxazole (trimethoprim at 160 mg and sulfamethoxazole at 800 mg), given as a dose of 3 tablets per day for 12 days, was used to treat a 31-year-old male patient >3 months after muscular pain began (18). He was already feeling slightly better when diagnosis was made and before medication was prescribed but greatly improved, except for some localized pain.
Of three patients who sought medical attention within 6 days of onset of fever and were treated with co-trimoxazole (a 960-mg dose twice each day), all improved clinically without an elevation of CK levels (30). Two other patients in a later phase of illness with elevated CK levels were also treated with co-trimoxazole, but one had to be re-treated with prednisone because of increasingly severe myalgia. A sixth patient, who had the longest interval from the onset of symptoms, was treated with prednisone. Both patients treated with prednisone improved rapidly.
Sulfathiazole, sulfamethazine, sulfamethoxazole, sulfadimethoxine, sulfadiazine, sulfachloropyridazine, trimethoprim, and pyrimethamine were tested at different concentrations in cell cultures against developing S. neurona merozoites (86). Pyrimethamine and trimethoprim were each cidal, but none of the sulfonamides had activity when used alone. Combinations of sulfonamides demonstrated improved activity.
What is lacking is a protocol that has been tested, found to be effective, and confirmed by replication. Because it is impractical and undesirable to use humans or primates for testing, it would be helpful as new patients are identified to report successful and unsuccessful treatment regimens. However, because symptoms usually wane with time, the efficacy of long-term medication might not be discerned from the natural course of the disease process.
PREVENTION
To prevent intestinal sarcocystosis, meat must be thoroughly cooked or frozen to kill the bradyzoites in the sarcocysts. Thorough cooking rendered bradyzoites noninfectious, as demonstrated in a volunteer study involving S. suihominis (40). If there is a toxic factor associated with the ingestion of sarcocysts, possibly as with S. suihominis (40), cooking also appears to destroy the effects. Sarcocystis meischeriana in pork was rendered noninfectious for dogs after meat was cooked at 60°C for 20 min, 70°C for 15 min, and 100°C for 5 min or frozen at −4°C for 48 h or at −20°C for 24 h (87). Likewise, whereas dogs fed uncooked chuck roast, round steak, hamburger, and rare roast beef became infected, other dogs fed cooked meat such as beef bologna and beef frankfurters or frozen meat such as hamburger or sandwich steaks did not become infected (45). Meat inspection might reduce some infections, but it would be costly and time-consuming, requiring the identification of organisms in meat. Unless heavily infected, sarcocysts would be difficult to detect by microscopic or antibody methods, and these tests would not determine the species and therefore could be misleading if the species does not infect humans. Molecular tests may determine the species, but obtaining infected tissues where sarcocysts are sparse would be impractical. To prevent infection of domesticated food animals, human feces containing sporocysts must not be permitted to contaminate water, bedding, and feed. Sanitation is the key; with the use of toilets and diligent hand washing, contamination can be reduced or eliminated.
To prevent humans from acquiring muscular sarcocystosis, the possible ingestion of sporocysts must be eliminated. Clean drinking water can reduce exposure to sporocysts, but recreational water and contact with soil are potential risk factors. Where contaminated drinking water is suspected, boiling will provide disinfection. Filters with pores small enough to remove bacteria from water can also remove sporocysts of Sarcocystis. Chemical disinfection with chlorine or other agents used for water treatments is not effective in killing sporocysts of Sarcocystis (88). Where available, drinking safe bottled water from sealed containers is recommended. Food can be contaminated at many places along the production, distribution, and preparation line. Where fresh produce is suspected to be contaminated with sporocysts in irrigation water or by food handlers, food should be painstakingly washed with clean water and/or thoroughly cooked before being eaten.
CONCLUSIONS
Since the first report of sarcocysts in the muscles of mice >170 years ago, >150 valid species of Sarcocystis have been named, and >2,000 reports have been published. Great contributions to our comprehension of the diversity and transmission of Sarcocystis species have been made by identification of hosts with intramuscular sarcocysts and others excreting sporocysts, by LM and TEM descriptions of sarcocyst and sporocyst morphology, by feeding experiments to determine the relationships among intermediate and final hosts, by identification of the asexual stages in tissues of intermediate or aberrant hosts and the sexual stages in the gut of the final host, and by the use of molecular markers to identify endogenous and exogenous stages. Clinical signs and outcomes of natural, experimental, and aberrant infections have raised awareness of muscular sarcocystosis from a biological curiosity, to an economically important disease in livestock and companion animals and a debilitating disease in some wildlife, to a serious disease in humans in certain geographic areas. Treatment strategies adapted from experimental animal studies and from treatment of related protists in humans can provide guidance for treatment of muscular sarcocystosis when diagnosis is made early in infection, before sarcocyst formation begins. The use of safe drinking water, filtered to remove particles larger than bacteria, and thorough cooking of meat should be effective to prevent most cases of sarcocystosis as well as most other enteric protist infections. The use of molecular methods has become essential for future progress on sarcocystosis, for identification and source tracking, for potential development of treatment strategies based on metabolic pathways, for development of immunological treatment strategies, and for unforeseen applications that follow the discovery of basic scientific data.
Biographies

Ronald Fayer is a Senior Scientist in the Agricultural Research Service, U.S. Department of Agriculture. He received his B.S. from the University of Alaska, Fairbanks, and his M.S. and Ph.D. from Utah State University. He has conducted research on protist parasites of veterinary and public health importance, including Sarcocystis, Cryptosporidium, Giardia, microsporidia, and Blastocystis, and currently studies the molecular epidemiology of zoonotic protists. He recently visited Tioman Island, the site of outbreaks of muscular sarcocystosis.

Douglas H. Esposito is a medical epidemiologist and pediatrician in the Division of Global Migration and Quarantine, Travelers' Health Branch, CDC. He received his bachelor's degree from the University of Rochester in New York and his M.D. and M.P.H. from the University of North Carolina at Chapel Hill, and he then trained in pediatrics at Children's Hospital and Medical Center in Seattle, WA. He worked for 13 years with the Indian Health Service in rural Arizona and Alaska. He completed an Epidemic Intelligence Service Fellowship at the Centers for Disease Control and Prevention. He is interested in the epidemiology of infectious diseases among international travelers and has been working with travelers with acute muscular sarcocystosis after they returned from Tioman Island, Malaysia. He recently visited the island.

Jitender P. Dubey is a senior scientist with the Animal Parasitic Diseases Laboratory, Agricultural Research Service, U.S. Department of Agriculture. He received his veterinary degree in 1960 in India and a Ph.D. in 1966 from the University of Sheffield, England. He has published extensively on cyst-forming zoonotic coccidian parasites, including Sarcocystis, Toxoplasma, and Neospora, and continues his research with these organisms. In 2010, he was elected to the U.S. National Academy of Sciences, Washington, DC.
REFERENCES
- 1.Dubey JP, Speer CA, Fayer R. 1989. Sarcocystosis of animals and man. CRC Press, Boca Raton, FL. [Google Scholar]
- 2.Senaud J. 1967. Contributuion a l'etude des sarcosporidies et des toxoplasmes Toxoplasma. Protistologica 3:169–232. [Google Scholar]
- 3.Fayer R. 1970. Sarcocystis: development in cultured avian and mammalian cells. Science 168:1104–1105. doi: 10.1126/science.168.3935.1104. [DOI] [PubMed] [Google Scholar]
- 4.Fayer R. 1972. Gametogony of Sarcocystis sp. in cell culture. Science 175:65–67. doi: 10.1126/science.175.4017.65. [DOI] [PubMed] [Google Scholar]
- 5.Heydorn AO, Rommel M. 1972. Beitrage zum Lebenszyklus der Sarkosporidien. II. Hund und Katze als Ubertrager ser Sarkosporidien des Rindes. Berl Munch Tierarztl Wochenschr 85:121–123. [PubMed] [Google Scholar]
- 6.Rommel M, Heydorn AO. 1972. Beitrage zum Lebenszyklus der Sarkosporidien. III. Isospora hominis (Railiet und Lucet, 1891) Wenyon 1923, eine Dauerform des Sarkosporidien des Rindes und des Schweins. Berl Munch Tierarztl Wochenschr 85:143–145. [PubMed] [Google Scholar]
- 7.Rommel M, Heydorn AO, Fischle B, Gestrich R. 1974. Beitrage zum Lebenszylus der Sarkosporidien. V. Weitere Enwirte der Sarkosporidien von Rind, Schaf und Schwein und die Bedentung des Zwischenwirtes fur die Verbreitung dieser Parasitose. Berl Munch Tierarztl Wochenschr 87:392–396. [PubMed] [Google Scholar]
- 8.Beaver PC, Gadgil RK, Morera P. 1979. Sarcocystis in man: a review and report of five cases. Am J Trop Med Hyg 28:819–844. [PubMed] [Google Scholar]
- 9.Pathmanathan R, Kan SP. 1981. Human Sarcocystis infection in Malaysia. Southeast Asian J Trop Med Public Health 12:247–250. [Google Scholar]
- 10.Agarwal PK, Srvastava AN. 1983. Sarcocystis in man: a report of two cases. Histopathology 7:783–787. doi: 10.1111/j.1365-2559.1983.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 11.Greve E. 1985. Sarcosporidiosis—an overlooked zoonosis. Man as intermediate and final host. Dan Med Bull 32:228–230. [PubMed] [Google Scholar]
- 12.Pathmanathan R, Kan SP. 1987. Two cases of human Sarcocystis in East Malaysia. Med J Malaysia 42:212–214. [PubMed] [Google Scholar]
- 13.Pathmanathan R, Jayalakshmi P, Kan SP. 1988. A case of human Sarcocystis infection in Malaysia. J Malays Soc Health 6:45–47. [Google Scholar]
- 14.Pamphlett R, O'Donoghue P. 1990. Sarcocystis infection of human muscle. Aust N Z J Med 20:705–707. doi: 10.1111/j.1445-5994.1990.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 15.Abdel Mawla MM. 1990. Ultrastructure of the cyst wall of S. lindemanni with pathological correlations. J Egypt Soc Parasitol 20:319–325. [PubMed] [Google Scholar]
- 16.Pathmanathan R, Kan SP. 1992. Three cases of human Sarcocystis infection with a review of human muscular sarcocystosis in Malaysia. Trop Geogr Med 44:102–108. [PubMed] [Google Scholar]
- 17.Wong KT, Pathmanathan R. 1992. High prevalence of human muscle sarcocystosis in south-east Asia. Trans R Soc Trop Med Hyg 86:631–632. doi: 10.1016/0035-9203(92)90161-5. [DOI] [PubMed] [Google Scholar]
- 18.Van den Enden E, Prae M, Joos R, Van Gompel A, Gigasse P. 1995. Eosinophilic myositis resulting from sarcocystosis. J Trop Med Hyg 98:273–276. [PubMed] [Google Scholar]
- 19.Mehrotra R, Bisht D, Singh PA, Gupta SC, Gupta RK. 1996. Diagnosis of human sarcocystis infection from biopsies of the skeletal muscle. Pathology 28:281–282. doi: 10.1080/00313029600169164. [DOI] [PubMed] [Google Scholar]
- 20.Shekhar KC, Pathmanathan R, Krishnan R. 1998. Human muscular sarcocystosis associated with neoplasms? Case report. Trop Biomed 15:61–64. [Google Scholar]
- 21.Arness MK, Brown JD, Dubey JP, Neafie RC, Granstrom DE. 1999. An outbreak of acute eosinophilic myositis attributed to human Sarcocystis parasitism. Am J Trop Med Hyg 61:548–553. [DOI] [PubMed] [Google Scholar]
- 22.Larbcharoensub N, Cheewaruangroj W, Nitiyanant P. 2011. Laryngeal sarcocystosis accompanying laryngeal squamous cell carcinoma: case report and literature review. Southeast Asian J Trop Med Public Health 42:1072–1076. [PubMed] [Google Scholar]
- 23.Makhija M. 2012. Histological identification of muscular sarcocystis: a report of two cases. Indian J Pathol Microbiol 55:552–554. doi: 10.4103/0377-4929.107813. [DOI] [PubMed] [Google Scholar]
- 24.Balakrishna JP, Chacko G, Manipadam MT, Ramyal. 2013. Glomerulopathy in a patient with sarcocystis infestation. Indian J Pathol Microbiol 56:285–287. doi: 10.4103/0377-4929.120400. [DOI] [PubMed] [Google Scholar]
- 25.AbuBakar S, Teoh BT, Sam SS, Chang LY, Johari J, Hooi PS, Lakhbeer-Singh HK, Italiano CM, Omar SF, Wong KT, Ramli N, Tan CT. 2013. Outbreak of human infection with Sarcocystis nesbitti, Malaysia, 2012. Emerg Infect Dis 19:1989–1991. doi: 10.3201/eid1912.120530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau YL, Chang PY, Tan CT, Fong MY, Mahmud R, Wong KT. 2014. Sarcocystis nesbitti infection in human skeletal muscle: possible transmission from snakes. Am J Trop Med Hyg 90:361–364. doi: 10.4269/ajtmh.12-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Italiano CM, Wong KT, AbuBakar S, Lau YL, Ramli N, Syed Omar SF, Kahar Bador M, Tan CT. 2014. Sarcocystis nesbitti causes acute relapsing febrile myositis with a high attack rate: description of a large outbreak of muscular sarcocystosis in Pangkor Island, Malaysia, 2012. PLoS Negl Trop Dis 8:e2876 doi: 10.1371/journal.pntd.0002876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esposito DH, Stich A, Epelboin L, Malvy D, Han PV, Bottieau E, da Silva A, Zanger P, Slesak G, van Genderen PJJ, Rosenthal BM, Camer JP, Visser LG, Munoz J, Drew CP, Goldsmith CS, Steiner F, Wagner N, Grobusch MP, Plier DA, Tappe , Sotir MJ, Brown C, Brunette GW, Fayer R, von Sonnenburg F, Freedman D, Neumayr A, Kozarsky PE. 2014. Acute muscular sarcocystosis: an international investigation among ill travelers returning from Tioman Island, Malaysia, 2011 and 2012. Clin Infect Dis 59:1401–1410. doi: 10.1093/cid/ciu622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slesak G, Tappe D, Keller C, Cramer J, Guthoff W, Zanger P, Frank M, Ernestus K, Rauthe S, Stic A, Schafer J. 2014. Muscular sarcocystosis after travel to Malaysia: a case series from Germany. Dtsch Med Wochenschr 139:990–995. doi: 10.1055/s-0034-1370004. [DOI] [PubMed] [Google Scholar]
- 30.Tappe D, Stich A, Langeheinecke A, von Sonnenburg F, Muntau B, Schafer J, Slesak G. 2014. Suspected new waves of muscular sarcocystosis in travelers returning from Tioman Island, Malaysia, May 2014. Euro Surveill 19(21):pii=20816 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20816. [DOI] [PubMed] [Google Scholar]
- 31.Slesak G, Schafer J, Langeheinecke A, Tappe D. 2015. Prolonged clinical course of muscular sarcocystosis and effectiveness of cotrimoxazole among travelers to Tioman Island, Malaysia, 2011-2014. Clin Infect Dis 60:329. doi: 10.1093/cid/ciu791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fayer R. 2004. Sarcocystis spp. in human infections. Clin Microbiol Rev 17:894–902. doi: 10.1128/CMR.17.4.894-902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang ZQ, Wei CG, Zen JS, Song JL, Zuo YX, He YS, Zhang HF, Attwood SW, Chen XW, Yang GC, Zhou X, Quan X, Li CY, Han D, Liu AW, Lin P. 2005. A taxonomic re-appraisal of Sarcocystis nesbitti (Protozoa: Sarcocystidae) from the monkey Macaca fascicularis in Yunnan, PR China. Parasitol Int 54:75–81. doi: 10.1016/j.parint.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Tian M, Chen Y, Wu L, Rosenthal BM, Liu X, He Y. 2012. Phylogenetic analysis of Sarcocystis nesbitti (Coccidia: Sarcocystidae) suggests a snake as its probable definitive host. Vet Parasitol 183:373–376. doi: 10.1016/j.vetpar.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 35.Laarman JJ. 1962. Isospora hominis (Railliet and Lucet, 1891) in the Netherlands. Acta Leiden 31:111–116. [PubMed] [Google Scholar]
- 36.Plotkowiak J, Klasa IA. 1973. Opis pierwszego przypadku inwazji Isospora hominis w Polsce. Wiad Parazytol 19:725–730. [PubMed] [Google Scholar]
- 37.Plotkowiak J. 1973. Inwazja Sarcocystis hominis nowy problem parazytologiczny w Polsce. Ann Acad Med Stetin 10:53–55. [PubMed] [Google Scholar]
- 38.Janitschke K. 1974. Coccidia infections in man in Germany, p 116 In Proceedings of the 3rd International Congress of Parasitology, Munich, August 25-31, 1974. Facta Publication, Munich, Germany. [Google Scholar]
- 39.Janitschke K. 1975. Neue Erkenntnisse uber die Kokzidien-inkstensionen des Menshen. II. Isospora Infektion. Bundesgesundheitsblatt 18:419. [Google Scholar]
- 40.Heydorn AO. 1977. Sarkosporidieninfiziertes Fleisch als mogliche Krankheitsursache fur den Menschen. Arch Lebensmittelhyg 28:27–31. [Google Scholar]
- 41.Tadros W, Laarman JJ. 1977. Studies on sarcosporidiosis and Sarocystis-induced coccidiosis in man, monkeys and other animals, abstr 137. Abstr 5th Int Congr Protozool, 26 June to 2 July 1977. [Google Scholar]
- 42.Flentje B, Jungmann R, Hiepe T. 1975. Vorkommen von Isospora-hominis Sporozysten beim Menschen. Dtsch Gesundheitsw 30:523–525. [Google Scholar]
- 43.Plotkowiak J. 1976. Wyniki dalsych badan nad wystepowaniem i epidemiologia inwazji Isospora hominis (Railliet and Lucet, 1891). Wiad Parazytol 22:137–147. [PubMed] [Google Scholar]
- 44.Giboda M, Rakár J. 1978. First record of “Isospora hominis” in Czechoslovakia. Folia Parasitol (Praha) 25:16. [PubMed] [Google Scholar]
- 45.Leek RG, Fayer R. 1978. Infectivity of Sarcocystis in beef and beef products from a retail food store. Proc Helminthol Soc Wash 45:135–136. [Google Scholar]
- 46.Tadros W, Hazelhoff W, Laarman JJ. 1979. The detection of circulating antibodies against Sarcocystis in human and bovine sera by the enzyme-linked immunosorbent assay (ELISA) technique. Acta Leiden 47:53–63. [PubMed] [Google Scholar]
- 47.Deluol AM, Mechali D, Cenac J, Savel J, Coulaud JP. 1980. Incidence and clinical aspects of intestinal coccidiosis in a tropical medicine practice. Bull Soc Pathol Exot Filiales 73:259–265. [PubMed] [Google Scholar]
- 48.Bunyaratvej S, Bunyawongwiro P, Nityanant P. 1982. Human intestinal sarcosporidiosis: report of six cases. Am J Trop Med Hyg 31:36–41. [DOI] [PubMed] [Google Scholar]
- 49.Zuo Y-X, Chen F-Q, Li W-Y. 1982. Two patients with Sarcocystis infection, p 52–53. In Jiang JB. (ed), Malaria and other protozoal infections. Chinese Society of Protozoologists, Zhongshan (Sun Yatsen) University, Guangzhou, China. [Google Scholar]
- 50.Li Y, Lian Z. 1986. Studies on man-pig cyclic infection of Sarcocystis suihominis found in Yunnan Province China. Acta Zool Sin 32:329–334. [Google Scholar]
- 51.Lian Z, Ma J, Wang Z, Fu L, Zhou Z, Li W, Wang X. 1990. Studies on man-cattle-man infection cycle of Sarcocystis hominis in Yunnan. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 8:50–53. (In Chinese.) [PubMed] [Google Scholar]
- 52.Straka S, Skracikova J, Konvit I, Szilagyiova M, Michal L. 1991. Sarcocystis species in Vietnamese workers. Cesk Epidemiol Microbiol Imunol 40:204–208. (In Slovak.) [PubMed] [Google Scholar]
- 53.Yu S. 1991. Field survey of Sarcocystis infection in the Tibet autonomous region. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 13:29–32. (In Chinese.) [PubMed] [Google Scholar]
- 54.Giboda M, Ditrich O, Scholz T, Viengsay T, Bouaphanh S. 1991. Current status of food borne parasitic zoonoses in Laos. Southeast Asian J Trop Med Public Health 22(Suppl):56–61. [PubMed] [Google Scholar]
- 55.Wilairatana P, Radomyos P, Radomyos B, Phraevanich R, Plooksawasdi W, Chanthavanich P, Viravan C, Looareesuwan S. 1996. Intestinal sarcocystosis in Thai laborers. Southeast Asian J Trop Med Public Health 27:43–46. [PubMed] [Google Scholar]
- 56.Meloni BP, Thompson RC, Hopkins RM, Reynoldson JA, Gracey M. 1993. The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. Med J Aust 158:157–159. [DOI] [PubMed] [Google Scholar]
- 57.Chen X, Zuo Y, Zuo W. 1999. Observation on the clinical symptoms and sporocysts excretion in human volunteers experimentally infected with Sarcocystis hominis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17:25–27. (In Chinese.) [PubMed] [Google Scholar]
- 58.Pena HFJ, Ogassawara S, Sinhorini IL. 2001. Occurrence of cattle Sarcocystis species in raw kibbe from Arabian food establishments in the city of Sao Paulo, Brazil, and experimental transmission to humans. J Parasitol 87:1459–1465. doi: 10.1645/0022-3395(2001)087[1459:OOCSSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 59.Clavel A, Doiz O, Varea M, Morales S, Castillo FJ, Rubio MC, Gómez-Lus R. 2001. Abdominal discomfort and soft stools in a habitual consumer of rare beef. Enferm Infecc Microbiol Clin 19:29–30. doi: 10.1016/S0213-005X(01)72545-1. [DOI] [PubMed] [Google Scholar]
- 60.Li JH, Lin Z, Tan YX, Du JF. 2004. Sarcocystis suihominis infection discovered in Guangxi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 22:82 (In Chinese.) [PubMed] [Google Scholar]
- 61.Tan YX, Li JH, Lin Z, Du JF. 2005. Investigation of the Sarcocystis suihominis infection status in the Zhuang ethnic population in part of Guangxi Province. Guangxi Med 11:295–296. (In Chinese.) [Google Scholar]
- 62.Li JH, Lin Z, Du JF, Qin YX. 2007. Experimental infection of Sarcocystis suihominis in pig and human volunteer in Guangxi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 25:466–468. (In Chinese.) [PubMed] [Google Scholar]
- 63.Tungtrongchitr A, Chiworaporn C, Praewanichm R, Radomyos P, Boitano JJ. 2007. The potential usefulness of the modified Kato thick smear technique in the detection of intestinal sarcocystosis during field surveys. Southeast Asian J Trop Med Public Health 38:232–238. [PubMed] [Google Scholar]
- 64.Velásquez JN, Di Risio C, Etchart CB, Chertcoff AV, Mendez N, Cabrera MG, Labbé JH, Carnevale S. 2008. Systemic sarcocystosis in a patient with acquired immune deficiency syndrome. Hum Pathol 39:1263–1267. doi: 10.1016/j.humpath.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 65.Chen X, Zuo Y, Rosenthal BM, He Y, Cui L, Yang Z. 2011. Sarcocystis sinensis is an ultrastructurally distinct parasite of water buffalo that can cause foodborne illness but cannot complete its life-cycle in human beings. Vet Parasitol 178:35–39. doi: 10.1016/j.vetpar.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 66.Boonjaraspinyo S, Boonmars T, Kaewsamut B, Ekobol N, Laummaunwai P, Aukanimart R, Wonkchalee N, Juasook A, Sriraj P. 2013. QA cross-sectional study on intestinal parasitic infections in rural communities, northeast Thailand. Korean J Parasitol 51:727–734. doi: 10.3347/kjp.2013.51.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nimri L. 2014. Unusual case presentation of intestinal Sarcocystis hominis infection in a healthy adult. JMM Case Rep 1:1–3. doi: 10.1099/jmmcr.0.T00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SC, Ngui R, Tan TK, Muhammad Aidil R, Lim YA. 2014. Neglected tropical diseases among two indigenous subtribes in peninsular Malaysia: highlighting differences in co-infection of helminthiasis and sarcocystosis. PLoS One 9:e107980. doi: 10.1371/journal.pone.0107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laarman JJ, Tadros W. 1982. Sarcosporidiosis of farm animals, p 81–88. In Walton JR, White EG, Hall SA (ed), Agriculture: some diseases of emerging importance to community trade. Luxembourg Commission of the European Communities, Luxembourg City, Luxembourg. [Google Scholar]
- 70.Solanki PK, Shrivastava HOP, Shah HL. 1991. Prevalence of Sarcocystis in naturally infected pigs in Madhya Pradesh with an epidemiological explanation for the higher prevalence of Sarcocystis suihominis. Indian J Anim Sci 61:820–821. [Google Scholar]
- 71.Banerjee PS, Bhatia BB, Pandit BA. 1994. Sarcosystis suihominis infection in human beings in India. J Vet Parasitol 8:57–58. [Google Scholar]
- 72.Tappe D, Abdullah S, Heo CC, Kannan Kutty M, Latif B. 2013. Human and animal invasive sarcocystosis in Malaysia—recent cases, review and hypotheses. Trop Biomed 30:355–366. [PubMed] [Google Scholar]
- 73.Jeffrey HC. 1974. Sarcosporidiosis in man. Trans R Soc Trop Med Hyg 68:17–29. doi: 10.1016/0035-9203(74)90247-8. [DOI] [PubMed] [Google Scholar]
- 74.McLeod R, Hirabayashi RN, Rothman W, Remington JS. 1980. Necrotizing vasculitis and Sarcocystis: a cause-and-effect relationship? South Med J 73:1380–1383. doi: 10.1097/00007611-198010000-00026. [DOI] [PubMed] [Google Scholar]
- 75.Poulson S, Stensvold CR. 2014. Current status of epidemiology and diagnosis of human sarcocystosis. J Clin Microbiol 52:3524–3530. doi: 10.1128/JCM.00955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heckeroth AR, Tenter AM. 1999. Development and validation of species-specific nested PCRs for diagnosis of acute sarcocystiosis in sheep. Int J Parasitol 29:1331–1349. doi: 10.1016/S0020-7519(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 77.Kutty MK, Dissanaike AS. 1975. A case of human Sarcocystis infection in west Malaysia. Trans R Soc Trop Med Hyg 69:503–504. doi: 10.1016/0035-9203(75)90108-X. [DOI] [PubMed] [Google Scholar]
- 78.Amarasinghe HK, Usgodaarachchi US, Johnson NW, Lalloo R, Warnakulasuriya S. 2010. Betel-quid chewing with or without tobacco is a major risk factor for oral potentially malignant disorders in Sri Lanka: a case-control study. Oral Oncol 46:297–301. doi: 10.1016/j.oraloncology.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 79.Warnakulasuriya S, Trivedy C, Peters TJ. 2002. Areca nut use: an independent risk factor for oral cancer. BMJ 324:799–800. doi: 10.1136/bmj.324.7341.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fayer R, Johnson AJ. 1975. Effect of amprolium on acute sarcocystosis in experimentally infected calves. J Parasitol 61:932–936. doi: 10.2307/3279240. [DOI] [PubMed] [Google Scholar]
- 81.Leek RG, Fayer R. 1980. Amprolium for prophylaxis of ovine Sarcocystis. J Parasitol 66:100–106. doi: 10.2307/3280598. [DOI] [PubMed] [Google Scholar]
- 82.Leek RG, Fayer R. 1983. Experimental Sarcocystis ovicanis infection in lambs: salinomycin chemoprophylaxis and protective immunity. J Parasitol 69:271–276. doi: 10.2307/3281218. [DOI] [PubMed] [Google Scholar]
- 83.Voigt WP, Heydorn AO. 1981. Chemotherapy of sarcosporidiosis and theileriosis in domestic animals. Zentralbl Bakteriol Mikrobiol Hyg A 250:256–259. [PubMed] [Google Scholar]
- 84.Husna Maizura AM, Khebir V, Chong CK, Shah A, Hakim L. 2012. Surveillance for sarcocystosis in Tioman Island, Malaysia. Malaysian J Public Health Med 12:39–44. [Google Scholar]
- 85.Sykes JE, Dubey JP, Lindsay LL, Prato P, Lappin MR, Guo LT, Mizisin AP, Shelton GD. 2011. Severe myositis associated with Sarcocystis spp. infection in 2 dogs. J Vet Intern Med 25:1277–1283. doi: 10.1111/j.1939-1676.2011.00828.x. [DOI] [PubMed] [Google Scholar]
- 86.Lindsay DS, Dubey JP. 1999. Determination of the activity of pyrimethamine, trimethoprim, sulfonamides, and combinations of pyrimethamine and sulfonamides against Sarcocystis neurona in cell cultures. Vet Parasitol 82:205–210. doi: 10.1016/S0304-4017(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 87.Saleque A, Juyal PD, Bhatia BB. 1990. Effect of temperature on the infectivity of Sarcocystis miescheriana cysts in pork. Vet Parasitol 36:343–346. doi: 10.1016/0304-4017(90)90047-F. [DOI] [PubMed] [Google Scholar]
- 88.Dubey JP, Saville WJ, Sreekumar C, Shen SK, Lindsay DS, Pena HF, Vianna MC, Gennari SM, Reed SM. 2002. Effects of high temperature and disinfectants on the viability of Sarcocystis neurona sporocysts. J Parasitol 88:1252–1254. doi: 10.1645/0022-3395(2002)088[1252:EOHTAD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]