SUMMARY
Actinomyces israelii has long been recognized as a causative agent of actinomycosis. During the past 3 decades, a large number of novel Actinomyces species have been described. Their detection and identification in clinical microbiology laboratories and recognition as pathogens in clinical settings can be challenging. With the introduction of advanced molecular methods, knowledge about their clinical relevance is gradually increasing, and the spectrum of diseases associated with Actinomyces and Actinomyces-like organisms is widening accordingly; for example, Actinomyces meyeri, Actinomyces neuii, and Actinomyces turicensis as well as Actinotignum (formerly Actinobaculum) schaalii are emerging as important causes of specific infections at various body sites. In the present review, we have gathered this information to provide a comprehensive and microbiologically consistent overview of the significance of Actinomyces and some closely related taxa in human infections.
INTRODUCTION
Human actinomycosis, a chronic, granulomatous infectious disease, has been recognized for a long time (1), and its causative agent, originally named Streptothrix israeli (currently Actinomyces israelii), was described in 1896 by Kruse (2). It was not until 1951 that another Actinomyces species, Actinomyces naeslundii, was implicated in actinomycotic lesions in humans (3), while Actinomyces odontolyticus and Actinomyces viscosus (first named as Odontomyces viscosus) were described in 1958 and 1969, respectively (4–6).
It is well established that actinomycosis is an endogenous infection. The causative Actinomyces species reside on mucosal surfaces and gain access to deeper tissues via trauma, surgical procedures, or foreign bodies, which disrupt the mucosal barrier. Inside the tissue, these bacteria form masses consisting of aggregates of branching, filamentous bacilli (7–9). Actinomycosis is defined as a hard mass-type lesion with a specific histopathological structure. There are a large number of case reports of actinomycosis in the literature, but in most cases, diagnosis has been based solely on clinical and histopathological findings. In the majority of early reports, microbiological confirmation of diagnosis was lacking. Even when microbiological assessment was included, culture was typically the only method used. If, however, antimicrobial treatment had been started before sample collection, the results of culture may be falsely negative. The increasing introduction of molecular bacterial detection and identification methods is helping to overcome such problems.
A large number of Actinomyces species have been described since the description of A. israelii, A. naeslundii, A. odontolyticus, and A. viscosus. In addition, reassignments within some species, such as A. naeslundii and A. viscosus, have occurred (10). However, only some human-associated Actinomyces species, including A. israelii, Actinomyces gerencseriae, and Actinomyces graevenitzii, may be involved in classical actinomycosis (11, 12). A wide range of Actinomyces species are being increasingly associated with infections at many body sites (11, 13, 14). Actinomyces meyeri, Actinomyces neuii, and Actinomyces turicensis are emerging as important causes of such infections.
Although actinomycosis is relatively rare, at least in Western populations (8), recently reported observations implicating A. meyeri in brain abscesses (15) and Actinobaculum schaalii (currently Actinotignum schaalii) in urosepsis (16) and the introduction of advanced microbiological techniques, which can identify even very fastidious organisms, have resulted in an increased awareness of Actinomyces and other Gram-positive, non-spore-forming bacilli in clinical microbiology.
To date, 25 validly published Actinomyces species from human material have been described (Table 1). Of these, the descriptions of 13 species occurred solely during this century. In the present review, we aim to give a comprehensive overview of human Actinomyces and closely related organisms and their roles in different types of actinomycoses and other infections.
TABLE 1.
Human Actinomyces species
| Species | Yr of description | Reference(s) |
|---|---|---|
| A. israelii | 1896 | 2 |
| A. naeslundii | 1951 | 3 |
| A. odontolyticus | 1958 | 4 |
| A. viscosus | 1969 | 5, 6 |
| A. meyeri | 1984 | 205 |
| A. georgiae | 1990 | 17 |
| A. gerencseriae | 1990 | 17 |
| A. neuii subsp. neuii and subsp. anitratus | 1994 | 207 |
| A. radingae | 1995 | 288 |
| A. turicensis | 1995 | 288 |
| A. europaeus | 1997 | 202 |
| A. graevenitzii | 1997 | 197 |
| A. radicidentis | 2000 | 188 |
| A. urogenitalis | 2000 | 45 |
| A. funkei | 2001 | 244 |
| A. cardiffensis | 2002 | 65 |
| A. hongkongensis | 2003 | 130 |
| A. nasicola | 2003 | 179 |
| A. oricola | 2003 | 189 |
| A. dentalis | 2005 | 190 |
| A. naeslundii (sensu stricto)a | 2009 | 10 |
| A. oris | 2009 | 10 |
| A. johnsonii | 2009 | 10 |
| A. massiliensis | 2009 | 246 |
| A. timonensis | 2010 | 224 |
| A. hominis | 2010 | 214 |
Emended description of A. naeslundii.
UPDATE ON TAXONOMY OF ACTINOMYCES AND CLOSELY RELATED TAXA
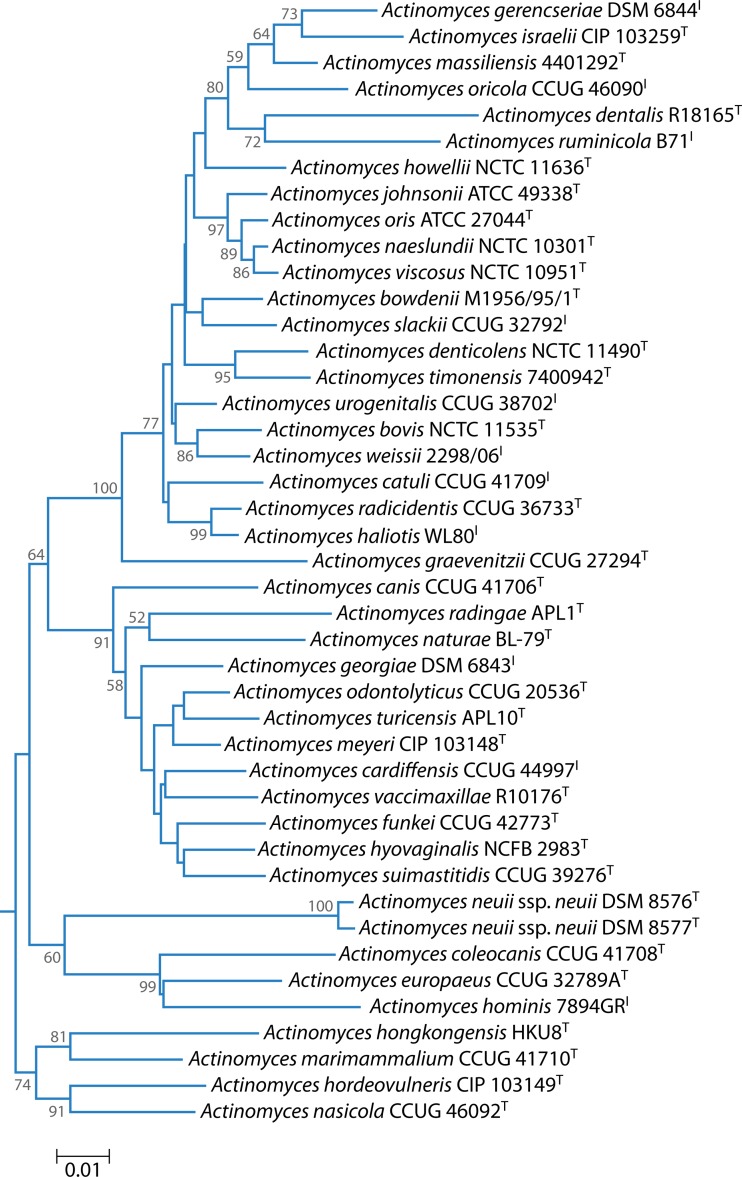
The clinically relevant Gram-positive anaerobic bacilli are found in two phyla: Actinobacteria and Firmicutes. The genus Actinomyces belongs to the Actinobacteria, is one of six genera within the family Actinomycetaceae, and is currently comprised of 42 validly published species (http://www.bacterio.net/actinomyces.html). The type species is Actinomyces bovis. A phylogenetic tree based on 16S rRNA gene sequence comparisons (Fig. 1) shows that there are three principal clusters albeit not particularly strongly supported by bootstrap analysis. The majority of species belong to the cluster that includes A. bovis, while the remaining clusters, which include A. neuii and Actinomyces hordeovulneris, respectively, should be investigated further to determine if they warrant proposal as novel genera.
FIG 1.
Phylogenetic tree based on 16S rRNA gene sequence comparisons over 1,260 aligned bases showing the relationship between species of the genus Actinomyces. The tree was reconstructed by using the neighbor-joining method from a distance matrix constructed from aligned sequences using the Jukes-Cantor correction. Numbers represent bootstrap values for each branch based on data for 1,000 trees.
The A. naeslundii/A. viscosus group has long been known to be heterogeneous. A. viscosus was originally isolated from a hamster (5), but human strains were subsequently isolated with sufficient phenotypic similarity to be assigned to this species. DNA-DNA hybridization studies showed that human A. viscosus strains were in fact more closely related to A. naeslundii genospecies 2 than A. viscosus and that A. viscosus includes strains resident in animals only (17). An additional genetic homology group, A. naeslundii genospecies WVA963, was also described. Multilocus sequence analysis has further clarified the relationships within this group, leading to a narrowing of the definition of A. naeslundii and the proposal of the new species A. oris for strains formerly assigned to A. naeslundii genospecies 2 and A. johnsonii for strains formerly assigned to genospecies WVA963 (10). Despite this work, human strains have continued to be assigned to A. viscosus, and it is often difficult to determine the taxon to which they belong in the current scheme. Most of these strains, however, are likely to be members of A. oris. For the remainder of this review, “A. viscosus” is denoted in quotation marks as a reminder of the taxonomic uncertainty regarding human strains identified as this species.
Taxonomic reassessment of members of this genus has led to some species being assigned to other genera, such as Actinobaculum suis (previously Actinomyces suis) (18), Cellulomonas humilata (formerly Actinomyces humiferus) (19), Trueperella bernardiae (formerly Actinomyces bernardiae and Arcanobacterium bernardiae) (20), and Trueperella pyogenes (formerly Actinomyces pyogenes and Arcanobacterium pyogenes) (20). In addition to Actinobaculum suis, which is of animal origin, three human Actinomyces-like species were also described as the novel species Actinobaculum schaalii (18), Actinobaculum massiliae (later corrected to A. massiliense) (21), and Actinobaculum urinale (22). Recently, A. schaalii and A. urinale were assigned to the new genus Actinotignum, and a strain from human blood was characterized and described as Actinotignum sanguinis (23). In the latter study, the type strain of Actinobaculum massiliense obtained from two culture collections was found to be phylogenetically distinct, on the basis of 16S rRNA gene sequence comparisons, from the strain originally described. The A. massiliense type strain currently deposited in culture collections appears to be closely related to A. schaalii (23). A group of Actinomyces-like strains isolated from human infections were found to represent a novel genus, Varibaculum, within the family Actinomycetaceae (24). The novel species “Actinomyces lingnae” and “Actinomyces houstonensis” were proposed by Clarridge and Zhang (25), but because the descriptions were incomplete and the strains have not been deposited with culture collections, the proposals have not been validated.
Until recently, all Actinomyces species described were isolated from microbiota associated with mammals. Rao et al. (26), however, isolated Actinomyces naturae from chlorinated solvent-contaminated groundwater. The optimal temperature for growth of this species, 30°C to 37°C, suggests that the primary source may have been an animal.
Although, as listed above, there have been a number of new Actinomyces species proposed in recent years, culture-independent studies directly targeting 16S rRNA genes have revealed sequences representing novel Actinomyces taxa. For the human mouth alone, the Human Oral Microbiome Database (http://www.homd.org/) lists 18 as-yet-unnamed species-level Actinomyces taxa (27).
NATURAL HABITATS OF ACTINOMYCES AND CLOSELY RELATED TAXA
Oral Cavity
Actinomyces is one of the predominant genera in the oral cavity. Indeed, at the age of 2 months, one-third of infants in one study were already colonized with Actinomyces (28). On oral mucosal surfaces of these edentulous infants, A. odontolyticus was the only representative of the genus found. The diversity of Actinomyces populations increased so that >90% of children harbored one or more Actinomyces species in their mouths from the age of 1 year onwards. During this 2-year longitudinal study, despite the appearance of A. naeslundii, “A. viscosus,” A. graevenitzii, and A. gerencseriae in the oral cavity at the time of the eruption of the primary teeth, A. odontolyticus clearly remained the most prominent oral Actinomyces species recovered from saliva in children (28). Actinomyces species play a central role in the initial stages of biofilm formation on teeth (i.e., dental plaque) both above (supragingival) and below (subgingival) the gumline (29). Indeed, A. odontolyticus, A. naeslundii, A. oris, and A. gerencseriae have been shown to participate in the formation of supragingival plaque on primary teeth of children aged 3 to 4 years (30). An interesting observation was that A. israelii, which is considered the major causative agent of human actinomycosis, is uncommon in the oral cavity of young children (28, 30). In the original description of A. georgiae (17), most characterized isolates came from gingival crevice samples from periodontally healthy children. A. odontolyticus has been shown to be one of the predominant Actinomyces species in developing biofilms on tooth surfaces in subjects of all ages, and the proportions found were not related to periodontal disease status (31). In a study characterizing 195 fresh Actinomyces isolates collected from supra- and subgingival plaque samples of five adults with chronic periodontitis, A. oris (formerly A. viscosus serotype II) was the isolate most commonly identified, followed by A. gerencseriae, A. naeslundii, A. georgiae, and A. israelii; A. meyeri was not found (32). In general, members of the genus Actinomyces are not considered to play a pathogenic role in periodontitis. Although the relative abundance of Actinomyces is decreased in diseased sites, its total biomass in subgingival plaque does not differ between periodontally healthy and diseased subjects (33). A. turicensis was reported to be the most common Actinomyces species on the tongue surface of eight subjects with oral malodor, followed by A. odontolyticus, A. israelii, and A. radingae (34). A recent study on the human oral microbiome, being part of the NIH-supported Human Microbiome Project and using comprehensive molecular tools, revealed A. georgiae, A. gerencseriae, A. israelii, A. meyeri, A. naeslundii, A. odontolyticus, A. oricola, A. radicidentis, as well as several not-yet-cultivated Actinomyces phylotypes as members of the resident microbiota in the human mouth (27).
Pharynx
Relatively few studies have characterized Actinomyces populations at nonoral human body sites. In nasopharyngeal specimens collected from infants during their first 2 years of life and examined by culture, Actinomyces organisms were common during acute otitis media episodes compared to their occasional presence during periods of health (35). In a recent study of the tonsillar crypt microbiota (36), oral species such as A. georgiae, A. israelii/A. gerencseriae, A. meyeri, A. naeslundii, A. odontolyticus, A. radicidentis, and the recently described A. cardiffensis and A. massiliensis were found. The bacterial diversity and composition within tonsillar crypts were compared on the one hand between 10 children and 10 adults and on the other hand between 10 subjects with recurrent tonsillitis and 10 corresponding healthy subjects. Interestingly, A. odontolyticus colonized all 20 subjects, and A. naeslundii colonized half of the subjects regardless of age or disease status, whereas A. cardiffensis and A. massiliensis were recovered mainly from healthy children (36).
Other Sites of Bacterial Colonization
It is often stated in reviews and case reports that in addition to the mouth, Actinomyces organisms are common inhabitants of the gut, genitourinary tract, and skin. It is true that at the phylum level, Actinobacteria are frequent colonizers of most ecological niches of the human body (37–39). At the genus level, however, the organisms found may belong to genera other than Actinomyces; for instance, until the introduction of accurate molecular methods of identification, many Bifidobacterium isolates from urine, cervix/uterus exudates, peritoneal wound, appendix wound, and blood were likely to have been misidentified as Actinomyces on the basis of their similarities in Gram stain and culture characteristics (40). Thus, organisms belonging to the phylum Actinobacteria may merely represent the genus Bifidobacterium in the gut or the genus Propionibacterium on skin and hair. The distal human esophagus has been reported to offer a relatively stable environment for bacterial colonization, including some Actinomyces species, such as A. odontolyticus (in particular), A. meyeri, and A. graevenitzii (41), although whether this site is truly colonized or whether the bacteria detected there are derived from saliva is unclear. A. graevenitzii was identified in biopsy specimens from the proximal small intestine of children with celiac disease, and an unidentified Actinomyces sp. was shown to be attached to the epithelial lining (38). Fecal samples were shown to harbor only a few clones identified as Actinomyces, with “A. viscosus” and A. odontolyticus being represented (42). Actinomyces species found in the human (female) urogenital tract in the absence of actinomycetal infection include A. meyeri, A. neuii, A. radingae, A. turicensis, and A. urogenitalis (43–47). After the detection of Actinobaculum schaalii (currently Actinotignum schaalii), a taxon closely related to the members of the genus Actinomyces, in urine, groin swabs, and vaginal swabs (18), it was recently suggested that this organism might be a relatively frequent commensal residing on the skin and mucosae of urogenital sites, but not in the colon, since no detection from feces was made (48).
Actinomyces at Normally Sterile Body Sites
Actinomyces species have been detected at body sites where microbes are not normally found. For example, Actinomyces was found to be a core component of the microbiota in sputum samples from 22 tuberculotic patients and 14 controls examined by 16S rRNA gene pyrosequencing (49). This is consistent with a report that Actinomyces was among the most prevalent genera in the sputum of cystic fibrosis patients (50). Urine has historically been considered a sterile fluid, but recent data have challenged this. Hilt et al. (51) used a combination of standard and expanded quantitative urine cultures and 16S rRNA gene sequencing to identify bacteria in urine specimens collected from 65 women (41 with overactive bladder and 24 controls). Actinomyces proved to be among the most prevalent genera, being found in 7% of the women. A. neuii, A. turicensis, A. urogenitalis, A. europaeus, A. odontolyticus, A. graevenitzii, A. naeslundii, and A. oris were detected, as were the closely related genus Actinobaculum (currently Actinotignum) and the species A. schaalii and A. urinale. Based on their detection of viable bacteria, those authors concluded that there is indeed a resident low-abundance microbiota in the adult female bladder (51). It is usually considered that overactive bladder is not of infectious origin; however, A. neuii and A. schaalii were recovered from symptomatic women only (11% and 16%, respectively) (52).
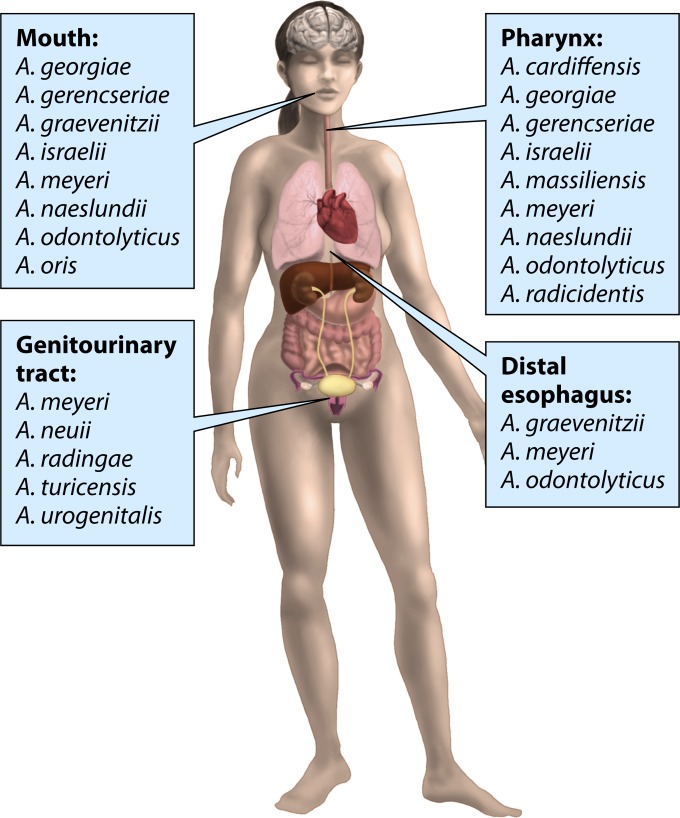
Figure 2 summarizes the distribution of Actinomyces species at various body sites of a healthy subject.
FIG 2.
Natural habitats of human Actinomyces species.
ACTINOMYCOSIS
Actinomycosis is generally considered an uncommon disease; however, current prevalence rates are not available, and furthermore, data from developing countries are incomplete (8). Actinomycosis affects subjects of all ages, although pediatric cases are much less frequent than cases in adults, where the disease is more common in men. The disease can appear in both immunocompetent and immunocompromised individuals (8, 9).
Typical actinomycosis is an indolent, slowly progressing granulomatous disease, which can be categorized, according to the body site, as orocervicofacial, thoracic, and abdominopelvic forms (7–9). The disease can also appear as cutaneous actinomycosis, musculoskeletal disease, pericarditis, infection of the central nervous system (CNS), or disseminated disease. Moreover, some actinomycosis cases have been linked to specific conditions, such as osteoradionecrosis or bisphosphonate-related osteonecrosis of the jaws (53, 54), the use of anti-inflammatory drugs (55, 56), or some hereditary diseases (57, 58). Also, unusual presentations of human actinomycosis have been reported, which presents a diagnostic challenge (59). Conversely, it is important to remember that Actinomyces species are found in a variety of polymicrobial infections, particularly associated with the head and neck, and only a minority of these are classical actinomycoses.
Early diagnosis of slowly progressing actinomycosis is difficult due to the nonspecific nature of the signs and symptoms of the disease, such as swelling, cough, low-grade fever, and weight loss, which leads to delays in patients seeking medical care. An additional diagnostic challenge is that a growing fibrotic mass, spreading through tissue planes, can resemble a malignant tumor (9). For example, in a recent case series of 94 thoracic actinomycosis cases (60), one-third of the cases were initially diagnosed as lung cancer, and only 6% were diagnosed as actinomycosis. Radiographic imaging techniques, such as computed tomography, and magnetic resonance imaging are valuable diagnostic tools for recognizing the location and size of the lesion(s) (61–63). Characteristic features of actinomycosis include chronic manifestations, abscess formation with sinus tracts, and purulent discharge (9). Hard macroscopic grains, “sulfur granules,” in pus have been considered among the confirmatory characteristics of actinomycotic lesions; however, they are not always present in pus samples (7, 12). When available, sulfur granules are of diagnostic value; in a histopathological examination, the granules are crushed, Gram stained, and viewed under a microscope, revealing typical Gram-positive branching filaments forming segment-like structures and being surrounded by inflammatory cells, mainly polymorphonuclear neutrophils (9, 14).
Orocervicofacial Actinomycosis
Orocervicofacial actinomycosis is the most common form of actinomycosis, consisting of more than half of all actinomycosis cases (7–9). According to the experience of Schaal and Lee (13) in Germany over a period of 25 years, cervicofacial actinomycosis cases were common, making up ∼25% of the odontogenic infections examined in their laboratory. This may not be surprising, since the mouth, particularly dental plaque, is the primary habitat of Actinomyces species. Indeed, poor oral hygiene is seen as an important predisposing factor for actinomycosis, as are smoking and heavy use of alcohol, reflecting the health behavior of the host. Actinomyces can easily gain access to oral tissues via invasive dental procedures such as tooth extraction (8).
To date, the most comprehensive data on the bacteriology of this form of actinomycosis come from a study conducted in two reference laboratories in Germany (12). Altogether, 12,253 specimens, collected between 1972 and 1999 from patients having cervicofacial inflammatory processes, were examined thoroughly by using culture-based techniques with a wide variety of growth media and incubation times of up to 14 days. Of these, 1,997 specimens yielded growth of filamentous bacteria, consisting primarily of A. israelii (42%) and A. gerencseriae (26.7%). In addition, A. naeslundii/“A. viscosus” was found in ∼9% of the specimens, while A. odontolyticus, A. meyeri, A. georgiae, and A. neuii subsp. neuii were occasionally recovered. Those authors suggested, however, that the latter Actinomyces findings could be due to these species being part of a polymicrobial infection rather than indicating their potential to cause true actinomycotic lesions, and the absence of real causative organisms may be explained by missing them in culture. Furthermore, 20% of Actinomyces isolates could not be identified to the species level, which is not surprising if the number of recently described novel species and the number of known unnamed species are considered. Noteworthy is that ∼90% of the 1,997 specimens also contained other bacterial species. In a reference laboratory in the United Kingdom, 88 clinical strains from unspecified infections of the neck-face area were identified; A. israelii, A. naeslundii, A. odontolyticus, and A. gerencseriae proved to be the main Actinomyces species, with each of them having a prevalence of ∼10% (64). Among the strain collection, several A. graevenitzii, A. meyeri, and A. turicensis as well as sporadic A. europaeus, A. georgiae, A. neuii, A. cardiffensis, and A. funkei strains were also identified (64, 65).
A recent report (66) reviewed 17 cases of pediatric cervicofacial actinomycosis, defined as a culture positive for Actinomyces or a biopsy specimen with “sulfur granules.” Of the 13 cases with a culture positive for Actinomyces, five isolates were identified as A. israelii, and three isolates each were identified as A. odontolyticus, “A. viscosus,” or A. bovis. It is not uncommon, however, that there are uncertainties in identifications in sporadic case reports. For instance, as nonhuman species, A. bovis and “A. viscosus” have probably been misidentified. Bacterial masses similar to those seen in actinomycosis, and from which A. israelii has been isolated, have also been recognized in pediatric osteomyelitis, and in the majority of cases, their location is the mandible (67).
Within the oral cavity, the hard palate is an uncommon site for actinomycosis, since only four cases have been described in the literature; in one of these cases, A. naeslundii was isolated from a diabetic patient (68). Another report described an ulcer-type actinomycotic lesion with A. odontolyticus on the oral mucosa of a patient with diabetes (69). Other locations for actinomycotic lesions categorized as cervicofacial actinomycosis include, for instance, the nasal and sinus region (70–72); pharynx (73, 74); larynx/tonsillae (75–78); middle ear, mastoid, and/or temporal bone (79–81); and skull base with the craniovertebral junction (82). A somewhat more distant location is the esophagus, from where actinomycotic lesions have also been recovered in both immunocompetent and immunocompromised individuals (83, 84). Except for actinomycotic cases with A. meyeri in the middle ear and mastoid (79) and A. israelii in the mastoid (81) and in the skull base (82), identified by molecular methods, as well as A. odontolyticus in the larynx (77), diagnoses were not based on microbiology but on clinical manifestations and histopathology with or without a presentation of sulfur granules. In some cases, culture was performed, resulting in “no growth,” or the species identification was not defined. Therefore, which specific Actinomyces species could have been involved in these actinomycotic lesions remains unclear.
Thoracic Actinomycosis
The main source of thoracic actinomycosis is considered to be the aspiration of oropharyngeal secretions, although hematogenous spread or direct spread from local infections can result in actinomycotic lesions at pulmonary sites (7, 8). Alternative causes to be considered in differential diagnoses include lung cancer, pneumonia, and tuberculosis (60). Since the spread of an actinomycotic lesion occurs despite anatomic barriers, invasion into the pleura or the chest wall can result in empyema or actinomycosis in the chest wall and surrounding bone structures (7, 9).
A. graevenitzii appears to have a predilection for respiratory sites (25, 64). Indeed, A. graevenitzii has been reported to be a causative organism in thoracic actinomycosis (56, 85, 86), multiple lung abscesses mimicking coccidioidomycosis (87), and organizing pneumonia with microabscesses (88). These observations are credible due to the possibility of aspiration, since A. graevenitzii colonizes the oral cavity in particular (28). A. meyeri (89–94), A. israelii (95, 96), A. odontolyticus (97, 98), and A. cardiffensis (99) have been recovered from actinomycotic lesions at pulmonary sites. A. cardiffensis has also been isolated from the blood of a patient with multiple lung abscesses and septicemia (100). Sporadic findings of A. naeslundii and “A. viscosus” from pediatric actinomycosis cases have been reported (95). Interestingly, a variety of typical oral species were present as concomitant bacteria in most specimens. Rarely, a progressing thoracic lesion extends to extrathoracic tissues, with abscess formation on the thoracic wall and pus eroding through the chest wall, causing “empyema necessitatis.” This is a severe condition, where A. odontolyticus, A. israelii, A. gerencseriae, and unspecified Actinomyces species have been detected as causative organisms besides mycobacteria and staphylococci (101–103). There are reports of cases where thoracic actinomycosis was found together with tuberculosis (85). In most of these cases, actinomycosis was due to A. israelii, while in one case, A. graevenitzii was identified as the causative organism.
Other Actinomyces species isolated from thorax specimens, collected in routine clinical laboratories and identified in a reference laboratory, were mainly A. meyeri and A. odontolyticus, but one isolate each of A. turicensis, A. cardiffensis, and A. funkei were also detected (64). Since there was no further clinical/histopathological information, it is not possible to confirm their connection specifically to actinomycosis.
Abdominal/Pelvic Actinomycosis
Abdominal actinomycosis is mainly a consequence of invasive procedures or abdominal infection such as appendicitis (8). Laparoscopic cholecystectomy with a lost gallstone(s) has been reported to be a potential complication leading to actinomycosis; A. naeslundii and an unspecified Actinomyces sp. were detected in two cases of abdominal abscesses (104), while A. meyeri was found in a case of abdominal actinomycosis extending from the kidney up to the thorax (105) and in an actinomycotic subphrenic abscess (106). A. israelii and A. meyeri have been identified in pus specimens from periappendical abscesses (107). A. meyeri was also implicated in splenic abscesses in a young girl with autoimmune hepatitis (108). In some abdominal actinomycosis cases arising from an abdominal source or even from the mouth, Actinomyces can result in pericarditis or the involvement of the liver; several Actinomyces species, particularly A. israelii (109–112) and A. meyeri (92, 113) but also A. funkei (114), A. odontolyticus (115), and A. turicensis (116), have been detected in liver abscesses. A. neuii has been detected in pericardial effusion samples of patients with chronic pericarditis (117). It is notable, however, that A. neuii is not considered to be involved in classical actinomycosis (118, 119).
Pelvic actinomycosis has usually been connected to Actinomyces present on an intrauterine contraceptive device (IUCD) after its prolonged use (8, 9). In a study conducted in Singapore, cervical smears of 1,108 women with IUCDs were screened for Actinomyces-like organisms by two cytologists (120). The prevalence of smears positive for target organisms was nearly 14%; however, no connection between positive smears and the duration of placement of the IUCD was found, contrary to most reports (121). Moreover, nearly all women, despite the presence of Actinomyces-like organisms, were asymptomatic (120, 121). When considering the frequency of use of IUCDs, the recovery of <100 actinomycotic specimens, most of those being tubo-ovarian abscesses, between 1926 and 1995 indicated that the risk of pelvic actinomycosis in relation to the use of IUCDs is very low (122). Among 130 Actinomyces isolates from clinical material associated with IUCDs sent to a reference laboratory for identification to the species level, one-third were identified as A. israelii, with its prevalence being double those of A. turicensis, A. naeslundii, A. odontolyticus, and A. gerencseriae, which were the next most common species (64). In an in vitro study, A. israelii grown in synthetic intrauterine medium was demonstrated to attach to and form spiderlike colonies and porous biofilm structures on copper plates, where the presence of sulfur was also confirmed (123). A. israelii has been found in IUCD users with pelvic manifestations (124, 125). Furthermore, A. odontolyticus (126) and some of the more recently isolated Actinomyces species, including A. urogenitalis (127, 128), A. hongkongensis (129, 130), A. cardiffensis (65), and A. turicensis (131, 132), appear to play a role in IUCD-associated pelvic actinomycosis. Certain gynecologic procedures may predispose an individual to complications with Actinomyces organisms; a case of bacteremia from a tubo-ovarian abscess caused by A. urogenitalis in a non-IUCD user exposed to a transvaginal oocyte retrieval procedure was reported (128), while in IUCD users, a similar procedure resulted in an infectious complication with A. israelii (124), and another gynecologic procedure, hysterectomy and salpingectomy, resulted in pelvic actinomycosis due to A. hongkongensis (129). In fact, the type strain of A. hongkongensis originates from a pus specimen from a pelvic actinomycosis case where ovarian tubes were described as being filled with pus (130).
The spread of causative organisms from pelvic sites to the abdominal region or vice versa can lead to abdominopelvic actinomycosis (7).
Other Types of Actinomycosis
Cutaneous actinomycosis.
Cutaneous actinomycosis is usually a secondary infectious process with an underlying focus at deeper tissues, or it appears as a result of hematogenous spread from actinomycotic lesion elsewhere in the body. Manifestations with a single or multiple draining sinuses can occur at various body sites, including the face, chest, midriff, hip, as well as upper and lower extremities. Primary cutaneous actinomycosis with multiple lesions has been described to be a first sign of a patient's HIV infection (133). A. meyeri and “A. viscosus” have been reported to be causative organisms of cutaneous actinomycosis (92, 133–135).
Musculoskeletal actinomycosis.
Musculoskeletal actinomycotic disease has been associated mainly with A. israelii. Typically, the patients' dentition and oral hygiene are poor, which are predisposing factors for the disease to occur. A recent report described an actinomycotic case with an involvement of the cervical spine, where A. meyeri was isolated from prevertebral pus samples and blood (136). Among 15 actinomycotic cases with an involvement of thoracic vertebral bone, however, A. israelii was detected in 9 of the cases, and A. meyeri was detected in 1 (137). In one A. israelii case, no signs of osteomyelitis in the spinal column were observed; the organism was detected in cerebrospinal fluid, and the entire spinal cord was involved, leaving the patient with severe neurological symptoms (138). Furthermore, A. israelii has been isolated from actinomycotic tissue biopsy specimens taken from the spine, together with Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans (139); iliac bone (140); and bones of a hand with extensive deformities (141). The latter was a most unusual case, initiated during the invasion of Normandy Beach in 1944, due to the persistence of the lesion despite long-term therapeutic interventions.
Cerebral actinomycosis.
Actinomycotic lesions in the CNS cause the most severe form of actinomycosis (7, 8). Actinomyces organisms usually gain access to this area either by hematogenous spread from remote sites or directly from local actinomycotic lesions of the head, and the disease usually appears as a single or multiple brain abscesses; among 70 cases of actinomycosis in the CNS, two-thirds proved to be brain abscesses (142). Actinomyces species isolated from cerebrospinal fluid include A. israelii, from a patient with meningitis (107), and A. naeslundii (sensu stricto) (10). Clinicians should be aware of the possibility of actinomycosis in the CNS, especially in patients with neurological symptoms who have a history of actinomycosis elsewhere in the body (143). Pediatric cerebral actinomycosis cases are very rare; A. israelii was detected in a 10-year-old boy with congenital heart disease (144), and “A. viscosus” together with Streptococcus constellatus were detected in an immunocompetent 7-year-old girl, who died due to subdural empyema (145). In the latter case, again, poor dental health was suspected to be a predisposing factor.
Disseminated actinomycosis.
Disseminated actinomycosis exhibits multiorgan involvement (9) and usually also has a polymicrobial nature; Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans is often among the coinfecting organisms (89, 146, 147). A. meyeri in particular has a tendency to be involved in disseminated infections (89, 146, 148). Despite the severity of disseminated infection, it can have clinically mild manifestations. For example, a patient with longstanding shoulder pain with gradual spread to the chest area was finally diagnosed with pericarditis and pneumonia, after many challenges. This infection was caused by Actinomyces and Actinobacillus (Aggregatibacter) actinomycetemcomitans, and 6 months later, the patient also developed a brain abscess (147). Notably, the patient's poor dentition was seen as a predisposing factor.
Actinomycosis Occurring in a Specific Context
Bisphosphonate-related osteonecrosis of the jaw.
Bisphosphonates are commonly used drugs in oncology. Bisphosphonate-related osteonecrosis of the jaws (BRONJ) is widely considered a specific disease entity where Actinomyces organisms may play an important role (54, 149). In three case series on BRONJ including a total of 96 patients, the rate of detection of Actinomyces varied between 53% and 86% (54, 150, 151). As known for most actinomycosis cases, concomitant or coinfecting organisms are usually present; therefore, it is conceivable that multiple bacterial morphotypes, located on active sites of bone resorption, were seen in BRONJ lesions examined by scanning electron microscopy (152).
Osteoradionecrosis.
Another type of therapy used in oncology, namely, irradiation of the head and neck area, can lead to devitalization and necrosis of the jaw bone. Out of 50 patients examined, 12% were diagnosed as having an actinomycotic bone lesion (150). It is notable that, already in the early 1980s, A. israelii was suggested to be an associated organism on the basis of immunocytological findings (153), but this was ignored at that time. Later, the impact of Actinomyces as an infectious organism under this condition was reinforced when Actinomyces, identified as A. israelii, was found prominently colonizing necrotic bone in the majority of 31 patients with osteoradionecrosis (53). Furthermore, it was shown that patients with bone biopsy specimens positive for Actinomyces were more susceptible to treatment failures than those with Actinomyces-negative biopsy specimens (154). In a study using sequencing of the 16S rRNA gene, however, only one A. israelii-positive specimen from osteoradionecrotic bone was found among six specimens examined (155), whereas the use of a DNA-DNA checkerboard method with targeted probes revealed the presence of Actinomyces species in all 12 resected jaw bone specimens examined (156). Ten specimens consisting of deep medullar bone of the jaw were positive for A. israelii, six for were positive “A. viscosus,” and five were positive for A. gerencseriae. These discrepancies in bacterial findings may be explained by different methodologies used in these studies.
Anti-tumor necrosis factor alpha drugs.
Anti-tumor necrosis factor alpha (TNF-α) drugs, which are increasingly used in the treatment of inflammatory diseases such as rheumatoid arthritis and Crohn's disease, have been linked to an increased susceptibility to bacterial infections. The most common infections resulting in hospitalization are pneumonia, skin/soft tissue infections, urinary tract infections, and bacteremia/sepsis (157). Sporadic actinomycosis cases have also been reported in this context, including thoracic actinomycosis due to A. graevenitzii (56), rapidly progressing pneumonia due to A. meyeri (93), as well as cutaneous actinomycosis, with one case due to A. neuii subsp. anitratus and another case due to coinfection with A. turicensis and A. urogenitalis (55).
Hereditary hemorrhagic telangiectasia.
Hereditary hemorrhagic telangiectasia is a vascular dysplasia with multiple-organ involvement. Recently, a case of multiple brain abscesses caused by A. israelii in a patient with hereditary hemorrhagic telangiectasia was described, and a review of such cases was conducted (57). Individuals with this syndrome are especially predisposed to brain abscesses, which are found in 75% of these individuals. In the literature between 1953 and 2013, ∼10 actinomycosis cases in telangiectasia patients are known, where A. israelii, A. meyeri, A. odontolyticus, and A. bovis were implicated (57, 158). Other bacteria were also present in half of the cases; for example, organisms isolated concomitantly with A. meyeri were Streptococcus intermedius, Fusobacterium nucleatum, Capnocytophaga spp., and Staphylococcus epidermidis (159), and in another case, organisms isolated concomitantly with A. odontolyticus included Haemophilus aphrophilus (now Aggregatibacter aphrophilus), peptostreptococci, and Bacteroides sp. (160). These findings suggest an oral source of these polymicrobial brain abscesses.
Chronic granulomatous disease.
The rare hereditary condition chronic granulomatous disease, which affects the clearance of phagocytosed microorganisms, can lead to severe infections, especially in the lungs, skin, lymph nodes, gastrointestinal tract, and liver, caused by fungi (e.g., Aspergillus) or aerobic bacteria (e.g., Staphylococcus aureus) (161). Interestingly, Actinomyces was detected in two pulmonary specimens from a total of 684 infectious episodes in 284 patients. Indeed, it was recently reported that patients suffering from chronic granulomatous disease could be especially vulnerable to actinomycosis (58). This case series of 10 patients consisted mainly of abscess specimens collected from the upper part of the body (submandibular region, neck, liver, and lung). Typically, recurrent infections in these patients are connected to catalase-producing bacteria and fungi (162), but here mainly catalase-negative Actinomyces species, including A. naeslundii (n = 5), A. gerencseriae (n = 1), A. meyeri (n = 1), an unspecified Actinomyces sp. (n = 1), and two cases specified as “actinomycosis,” were suggested to be causative organisms of chronic granulomatous disease-related actinomycosis (58). However, whether the described cases represented typical actinomycosis or another type of Actinomyces-associated infection remained unclear.
OTHER INFECTIONS WITH INVOLVEMENT OF ACTINOMYCES
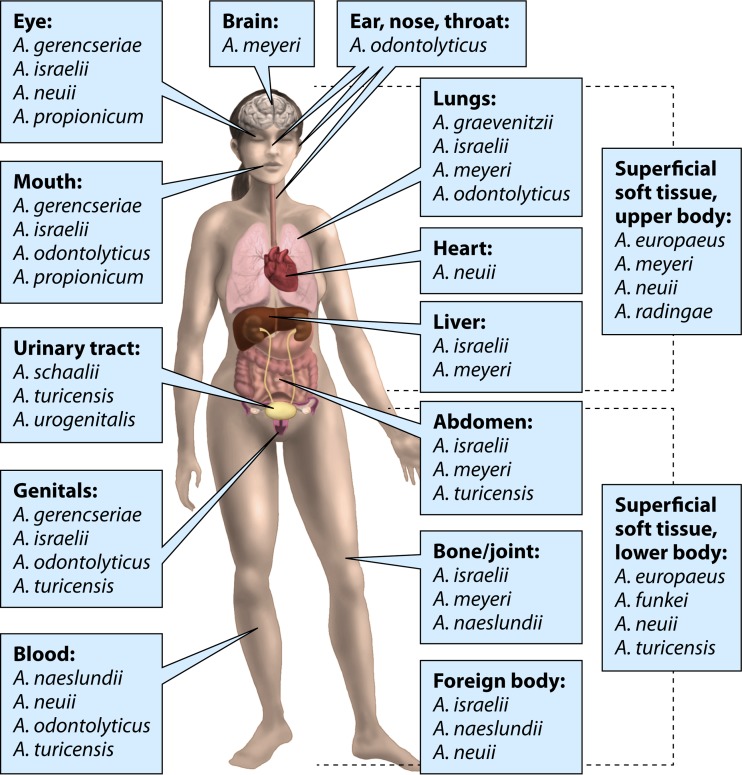
The range of clinical infections with an involvement of Actinomyces species is widening due to the introduction of novel species that have been described during this century. Figure 3 presents infectious sites and major Actinomyces recoveries from human infections besides classical actinomycosis.
FIG 3.
Major Actinomyces findings in human infections at different body sites.
Brain Abscesses
In a recent comprehensive nationwide study conducted in Norway, the bacteriology of brain abscess specimens collected over a 2-year period was examined by culture and direct 16S rRNA gene sequencing, and positive specimens (n = 52) were reanalyzed by using massive parallel sequencing (15). Of these specimens, 37 came from spontaneous brain abscesses, 3 were from spontaneous subdural infections, and 12 were from postoperative infections. That study revealed a bacterial detection rate by massive parallel sequencing that was three times higher than that obtained by standard culture-based routine diagnostics. The vast majority of abscess specimens yielded polymicrobial growth, where Aggregatibacter aphrophilus, Fusobacterium nucleatum, Streptococcus intermedius, and Actinomyces were among the predominant organisms. A very interesting observation was that nearly all Actinomyces isolates recovered from 37 spontaneous brain abscesses were identified as A. meyeri (n = 12), while other findings included A. georgiae (n = 2), A. israelii (n = 1), and Actinomyces sp. (n = 1) (15). Indeed, there are several reported cases of the involvement of A. meyeri in brain abscesses and/or other types of CNS infections (64, 92, 163), indicating the predilection of this organism for severe infections in the human CNS.
Eye Infections
Actinomyces species have been isolated from cases of endophthalmitis, keratitis, and canaliculitis. Of these, the most common infection is postoperative endophthalmitis after an ocular procedure, where A. neuii (164–166), A. meyeri (167), and “A. viscosus” (168) have been detected, while in cases of endogenous endophthalmitis, which is far less common, A. neuii (169) and A. israelii (170) have been detected. A. israelii has also been isolated from cases of postoperative keratitis (171) and primary infections of the lacrimal duct, i.e., canaliculitis (172–174). In addition, A. meyeri and A. georgiae have been found in specimens from cases of canaliculitis (172, 175), while A. naeslundii was identified in a swab specimen taken from a cornea after trauma leading to keratitis (107). Of 59 eye secretions/tear fluid examined in a reference laboratory in Germany, 22% were positive for “A. viscosus,” 19% were positive for A. israelii, 14% were positive for A. naeslundii, and 10% were positive for A. gerencseriae and A. odontolyticus each; A. israelii and A. gerencseriae were isolated from canaliculitis cases, whereas “A. viscosus,” A. naeslundii, and A. odontolyticus were more common in cases of conjunctivitis (13). Among 15 clinical strains collected from eye infections (infection not specified) and sent to a reference laboratory in the United Kingdom for identification, A. gerencseriae, A. israelii, A. odontolyticus, A. naeslundii, and A. georgiae, in descending order, were detected (64).
Ear, Nose, and Throat Infections
Actinomyces has been detected at increased frequencies in the nasopharynx of children suffering from recurrent otitis media during their first 2 years of life (35). In nasopharyngeal aspirates collected during acute otitis media episodes, one-quarter were colonized by A. odontolyticus, but A. gerencseriae was also found (176). In a culture-based study of peritonsillar abscesses, A. odontolyticus was found in one-quarter of 124 pus aspirates collected from young adults (177). A. turicensis has been detected in chronic otitis media and mastoiditis cases (47, 178), while one A. cardiffensis strain was found in pus collected from temporal, large, and small parietal and ear abscesses in a patient after mastoidectomy (65). Moreover, another A. cardiffensis strain was obtained from an antral washout specimen of a patient with sinusitis (65), and a novel A. nasicola strain was obtained from pus collected from the nasal antrum (179).
Oral Infections
Actinomyces species are involved in infectious processes of the mouth, including a wide variety of diseases induced by polymicrobial consortia living in biofilms.
Dental caries.
The role of Actinomyces in dental caries is well known; in this context, the most important species are A. gerencseriae and A. israelii (30, 180, 181). A. gerencseriae in particular is among the most active species in dental plaque of children suffering from severe early childhood caries, which is a chronic disease causing extensive destruction of the primary dentition (181). Elderly subjects with exposed root surfaces (usually due to treated or untreated advanced periodontitis) are susceptible to root caries, where certain Actinomyces species are considered important causative organisms, particularly A. israelii and A. gerencseriae, but A. naeslundii, A. odontolyticus, and A. georgiae have also been isolated (180). Both A. naeslundii genospecies 1 (A. naeslundii sensu stricto) and genospecies 2 (now A. oris) colonize active root caries lesions (182).
Endodontic infections.
The primary cause of endodontic infection is dental caries, which, when untreated, progresses into the pulp cavum and root canal, infecting the pulp and eventually causing necrosis of the pulpal tissue. Since the infectious process can be asymptomatic, if no root canal treatment is given, microorganisms may gain access to even periapical sites. Actinomyces species are rarely involved in early stages of endodontic infections but are typically found in persistent infections and extraradicular lesions (183–187). Rates of detection of Actinomyces in clinical endodontic samples varied from 9% among 53 specimens examined by DNA-DNA checkerboard analysis (185) to 56% among 129 specimens examined by PCR (187). There were also obvious differences in the species distribution: A. gerencseriae and A. israelii (185) on the one hand and “A. viscosus” and A. israelii (187) on the other hand were reported as major Actinomyces species in teeth with abscesses, whereas A. naeslundii was either absent (185) or present at low levels (187). In a culture-based study of refractory periapical infections, sulfur granules were found in 9 of 36 periapical lesions (186); five of the seven culture-positive granules grew Actinomyces, including A. israelii, “A. viscosus,” A. naeslundii, and A. meyeri. Also, A. radicidentis isolates have been recovered sporadically from endodontic specimens (184, 188). Most, if not all, endodontic infections are polymicrobial (183–187). Two novel Actinomyces species, A. oricola and A. dentalis, with one strain each, have been isolated from dental abscesses (189, 190).
Oral infections in tissues surrounding teeth/implants.
Actinomyces organisms, particularly A. naeslundii and A. oris, are common in dental plaque (10). Indeed, A. naeslundii has been found in cases of dental plaque-induced gingivitis, although A. naeslundii and A. gerencseriae were also reported to be associated with periodontal health (191). Somewhat surprising was the detection of A. gerencseriae as one of the dominant species of the microbiota at the gingival margin in relation to a severe form of gingival disease, necrotizing ulcerative gingivitis (192). Typically, Gram-negative anaerobes, such as fusobacteria, Prevotella intermedia, and spirochetes, are considered the etiologic organisms of this acute and painful disease, which destroys soft tissues around affected teeth, especially interdental gingival papillae. Elevated levels of A. neuii in subgingival sites of women with gingivitis were reported in a study where the DNA-DNA checkerboard technique was used as a detection method (193). Otherwise, A. neuii is seldom detected in oral biofilms. The known cross-reactivity of the checkerboard method may explain this unusual finding. Furthermore, A. odontolyticus, A. israelii, A. naeslundii/“A. viscosus,” A. meyeri, and A. gerencseriae, in descending order, have been recovered from a case of pericoronitis of wisdom teeth (194), while A. odontolyticus, A. naeslundii, “A. viscosus,” A. israelii, A. georgiae, A. gerencseriae, and A. graevenitzii, in descending order, were recovered from 33 failed dental implant fixtures, which had been removed due to infection (195). When bone was harvested from jawbone(s) for augmentation procedures, which may occasionally be needed for successful implantation of dental implant fixtures, A. odontolyticus was found to be among the most frequent contaminants of bone debris (196).
Pulmonary Infections
On the basis of the literature, it is unclear whether reported pneumonia or other disease cases with an involvement of Actinomyces represent thoracic actinomycosis or lung infection without specific actinomycotic lesions. For instance, in the original description of A. graevenitzii, three out of four strains characterized came from respiratory material, including bronchus brush, bronchial secretion, and sputum samples; however, no information on the clinical situation regarding these strains was given (197). This was also the case in another study, where four A. graevenitzii isolates were obtained from similar pulmonary sources (25). In a recent epidemiological investigation, A. graevenitzii proved to be the predominant species identified in Actinomyces-positive bronchoscopy cultures (198). The 18 case patients had abnormalities on chest radiography but no biopsy findings typical of pulmonary actinomycosis; 12 of the patients were positive for A. graevenitzii, 3 were positive for A. odontolyticus, 1 was positive for both A. graevenitzii and A. odontolyticus, and 2 were positive for unspecified Actinomyces species. An interesting observation was the significant increase in the number of Actinomyces-positive pulmonary specimens after the culture protocol was modified (198). Previously, A. israelii had been identified as the main Actinomyces species recovered in bronchial secretions (13). A. meyeri was isolated from a pus specimen related to an empyema that developed after pneumonia (107). Indeed, several A. meyeri cases in connection to pneumonia have been reported (92). Besides A. meyeri, A. odontolyticus, A. turicensis, A. cardiffensis, and A. funkei were among thorax specimens that were identified in a reference laboratory in the United Kingdom (64); due to the lack of other information, it was not possible to estimate whether they were associated with thoracic actinomycosis or other pulmonary infections.
Superficial Infections
The upper body.
Many Actinomyces species have been identified in soft tissue abscesses of the body above the waistline. Breast tissue is a site commonly affected by actinomycosis, with A. neuii especially being identified as a causative organism (11, 64, 119, 175, 199–201). Also, A. europaeus (25, 64, 202, 203), A. radingae (25, 64, 204), A. meyeri (92, 205), and A. turicensis (25, 204, 206) have been recovered. In one breast abscess case, A. turicensis was isolated in pure culture (25). Nipple piercing was suggested to be a predisposing factor for Actinomyces infection of the breast in two patients, one due to A. radingae (204) and another due to A. turicensis together with a Gram-positive anaerobic coccus, Peptoniphilus harei (206). Other sites of the upper body with abscesses with an involvement of Actinomyces organisms include the face, neck, axilla, armpit, chest, and/or back, with A. europaeus, A. georgiae, A. meyeri, A. neuii (both subspecies), and A. radingae being the identified species (25, 64, 92, 107, 175, 205). In addition, A. turicensis has been isolated from necrotic tissue associated with cervicofacial fasciitis (107), and A. neuii has been isolated from a mammary hematoma (207).
The lower body.
The majority of skin-related infections caused by Actinomyces below the waistline are due to A. turicensis (25, 64, 107, 178, 208, 209). Abscesses are typically found in the groin, buttock, rectal area, and skin of the genitals. Other Actinomyces species commonly detected include A. europaeus, A. funkei, A. neuii (both subspecies), and A. radingae (25, 47, 64, 107, 114, 175, 178, 202, 209). It has been suggested that lipid-rich areas are favorable for the growth of these Actinomyces species (178). Furthermore, A. europaeus, A. funkei, and A. turicensis have been isolated from decubitus ulcers (25, 114, 202); A. neuii and A. radingae have been isolated from diabetic ulcers (118, 175); and A. europaeus, A. turicensis, and A. odontolyticus have been isolated from skin-related infections in lower extremities (25, 202, 210). It was suggested that the latter species, isolated from an intravenous drug abuser, originated from the oral cavity due to licking of an injection needle (210). This is consistent with the results of a study comparing bacterial recoveries from soft tissue abscesses of intravenous drug abusers and nonusers (211). Oral-type bacteria, including A. odontolyticus, dominated in the majority of abscesses of drug abusers but not nonusers. In another case, A. odontolyticus together with Eikenella corrodens, both oral species, caused infection in a foot due to trauma by toothpick puncture (212). A coinfection by two Actinomyces species, A. europaeus and A. turicensis, resulting in subcutaneous fistulae in association with an irritative exoprosthesis of a leg was reported (213). Sporadic Actinomyces findings include A. neuii and A. europaeus in infected atheromas (118, 202) and an A. hominis strain in a wound specimen (214), but their location was not specified. A case with a large vulvar lesion was connected to A. israelii, present together with Propionibacterium acnes and Peptostreptococcus sp. (215). Recently, a polymicrobial case of Fournier's gangrene affecting an immunocompetent elderly man was analyzed by using 16S rRNA gene sequencing, which revealed the presence of A. funkei together with Fusobacterium gonidiaformans and Clostridium hathewayi as etiologic organisms (216). Seven cases of A. funkei infection were also detected recently in Spain by using sequencing-based isolate identification. Positive specimens included specimens from three abscesses, three wounds, and one bronchial aspirate (216).
Genitourinary Infections
Genital tract.
The most common infections due to Actinomyces species in the genital tract are associated with the use of an IUCD. Among 130 isolates from IUCD-related infections, A. israelii in particular as well as A. turicensis, the A. naeslundii-“A. viscosus” complex, A. odontolyticus, and A. gerencseriae were identified as the main species in a reference laboratory in the United Kingdom, but A. cardiffensis and A. funkei were also not uncommon (64, 65). According to data from a reference laboratory in Germany (13), A. israelii was the most frequently detected isolate from IUCDs and cervical secretions. Also “A. viscosus” was frequently found, whereas A. gerencseriae, A. naeslundii, and A. odontolyticus were identified in only 5% of 82 specimens. In addition, A. urogenitalis and A. radingae have been isolated from IUCDs and vaginal secretions (11, 45, 175). A. urogenitalis has also been detected in high numbers in vaginal samples from patients with bacterial vaginosis (45). A. turicensis can be detected in a variety of infections of the female genital tract, such as adnexitis, endometritis, cervicitis, vaginitis, and vulvitis (178). In pregnancy, A. neuii has been found in infected amniotic fluid (118) and in severe cases of chorioamnionitis, leading to sepsis of the neonate (217). A. meyeri has been found to cause chorioamnionitis, leading to necrotizing funisitis and preterm birth (218). In infections of the genital tract in males, A. neuii has been detected in prostatitis cases (118), and A. turicensis has been detected in balanitis, penile abscess, and prostatitis cases (64, 175, 178).
Urinary tract.
In both males and females, A. turicensis has been detected in connection with urethritis and cystitis (25, 107, 178). Other Actinomyces organisms found in the urinary tract include A. urogenitalis from urethra and urine (45), A. neuii in urinary tract infection (118), and A. europaeus in patients with cystitis or purulent urethritis (178). There are often other bacteria present, and only slightly elevated levels of leukocytes are observed in urine (178).
Bone/Joint Infections
Actinomyces species have been recovered from infections affecting bone, including osteomyelitis with an involvement of A. meyeri in the jaw, symphysis pubis, leg (tibia), and foot (92); A. israelii in osteomyelitis of the sternum (219); A. naeslundii in chronic osteomyelitis with thickening of the periosteum in the lower leg (220); and A. graevenitzii in an osteitis lesion of the jaw (197). There are also reports where two causative organisms of chronic osteomyelitis have been described, including A. meyeri together with Fusobacterium nucleatum in the leg (fibula) (221) and A. neuii subsp. neuii together with Dermabacter hominis in the calcaneus (222). Spondylodiscitis can rarely be due to Actinomyces organisms, such as A. meyeri (92) and A. israelii (223). A strain representing the novel Actinomyces species A. timonensis was isolated from an osteoarticular specimen of a 13-year-old girl suffering from chronic sacroiliitis (224).
Foreign-Body Infections
Different types of devices and materials are increasingly used in modern medicine to support defective or lost functions of the human body. According to recent reports, A. neuii, especially A. neuii subsp. neuii, seems to be the most frequently detected Actinomyces species in infected tissues around different types of prostheses, including mammary prostheses (225), penile prostheses (226), prosthetic valves (227), and hip prostheses (228), but also in connection to medical devices such as ventriculoperitoneal shunts (229, 230) and peritoneal dialysis catheters (231). In addition, A. israelii and A. naeslundii have been identified as causative agents of periprosthetic infections of the hip or knee joints (232–234). Moreover, A. odontolyticus was isolated from a subcutaneous abscess connected to an exit site of a catheter in a patient treated with continuous ambulatory peritoneal dialysis (235). Recently, A. meyeri was found to be the cause of a disseminated infection resulting in fatal mediastinitis after an esophageal stent operation (236).
Infective Endocarditis
Although Actinomyces species are rarely considered causative organisms in cases of endocarditis, isolation of A. neuii (119, 227, 237), A. funkei (238), A. israelii (239, 240), “A. viscosus” (240–242), and A. meyeri (92, 243) has been reported. One reason for the rarity of reports of Actinomyces in blood specimens could be due to considering the finding to be insignificant or, if an organism is isolated, to the difficulty of identification of members of this group. Methodological improvements in identification and the availability of these methods in clinical microbiology laboratories, e.g., matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry and 16S rRNA gene sequencing, may allow more definitive data to be collected.
Bacteremia/Sepsis
Several Actinomyces species have been detected in blood, including A. funkei (244), A. georgiae (64, 175), A. graevenitzii (245), A. massiliensis (246), A. naeslundii (10, 64, 247), A. neuii (107, 119, 207), A. odontolyticus (25, 64, 97, 107), A. turicensis (64, 175, 178), and “A. viscosus” (107). An important source of Actinomyces bacteremias is the mouth in particular. Since bacteremia is common after invasive dental procedures and after tooth brushing in subjects with gingivitis and periodontitis, who typically have inflamed bleeding gums, oral actinobacteria can easily gain access to the bloodstream; A. georgiae, A. meyeri, A. odontolyticus, A. cardiffensis, and a number of unnamed Actinomyces phylotypes were detected in blood samples particularly following the extraction of a tooth without antibiotic prophylaxis (248).
INFECTIOUS ROLE OF SOME ACTINOMYCES-RELATED ORGANISMS
Actinotignum (Formerly Actinobaculum) Species in the Urogenital Tract
Five Actinomyces-like strains characterized as A. schaalii were isolated from either human blood or urine in the original description of the novel genus Actinobaculum (18). Subsequently, two novel Actinobaculum strains were detected in urine samples from women with urinary tract infections, namely, A. massiliae (A. massiliense) and A. urinale (21, 22). Following these reports, it remained unclear as to whether these species played a significant role as human pathogens. This may have been because routine aerobic culture of urine samples, with an incubation time of 18 to 24 h, was not adequate for the detection of these fastidious and slow-growing species. In addition, if they had been cultivated, they may have been regarded as being insignificant commensals. Therefore, the first reports on their involvement in infections of the urinary tract came from laboratories with a special interest in unusual organisms and expertise in molecular detection methods. Among these organisms, A. schaalii was found to be a causative organism in several cystitis cases (249) and, more significantly, in patients with disseminated infection (249–252). The first described pediatric case of A. schaalii infection was that of a child with purulent pyelonephritis (253). Sporadic findings of A. urinale and A. massiliense were also reported (250, 252, 254). Before the 2010s, there were a total of ∼30 cases caused by human Actinobaculum (currently Actinotignum) species in the literature. Since then, an extensive number of reports, especially on A. schaalii as an important uropathogen, have been published.
Typically, individuals affected by A. schaalii have an underlying condition in the urinary tract and are >60 years of age (255–257). Interestingly, considerable proportions of individuals without symptoms can also be positive for this organism; urine specimens from 13% of 38 healthy controls (aged between 63 and 81 years) proved to be positive by real-time PCR (255), and 22% of 55 culture-positive elderly subjects were characterized as having asymptomatic bacteriuria (256). A. schaalii is thus considered a uropathogen in the elderly, but the significance of this species in children is receiving attention (258, 259). Cystitis, pyelonephritis, and urosepsis are the major forms of Actinotignum (formerly Actinobaculum)-associated infections; however, other types of infections, especially with A. schaalii, are increasingly being reported, including bacteremia, abscesses, cellulitis, spondylodiscitis, bladder necrosis, epididymitis, and endocarditis (16, 260–264). In a case of Fournier's gangrene, only A. schaalii was detected by 16S rRNA gene sequencing (265). Despite improved techniques and targeted searches for Actinotignum (formerly Actinobaculum) species in urine specimens, only single findings of A. massiliense and A. urinale in urine or blood have been made (256, 261, 266), differing from the obvious involvement of A. schaalii in infections in, and occasionally outside, the urogenital tract.
Varibaculum cambriense as a Soft Tissue Pathogen
Fifteen Actinomyces-like strains from infections at a variety of body sites in 14 subjects were characterized and described as belonging to the genus Varibaculum, including one species, V. cambriensis (later corrected to V. cambriense) (24). Most cases were abscesses, including sites such as brain, ear, cheek, jaw, breast, and the ischiorectal area, but were also infections of the female genital tract with or without an IUCD. In addition, a study performed in Hong Kong in 2006 reported four cases caused by V. cambriense (267). All four subjects suffered from superficial abscesses, with the pus specimens originating from a recurrent umbilical scar with abscess formation on the skin, an abscess in the groin, a persistent lump in the groin, and an abscess on the back. Taken together, V. cambriense was proven to be a pathogen involved in superficial soft tissue infections.
Propionibacterium propionicum Infections
Propionibacterium propionicum is an organism resembling Actinomyces (formerly Actinomyces propionicus and then Arachnia propionica), but it differs from Actinomyces by producing large amounts of propionic acid as its metabolic end product in glucose fermentation (14). The morphological and biochemical resemblance to A. israelii is particularly striking. P. propionicum can cause infections similar to those with an involvement of Actinomyces organisms, such as actinomycotic lesions and abscesses (12, 13). In addition, this organism has been associated mainly with infections of the eye (13, 172, 268) and various endodontic infections (183, 184, 269, 270). In a German reference laboratory, 22% of 59 specimens from eye secretions/tear fluid and 12% of 43 bronchial secretion specimens were found to be positive for P. propionicum (13). Of 26 P. propionicum isolates recovered from patients with eye infections by a United Kingdom reference laboratory, the majority originated from cases of canaliculitis in the elderly (268). A study using a nested-PCR assay targeted to P. propionicum and A. radicidentis revealed high rates of detection of P. propionicum in primary endodontic infections: 50% in teeth with acute apical periodontitis, 37% in teeth with periradicular abscesses, and 29% in teeth with chronic periradicular lesions (184). Moreover, a different disease pattern was observed for A. radicidentis. P. propionicum was also among the species related to endodontic treatment failures (270). A pulmonary infection with multiple microabscesses in a 7-year-old child with chronic granulomatous disease (271) and a brain abscess in a young male with congenital cyanotic heart disease (272) due to P. propionicum have been described. The involvement of P. propionicum in brain abscesses is very rare, with only two reports in the current literature (272, 273). The first case of pelvic actinomycosis caused by P. propionicum was that of a woman who had long-term use of an IUCD as a predisposing factor (274). In addition to P. propionicum, two peptostreptococcal organisms were isolated from a pus specimen from the right ovary, and their identification was confirmed by 16S rRNA gene sequencing. Although three different microorganisms were present, of those, P. propionicum was considered the most significant (274). Recently, a psoas abscess caused by P. propionicum in a woman with no history of IUCD, other foreign bodies, or previous surgery was reported (275).
VIRULENCE PROPERTIES OF ACTINOMYCES
Little is known regarding virulence factors of Actinomyces species. They do not produce classical exotoxins, and the virulence determinants that allow A. israelii, A. gerencseriae, and others to cause actinomycosis are unknown. Presumably, they possess the ability to evade clearance by the host immune system and, thus, cause a chronic lesion. An A. israelii strain that was able to cause infection in an animal model was rapidly phagocytosed in vitro, and it was hypothesized that the ability of this organism to form a dense mass of interlinked branched chains of bacilli inhibited phagocytic clearance in vivo (276).
The virulence factors involved in polymicrobial soft tissue infections are also poorly characterized, but it is thought that multispecies communities can work together to evade the host, for example, by the production of a capsule (277) and serially degrade host tissues to provide nutrients for the whole community (278). Since Actinomyces species are frequently isolated from polymicrobial infections, they must be assumed to contribute to the pathogenetic processes involved in such situations.
A. oris and A. naeslundii play an important role in dental plaque (biofilm) formation. They are early colonizers and produce fimbriae that bind to saliva proline-rich proteins and statherin, which adsorb onto the tooth surface (279, 280). They also interact with a range of other dental plaque bacteria, including representatives of the genera Fusobacterium, Prevotella, and Veillonella, by coaggregation (281), which provides structural integrity to the plaque (282). Once a biofilm has formed, and in the presence of a fermentable carbohydrate, many plaque bacteria, such as Actinomyces species, can produce acid, which may lead to dental caries (182, 283).
CONCOMITANT/COINFECTING MICROBES
It is noteworthy that the vast majority of actinomycotic lesions as well as other Actinomyces-associated infections are polymicrobial, with the rate of occurrence of concomitants varying between 75 and 95% (12, 13, 178). The role of concomitant organisms is considered to synergistically enhance the infectious process. Bacteria frequently occurring together with Actinomyces species include strict anaerobes, such as Fusobacterium spp.; members of the family Bacteroidaceae; and Gram-positive anaerobic cocci (GPAC), especially Parvimonas micra, microaerophilic anginosus group streptococci (formerly “Streptococcus milleri”), and the capnophilic Aggregatibacter species A. actinomycetemcomitans (formerly Actinobacillus actinomycetemcomitans) and A. aphrophilus (formerly Haemophilus paraphrophilus), and aerobic coagulase-negative staphylococci.
Analysis of material from sulfur granules and/or pus of 1,997 specimens from cervicofacial actinomycotic lesions, collected by incision or needle aspiration and thoroughly examined under various culture conditions and with prolonged incubation, revealed that 95.5% of specimens were positive for not only fermentative actinomycetes, mainly A. israelii and A. gerencseriae, but also aerobic and/or anaerobic companions (12). “Microaerophilic and anaerobic streptococci” were identified in 49.7% of the specimens. Based on previously reported data from the same laboratories in Germany, it can be estimated that 60% of these organisms were anginosus group streptococci and that 40% were GPAC (13). Other common findings, in descending order, were coagulase-negative staphylococci (39.1%); Fusobacterium spp. (37.7%); Propionibacterium spp. other than P. propionicum (27.5%); pigmented and nonpigmented Prevotella-Porphyromonas spp. (25.1% and 21%, respectively); corroding Gram-negative rods (18.5%), including Campylobacter gracilis, Capnocytophaga spp., and Eikenella corrodens; and Aggregatibacter actinomycetemcomitans (14.2%) (12).
Data on pediatric cases include data from 10 case reports of cervicofacial actinomycosis, 8 of which reported the presence of concomitant organisms (66), and 14 case reports of thoracic actinomycosis, with all specimens being positive for concomitants (95). In cases of cervicofacial actinomycosis, except for a recent report of concomitants representing the genera Capnocytophaga, Prevotella, Enterococcus, and Streptococcus, only a few, poorly defined bacterial taxa have been identified (66). Among 14 thoracic actinomycosis cases involving children, Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum were the most commonly detected organisms, being detected in 9 and 5 cases, respectively (95).
Brain abscesses with an involvement of A. meyeri seem to harbor striking similarities in the compositions of their concomitant and/or coinfecting microbiota. Based on data reported recently by Kommedal et al. (15) (Table 2), 4 to 10 species were found in specimens from 12 spontaneous brain abscesses with an involvement of A. meyeri. In these specimens, Fusobacterium nucleatum, Parvimonas micra, and Streptococcus intermedius were the major concomitants, but also, Aggregatibacter aphrophilus, Campylobacter gracilis, Eikenella corrodens, and Eubacterium brachy were each detected in at least half of the specimens (15). All these organisms are inhabitants of the oral cavity. Interestingly, Aggregatibacter aphrophilus has been linked to invasive infections of the CNS, and its frequency in brain abscesses was considered disproportional to its presence in its natural habitat, the oropharynx (284). Among 26 various types of actinomycosis cases caused by A. meyeri identified from 1960 to 1995, 17 cases involved concomitants; of these, Aggregatibacter actinomycetemcomitans was the most common (89). This is, however, contradictory to the assumption that Aggregatibacter actinomycetemcomitans acts as a concomitant solely for A. israelii (25).
TABLE 2.
Concomitant/coinfecting organisms in 12 brain abscess cases with an involvement of Actinomyces meyeria
| Case | Concomitant/coinfecting organism |
|||||||
|---|---|---|---|---|---|---|---|---|
| Aggregatibacter | Campylobacter | Eikenella | Eubacterium | Fusobacterium | Parvimonas | Streptococcus | Other | |
| A | A. aphrophilus | C. gracilis | E. corrodens | F. nucleatum | Parvimonas sp. | |||
| C. rectus | ||||||||
| B | A. aphrophilus | C. gracilis | E. brachy | Fusobacterium sp. | P. micra | S. intermedius | Actinomyces georgiae | |
| Parvimonas sp. | ||||||||
| C | A. aphrophilus | F. nucleatum | P. micra | S. constellatus | Anaeroglobus geminatus | |||
| Parvimonas sp. | ||||||||
| D | A. aphrophilus | S. intermedius | Actinomyces georgiae | |||||
| Capnocytophaga | ||||||||
| E | A. aphrophilus | C. gracilis | E. corrodens | E. brachy | F. nucleatum | P. micra | S. intermedius | Capnocytophaga |
| E. yurii | Tannerella forsythia | |||||||
| F | Fusobacterium sp. | Parvimonas sp. | S. intermedius | |||||
| G | C. gracilis | E. corrodens | F. nucleatum | P. micra | S. intermedius | Gemella morbillorum | ||
| Parvimonas sp. | ||||||||
| H | E. brachy | F. nucleatum | P. micra | S. intermedius | ||||
| I | C. gracilis | E. corrodens | E. brachy | F. nucleatum | P. micra | S. intermedius | Prevotella oris | |
| E. yurii | Prevotella timonensis | |||||||
| Tannerella forsythia | ||||||||
| J | C. gracilis | E. corrodens | F. nucleatum | P. micra | S. intermedius | Prevotella sp. | ||
| Fusobacterium sp. | ||||||||
| K | C. rectus | E. brachy | F. nucleatum | P. micra | S. intermedius | |||
| L | A. aphrophilus | C. gracilis | E. corrodens | E. brachy | Fusobacterium sp. | Parvimonas sp. | S. intermedius | Johnsonella ignava |
Data adapted from reference 15.
In a case report of a polybacterial brain abscess, Capnocytophaga spp. and Streptococcus intermedius were found together with Actinomyces, i.e., all typical inhabitants of the mouth, thus indicating an oral source, since the 25-year-old patient had severe gingivitis and three infected wisdom teeth (285). The authors of that report underlined the importance of prolonged incubation in detection of these fastidious organisms. In another case, after 14 days of incubation, nonpigmented A. odontolyticus was isolated together with Haemophilus paraphrophilus (i.e., Aggregatibacter aphrophilus), Fusobacterium nucleatum, and Peptostreptococcus micros (now Parvimonas micra) (286). A case of a cerebellar abscess where Actinomyces (“non-israelii” Actinomyces) was found among other oral-type bacteria, namely, alpha-hemolytic streptococci, Eikenella corrodens, and P. micros (i.e., P. micra), was suggested to be linked to tongue piercing (287). On the other hand, according to a report on five intracranial cases of Actinomyces infection in immunocompetent individuals, three cases were coinfected with another bacterium, including Escherichia coli, Pseudomonas aeruginosa, or Staphylococcus warneri (61). These findings obviously reflect an origin other than the oral cavity. Aggregatibacter species have not been detected together with A. funkei, A. radingae, or A. turicensis. Instead, their typical concomitants are, for example, Bacteroides spp., especially B. fragilis; enterococci; and coagulase-negative staphylococci but also GPAC (25, 114, 288). Together with A. europaeus, coagulase-negative staphylococci and corynebacteria, commonly found on the skin, are typical (25).
Similar to infections with Actinomyces, those with P. propionicum and A. schaalii are often polymicrobial (13, 257). For example, together with A. schaalii, other bacteria were isolated from 5 of 12 (42%) urine and 5 of 21 (24%) blood specimens examined, while polymicrobial infection was detected in all 7 abscess specimens (257). In polybacterial blood cultures, Pseudomonas aeruginosa, Finegoldia magna, Clostridium clostridioforme, Bacteroides fragilis, and/or Veillonella spp. were identified.
IDENTIFICATION IN CLINICAL LABORATORIES
Direct microscopic examination of samples collected from suspected cases of actinomycosis should be performed. The presence of masses of Gram-positive branching filaments is characteristic of the disease, as are sulfur granules visible with the naked eye, although, as discussed above, these may not always be present. The presence of branching Gram-positive organisms in cervical smear specimens from women with an IUCD suggests an infection with Actinomyces (122). Results of microscopy of samples from superficial infections at mucosal surfaces where Actinomyces and other Gram-positive bacilli are commonly found should be interpreted with caution. It is important not to imply a diagnosis of actinomycosis in the absence of a relevant clinical history, signs, and symptoms.
Identification of presumptive Actinomyces species using conventional biochemical tests is possible for most species but challenging (14). Strains frequently exhibit indifferent growth in test media, leading to false-negative results and poor reproducibility. Recent taxonomic changes have compounded these problems. Many species descriptions have been based on a single strain, so the natural variation of test results within species is unknown. Furthermore, the taxonomy of some species, e.g., members of the A. naeslundii group, have been clarified on the basis of multilocus gene sequence analysis, and there are no phenotypic characteristics that differentiate certain species (10).
Commercially available identification kits that test for preformed enzymes can be used to identify the members of this group. Although kits offer a convenient method for isolate identification, they are typically supported by databases, which are incomplete in that either representatives of recently described species are not included or profiles for named species are inaccurate (175, 289).
Because it is now recognized that the use of conventional and biochemical tests may result in misidentification of clinical isolates of Actinomyces and related taxa, alternative methods with greater precision are increasingly being used. 16S rRNA gene sequence analysis, which was originally used to reconstruct phylogenetic relationships between organisms, is now frequently used for isolate identification. Far more precise identifications can be obtained by 16S rRNA gene sequence analysis (290). Genomic DNA can be extracted easily from isolates by using commercially available kits and the 16S rRNA gene amplified with “universal” primers that amplify all members of the domain Bacteria (291). A partial sequence of ∼500 bp will allow the identification of most Actinomyces species. Sequences can be submitted via the Internet to databases such as the Ribosomal Database Project (292) for identification. Although this method is extremely powerful and can be relied upon for genus-level identification, some validly published species have highly similar 16S rRNA sequences, and there has been extensive recombination in the genomes, including the 16S rRNA gene, among certain groups of organisms, notably those that are naturally competent, such as the genus Neisseria (293). The A. naeslundii group presents similar problems, and 16S rRNA gene sequence analysis does not allow unequivocal identification of some of the recently described members of this group (10).
A bacterial identification method based on MALDI-TOF mass spectrometry is being widely adopted by clinical laboratories (294). Evaluations of its use with Actinomyces species have yielded positive results. In a comparative study, MALDI-TOF mass spectrometry correctly identified 97% of 32 strains to the species level, while a commercially available biochemical kit achieved only 33% success (209). This method was also shown to correctly identify five clinical isolates of A. neuii (295).
CLINICAL ANTIMICROBIAL SUSCEPTIBILITY TESTING
Susceptibility testing of anaerobes is seldom performed in clinical microbiology laboratories. In general, this may not be an important issue with Actinomyces species, since they are rarely, if ever, resistant to beta-lactam agents (14). As Gram-positive organisms, they are also susceptible to vancomycin. Whenever isolates originate from serious invasive infections, however, antimicrobial susceptibility testing is indicated. Standard methods and interpretations, as for other anaerobic Gram-positive nonsporing rods, can be used. Recently, an excellent review on susceptibility testing of anaerobic bacteria was reported, describing the methods to be used and their challenges (296). For testing of single clinical isolates, a commercially available MIC gradient diffusion method, which gives results equivalent to those generated by using the Clinical and Laboratory Standards Institute (CLSI) agar dilution method (296), is the most appropriate choice.
CONCEPTS OF TREATMENT
An appropriate diagnosis is the cornerstone to be able to choose a proper treatment modality and, conceivably, to have a successful treatment outcome. In the case of actinomycosis, it has been considered that a long duration of antimicrobial therapy with high doses is necessary, with treatment extending up to 1 year (or even longer). This concept is changing, and medications are now adjusted on the basis of individual treatment needs (8). The same is valid for surgery, which was previously used routinely for treatment of actinomycotic lesions; however, the current trend is to limit invasive procedures and to rely on a targeted antibiotic regimen instead (8, 9). Treatment of abscesses usually requires drainage, whereas resective surgery may be indicated only in cases with extensive necrotic lesions or when antimicrobial therapy fails.
In general, Actinomyces species are susceptible to penicillin and other beta-lactam antibiotics as well as to most agents used against Gram-positive anaerobic rods; however, it must be noted that they are intrinsically resistant to metronidazole (14). In a recent survey on antimicrobial susceptibilities of anaerobic bacteria conducted in laboratories in Ontario, Canada, nearly 400 Actinomyces and 30 Actinobaculum (currently Actinotignum) strains were tested against six antimicrobial agents, including penicillin, piperacillin-tazobactam, meropenem, cefoxitin, clindamycin, and metronidazole (297). Except for high rates of resistance to metronidazole (>80% for both genera) and clindamycin (18% for Actinomyces and 53% for Actinobaculum), the strains were fully susceptible to the other antimicrobial agents examined. Moreover, it is noteworthy that there are considerable differences in MICs among Actinomyces species, with A. europaeus and A. turicensis being the most resistant (298). A. turicensis strains showed resistance to clindamycin, tetracyclines (doxycycline and tetracycline), macrolides (clarithromycin and erythromycin), ciprofloxacin, and/or linezolid, whereas A. europaeus strains showed resistance to ceftriaxone, clindamycin, macrolides (clarithromycin and erythromycin), ciprofloxacin, and/or tazobactam (298). In addition, sporadic strains with increased MIC values to tested antimicrobial agents have been observed for other Actinomyces species, such as A. funkei (tetracycline), A. graevenitzii (doxycycline and tetracycline), A. israelii (linezolid), A. odontolyticus (clindamycin), and “A. viscosus” (clindamycin) (298–301).
For the treatment of A. schaalii infections of the urinary tract, beta-lactam antibiotics are recommended (16). In contrast, fluoroquinolones are considered ineffective despite their relatively good (except for ciprofloxacin) in vitro activities. Indeed, A. schaalii is resistant to typical antibiotics used for the treatment of urinary tract infections (262). Although there are no clear guidelines for the length of antimicrobial therapy of A. schaalii-associated infections, it has been suggested that the duration should be 2 weeks or more (16).
The impact of concomitant/coinfecting organisms is not well known. Despite the lack of data, it is generally accepted that targeted therapy against Actinomyces organisms, P. propionicum, or A. schaalii will result in the expected outcome.
FUTURE CONSIDERATIONS
It is clear from this review that there are still a number of uncertainties regarding the role of Actinomyces and related organisms in human infections. On the one hand, actinomycosis is relatively rare and often diagnosed by clinical and histopathological means, with accurate microbiological analysis not being performed. Multicenter studies should be performed in order to be able to include a sufficiently high number of cases. At the very least, major centers should store the strains isolated from confirmed cases of actinomycosis so that detailed microbiological investigations can be performed later. On the other hand, there needs to be enhanced educational efforts to make health care professionals aware that Actinomyces species are members of the healthy core microbiome, particularly in the mouth, and are thus frequently isolated from samples collected from mucous membranes. Whether these organisms can or do play a pathogenic role in these circumstances should also be the focus of research. As discussed above, little is known regarding the virulence properties of A. israelii and other disease-associated Actinomyces species. The increasing availability of genome sequences of strains representing many Actinomyces species should be exploited, in order to better understand their pathogenesis in human infection and to enable the development of new methods of treatment.
Recent taxonomic advances, including the naming of a number of novel Actinomyces species, have led to interesting and specific disease associations. This demonstrates the value of and need for taxonomic studies; the high number of known unnamed Actinomyces species-level taxa suggests that this genus should be a priority.
ACKNOWLEDGMENT
We thank Klaus Könönen for generating the anatomical figures for this article.
Biographies

Eija Könönen, D.D.S., Ph.D., has been Professor and Chair of Periodontology at the University of Turku, Turku, Finland, since 2007. She completed her undergraduate and specialist (periodontology) education and received her Ph.D. (oral microbiology) at the University of Helsinki, where she is an Adjunct Professor of Oral Microbiology. Before her current position within Periodontology, she worked with anaerobic bacteria at the National Public Health Institute (now the National Institute for Health and Welfare), Helsinki, Finland, for nearly 20 years, first as a Ph.D. student and then as a postdoctoral student, Senior investigator, and Head of the Anaerobe Reference Laboratory. She is still active within anaerobic bacteriology, for instance, in writing book chapters for international manuals/textbooks (e.g., Manual of Clinical Microbiology and Principles and Practice of Infectious Diseases). She has over 100 scientific articles in international peer-review journals; most publications deal with anaerobic bacteria.

William G. Wade, Ph.D., is Professor of Oral Microbiology at Barts and The London School of Medicine and Dentistry, Queen Mary University of London, and an Honorary Senior Research Investigator at the Forsyth Institute, Cambridge, MA. He qualified in Microbiology in 1978 at the University of East Anglia. He pursued a Ph.D. in Oral Microbiology at Cardiff Dental School and was appointed to a Lectureship there in 1987. He then moved in 1993 to the University of Bristol to take up a Senior Lectureship in Oral Microbiology, and in 1996, he was appointed Professor of Oral Microbiology at King's College London. His current interests include the molecular characterization of the oral microbiome in health and disease, the cultivation of “noncultivable” bacteria, and the development and evaluation of antimicrobials and probiotics for the prevention and treatment of oral diseases.
REFERENCES
- 1.Israel J. 1878. Neue Beobachtungen auf dem Gebiete der Mykosen des Menschen. Arch Pathol Anat 74:15–53. [Google Scholar]
- 2.Kruse W. 1896. Systematik der Streptothricheen und Bakterien, p 48–96. In Flugge C. (ed), Die Mikroorganismen, 3rd ed, vol 2 Vogel, Leipzig, Germany. [Google Scholar]
- 3.Thompson L, Lovestedt SA. 1951. An Actinomyces-like organism obtained from the human mouth. Proc Staff Meet Mayo Clin 26:169–175. [PubMed] [Google Scholar]
- 4.Batty I. 1958. Actinomyces odontolyticus, a new species of actinomycete regularly isolated from deep carious dentine. J Pathol Bacteriol 75:455–459. doi: 10.1002/path.1700750225. [DOI] [PubMed] [Google Scholar]
- 5.Howell A Jr, Jordan HV, Georg LK, Pine L. 1965. Odontomyces viscosus, gen. nov., spec. nov., a filamentous microorganism isolated from periodontal plaque in hamsters. Sabouraudia 4:65–68. doi: 10.1080/00362176685190181. [DOI] [PubMed] [Google Scholar]
- 6.Georg LK, Pine L, Gerencser MA. 1969. Actinomyces viscosus, comb. nov., a catalase positive, facultative member of the genus Actinomyces. Int J Syst Bacteriol 19:291–293. doi: 10.1099/00207713-19-3-291. [DOI] [Google Scholar]
- 7.Smego RA, Foglia G. 1998. Actinomycosis. Clin Infect Dis 26:1255–1261. [DOI] [PubMed] [Google Scholar]
- 8.Wong VK, Turmezei TD, Weston VC. 2011. Actinomycosis. BMJ 343:d6099. doi: 10.1136/bmj.d6099. [DOI] [PubMed] [Google Scholar]
- 9.Russo TA. 2009. Agents of actinomycosis, p 2864–2873. In Mandell GL, Bennett JE, Dolin R (ed), Principles and practice in infectious diseases, 7th ed Elsevier, Philadelphia, PA. [Google Scholar]
- 10.Henssge U, Do T, Radford DR, Gilbert SC, Clark D, Beighton D. 2009. Emended description of Actinomyces naeslundii and descriptions of Actinomyces oris sp. nov. and Actinomyces johnsonii sp. nov., previously identified as Actinomyces naeslundii genospecies 1, 2 and WVA 963. Int J Syst Evol Microbiol 59:509–516. doi: 10.1099/ijs.0.000950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall V. 2008. Actinomyces—gathering evidence of human colonization and infection. Anaerobe 14:1–7. doi: 10.1016/j.anaerobe.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Pulverer G, Schutt-Gerowitt H, Schaal KP. 2003. Human cervicofacial actinomycoses: microbiological data for 1997 cases. Clin Infect Dis 37:490–497. doi: 10.1086/376621. [DOI] [PubMed] [Google Scholar]
- 13.Schaal KP, Lee HJ. 1992. Actinomycete infections in humans—a review. Gene 115:201–211. doi: 10.1016/0378-1119(92)90560-C. [DOI] [PubMed] [Google Scholar]
- 14.Wade WG, Könönen E. 2011. Propionibacterium, Lactobacillus, Actinomyces, and other non-spore-forming anaerobic gram-positive rods, p 817–833. In Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (ed), Manual of clinical microbiology, 10th ed ASM Press, Washington, DC. [Google Scholar]
- 15.Kommedal Ø, Wilhelmsen MT, Skrede S, Meisal R, Jakovljev A, Gaustad P, Hermansen NO, Vik-Mo E, Solheim O, Ambur OH, Sæbø Ø, Høstmælingen CT, Helland C. 2014. Massive parallel sequencing provides new perspectives on bacterial brain abscesses. J Clin Microbiol 52:1990–1997. doi: 10.1128/JCM.00346-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cattoir V. 2012. Actinobaculum schaalii: review of an emerging uropathogen. J Infect 64:260–267. doi: 10.1016/j.jinf.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JL, Moore LV, Kaneko B, Moore WE. 1990. Actinomyces georgiae sp. nov., Actinomyces gerencseriae sp. nov., designation of two genospecies of Actinomyces naeslundii, and inclusion of A. naeslundii serotypes II and III and Actinomyces viscosus serotype II in A. naeslundii genospecies 2. Int J Syst Bacteriol 40:273–286. doi: 10.1099/00207713-40-3-273. [DOI] [PubMed] [Google Scholar]
- 18.Lawson PA, Falsen E, Akervall E, Vandamme P, Collins MD. 1997. Characterization of some Actinomyces-like isolates from human clinical specimens: reclassification of Actinomyces suis (Soltys and Spratling) as Actinobaculum suis comb. nov. and description of Actinobaculum schaalii sp. nov. Int J Syst Bacteriol 47:899–903. doi: 10.1099/00207713-47-3-899. [DOI] [PubMed] [Google Scholar]
- 19.Collins MD, Pascual C. 2000. Reclassification of Actinomyces humiferus (Gledhill and Casida) as Cellulomonas humilata nom. corrig., comb. nov. Int J Syst Evol Microbiol 50:661–663. doi: 10.1099/00207713-50-2-661. [DOI] [PubMed] [Google Scholar]
- 20.Yassin AF, Hupfer H, Siering C, Schumann P. 2011. Comparative chemotaxonomic and phylogenetic studies on the genus Arcanobacterium Collins et al. 1982 emend. Lehnen et al. 2006: proposal for Trueperella gen. nov. and emended description of the genus Arcanobacterium. Int J Syst Evol Microbiol 61:1265–1274. doi: 10.1099/ijs.0.020032-0. [DOI] [PubMed] [Google Scholar]
- 21.Greub G, Raoult D. 2002. “Actinobaculum massiliae,” a new species causing chronic urinary tract infection. J Clin Microbiol 40:3938–3941. doi: 10.1128/JCM.40.11.3938-3941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall V, Collins MD, Hutson RA, Falsen E, Inganas E, Duerden BI. 2003. Actinobaculum urinale sp. nov., from human urine. Int J Syst Evol Microbiol 53:679–682. doi: 10.1099/ijs.0.02422-0. [DOI] [PubMed] [Google Scholar]
- 23.Yassin AA, Spröer C, Pukall R, Sylvester M, Siering C, Schumann P. 18 November 2014. Dissection of the genus Actinobaculum: reclassification of Actinobaculum schaalii Lawson et al. 1997 and Actinobaculum urinale Hall et al. 2003 as Actinotignum schaalii gen. nov., comb. nov. and Actinotignum urinale comb. nov., description of Actinotignum sanguinis sp. nov. and emended description of the genus Actinobaculum. Re-examination of Actinobaculum massiliense culture deposited as CCUG 47753T (=DSM 19118T) revealed that it does not represent a strain of this species. Int J Syst Evol Microbiol doi: 10.1099/ijs.0.069294-0. [DOI] [PubMed] [Google Scholar]
- 24.Hall V, Collins MD, Lawson PA, Hutson RA, Falsen E, Inganas E, Duerden B. 2003. Characterization of some Actinomyces-like isolates from human clinical sources: description of Varibaculum cambriensis gen. nov., sp. nov. J Clin Microbiol 41:640–644. doi: 10.1128/JCM.41.2.640-644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarridge JE, Zhang Q. 2002. Genotypic diversity of clinical Actinomyces species: phenotype, source, and disease correlation among genospecies. J Clin Microbiol 40:3442–3448. doi: 10.1128/JCM.40.9.3442-3448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao JU, Rash BA, Nobre MF, da Costa MS, Rainey FA, Moe WM. 2012. Actinomyces naturae sp. nov., the first Actinomyces sp. isolated from a non-human or animal source. Antonie Van Leeuwenhoek 101:155–168. doi: 10.1007/s10482-011-9644-4. [DOI] [PubMed] [Google Scholar]
- 27.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol 192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkonen N, Könönen E, Summanen P, Kanervo A, Takala A, Jousimies-Somer H. 2000. Oral colonization with Actinomyces species in infants by two years of age. J Dent Res 79:864–867. doi: 10.1177/00220345000790031301. [DOI] [PubMed] [Google Scholar]
- 29.Zijnge V, van Leeuwen MBM, Degener JE, Abbas F, Thurnheer T, Gmür R, Harmsen HJM. 2010. Oral biofilm architecture on natural teeth. PLoS One 5:e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang G, Yip HK, Samaranayake LP, Luo G, Lo ECM, Teo CS. 2003. Actinomyces spp. in supragingival plaque of ethnic Chinese preschool children with and without active dental caries. Caries Res 37:381–390. doi: 10.1159/000072172. [DOI] [PubMed] [Google Scholar]
- 31.Liljemark WF, Bloomquist CG, Bandt CL, Pihlstrom BL, Hinrichs JE, Wolff LF. 1993. Comparison of the distribution of Actinomyces in dental plaque on inserted enamel and natural tooth surfaces in periodontal health and disease. Oral Microbiol Immunol 8:5–15. doi: 10.1111/j.1399-302X.1993.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 32.Ximénez-Fyvie LA, Haffajee AD, Martin L, Tanner A, Macuch P, Socransky SS. 1999. Identification of oral Actinomyces species using DNA probes. Oral Microbiol Immunol 14:257–265. doi: 10.1034/j.1399-302X.1999.140410.x. [DOI] [PubMed] [Google Scholar]
- 33.Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. 2013. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J 7:1016–1025. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyrrell KL, Citron DM, Warren YA, Nachnani S, Goldstein EJC. 2003. Anaerobic bacteria cultured from the tongue dorsum of subjects with oral malodor. Anaerobe 9:243–246. doi: 10.1016/S1075-9964(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 35.Könönen E, Syrjänen R, Takala A, Jousimies-Somer H. 2003. Nasopharyngeal carriage of anaerobes during health and acute otitis media by two years of age. Diagn Microbiol Infect Dis 46:167–172. doi: 10.1016/S0732-8893(03)00049-X. [DOI] [PubMed] [Google Scholar]
- 36.Jensen A, Fagö-Olsen H, Sørensen CH, Kilian M. 2013. Molecular mapping to species level of the tonsillar crypt microbiota associated with health and recurrent tonsillitis. PLoS One 8:e56418. doi: 10.1371/journal.pone.0056418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho I, Blaser MJ. 2012. The human microbiome: at the interface of health and disease. Nat Rev Genet 13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou G, Hedberg M, Hörstedt P, Baranov V, Forsberg G, Drobni M, Sandström O, Wai SN, Johansson I, Hammarström M-L, Hernell O, Hammarström S. 2009. Proximal small intestinal microbiota and identification of rod-shaped bacteria associated with childhood celiac disease. Am J Gastroenterol 104:3058–3067. doi: 10.1038/ajg.2009.524. [DOI] [PubMed] [Google Scholar]
- 39.Stearns JC, Lynch MDJ, Senadheera DB, Tenenbaum HC, Goldberg MB, Cvitkovitch DG, Croitoru K, Moreno-Hagelsieb G, Neufeld JD. 2011. Bacterial biogeography of the human digestive tract. Sci Rep 1:170. doi: 10.1038/srep00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahlen SD, Clarridge JE. 2009. Site and clinical significance of Alloscardovia omnicolens and Bifidobacterium species isolated in the clinical laboratory. J Clin Microbiol 47:3289–3293. doi: 10.1128/JCM.00555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. 2004. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci U S A 101:4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoyles L, Clear JA, McCartney AL. 2013. Use of denaturing gradient gel electrophoresis to detect Actinobacteria associated with the human faecal microbiota. Anaerobe 22:90–96. doi: 10.1016/j.anaerobe.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 43.El Aila NA, Tency I, Claeys G, Verstraelen H, Saerens B, Santiago GLDS, De Backer E, Cools P, Temmerman M, Verhelst R, Vaneechoutte M. 2009. Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC Infect Dis 9:167. doi: 10.1186/1471-2334-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikolaitchouk N, Andersch B, Falsen E, Strömbeck L, Mattsby-Baltzer I. 2008. The lower genital tract microbiota in relation to cytokine-, SLPI- and endotoxin levels: application of checkerboard DNA-DNA hybridization (CDH). APMIS 116:263–277. doi: 10.1111/j.1600-0463.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- 45.Nikolaitchouk N, Hoyles L, Falsen E, Grainger JM, Collins MD. 2000. Characterization of Actinomyces isolates from samples from the human urogenital tract: description of Actinomyces urogenitalis sp. nov. Int J Syst Evol Microbiol 50:1649–1654. doi: 10.1099/00207713-50-4-1649. [DOI] [PubMed] [Google Scholar]
- 46.Santiago GLDS, Cools P, Verstraelen H, Trog M, Missine G, El Aila N, Verhelst R, Tency I, Claeys G, Temmerman M, Vaneechoutte M. 2011. Longitudinal study of the dynamics of vaginal microflora during two consecutive menstrual cycles. PLoS One 6:e28180. doi: 10.1371/journal.pone.0028180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandamme P, Falsen E, Vancanneyt M, Van Esbroeck M, Van de Merwe D, Bergmans A, Schouls L, Sabbe L. 1998. Characterization of Actinomyces turicensis and Actinomyces radingae strains from human clinical samples. Int J Syst Bacteriol 48:503–510. doi: 10.1099/00207713-48-2-503. [DOI] [PubMed] [Google Scholar]
- 48.Olsen AB, Andersen PK, Bank S, Søby KM, Lund L, Prag J. 2013. Actinobaculum schaalii, a commensal of the urogenital area. BJU Int 112:394–397. doi: 10.1111/j.1464-410X.2012.11739.x. [DOI] [PubMed] [Google Scholar]
- 49.Cheung MK, Lam WY, Fung WYW, Law PTW, Au CH, Nong W, Kam KM, Kwan HS, Tsui SKW. 2013. Sputum microbiota in tuberculosis as revealed by 16S rRNA pyrosequencing. PLoS One 8:e54574. doi: 10.1371/journal.pone.0054574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tunney MM, Klem ER, Fodor AA, Gilpin DF, Moriarty TF, McGrath SJ, Muhlebach MS, Boucher RC, Cardwell C, Doering G, Elborn JS, Wolfgang MC. 2011. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax 66:579–584. doi: 10.1136/thx.2010.137281. [DOI] [PubMed] [Google Scholar]
- 51.Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, Gai X, Wolfe AJ, Schreckenberger PC. 2014. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol 52:871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, Kliethermes S, Schreckenberger PC, Brubaker L, Gai X, Wolfe AJ. 2014. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio 5(4):e01283-14. doi: 10.1128/mBio.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen T, Kunkel M, Kirkpatrick CJ, Weber A. 2006. Actinomyces in infected osteoradionecrosis—underestimated? Hum Pathol 37:61–67. doi: 10.1016/j.humpath.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Schipmann S, Metzler P, Rössle M, Zemann W, von Jackowski J, Obwegeser JA, Grätz KW, Jacobsen C. 2013. Osteopathology associated with bone resorption inhibitors—which role does Actinomyces play? A presentation of 51 cases with systematic review of the literature. J Oral Pathol Med 42:587–593. doi: 10.1111/jop.12038. [DOI] [PubMed] [Google Scholar]
- 55.Breton AL, Lamblin G, Pariset C, Jullien D. 2014. Cutaneous actinomycosis associated with anti-TNF-alpha therapy: report of two cases. Dermatology 228:112–114. doi: 10.1159/000357522. [DOI] [PubMed] [Google Scholar]
- 56.Cohen RD, Bowie WR, Enns R, Flint J, Fitzgerald JM. 2007. Pulmonary actinomycosis complicating infliximab therapy for Crohn's disease. Thorax 62:1013–1014. doi: 10.1136/thx.2006.075150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koubaa M, Lahiani D, Mâaloul I, Fourati H, Chaari L, Marrakchi C, Mnif Z, Boudawara Z, Ben Jemâa M. 2013. Actinomycotic brain abscess as the first clinical manifestation of hereditary hemorrhagic telangiectasia—case report and review of the literature. Ann Hematol 92:1141–1143. doi: 10.1007/s00277-012-1666-0. [DOI] [PubMed] [Google Scholar]
- 58.Reichenbach J, Lopatin U, Mahlaoui N, Beovic B, Siler U, Zbinden R, Seger RA, Galmiche L, Brousse N, Kayal S, Gungor T, Blanche S, Holland SM. 2009. Actinomyces in chronic granulomatous disease: an emerging and unanticipated pathogen. Clin Infect Dis 49:1703–1710. doi: 10.1086/647945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Acevedo F, Baudrand R, Letelier LM, Gaete P. 2008. Actinomycosis: a great pretender. Case reports of unusual presentations and a review of the literature. Int J Infect Dis 12:358–362. doi: 10.1016/j.ijid.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Kim SR, Jung LY, Oh I-J, Kim Y-C, Shin K-C, Lee MK, Yang S-H, Park HS, Kim M-K, Kwak JY, Um S-J, Ra SW, Kim WJ, Kim S, Choi E-G, Lee YC. 2013. Pulmonary actinomycosis during the first decade of 21st century: cases of 94 patients. BMC Infect Dis 13:216. doi: 10.1186/1471-2334-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akhaddar A, Elouennass M, Baallal H, Boucetta M. 2010. Focal intracranial infections due to Actinomyces species in immunocompetent patients: diagnostic and therapeutic challenges. World Neurosurg 74:346–350. doi: 10.1016/j.wneu.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 62.Han J-Y, Lee K-N, Lee JK, Kim YH, Choi SJ, Jeong YJ, Roh M-S, Choi PJ. 2013. An overview of thoracic actinomycosis: CT features. Insights Imaging 4:245–252. doi: 10.1007/s13244-012-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heo SH, Shin SS, Kim JW, Lim HS, Seon HJ, Jung S-I, Jeong YY, Kang HK. 2014. Imaging of actinomycosis in various organs: a comprehensive review. Radiographics 34:19–33. doi: 10.1148/rg.341135077. [DOI] [PubMed] [Google Scholar]
- 64.Hall V, Talbot PR, Stubbs SL, Duerden BI. 2001. Identification of clinical isolates of Actinomyces species by amplified 16S ribosomal DNA restriction analysis. J Clin Microbiol 39:3555–3562. doi: 10.1128/JCM.39.10.3555-3562.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hall V, Collins MD, Hutson R, Falsen E, Duerden BI. 2002. Actinomyces cardiffensis sp. nov. from human clinical sources. J Clin Microbiol 40:3427–3431. doi: 10.1128/JCM.40.9.3427-3431.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thacker SA, Healy CM. 2014. Pediatric cervicofacial actinomycosis: an unusual cause of head and neck masses. J Pediatr Infect Dis 3:e15–e19. doi: 10.1093/jpids/pit016. [DOI] [PubMed] [Google Scholar]
- 67.Robinson JL, Vaudry WL, Dobrovolsky W. 2005. Actinomycosis presenting as osteomyelitis in the pediatric population. Pediatr Infect Dis J 24:365–369. doi: 10.1097/01.inf.0000157215.15152.c2. [DOI] [PubMed] [Google Scholar]
- 68.de Andrade AL, Novaes MM, Germano AR, Luz KG, de Almeida Freitas R, Galvao HC. 2014. Acute primary actinomycosis involving the hard palate of a diabetic patient. J Oral Maxillofac Surg 72:537–541. doi: 10.1016/j.joms.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Alamillos-Granados FJ, Dean-Ferrer A, Garcia-Lopez A, Lopez-Rubio F. 2000. Actinomycotic ulcer of the oral mucosa: an unusual presentation of oral actinomycosis. Br J Oral Maxillofac Surg 38:121–123. doi: 10.1054/bjom.1997.0373. [DOI] [PubMed] [Google Scholar]
- 70.Özcan C, Talas D, Görur K, Aydin Ö, Yildiz A. 2005. Actinomycosis of the middle turbinate: an unusual cause of nasal obstruction. Eur Arch Otorhinolaryngol 262:412–415. doi: 10.1007/s00405-004-0832-y. [DOI] [PubMed] [Google Scholar]
- 71.Vorasubin N, Wu AW, Day C, Suh JD. 2013. Invasive sinonasal actinomycosis: case report and literature review. Laryngoscope 123:334–338. doi: 10.1002/lary.23477. [DOI] [PubMed] [Google Scholar]
- 72.Woo H-J, Bae CH, Song S-Y, Choi YS, Kim Y-D. 2008. Actinomycosis of the paranasal sinus. Otolaryngol Head Neck Surg 139:460–462. doi: 10.1016/j.otohns.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Daamen N, Johnson JT. 2004. Nasopharyngeal actinomycosis: a rare cause of nasal airway obstruction. Laryngoscope 114:1403–1405. doi: 10.1097/00005537-200408000-00016. [DOI] [PubMed] [Google Scholar]
- 74.Zheng Y, Tang J. 2013. Retropharyngeal abscess due to Actinomyces species in an immunocompromised patient: case report. J Oral Maxillofac Surg 71:e147–e150. doi: 10.1016/j.joms.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Batur Calis A, Özbal AE, Basak T, Turgut S. 2006. Laryngeal actinomycosis accompanying laryngeal carcinoma: report of two cases. Eur Arch Otorhinolaryngol 263:783–785. doi: 10.1007/s00405-006-0057-3. [DOI] [PubMed] [Google Scholar]
- 76.Cohen PR, Tschen JA. 2010. Tonsillar actinomycosis mimicking a tonsillolith: colonization of the palantine tonsil presenting as a foul-smelling, removable, unilateral, giant tonsillar concretion. Int J Dermatol 49:1165–1168. doi: 10.1111/j.1365-4632.2009.04432.x. [DOI] [PubMed] [Google Scholar]
- 77.Lensing F, Abele T, Wiggins R III, Quigley E. 2014. Laryngeal actinomycosis. Proc Bayl Univ Med Cent 27:35–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takasaki K, Kitaoka K, Kaieda S, Hayashi T, Abe K, Takahashi H. 2006. A case of actinomycosis causing unilateral tonsillar hypertrophy. Acta Otolaryngol 126:1001–1004. doi: 10.1080/00016480600590604. [DOI] [PubMed] [Google Scholar]
- 79.Kakuta R, Hidaka H, Yano H, Miyazaki H, Suzaki H, Nakamura Y, Kanamori H, Endo S, Hirakata Y, Kaku M, Kobayashi T. 2013. Identification of Actinomyces meyeri actinomycosis in middle ear and mastoid by 16S rRNA analysis. J Med Microbiol 62:1245–1248. doi: 10.1099/jmm.0.051698-0. [DOI] [PubMed] [Google Scholar]
- 80.Mehta D, Statham M, Choo D. 2007. Actinomycosis of the temporal bone with labyrinthine and facial nerve involvement. Laryngoscope 117:1999–2001. doi: 10.1097/MLG.0b013e318133a127. [DOI] [PubMed] [Google Scholar]
- 81.Salipante SJ, Hoogestraat DR, Abbott AN, SenGupta DJ, Cummings LA, Butler-Wu SM, Stephens K, Cookson BT, Hoffman NG. 2014. Coinfection of Fusobacterium nucleatum and Actinomyces israelii in mastoiditis diagnosed by next-generation DNA sequencing. J Clin Microbiol 52:1789–1792. doi: 10.1128/JCM.03133-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nomura M, Shin M, Ohta M, Nukui Y, Ohkusu K, Saito N. 2011. Atypical osteomyelitis of the skull base and craniovertebral junction caused by Actinomyces infection—case report. Neurol Med Chir (Tokyo) 51:64–66. doi: 10.2176/nmc.51.64. [DOI] [PubMed] [Google Scholar]
- 83.Abdalla J, Myers J, Moorman J. 2005. Actinomycotic infection of the oesophagus. J Infect 51:E39–E43. doi: 10.1016/j.jinf.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 84.Murchan EM, Redelman-Sidi G, Patel M, Dimaio C, Seo SK. 2010. Esophageal actinomycosis in a fifty-three-year-old man with HIV: case report and review of the literature. AIDS Patient Care STDs 24:73–78. doi: 10.1089/apc.2009.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tietz A, Aldridge KE, Figueroa JE. 2005. Disseminated coinfection with Actinomyces graevenitzii and Mycobacterium tuberculosis: case report and review of the literature. J Clin Microbiol 43:3017–3022. doi: 10.1128/JCM.43.6.3017-3022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gliga S, Devaux M, Gosset Woimant M, Mompoint D, Perronne C, Davido B. 2014. Actinomyces graevenitzii pulmonary abscess mimicking tuberculosis in a healthy young man. Can Respir J 21:e75–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagaoka K, Izumikawa K, Yamamoto Y, Yanagihara K, Ohkusu K, Kohno S. 2012. Multiple lung abscesses caused by Actinomyces graevenitzii mimicking acute pulmonary coccidioidomycosis. J Clin Microbiol 50:3125–3128. doi: 10.1128/JCM.00761-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fujita Y, Iikura M, Horio Y, Ohkusu K, Kobayashi N. 2012. Pulmonary Actinomyces graevenitzii infection presenting as organizing pneumonia diagnosed by PCR analysis. J Med Microbiol 61:1156–1158. doi: 10.1099/jmm.0.040394-0. [DOI] [PubMed] [Google Scholar]
- 89.Apothéloz C, Regamey C. 1996. Disseminated infection due to Actinomyces meyeri: case report and review. Clin Infect Dis 22:621–625. doi: 10.1093/clinids/22.4.621. [DOI] [PubMed] [Google Scholar]
- 90.Attaway A, Flynn T. 2013. Actinomyces meyeri: from “lumpy jaw” to empyema. Infection 41:1025–1027. doi: 10.1007/s15010-013-0453-8. [DOI] [PubMed] [Google Scholar]
- 91.Costiniuk CT, Voduc N, de Souza C. 2011. Pulmonary actinomycosis in a male patient with a tracheal bronchus. Can Respir J 18:84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fazili T, Blair D, Riddell S, Kiska D, Nagra S. 2012. Actinomyces meyeri infection: case report and review of the literature. J Infect 65:357–361. doi: 10.1016/j.jinf.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 93.Marie I, Lahaxe L, Levesque H, Heliot P. 2008. Pulmonary actinomycosis in a patient with diffuse systemic sclerosis treated with infliximab. QJM 101:419–421. doi: 10.1093/qjmed/hcn028. [DOI] [PubMed] [Google Scholar]
- 94.Vallet C, Pezzetta E, Nicolet-Chatelin G, El Lamaa Z, Martinet O, Ris H-B. 2004. Stage III empyema caused by Actinomyces meyeri: a plea for decortication. J Thorac Cardiovasc Surg 127:1511–1513. doi: 10.1016/j.jtcvs.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 95.Bartlett AH, Rivera AL, Krishnamurthy R, Baker CJ. 2008. Thoracic actinomycosis in children: case report and review of the literature. Pediatr Infect Dis J 27:165–169. doi: 10.1097/INF.0b013e3181598353. [DOI] [PubMed] [Google Scholar]
- 96.Huits RM, Winter HL, Slebos DJ. 2006. A painful swelling on the chest. Thoracic actinomycosis. Clin Infect Dis 42:100–102, 148–150. doi: 10.1086/498513. [DOI] [PubMed] [Google Scholar]
- 97.Cone LA, Leung MM, Hirschberg J. 2003. Actinomyces odontolyticus bacteremia. Emerg Infect Dis 9:1629–1632. doi: 10.3201/eid0912.020646. [DOI] [PubMed] [Google Scholar]
- 98.Takiguchi Y, Terano T, Hirai A. 2003. Lung abscess caused by Actinomyces odontolyticus. Intern Med 42:723–725. doi: 10.2169/internalmedicine.42.723. [DOI] [PubMed] [Google Scholar]
- 99.Wakabayashi K, Yano S, Kadowaki T, Tokushima T, Kanda H, Kobayashi K, Kimura M, Ishikawa S, Ikeda T. 2012. Pulmonary actinomycosis caused by Actinomyces cardiffensis. Intern Med 51:2929–2931. doi: 10.2169/internalmedicine.51.7997. [DOI] [PubMed] [Google Scholar]
- 100.Seo JY, Yeom J-S, Ko KS. 2012. Actinomyces cardiffensis septicemia: a case report. Diagn Microbiol Infect Dis 73:86–88. doi: 10.1016/j.diagmicrobio.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 101.Gupta A, Lodato RF. 2012. Empyema necessitatis due to Actinomyces israelii. Am J Respir Crit Care Med 185:e16. doi: 10.1164/rccm.201108-1532CR. [DOI] [PubMed] [Google Scholar]
- 102.Llamas-Velasco M, Domínguez I, Ovejero E, Pérez-Gala S, García-Diez A. 2010. Empyema necessitatis revisited. Eur J Dermatol 20:115–119. doi: 10.1684/ejd.2010.0809. [DOI] [PubMed] [Google Scholar]
- 103.Pérez-Castrillón JL, Gonzalez-Castâneda C, del Campo-Matias F, Bellido-Casado J, Diaz G. 1997. Empyema necessitatis due to Actinomyces odontolyticus. Chest 111:1144. [DOI] [PubMed] [Google Scholar]
- 104.Vyas JM, Kasmar A, Chang HR, Holden J, Hohmann E. 2007. Abdominal abscesses due to actinomycosis after laparoscopic cholecystectomy: case reports and review. Clin Infect Dis 44:e1–e4. doi: 10.1086/510077. [DOI] [PubMed] [Google Scholar]
- 105.Ladic A, Petrovic I, Augustin G, Puretic H, Skegro M, Gojevic A, Nikolic I. 2013. Hemoptysis as an early symptom of abdominal actinomycosis with thoracic extension ten years after cholecystectomy with retained gallstone. Surg Infect 14:408–411. doi: 10.1089/sur.2012.027. [DOI] [PubMed] [Google Scholar]
- 106.Zbar AP, Ranasinghe W, Kennedy PJ. 2009. Subphrenic abscess secondary to Actinomycosis meyeri and Klebsiella ozaenae following laparoscopic cholecystectomy. South Med J 102:725–727. doi: 10.1097/SMJ.0b013e3181abddc5. [DOI] [PubMed] [Google Scholar]
- 107.Hansen JM, Fjeldsøe-Nielsen H, Sulim S, Kemp M, Christensen JJ. 2009. Actinomyces species: a Danish survey on human infections and microbiological characteristics. Open Microbiol J 3:113–120. doi: 10.2174/1874285800903010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garduño E, Rebollo M, Asencio MA, Carro J, Pascasio JM, Blanco J. 2000. Splenic abscesses caused by Actinomyces meyeri in a patient with autoimmune hepatitis. Diagn Microbiol Infect Dis 37:213–214. doi: 10.1016/S0732-8893(00)00133-4. [DOI] [PubMed] [Google Scholar]
- 109.Llenas-García J, Lalueza-Blanco A, Fernández-Ruiz M, Villar-Silva J, Ochoa M, Lozano F, Lizasoain M, Aguado JM. 2012. Primary hepatic actinomycosis presenting as purulent pericarditis with cardiac tamponade. Infection 40:339–341. doi: 10.1007/s15010-011-0200-y. [DOI] [PubMed] [Google Scholar]
- 110.Makaryus AN, Latzman J, Yang R, Rosman D. 2005. A rare case of Actinomyces israelii presenting as pericarditis in a 75-year-old man. Cardiol Rev 13:125–127. doi: 10.1097/01.crd.0000148846.97618.aa. [DOI] [PubMed] [Google Scholar]
- 111.Saad M, Moorman J. 2005. Images in clinical medicine. Actinomyces hepatic abscess with cutaneous fistula. N Engl J Med 353:e16. doi: 10.1056/NEJMicm050528. [DOI] [PubMed] [Google Scholar]
- 112.Uehara Y, Takahashi T, Yagoshi M, Shimoguchi K, Yanai M, Kumasaka K, Kikuchi K. 2010. Liver abscess of Actinomyces israelii in a hemodialysis patient: case report and review of the literature. Intern Med 49:2017–2020. doi: 10.2169/internalmedicine.49.3700. [DOI] [PubMed] [Google Scholar]
- 113.Harsch IA, Benninger J, Niedobitek G, Schindler G, Schneider HT, Hahn EG, Nusko G. 2001. Abdominal actinomycosis: complication of endoscopic stenting in chronic pancreatitis? Endoscopy 33:1065–1069. doi: 10.1055/s-2001-18930. [DOI] [PubMed] [Google Scholar]
- 114.Hinić V, Straub C, Schultheiss E, Kaempfer P, Frei R, Goldenberger D. 2013. Identification of a novel 16S rRNA gene variant of Actinomyces funkei from six patients with purulent infections. Clin Microbiol Infect 19:E312–E314. doi: 10.1111/1469-0691.12201. [DOI] [PubMed] [Google Scholar]
- 115.Chao CT, Liao CH, Lai CC, Hsueh PR. 2011. Liver abscess due to Actinomyces odontolyticus in an immunocompetent patient. Infection 39:77–79. doi: 10.1007/s15010-010-0063-7. [DOI] [PubMed] [Google Scholar]
- 116.Riegert-Johnson DL, Sandhu N, Rajkumar SV, Patel R. 2002. Thrombotic thrombocytopenic purpura associated with a hepatic abscess due to Actinomyces turicensis. Clin Infect Dis 35:636–637. doi: 10.1086/342327. [DOI] [PubMed] [Google Scholar]
- 117.Levy P-Y, Fournier P-E, Charrel R, Metras D, Habib G, Raoult D. 2006. Molecular analysis of pericardial fluid: a 7-year experience. Eur Heart J 27:1942–1946. doi: 10.1093/eurheartj/ehl025. [DOI] [PubMed] [Google Scholar]
- 118.Funke G, von Graevenitz A. 1995. Infections due to Actinomyces neuii (former “CDC coryneform group 1” bacteria). Infection 23:73–75. doi: 10.1007/BF01833868. [DOI] [PubMed] [Google Scholar]
- 119.von Graevenitz A. 2011. Actinomyces neuii: review of an unusual infectious agent. Infection 39:97–100. doi: 10.1007/s15010-011-0088-6. [DOI] [PubMed] [Google Scholar]
- 120.Kalaichelvan V, Maw AA, Singh K. 2006. Actinomyces in cervical smears of women using the intrauterine device in Singapore. Contraception 73:352–355. doi: 10.1016/j.contraception.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 121.Westhoff C. 2007. IUDs and colonization or infection with Actinomyces. Contraception 75:S48–S50. doi: 10.1016/j.contraception.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 122.Fiorino AS. 1996. Intrauterine contraceptive device-associated actinomycotic abscess and Actinomyces detection on cervical smear. Obstet Gynecol 87:142–149. doi: 10.1016/0029-7844(95)00350-9. [DOI] [PubMed] [Google Scholar]
- 123.Carrillo M, Valdez B, Vargas L, Alvarez L, Schorr M, Zlatev R, Stoytcheva M. 2010. In vitro Actinomyces israelii biofilm development on IUD copper surfaces. Contraception 81:261–264. doi: 10.1016/j.contraception.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 124.Asemota OA, Girda E, Dueñas O, Neal-Perry G, Pollack SE. 2013. Actinomycosis pelvic abscess after in vitro fertilization. Fertil Steril 100:408–411. doi: 10.1016/j.fertnstert.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 125.Elhag KM, Bahar AM, Mubarak AA. 1988. The effect of a copper intra-uterine contraceptive device on the microbial ecology of the female genital tract. J Med Microbiol 25:245–251. doi: 10.1099/00222615-25-4-245. [DOI] [PubMed] [Google Scholar]
- 126.Woo PCY, Fung AMY, Lau SKP, Hon E, Yuen K. 2002. Diagnosis of pelvic actinomycosis by 16S ribosomal RNA gene sequencing and its clinical significance. Diagn Microbiol Infect Dis 43:113–118. doi: 10.1016/S0732-8893(02)00375-9. [DOI] [PubMed] [Google Scholar]
- 127.Elsayed S, George A, Zhang K. 2006. Intrauterine contraceptive device-associated pelvic actinomycosis caused by Actinomyces urogenitalis. Anaerobe 12:67–70. doi: 10.1016/j.anaerobe.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 128.Van Hoecke F, Beuckelaers E, Lissens P, Boudewijns M. 2013. Actinomyces urogenitalis bacteremia and tubo-ovarian abscess after an in vitro fertilization (IVF) procedure. J Clin Microbiol 51:4252–4254. doi: 10.1128/JCM.02142-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Flynn AN, Lyndon CA, Church DL. 2013. Identification by 16S rRNA gene sequencing of an Actinomyces hongkongensis isolate recovered from a patient with pelvic actinomycosis. J Clin Microbiol 51:2721–2723. doi: 10.1128/JCM.00509-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Woo PC, Fung AM, Lau SK, Teng JL, Wong BH, Wong MK, Hon E, Tang GW, Yuen KY. 2003. Actinomyces hongkongensis sp. nov. a novel Actinomyces species isolated from a patient with pelvic actinomycosis. Syst Appl Microbiol 26:518–522. doi: 10.1078/072320203770865819. [DOI] [PubMed] [Google Scholar]
- 131.Matsuda K, Nakajima H, Khan KN, Tanigawa T, Hamaguchi D, Kitajima M, Hiraki K, Moriyama S, Masuzaki H. 2012. Preoperative diagnosis of pelvic actinomycosis by clinical cytology. Int J Womens Health 4:527–533. doi: 10.2147/IJWH.S35573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ong C, Barnes S, Senanayake S. 2012. Actinomyces turicensis infection mimicking ovarian tumour. Singapore Med J 53:e9–e11. [PubMed] [Google Scholar]
- 133.Gomes J, Pereira T, Carvalho A, Brito C. 2011. Primary cutaneous actinomycosis caused by Actinomyces meyeri as first manifestation of HIV infection. Dermatol Online J 17:5. [PubMed] [Google Scholar]
- 134.Capobianco G, Dessole S, Becchere MP, Profili S, Cosmi E, Cherchi PL, Meloni GB. 2005. A rare case of primary actinomycosis of the breast caused by Actinomyces viscosus: diagnosis by fine-needle aspiration cytology under ultrasound guidance. Breast J 11:57–59. doi: 10.1111/j.1075-122X.2005.21613.x. [DOI] [PubMed] [Google Scholar]
- 135.Hermida MD, Della Giovanna P, Lapadula M, García S, Cabrera HN. 2009. Actinomyces meyeri cutaneous actinomycosis. Int J Dermatol 48:154–156. doi: 10.1111/j.1365-4632.2009.03798.x. [DOI] [PubMed] [Google Scholar]
- 136.Duvignaud A, Ribeiro E, Moynet D, Longy-Boursier M, Malvy D. 2014. Cervical spondylitis and spinal abscess due to Actinomyces meyeri. Braz J Infect Dis 18:106–109. doi: 10.1016/j.bjid.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Honda H, Bankowski MJ, Kajioka EHN, Chokrungvaranon N, Kim W, Gallacher ST. 2008. Thoracic vertebral actinomycosis: Actinomyces israelii and Fusobacterium nucleatum. J Clin Microbiol 46:2009–2014. doi: 10.1128/JCM.01706-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gaïni S, Røge BT, Pedersen C, Pedersen SS, Brenøe A-S. 2006. Severe Actinomyces israelii infection involving the entire spinal cord. Scand J Infect Dis 38:211–213. doi: 10.1080/00365540500322312. [DOI] [PubMed] [Google Scholar]
- 139.Ghafghaichi L, Troy S, Budvytiene I, Banaei N, Baron EJ. 2010. Mixed infection involving Actinomyces, Aggregatibacter, and Fusobacterium species presenting as perispinal tumor. Anaerobe 16:174–178. doi: 10.1016/j.anaerobe.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 140.Edwards TM, Demos TC, Lomasney LM. 2011. Radiologic case study. Musculoskeletal actinomycosis. Orthopedics 34:641, 730–733. doi: 10.3928/01477447-20110714-49. [DOI] [PubMed] [Google Scholar]
- 141.Musher DM. 1998. Actinomycosis of 54 years' duration. Clin Infect Dis 27:889. doi: 10.1086/514965. [DOI] [PubMed] [Google Scholar]
- 142.Smego RA., Jr 1987. Actinomycosis of the central nervous system. Rev Infect Dis 9:855–865. doi: 10.1093/clinids/9.5.855. [DOI] [PubMed] [Google Scholar]
- 143.Van Dellen JR. 2010. Actinomycosis: an ancient disease difficult to diagnose. World Neurosurg 74:263–264. doi: 10.1016/j.wneu.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 144.Olah E, Berger C, Boltshauser E, Nadal D. 2004. Cerebral actinomycosis before adolescence. Neuropediatrics 35:239–241. doi: 10.1055/s-2004-820895. [DOI] [PubMed] [Google Scholar]
- 145.Bouziri A, Khaldi A, Smaoui H, Menif K, Ben Jaballah N. 2011. Fatal subdural empyema caused by Streptococcus constellatus and Actinomyces viscosus in a child—case report. J Microbiol Immunol Infect 44:394–396. doi: 10.1016/j.jmii.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 146.Kuijper EJ, Wiggerts HO, Jonker GJ, Schaal KP, de Gans J. 1992. Disseminated actinomycosis due to Actinomyces meyeri and Actinobacillus actinomycetemcomitans. Scand J Infect Dis 24:667–672. doi: 10.3109/00365549209054655. [DOI] [PubMed] [Google Scholar]
- 147.Zijlstra EE, Swart GR, Godfroy FJ, Degener JE. 1992. Pericarditis, pneumonia and brain abscess due to a combined Actinomyces-Actinobacillus actinomycetemcomitans infection. J Infect 25:83–87. doi: 10.1016/0163-4453(92)93633-2. [DOI] [PubMed] [Google Scholar]
- 148.Colmegna I, Rodriguez-Barradas M, Rauch R, Clarridge J, Young EJ. 2003. Disseminated Actinomyces meyeri infection resembling lung cancer with brain metastases. Am J Med Sci 326:152–155. doi: 10.1097/00000441-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 149.Naik NH, Russo TA. 2009. Bisphosphonate-related osteonecrosis of the jaw: the role of Actinomyces. Clin Infect Dis 49:1729–1732. doi: 10.1086/648075. [DOI] [PubMed] [Google Scholar]
- 150.Curi MM, Dib LL, Kowalski LP, Landman G, Mangini C. 2000. Opportunistic actinomycosis in osteoradionecrosis of the jaws in patients affected by head and neck cancer: incidence and clinical significance. Oral Oncol 36:294–299. doi: 10.1016/S1368-8375(99)00080-9. [DOI] [PubMed] [Google Scholar]
- 151.Kos M, Kuebler JF, Luczak K, Engelke W. 2010. Bisphosphonate-related osteonecrosis of the jaws: a review of 34 cases and evaluation of risk. J Craniomaxillofac Surg 38:255–259. doi: 10.1016/j.jcms.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 152.Sedghizadeh PP, Kumar SKS, Gorur A, Schaudinn C, Shuler CF, Costerton JW. 2009. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J Am Dent Assoc 140:1259–1265. doi: 10.14219/jada.archive.2009.0049. [DOI] [PubMed] [Google Scholar]
- 153.Happonen RP, Viander M, Pelliniemi L, Aitasalo K. 1983. Actinomyces israelii in osteoradionecrosis of the jaws. Histopathologic and immunocytochemical study of five cases. Oral Surg Oral Med Oral Pathol 55:580–588. doi: 10.1016/0030-4220(83)90374-2. [DOI] [PubMed] [Google Scholar]
- 154.Hansen T, Wagner W, Kirkpatrick CJ, Kunkel M. 2006. Infected osteoradionecrosis of the mandible: follow-up study suggests deterioration in outcome for patients with Actinomyces-positive bone biopsies. Int J Oral Maxillofac Surg 35:1001–1004. doi: 10.1016/j.ijom.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 155.Aas JA, Reime L, Pedersen K, Eribe ERK, Abesha-Belay E, Støre G, Olsen I. 13 July 2010. Osteoradionecrosis contains a wide variety of cultivable and non-cultivable bacteria. J Oral Microbiol doi: 10.3402/jom.v2i0.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Støre G, Eribe ERK, Olsen I. 2005. DNA-DNA hybridization demonstrates multiple bacteria in osteoradionecrosis. Int J Oral Maxillofac Surg 34:193–196. doi: 10.1016/j.ijom.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 157.Curtis JR, Yang S, Patkar NM, Chen L, Singh JA, Cannon GW, Mikuls TR, Delzell E, Saag KG, Safford MM, DuVall S, Alexander K, Napalkov P, Winthrop KL, Burton MJ, Kamauu A, Baddley JW. 2014. Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care Res 66:990–997. doi: 10.1002/acr.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen KH, Lin CH. 2013. Brain abscess as an initial presentation in a patient of hereditary haemorrhagic telangiectasia caused by a novel ENG mutation. BMJ Case Rep 2013:bcr2013008802. doi: 10.1136/bcr-2013-008802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hall WA. 1994. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease) presenting with polymicrobial brain abscess Case report. J Neurosurg 81:294–296. [DOI] [PubMed] [Google Scholar]
- 160.Woods ML. 1998. Photo quiz I. Hereditary hemorrhagic telangiectasia, pulmonary arteriovenous malformations, and brain abscess. Clin Infect Dis 26:1071, 1220–1221. [DOI] [PubMed] [Google Scholar]
- 161.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Español T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia M-J, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. 2009. Chronic granulomatous disease: the European experience. PLoS One 4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Agger WA, Kowalski TJ. 2010. Chronic granulomatous disease, catalase, and Actinomyces. Clin Infect Dis 50:1325–1326. doi: 10.1086/651690. [DOI] [PubMed] [Google Scholar]
- 163.Hagiya H, Otsuka F. 2014. Actinomyces meyeri meningitis: the need for anaerobic cerebrospinal fluid cultures. Intern Med 53:67–71. doi: 10.2169/internalmedicine.53.0403. [DOI] [PubMed] [Google Scholar]
- 164.Garelick JM, Khodabakhsh AJ, Josephberg RG. 2002. Acute postoperative endophthalmitis caused by Actinomyces neuii. Am J Ophthalmol 133:145–147. doi: 10.1016/S0002-9394(01)01206-5. [DOI] [PubMed] [Google Scholar]
- 165.Pérez-Santonja JJ, Campos-Mollo E, Fuentes-Campos E, Samper-Giménez J, Alió JL. 2007. Actinomyces neuii subspecies anitratus chronic endophthalmitis after cataract surgery. Eur J Ophthalmol 17:445–447. [DOI] [PubMed] [Google Scholar]
- 166.Raman VS, Evans N, Shreshta B, Cunningham R. 2004. Chronic postoperative endophthalmitis caused by Actinomyces neuii. J Cataract Refract Surg 30:2641–2643. doi: 10.1016/j.jcrs.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 167.Peponis VG, Chalkiadakis SE, Parikakis EA, Mitropoulos PG. 2011. Chronic postoperative endophthalmitis caused by Actinomyces meyeri. Case Rep Ophthalmol 2:95–98. doi: 10.1159/000326062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Scarano FJ, Ruddat MS, Robinson A. 1999. Actinomyces viscosus postoperative endophthalmitis. Diagn Microbiol Infect Dis 34:115–117. doi: 10.1016/S0732-8893(99)00009-7. [DOI] [PubMed] [Google Scholar]
- 169.Graffi S, Peretz A, Naftali M. 2012. Endogenous endophthalmitis with an unusual infective agent: Actinomyces neuii. Eur J Ophthalmol 22:834–835. doi: 10.5301/ejo.5000106. [DOI] [PubMed] [Google Scholar]
- 170.Milman T, Mirani N, Gibler T, Van Gelder RN, Langer PD. 2008. Actinomyces israelii endogenous endophthalmitis. Br J Ophthalmol 92:427–428. doi: 10.1136/bjo.2007.123596. [DOI] [PubMed] [Google Scholar]
- 171.Karimian F, Feizi S, Nazari R, Zarin-Bakhsh P. 2008. Delayed-onset Actinomyces keratitis after laser in situ keratomileusis. Cornea 27:843–846. doi: 10.1097/ICO.0b013e31816a624a. [DOI] [PubMed] [Google Scholar]
- 172.Hussain I, Bonshek RE, Loudon K, Armstrong M, Tullo AB. 1993. Canalicular infection caused by Actinomyces. Eye 7:542–544. doi: 10.1038/eye.1993.118. [DOI] [PubMed] [Google Scholar]
- 173.Olender A, Matysik-Woźniak A, Rymgayłło-Jankowska B, Rejdak R. 2013. The cause of Actinomyces canalictulis—a case study. Ann Agric Environ Med 20:742–744. [PubMed] [Google Scholar]
- 174.Yuksel D, Hazirolan D, Sungur G, Duman S. 2012. Actinomyces canaliculitis and its surgical treatment. Int Ophthalmol 32:183–186. doi: 10.1007/s10792-012-9531-7. [DOI] [PubMed] [Google Scholar]
- 175.Kerttula AM, Carlson P, Sarkonen N, Hall V, Könönen E. 2005. Enzymatic/biochemical analysis of Actinomyces with commercial test kits with an emphasis on newly described species. Anaerobe 11:99–108. doi: 10.1016/j.anaerobe.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 176.Könönen E, Kanervo A, Bryk A, Aino T, Syrjänen R, Jousimies-Somer H. 1999. Anaerobes in the nasopharynx during acute otitis media episodes in infancy. Anaerobe 5:237–239. doi: 10.1006/anae.1999.0225. [DOI] [Google Scholar]
- 177.Jousimies-Somer H, Savolainen S, Mäkitie A, Ylikoski J. 1993. Bacteriologic findings in peritonsillar abscesses in young adults. Clin Infect Dis 16(Suppl 4):S292–S298. doi: 10.1093/clinids/16.Supplement_4.S292. [DOI] [PubMed] [Google Scholar]
- 178.Sabbe LJ, Van De Merwe D, Schouls L, Bergmans A, Vaneechoutte M, Vandamme P. 1999. Clinical spectrum of infections due to the newly described Actinomyces species A. turicensis, A. radingae, and A. europaeus. J Clin Microbiol 37:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hall V, Collins MD, Lawson PA, Falsen E, Duerden BI. 2003. Actinomyces nasicola sp. nov., isolated from a human nose. Int J Syst Evol Microbiol 53:1445–1448. doi: 10.1099/ijs.0.02582-0. [DOI] [PubMed] [Google Scholar]
- 180.Brailsford SR, Tregaskis RB, Leftwich HS, Beighton D. 1999. The predominant Actinomyces spp. isolated from infected dentin of active root caries lesions. J Dent Res 78:1525–1534. doi: 10.1177/00220345990780090701. [DOI] [PubMed] [Google Scholar]
- 181.Tanner ACR, Mathney JMJ, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, Papadopolou E, Dewhirst FE. 2011. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol 49:1464–1474. doi: 10.1128/JCM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bowden GH, Nolette N, Ryding H, Cleghorn BM. 1999. The diversity and distribution of the predominant ribotypes of Actinomyces naeslundii genospecies 1 and 2 in samples from enamel and from healthy and carious root surfaces of teeth. J Dent Res 78:1800–1809. doi: 10.1177/00220345990780120601. [DOI] [PubMed] [Google Scholar]
- 183.Signoretti FGC, Endo MS, Gomes BPFA, Montagner F, Tosello FB, Jacinto RC. 2011. Persistent extraradicular infection in root-filled asymptomatic human tooth: scanning electron microscopic analysis and microbial investigation after apical microsurgery. J Endod 37:1696–1700. doi: 10.1016/j.joen.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 184.Siqueira JF, Rôças IN. 2003. Polymerase chain reaction detection of Propionibacterium propionicus and Actinomyces radicidentis in primary and persistent endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 96:215–222. doi: 10.1016/S1079-2104(03)00158-6. [DOI] [PubMed] [Google Scholar]
- 185.Siqueira JF, Rôças IN, Souto R, de Uzeda M, Colombo AP. 2002. Actinomyces species, streptococci, and Enterococcus faecalis in primary root canal infections. J Endod 28:168–172. doi: 10.1097/00004770-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 186.Sunde PT, Olsen I, Debelian GJ, Tronstad L. 2002. Microbiota of periapical lesions refractory to endodontic therapy. J Endod 28:304–310. doi: 10.1097/00004770-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 187.Xia T, Baumgartner JC. 2003. Occurrence of Actinomyces in infections of endodontic origin. J Endod 29:549–552. doi: 10.1097/00004770-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 188.Collins MD, Hoyles L, Kalfas S, Sundquist G, Monsen T, Nikolaitchouk N, Falsen E. 2000. Characterization of Actinomyces isolates from infected root canals of teeth: description of Actinomyces radicidentis sp. nov. J Clin Microbiol 38:3399–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hall V, Collins MD, Hutson RA, Inganäs E, Falsen E, Duerden BI. 2003. Actinomyces oricola sp. nov., from a human dental abscess. Int J Syst Evol Microbiol 53:1515–1518. doi: 10.1099/ijs.0.02576-0. [DOI] [PubMed] [Google Scholar]
- 190.Hall V, Collins MD, Lawson PA, Falsen E, Duerden BI. 2005. Actinomyces dentalis sp. nov., from a human dental abscess. Int J Syst Evol Microbiol 55:427–431. doi: 10.1099/ijs.0.63376-0. [DOI] [PubMed] [Google Scholar]
- 191.Tanner A, Maiden MF, Macuch PJ, Murray LL, Kent RL. 1998. Microbiota of health, gingivitis, and initial periodontitis. J Clin Periodontol 25:85–98. doi: 10.1111/j.1600-051X.1998.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 192.Gmür R, Wyss C, Xue Y, Thurnheer T, Guggenheim B. 2004. Gingival crevice microbiota from Chinese patients with gingivitis or necrotizing ulcerative gingivitis. Eur J Oral Sci 112:33–41. doi: 10.1111/j.0909-8836.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- 193.Persson GR, Hitti J, Paul K, Hirschi R, Weibel M, Rothen M, Persson RE. 2008. Tannerella forsythia and Pseudomonas aeruginosa in subgingival bacterial samples from parous women. J Periodontol 79:508–516. doi: 10.1902/jop.2008.070350. [DOI] [PubMed] [Google Scholar]
- 194.Sixou J-L, Magaud C, Jolivet-Gougeon A, Cormier M, Bonnaure-Mallet M. 2003. Evaluation of the mandibular third molar pericoronitis flora and its susceptibility to different antibiotics prescribed in France. J Clin Microbiol 41:5794–5797. doi: 10.1128/JCM.41.12.5794-5797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sarkonen N, Könönen E, Eerola E, Könönen M, Jousimies-Somer H, Laine P. 2005. Characterization of Actinomyces species isolated from failed dental implant fixtures. Anaerobe 11:231–237. doi: 10.1016/j.anaerobe.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 196.Young MP, Carter DH, Worthington H, Korachi M, Drucker DB. 2001. Microbial analysis of bone collected during implant surgery: a clinical and laboratory study. Clin Oral Implants Res 12:95–103. doi: 10.1034/j.1600-0501.2001.012002095.x. [DOI] [PubMed] [Google Scholar]
- 197.Ramos CP, Falsen E, Alvarez N, Akervall E, Sjödén B, Collins MD. 1997. Actinomyces graevenitzii sp. nov., isolated from human clinical specimens. Int J Syst Bacteriol 47:885–888. doi: 10.1099/00207713-47-3-885. [DOI] [PubMed] [Google Scholar]
- 198.Peaper DR, Havill NL, Aniskiewicz M, Callan D, Pop O, Towle D, Boyce JM. 2015. Pseudo-outbreak of Actinomyces graevenitzii associated with bronchoscopy. J Clin Microbiol 53:113–117. doi: 10.1128/JCM.02302-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gómez-Garcés JL, Burillo A, Gil Y, Sáez-Nieto JA. 2010. Soft tissue infections caused by Actinomyces neuii, a rare pathogen. J Clin Microbiol 48:1508–1509. doi: 10.1128/JCM.02139-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lacoste C, Escande M-C, Jammet P, Nos C. 2009. Breast Actinomyces neuii abscess simulating primary malignancy: a case diagnosed by fine-needle aspiration. Diagn Cytopathol 37:311–312. doi: 10.1002/dc.21044. [DOI] [PubMed] [Google Scholar]
- 201.Olson JM, Vary JC. 2013. Primary cutaneous Actinomyces neuii infection of the breast successfully treated with doxycycline. Cutis 92:E3–E4. [PubMed] [Google Scholar]
- 202.Funke G, Alvarez N, Pascual C, Falsen E, Akervall E, Sabbe L, Schouls L, Weiss N, Collins MD. 1997. Actinomyces europaeus sp. nov., isolated from human clinical specimens. Int J Syst Bacteriol 47:687–692. doi: 10.1099/00207713-47-3-687. [DOI] [PubMed] [Google Scholar]
- 203.Silva WA, Pinheiro AM, Jahns B, Bögli-Stuber K, Droz S, Zimmerli S. 2011. Breast abscess due to Actinomyces europaeus. Infection 39:255–258. doi: 10.1007/s15010-011-0119-3. [DOI] [PubMed] [Google Scholar]
- 204.Attar KH, Waghorn D, Lyons M, Cunnick G. 2007. Rare species of Actinomyces as causative pathogens in breast abscess. Breast J 13:501–505. doi: 10.1111/j.1524-4741.2007.00472.x. [DOI] [PubMed] [Google Scholar]
- 205.Cato EP, Moore WEC, Nygaard G, Holdeman LV. 1984. Actinomyces meyeri sp. nov., specific epithet rev. Int J Syst Bacteriol 34:487–489. doi: 10.1099/00207713-34-4-487. [DOI] [Google Scholar]
- 206.Abdulrahman GO Jr, Gateley CA. 2015. Primary actinomycosis of the breast caused by Actinomyces turicensis with associated Peptoniphilus harei. Breast Dis 35:45–47. doi: 10.3233/BD-140381. [DOI] [PubMed] [Google Scholar]
- 207.Funke G, Stubbs S, von Graevenitz A, Collins MD. 1994. Assignment of human-derived CDC group 1 coryneform bacteria and CDC group 1-like coryneform bacteria to the genus Actinomyces as Actinomyces neuii subsp. neuii sp. nov., subsp. nov., and Actinomyces neuii subsp. anitratus, subsp. nov. Int J Syst Bacteriol 44:167–171. [DOI] [PubMed] [Google Scholar]
- 208.Chudackova E, Geigerova L, Hrabak J, Bergerova T, Liska V, Scharfen J Jr. 2010. Seven isolates of Actinomyces turicensis from patients with surgical infections of the anogenital area in a Czech hospital. J Clin Microbiol 48:2660–2661. doi: 10.1128/JCM.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ng LS, Sim JH, Eng LC, Menon S, Tan TY. 2012. Comparison of phenotypic methods and matrix-assisted laser desorption ionisation time-of-flight mass spectrometry for the identification of aero-tolerant Actinomyces spp. isolated from soft-tissue infections. Eur J Clin Microbiol Infect Dis 31:1749–1752. doi: 10.1007/s10096-011-1496-3. [DOI] [PubMed] [Google Scholar]
- 210.Sofianou D, Avgoustinakis E, Dilopoulou A, Pournaras S, Tsirakidis G, Tsakris A. 2004. Soft-tissue abscess involving Actinomyces odontolyticus and two Prevotella species in an intravenous drug abuser. Comp Immunol Microbiol Infect Dis 27:75–79. doi: 10.1016/S0147-9571(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 211.Summanen PH, Talan DA, Strong C, McTeague M, Bennion R, Thompson JE, Väisänen ML, Moran G, Winer M, Finegold SM. 1995. Bacteriology of skin and soft-tissue infections: comparison of infections in intravenous drug users and individuals with no history of intravenous drug use. Clin Infect Dis 20(Suppl 2):S279–S282. doi: 10.1093/clinids/20.Supplement_2.S279. [DOI] [PubMed] [Google Scholar]
- 212.Davanos E, Rahman SM, Nogid B. 2008. Treatment of Eikenella corrodens and Actinomyces odontolyticus foot abscess in a penicillin-allergic patient. Ann Pharmacother 42:1706–1710. doi: 10.1345/aph.1L257. [DOI] [PubMed] [Google Scholar]
- 213.Zautner AE, Schmitz S, Aepinus C, Schmialek A, Podbielski A. 2009. Subcutaneous fistulae in a patient with femoral hypoplasia due to Actinomyces europaeus and Actinomyces turicensis. Infection 37:289–291. doi: 10.1007/s15010-008-7392-9. [DOI] [PubMed] [Google Scholar]
- 214.Funke G, Englert R, Frodl R, Bernard KA, Stenger S. 2010. Actinomyces hominis sp. nov., isolated from a wound swab. Int J Syst Evol Microbiol 60:1678–1681. doi: 10.1099/ijs.0.015818-0. [DOI] [PubMed] [Google Scholar]
- 215.McElroy JY, Gorens ME, Jackson LN, Stigger D, Becker T, Sheiner E. 2006. Actinomyces israelii may produce vulvar lesions suspicious for malignancy. Infect Dis Obstet Gynecol 2006:48269. doi: 10.1155/IDOG/2006/48269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tena D, Losa C, Medina-Pascual MJ, Saez-Nieto JA. 2014. Fournier's gangrene caused by Actinomyces funkei, Fusobacterium gonidiaformans and Clostridium hathewayi. Anaerobe 27:14–16. doi: 10.1016/j.anaerobe.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 217.Mann C, Dertinger S, Hartmann G, Schurz R, Simma B. 2002. Actinomyces neuii and neonatal sepsis. Infection 30:178–180. doi: 10.1007/s15010-002-2165-3. [DOI] [PubMed] [Google Scholar]
- 218.Wright JR, Stinson D, Wade A, Haldane D, Heifetz SA. 1994. Necrotizing funisitis associated with Actinomyces meyeri infection: a case report. Pediatr Pathol 14:927–934. doi: 10.3109/15513819409037689. [DOI] [PubMed] [Google Scholar]
- 219.Pinilla I, Martín-Hervás C, Gil-Garay E. 2006. Primary sternal osteomyelitis caused by Actinomyces israelii. South Med J 99:96–97. doi: 10.1097/01.smj.0000197512.82441.41. [DOI] [PubMed] [Google Scholar]
- 220.Vandevelde AG, Jenkins SG, Hardy PR. 1995. Sclerosing osteomyelitis and Actinomyces naeslundii infection of surrounding tissues. Clin Infect Dis 20:1037–1039. doi: 10.1093/clinids/20.4.1037. [DOI] [PubMed] [Google Scholar]
- 221.Lee MJ, Ha YE, Park HY, Lee JH, Lee YJ, Sung KS, Kang C-I, Chung DR, Song J-H, Peck KR. 2012. Osteomyelitis of a long bone due to Fusobacterium nucleatum and Actinomyces meyeri in an immunocompetent adult: a case report and literature review. BMC Infect Dis 12:161. doi: 10.1186/1471-2334-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Van Bosterhaut B, Boucquey P, Janssens M, Wauters G, Delmée M. 2002. Chronic osteomyelitis due to Actinomyces neuii subspecies neuii and Dermabacter hominis. Eur J Clin Microbiol Infect Dis 21:486–487. doi: 10.1007/s10096-002-0747-8. [DOI] [PubMed] [Google Scholar]
- 223.Lecouvet F, Irenge L, Vandercam B, Nzeusseu A, Hamels S, Gala J-L. 2004. The etiologic diagnosis of infectious discitis is improved by amplification-based DNA analysis. Arthritis Rheum 50:2985–2994. doi: 10.1002/art.20462. [DOI] [PubMed] [Google Scholar]
- 224.Renvoise A, Raoult D, Roux V. 2010. Actinomyces timonensis sp. nov., isolated from a human clinical osteo-articular sample. Int J Syst Evol Microbiol 60:1516–1521. doi: 10.1099/ijs.0.012914-0. [DOI] [PubMed] [Google Scholar]
- 225.Brunner S, Graf S, Riegel P, Altwegg M. 2000. Catalase-negative Actinomyces neuii subsp. neuii isolated from an infected mammary prosthesis. Int J Med Microbiol 290:285–287. doi: 10.1016/S1438-4221(00)80128-9. [DOI] [PubMed] [Google Scholar]
- 226.Hsi RS, Hotaling JM, Spencer ES, Bollyky PL, Walsh TJ. 2011. Isolated infection of a decommissioned penile prosthesis reservoir with Actinomyces neuii. J Sex Med 8:923–926. doi: 10.1111/j.1743-6109.2010.02144.x. [DOI] [PubMed] [Google Scholar]
- 227.Grundmann S, Huebner J, Stuplich J, Koch A, Wu K, Geibel-Zehender A, Bode C, Brunner M. 2010. Prosthetic valve endocarditis due to Actinomyces neuii successfully treated with antibiotic therapy. J Clin Microbiol 48:1008–1011. doi: 10.1128/JCM.01106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rieber H, Schwarz R, Krämer O, Cordier W, Frommelt L. 2009. Actinomyces neuii subsp. neuii associated with periprosthetic infection in total hip arthroplasty as causative agent. J Clin Microbiol 47:4183–4184. doi: 10.1128/JCM.01249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Anderson IA, Jarral F, Sethi K, Chumas PD. 2014. Paediatric ventriculoperitoneal shunt infection caused by Actinomyces neuii. BMJ Case Rep 2014:bcr2014204576. doi: 10.1136/bcr-2014-204576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Watkins RR, Anthony K, Schroder S, Hall GS. 2008. Ventriculoperitoneal shunt infection caused by Actinomyces neuii subsp. neuii. J Clin Microbiol 46:1888–1889. doi: 10.1128/JCM.02141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Varughese S, Bargman J. 2014. Actinomyces neuii PD peritonitis—resolution of infection without catheter removal. Perit Dial Int 34:815–816. doi: 10.3747/pdi.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hedke J, Skripitz R, Ellenrieder M, Frickmann H, Köller T, Podbielski A, Mittelmeier W. 2012. Low-grade infection after a total knee arthroplasty caused by Actinomyces naeslundii. J Med Microbiol 61:1162–1164. doi: 10.1099/jmm.0.030395-0. [DOI] [PubMed] [Google Scholar]
- 233.Wüst J, Steiger U, Vuong H, Zbinden R. 2000. Infection of a hip prosthesis by Actinomyces naeslundii. J Clin Microbiol 38:929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zaman R, Abbas M, Burd E. 2002. Late prosthetic hip joint infection with Actinomyces israelii in an intravenous drug user: case report and literature review. J Clin Microbiol 40:4391–4392. doi: 10.1128/JCM.40.11.4391-4392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Siu YP, Tong MK, Lee MK, Leung KT, Kwan TH. 2004. Exit-site infection caused by Actinomyces odontolyticus in a CAPD patient. Perit Dial Int 24:602–603. [PubMed] [Google Scholar]
- 236.Branquinho DF, Andrade DR, Almeida N, Sofia C. 2014. Mediastinitis by Actinomyces meyeri after oesophageal stent placement. BMJ Case Rep 2014:bcr2014204499. doi: 10.1136/bcr-2014-204499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cohen E, Bishara J, Medalion B, Sagie A, Garty M. 2007. Infective endocarditis due to Actinomyces neuii. Scand J Infect Dis 39:180–183. doi: 10.1080/00365540600802007. [DOI] [PubMed] [Google Scholar]
- 238.Westling K, Lidman C, Thalme A. 2002. Tricuspid valve endocarditis caused by a new species of actinomyces: Actinomyces funkei. Scand J Infect Dis 34:206–207. doi: 10.1080/00365540110077425. [DOI] [PubMed] [Google Scholar]
- 239.Adalja AA, Vergis EN. 2010. Actinomyces israelii endocarditis misidentified as “diptheroids.” Anaerobe 16:472–473. doi: 10.1016/j.anaerobe.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 240.Lam S, Samraj J, Rahman S, Hilton E. 1993. Primary actinomycotic endocarditis: case report and review. Clin Infect Dis 16:481–485. doi: 10.1093/clind/16.4.481. [DOI] [PubMed] [Google Scholar]
- 241.Julian KG, de Flesco L, Clarke LE, Parent LJ. 2005. Actinomyces viscosus endocarditis requiring aortic valve replacement. J Infect 50:359–362. doi: 10.1016/j.jinf.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 242.Mardis JS, Many WJ. 2001. Endocarditis due to Actinomyces viscosus. South Med J 94:240–243. [PubMed] [Google Scholar]
- 243.Moffatt S, Ahmen AR, Forward K. 1996. First reported case of bacterial endocarditis attributable to Actinomyces meyeri. Can J Infect Dis 7:71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lawson PA, Nikolaitchouk N, Falsen E, Westling K, Collins MD. 2001. Actinomyces funkei sp. nov., isolated from human clinical specimens. Int J Syst Evol Microbiol 51:853–855. doi: 10.1099/00207713-51-3-853. [DOI] [PubMed] [Google Scholar]
- 245.Hwang SS, Park SD, Jang IH, Uh Y, Yoon KJ, Kim HY. 2011. Actinomyces graevenitzii bacteremia in a patient with alcoholic liver cirrhosis. Anaerobe 17:87–89. doi: 10.1016/j.anaerobe.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 246.Renvoise A, Raoult D, Roux V. 2009. Actinomyces massiliensis sp. nov., isolated from a patient blood culture. Int J Syst Evol Microbiol 59:540–544. doi: 10.1099/ijs.0.001503-0. [DOI] [PubMed] [Google Scholar]
- 247.Topic MB, Desnica B, Vickovic N, Skuhala T, Bayer K, Bukovski S. 2014. The polymicrobial Actinomyces naeslundii and Pseudomonas aeruginosa sepsis in a patient with ulcerative colitis 2 months after colonoscopy. Wien Klin Wochenschr 126:130–132. doi: 10.1007/s00508-013-0471-7. [DOI] [PubMed] [Google Scholar]
- 248.Bahrani-Mougeot FK, Paster BJ, Coleman S, Ashar J, Barbuto S, Lockhart PB. 2008. Diverse and novel oral bacterial species in blood following dental procedures. J Clin Microbiol 46:2129–2132. doi: 10.1128/JCM.02004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Reinhard M, Prag J, Kemp M, Andresen K, Klemmensen B, Højlyng N, Sørensen SH, Christensen JJ. 2005. Ten cases of Actinobaculum schaalii infection: clinical relevance, bacterial identification, and antibiotic susceptibility. J Clin Microbiol 43:5305–5308. doi: 10.1128/JCM.43.10.5305-5308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fendukly F, Osterman B. 2005. Isolation of Actinobaculum schaalii and Actinobaculum urinale from a patient with chronic renal failure. J Clin Microbiol 43:3567–3569. doi: 10.1128/JCM.43.7.3567-3569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Haller P, Bruderer T, Schaeren S, Laifer G, Frei R, Battegay M, Flückiger U, Bassetti S. 2007. Vertebral osteomyelitis caused by Actinobaculum schaalii: a difficult-to-diagnose and potentially invasive uropathogen. Eur J Clin Microbiol Infect Dis 26:667–670. doi: 10.1007/s10096-007-0345-x. [DOI] [PubMed] [Google Scholar]
- 252.Sturm PDJ, Van Eijk J, Veltman S, Meuleman E, Schülin T. 2006. Urosepsis with Actinobaculum schaalii and Aerococcus urinae. J Clin Microbiol 44:652–654. doi: 10.1128/JCM.44.2.652-654.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pajkrt D, Simoons-Smit AM, Savelkoul PHM, van den Hoek J, Hack WWM, van Furth AM. 2003. Pyelonephritis caused by Actinobaculum schaalii in a child with pyeloureteral junction obstruction. Eur J Clin Microbiol Infect Dis 22:438–440. doi: 10.1007/s10096-003-0933-3. [DOI] [PubMed] [Google Scholar]
- 254.Waghorn DJ. 2004. Actinobaculum massiliae: a new cause of superficial skin infection. J Infect 48:276–277. doi: 10.1016/j.jinf.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 255.Bank S, Jensen A, Hansen TM, Soby KM, Prag J. 2010. Actinobaculum schaalii, a common uropathogen in elderly patients, Denmark. Emerg Infect Dis 16:76–80. doi: 10.3201/eid1601.090761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Nielsen HL, Søby KM, Christensen JJ, Prag J. 2010. Actinobaculum schaalii: a common cause of urinary tract infection in the elderly population. Bacteriological and clinical characteristics Scand J Infect Dis 42:43–47. doi: 10.3109/00365540903289662. [DOI] [PubMed] [Google Scholar]
- 257.Tschudin-Sutter S, Frei R, Weisser M, Goldenberger D, Widmer AF. 2011. Actinobaculum schaalii—invasive pathogen or innocent bystander? A retrospective observational study. BMC Infect Dis 11:289. doi: 10.1186/1471-2334-11-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Andersen LB, Bank S, Hertz B, Søby KM, Prag J. 2012. Actinobaculum schaalii, a cause of urinary tract infections in children? Acta Paediatr 101:e232–e234. doi: 10.1111/j.1651-2227.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- 259.Zimmermann P, Berlinger L, Liniger B, Grunt S, Agyeman P, Ritz N. 2012. Actinobaculum schaalii an emerging pediatric pathogen? BMC Infect Dis 12:201. doi: 10.1186/1471-2334-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lotte R, Durand M, Mbeutcha A, Ambrosetti D, Pulcini C, Degand N, Loeffler J, Ruimy R, Amiel J. 2014. A rare case of histopathological bladder necrosis associated with Actinobaculum schaalii: the incremental value of an accurate microbiological diagnosis using 16S rDNA sequencing. Anaerobe 26:46–48. doi: 10.1016/j.anaerobe.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 261.Salvado M, Plasencia V, Segura C, Gomez J, Medina MJ, Saez-Nieto JA, Castellanos S, Horcajada JP. 2013. Infection due to Actinobaculum spp: report of 12 patients in Spain. J Infect 66:107–109. doi: 10.1016/j.jinf.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 262.Sandlund J, Glimaker M, Svahn A, Brauner A. 2014. Bacteraemia caused by Actinobaculum schaalii: an overlooked pathogen? Scand J Infect Dis 46:605–608. doi: 10.3109/00365548.2014.913306. [DOI] [PubMed] [Google Scholar]
- 263.Tena D, Fernández C, Lago MR, Arias M, Medina MJ, Sáez-Nieto JA. 2014. Skin and soft-tissue infections caused by Actinobaculum schaalii: report of two cases and literature review. Anaerobe 28:95–97. doi: 10.1016/j.anaerobe.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 264.van Aarle S, Arents NLA, de Laet K. 2013. Actinobaculum schaalii causing epididymitis in an elderly patient. J Med Microbiol 62:1092–1093. doi: 10.1099/jmm.0.048611-0. [DOI] [PubMed] [Google Scholar]
- 265.Vanden Bempt I, Van Trappen S, Cleenwerck I, De Vos P, Camps K, Celens A, Van De Vyvere M. 2011. Actinobaculum schaalii causing Fournier's gangrene. J Clin Microbiol 49:2369–2371. doi: 10.1128/JCM.00272-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gomez E, Gustafson DR, Rosenblatt JE, Patel R. 2011. Actinobaculum bacteremia: a report of 12 cases. J Clin Microbiol 49:4311–4313. doi: 10.1128/JCM.00798-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chu YW, Wong CH, Chu MY, Cheung CPF, Cheung TKM, Tse C, Luk WK, Lo JYC. 2009. Varibaculum cambriense infections in Hong Kong, China, 2006. Emerg Infect Dis 15:1137–1139. doi: 10.3201/eid1507.081291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Brazier JS, Hall V. 1993. Propionibacterium propionicum and infections of the lacrimal apparatus. Clin Infect Dis 17:892–893. doi: 10.1093/clinids/17.5.892. [DOI] [PubMed] [Google Scholar]
- 269.Chávez de Paz LE, Molander A, Dahlén G. 2004. Gram-positive rods prevailing in teeth with apical periodontitis undergoing root canal treatment. Int Endod J 37:579–587. doi: 10.1111/j.1365-2591.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 270.Siqueira JF, Rôças IN. 2004. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:85–94. doi: 10.1016/S1079-2104(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 271.Pasic S, Savic D, Milovic I, Vasiljevic Z, Djuricic S. 2004. Propionibacterium propionicus infection in chronic granulomatous disease. Clin Infect Dis 38:459. doi: 10.1086/381030. [DOI] [PubMed] [Google Scholar]
- 272.Chau AMT, Xu LL, Fairhall JM, Chaganti J, McMullan BJ. 2012. Brain abscess due to Propionibacterium propionicum in Eisenmenger syndrome. Med J Aust 196:525–526. doi: 10.5694/mja11.10768. [DOI] [PubMed] [Google Scholar]
- 273.Riley TV, Ott AK. 1981. Brain abscess due to Arachnia propionica. BMJ 282:1035. doi: 10.1136/bmj.282.6269.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wunderink HF, Lashley EELO, van Poelgeest MIE, Gaarenstroom KN, Claas ECJ, Kuijper EJ. 2011. Pelvic actinomycosis-like disease due to Propionibacterium propionicum after hysteroscopic removal of an intrauterine device. J Clin Microbiol 49:466–468. doi: 10.1128/JCM.01772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yonetani S, Ohnishi H, Araki K, Hiroi M, Takagi Y, Ichimura S, Watanabe T. 2014. A psoas abscess caused by Propionibacterium propionicum. J Infect Chemother 20:650–652. doi: 10.1016/j.jiac.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 276.Figdor D, Sjogren U, Sorlin S, Sundqvist G, Nair PN. 1992. Pathogenicity of Actinomyces israelii and Arachnia propionica: experimental infection in guinea pigs and phagocytosis and intracellular killing by human polymorphonuclear leukocytes in vitro. Oral Microbiol Immunol 7:129–136. doi: 10.1111/j.1399-302X.1992.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 277.Brook I. 1994. The role of encapsulated anaerobic bacteria in synergistic infections. FEMS Microbiol Rev 13:65–74. doi: 10.1111/j.1574-6976.1994.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 278.Wade WG. 2013. The oral microbiome in health and disease. Pharmacol Res 69:137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 279.Gibbons RJ, Hay DI. 1988. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun 56:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Li T, Khah MK, Slavnic S, Johansson I, Stromberg N. 2001. Different type 1 fimbrial genes and tropisms of commensal and potentially pathogenic Actinomyces spp. with different salivary acidic proline-rich protein and statherin ligand specificities. Infect Immun 69:7224–7233. doi: 10.1128/IAI.69.12.7224-7233.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shen S, Samaranayake LP, Yip HK. 2005. Coaggregation profiles of the microflora from root surface caries lesions. Arch Oral Biol 50:23–32. doi: 10.1016/j.archoralbio.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 282.Kolenbrander PE. 2000. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol 54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- 283.Takahashi N, Nyvad B. 2011. The role of bacteria in the caries process: ecological perspectives. J Dent Res 90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 284.Norskov-Lauritsen N. 2014. Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin Microbiol Rev 27:214–240. doi: 10.1128/CMR.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Engelhardt K, Kampfl A, Spiegel M, Pfausler B, Hausdorfer H, Schmutzhard E. 2002. Brain abscess due to Capnocytophaga species, Actinomyces species, and Streptococcus intermedius in a patient with cyanotic congenital heart disease. Eur J Clin Microbiol Infect Dis 21:236–237. doi: 10.1007/s10096-002-0696-2. [DOI] [PubMed] [Google Scholar]
- 286.Simpson AJ, Das SS, Mitchelmore IJ. 1996. Polymicrobial brain abscess involving Haemophilus paraphrophilus and Actinomyces odontolyticus. Postgrad Med J 72:297–298. doi: 10.1136/pgmj.72.847.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Martinello RA, Cooney EL. 2003. Cerebellar brain abscess associated with tongue piercing. Clin Infect Dis 36:e32–e34. doi: 10.1086/345755. [DOI] [PubMed] [Google Scholar]
- 288.Wüst J, Stubbs S, Weiss N, Funke G, Collins MD. 1995. Assignment of Actinomyces pyogenes-like (CDC coryneform group E) bacteria to the genus Actinomyces as Actinomyces radingae sp. nov. and Actinomyces turicensis sp. nov. Lett Appl Microbiol 20:76–81. doi: 10.1111/j.1472-765X.1995.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 289.Santala A-M, Sarkonen N, Hall V, Carlson P, Jousimies-Somer H, Könönen E. 2004. Evaluation of four commercial test systems for identification of Actinomyces and some closely related species. J Clin Microbiol 42:418–420. doi: 10.1128/JCM.42.1.418-420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kolbert CP, Persing DH. 1999. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr Opin Microbiol 2:299–305. doi: 10.1016/S1369-5274(99)80052-6. [DOI] [PubMed] [Google Scholar]
- 291.Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 292.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM. 2014. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Corander J, Connor TR, O'Dwyer CA, Kroll JS, Hanage WP. 2012. Population structure in the Neisseria, and the biological significance of fuzzy species. J R Soc Interface 9:1208–1215. doi: 10.1098/rsif.2011.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, Raoult D. 2009. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 295.De Vreese K, Verhaegen J. 2013. Identification of coryneform Actinomyces neuii by MALDI-TOF MS: 5 case reports and review of literature. Acta Clin Belg 68:210–214. doi: 10.2143/ACB.3224. [DOI] [PubMed] [Google Scholar]
- 296.Schuetz AN. 2014. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Clin Infect Dis 59:698–705. doi: 10.1093/cid/ciu395. [DOI] [PubMed] [Google Scholar]
- 297.Marchand-Austin A, Rawte P, Toye B, Jamieson FB, Farrell DJ, Patel SN. 2014. Antimicrobial susceptibility of clinical isolates of anaerobic bacteria in Ontario, 2010-2011. Anaerobe 28:120–125. doi: 10.1016/j.anaerobe.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 298.Smith AJ, Hall V, Thakker B, Gemmell CG. 2005. Antimicrobial susceptibility testing of Actinomyces species with 12 antimicrobial agents. J Antimicrob Chemother 56:407–409. doi: 10.1093/jac/dki206. [DOI] [PubMed] [Google Scholar]
- 299.Goldstein EJ, Citron DM, Merriam CV, Warren YA, Tyrrell KL, Fernandez HT. 2006. Comparative in vitro susceptibilities of 396 unusual anaerobic strains to tigecycline and eight other antimicrobial agents. Antimicrob Agents Chemother 50:3507–3513. doi: 10.1128/AAC.00499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Goldstein EJC, Citron DM, Merriam CV, Warren YA, Tyrrell KL, Fernandez HT, Bryskier A. 2005. Comparative in vitro activities of XRP 2868, pristinamycin, quinupristin-dalfopristin, vancomycin, daptomycin, linezolid, clarithromycin, telithromycin, clindamycin, and ampicillin against anaerobic gram-positive species, actinomycetes, and lactobacilli. Antimicrob Agents Chemother 49:408–413. doi: 10.1128/AAC.49.1.408-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tyrrell KL, Citron DM, Warren YA, Goldstein EJC. 2012. In vitro activity of TD-1792, a multivalent glycopeptide-cephalosporin antibiotic, against 377 strains of anaerobic bacteria and 34 strains of Corynebacterium species. Antimicrob Agents Chemother 56:2194–2197. doi: 10.1128/AAC.06274-11. [DOI] [PMC free article] [PubMed] [Google Scholar]