SUMMARY
Substandard/counterfeit antimicrobial drugs are a growing global problem. The most common substandard/counterfeit antimicrobials include beta-lactams (among antibiotics) and chloroquine and artemisin derivatives (among antimalarials). The most common type of substandard/counterfeit antimicrobial drugs have a reduced amount of the active drug, and the majority of them are manufactured in Southeast Asia and Africa. Counterfeit antimicrobial drugs may cause increased mortality and morbidity and pose a danger to patients. Here we review the literature with regard to the issue of substandard/counterfeit antimicrobials and describe the prevalence of this problem, the different types of substandard/counterfeit antimicrobial drugs, and the consequences for the individuals and global public health. Local, national, and international initiatives are required to combat this very important public health issue.
INTRODUCTION
Counterfeit and substandard drugs are an emerging menace (1–9). Antimicrobials, especially antibiotics and antimalarial agents, are most often reported as substandard/counterfeit in less-developed countries (10–14). The counterfeiting of antimicrobials needs to be studied further, but journalism is the main source of information compared with limited available information in the scientific literature. Only few systematic reviews of evidence regarding low-quality antimicrobials have been published (15–17). Here we describe the published scientific evidence regarding counterfeit/substandard antimicrobials, the different types of substandard antimicrobials and how they differ from counterfeits, their consequences to individuals and to the public health, and the strategies to detect them and combat them.
CHARACTERISTICS OF SUBSTANDARD/COUNTERFEIT DRUGS
Substandard medicines have not been defined uniformly (18). Substandard drugs are “genuine medicines which have not passed the standards and quality testing protocols set for them” (19–22). The World Health Organization (WHO) and International Pharmacopoeias have previously determined these standards and quality tests (23). Counterfeit medicines are a type of substandard drugs. There is no international consensus regarding the definition of counterfeit medicines (18), and the WHO defines them as “drugs that are deliberately and fraudulently mislabeled with respect to identity and/or source” (12).
The characteristics of antimicrobials with poor quality are summarized in Table 1 (1, 2, 7, 9, 15, 17, 24–60). Counterfeit medicines may be brand and/or generic products with falsified packaging and may have too little, wrong, or no active ingredient (12, 61). Both poor manufacturing and counterfeiting may lead to variations in the quality and quantity of active ingredients in substandard drugs (15). Although it is important to distinguish among the different types of suboptimal drugs, such as “fake,” “counterfeit,” and “substandard” medicines, in most of the studies that have addressed this topic it was not determined whether poor production, storage, or counterfeiting led to the low quality of the drug (2, 7). In most studies, the cause for the low quality of the drugs was not identified (57, 62), and the terms “substandard” and “counterfeit” were used interchangeably. Determining whether substandard drugs are also counterfeit is challenging, based on the limited available evidence (59). In most cases, it is not known if the wrong concentration of the active component in a drug is the result of fraud, even after chemical analysis of a drug (59). In 2011, the WHO proposed the use of the term “substandard/spurious/falsely labeled/falsified/counterfeit drugs” (SSFFC) (59); this is ambiguous (18) since it cannot differentiate the different causes of poor drug quality (counterfeit versus substandard), which need to be addressed using different approaches. In this article the term “substandard/counterfeit” drug is used when the cause for the poor-quality drug has not been determined.
TABLE 1.
Characteristics of substandard and counterfeit antimicrobials
| Group and characteristic | Comments (references) |
|---|---|
| Substandard antimicrobials | |
| Reduced concn of AIa | Quantification of the AI content of an antimicrobial agent shows that the concn of the AI is lower than the claimed content declared on the packaging |
| Poor manufacturing or transportation, decomposition, and poor storage conditions (2, 34, 35) and dilution of drugs with other chemicals (25, 26, 27) may lead to low concn of the AI | |
| In a recent report of published prevalence studies regarding substandard/counterfeit antimicrobials, 14/15 (93%) studies reported this problem (59) | |
| Reduced stability (dissolution failure) and bioavailability | Solubility or release of AI is not within the specified time range due to reduced stability of the drug, and this leads to reduced bioavailability of the antibiotic (59) |
| Most of the limited no. of studies that have assessed the stability of antimicrobials had suboptimal design (29–33, 35, 36) | |
| Antibiotics such as ampicillin (34), but not others such as penicillins and tetracyclines (7, 35), may degrade with high temperatures and humidity (30) | |
| Reduced bioavailability may lead to suboptimal activity of antimicrobials (36); examples include antibacterials such as co-trimoxazole (38), tetracyclines (37), and metronidazole (38) and antiparasitic agents such as chloroquine (39), mefloquine (40), and pyrimethamine (41–43) | |
| In a recent report of published prevalence studies regarding substandard/counterfeit antimicrobials, 5/15 (33%) studies reported this problem (59) | |
| Impurities/unknown ingredient | Substandard wt of a tablet or capsule (59) |
| Altered odor due to diluted AI or harmful additives | |
| Inactive or harmful ingredients, impurities, or contamination such as mold | |
| In a recent report of published prevalence studies regarding substandard/counterfeit antimicrobials, 2/15 (13%) studies reported this problem (59) | |
| Counterfeit antimicrobials | |
| Absence of pharmaceutical AI | Quantification of the AI content of an antimicrobial agent shows that the AI as declared on the packaging is absent (59) |
| Antibiotics may contain no AI (9, 44, 48, 49) | |
| The AI is replaced by cheap substances, such as flour in oral presentations and water in drinkable or injectable presentations (15, 17) | |
| In a recent report of published prevalence studies regarding substandard/counterfeit antimicrobials, 7/15 (47%) studies reported this problem (59) | |
| Reduced amt of AI | The antimicrobial may be considered counterfeit when the substandard amt of the AI is included deliberately in the drug (59) |
| Increased amt of AI | The concn of the AI may be higher than the amt reported in the packaging (59) |
| In a recent report of published prevalence studies regarding substandard/counterfeit antimicrobials, 6/15 (40%) studies reported this problem (59) | |
| Altered chemical content/wrong ingredient | Detection of AI in the drug that is not declared on the packaging (59) |
| Examples of wrong ingredients include erythromycin (45), flour, starch, or powder (26, 45–47), and tap water (9, 44, 48, 49) | |
| These products may contain toxic chemical impurities (15, 17) | |
| In a recent report of published prevalence studies regarding substandard/counterfeit antimicrobials, 4/15 (27%) studies reported this problem (59) | |
| Impurities/unknown ingredient | Extraneous contaminants that should not be present in a drug (if done deliberately and not the result of poor manufacturing) (59) |
| In a recent report of published prevalence studies regarding substandard/counterfeit antimicrobials, 2/15 (13%) studies reported this problem (59) | |
| Inappropriate packaging | Packaging has incorrect labeling information about a drug origin or authenticity, and the color, size of pills, and bar codes are often similar to those for the original drug (25) |
| False representation of identity is commonly used, by copying the packaging of another marketed product; the brand name may be modified to try to escape laws on infringing intellectual property | |
| They are generally undisguisable in their outward packaging | |
| In developing countries, many of the purchased drugs without packaging were counterfeit (57) | |
| Antimicrobials with false packaging and labeling include penicillins (24), co-trimoxazole (50), tetracyclines (1, 51–54), chloramphenicol (1), quinolones (51), aminoglycosides (55, 56), and antimalarials (40, 57, 60). | |
| Very few studies performed packaging analysis of the samples collected (40, 58, 59) | |
| Mass uniformity test failure | The wt of a tablet or capsule is not within the avg range specified (if done deliberately and not the result of poor manufacturing) (59) |
AI, active ingredient.
EPIDEMIOLOGY OF SUBSTANDARD/COUNTERFEIT ANTIMICROBIALS
Determination of the Epidemiology of Low-Quality Antimicrobials Is Challenging
The number of published research studies regarding counterfeit drugs is limited, and the problem is reported mostly in newspapers and online resources rather than the biomedical literature. A large randomized, blinded study with random and adequate sampling is needed to accurately determine the epidemiology of low-quality antimicrobials. Only a limited number of studies had adequate methodology and random sampling (2, 15, 59), whereas sampling bias, inappropriate storage conditions (38), and instability of certain antibiotics (28) limited the value of the reported data in most studies. Other reports were restricted or not published (59). Many studies used only a small sample of antibiotics that did not represent the magnitude of the problem (5, 63). In many studies there were inconsistencies with regard to the definitions, pharmacopoeias, and drug sampling methods that were used. Thus, direct comparison between different studies is difficult. Finally, the individual contribution of each factor, such as falsified packaging analysis, to the epidemiology of low-quality antimicrobials has not been adequately studied. However, epidemiological data from national and international organizations and from prevalence studies can better define the magnitude of the problem.
Epidemiological Data from National and International Organizations
According to the WHO, up to 10% of the drugs worldwide may be counterfeits (13, 64); 50% of them involved antimicrobial drugs, and 78% were from developing countries. Moreover, 59% of cases with available information on the quality of drugs were fraudulent, and only 7% had the standard concentration of the active drug (1, 65, 66). However, reporting of counterfeit drugs within WHO is <15% (22, 67). A WHO study of drug product quality in developing countries in Africa found that 7.6% of major antibiotic formulations contained no active ingredient, whereas 17.8% of antibiotics and 13% of antiparasitic products were substandard by WHO criteria but not necessarily counterfeit (27). However, well-designed studies to define the problem are lacking, and this has led to significant variability in the estimates of counterfeit drugs among other developing countries (68–70). According to the U.S. Food and Drug Administration (FDA), up to 25% of all medicines in developing countries and >10% of drugs worldwide have low quality (71). The Pharmaceutical Security Institute data indicate an increase in the reports of fake drugs of more than 10-fold within 2002 to 2012 (72). The data reflect the regulatory oversight in countries where the counterfeit antimicrobials have been studied (73). Since pharmaceutical companies and regulatory authorities have not published most of their data on counterfeit antibiotics (18, 73), published prevalence studies may provide useful epidemiology data (73).
Counterfeit Drugs Have Been Reported Worldwide
Counterfeit drugs are less than 1% of the market in higher-income countries (74) but have been reported 10 times more often within the last 5 years in the United States (17). In Europe within 1 year (2007 to 2008), counterfeit products increased by 118% and their confiscations had a relative increase of 57% (17). In developing countries, with insufficient public resources and monitoring (13, 64), counterfeit drugs account for between 10% and 30% of all drugs sold (75). The rate of counterfeiting is as high as 20% of all drugs in the countries of the former Soviet Union. According to the World Trade Organization, the United Nations Office on Drugs and Crime (UNODC), and data that are often based on package labels and may be incomplete, India manufactured most of the counterfeit drugs worldwide in 2006, followed by China and Thailand (75, 76). In a recent literature review, 44%, 30%, and 9% of 163 counterfeit antibiotics were detected in Asia, Africa, and Europe and North America, respectively (17). Overall, up to 60% of antimicrobials in Africa and Asia may have low quality (75).
Published Prevalence Studies Regarding Low-Quality Antimicrobials Provide a Better Estimate of the Magnitude of the Problem
The problem of low-quality pharmaceuticals has increasingly been reported (77, 78). In our previous review of the literature (15), the prevalence of substandard/counterfeit antimicrobials varied from 10% to more than 50% in some studies (2, 7, 38, 79, 80), whereas in another report it was 28.5% (range, 11 to 48%) and was similar in developing countries (22 to 38%). In the latter review, out of a total of 44 identified studies, only 15 studies were included in the analysis based on quality criteria (59), four used random sampling methods (7, 28, 81, 82), and only one included sample size estimates (81). Packaging analysis of the collected samples was performed in only two studies from Southeast Asia (40, 58). Five of these studies reported the antimicrobials as possible counterfeit (7, 28, 57, 82). Overall, the problem of low-quality antimicrobials was prevalent in Africa and Asia, but the epidemiology of these drugs in upper-income countries is unknown.
Factors That Contribute to the Spread of Low-Quality Medicines
The WHO has determined factors that may lead to spread of substandard drugs, such as counterfeiting (44), chemical instability (29), and poor quality control (83) (summarized in Table 2) (12). Corruption, crime, and poorly regulated pharmaceutical companies are major contributors to counterfeiting (26, 84, 85). The counterfeit drug market is more profitable for counterfeiters than that of illicit drugs, according to the International Drug Industry Federation (17). Financial interests counteracted international efforts to combat counterfeiting (86). In a recent review of 5 studies regarding counterfeit antimicrobials, 51% and 24% of the counterfeit drugs were derived from unlicensed retailers and licensed authorities, respectively (40, 87–89). Thus, the unauthorized sale of substandard drugs is common in less-developed countries (57, 90). Authentic medicines are often expensive, and people buy inexpensive medicines from unauthorized retailers (57), which are often considered of good quality by the public (90). Future studies need to further determine the spread of low-quality antimicrobials in the unauthorized market.
TABLE 2.
Factors that contribute to poor-quality drugs
| Factor(s) contributing to poor-quality drugs |
|---|
| Substandard drugs |
| Reduced stability of drugs in developing countries due to environmental conditions and poor storage |
| No good manufacturing process in developing countries |
| Poor quality control during manufacture |
| Poor surveillance about expiration dates and storage conditions in poor settings |
| Use of nonstandardized pharmacopoeias by many developing countries |
| Counterfeit drugs |
| Financial interests: crime, corruption of politicians and industry |
| Inadequate resources dedicated to control manufacture, import, and export activities, complex transactions, inefficient cooperation among stakeholders |
| High demand for antimicrobials and vaccines exceeds supply |
| High prices of original drugs |
| Development of Internet |
| High rate of illiteracy and very low income of population in less-developed countries |
| Lack of sensitization of people in less-developed countries to the impact and dangers of counterfeit drugs purchased from nonauthorized dealers |
METHODS USED TO DETECT COUNTERFEIT/SUBSTANDARD ANTIMICROBIALS
Substandard drugs can be detected by different methods, including inspection, colorimetric methods, chromatography, and spectrometry (Table 3) (9, 12, 31, 43, 45, 91–106). A three-level approach (107) consisting of different quality control procedures has been proposed to detect substandard/counterfeit antimicrobials. Level 1 includes inspection to determine the quality of packaging and labeling. Level 2 encompasses methods that can be done in the field. Level 3 requires the equipment of an established laboratory to determine drug quality according to established specifications. New technologies such as spectroscopy (108), X-ray methods (109), data matrix type, radio frequency identification (RFID) labels, holograms, engraving, invisible prints, and nanotechnologies have increasingly been used to determine quality of antimicrobials and may help increase the efficacy of detection of substandard/counterfeit antimicrobials. However, such solutions are expensive and often available only to higher-income countries. Thus, since technology may improve the detection of low-quality drugs, it would be important that these methods will also be available in developing countries, where counterfeit products are the most common.
TABLE 3.
Methods for detection of counterfeit/substandard antibioticsa
| Method | Advantage(s) | Disadvantage(s) |
|---|---|---|
| Diagnostic methods to determine quality of medicines widely used in developing countries | ||
| Inspection: physical properties (printing, embossing, shape, odor, taste, consistency) and secure labeling of products (12) | Quickest and cheapest way to detect a counterfeit drug | Neither sensitive nor specific since counterfeits have become increasingly sophisticated (45, 91) |
| Characteristic physical and chemical properties: wt, density, refractive index, viscosity, osmolarity, pH, crystal morphology, solubility | Low cost | Nonspecific |
| Can provide simple tests for detecting counterfeit drugs such as antimalarials | ||
| Colorimetric techniques (92, 93) | One of the most widely used methods to evaluate drug quality (9, 94) | Less sensitive and less specific than other, more sophisticated techniques such as HPLC |
| Can be done by untrained personnel | ||
| Rapid and highly specific | ||
| Improved colorimetric methods incorporate colorimetric reactions onto a paper-based device (95) | ||
| Dissolution/disintegration assays | Widely used (31, 43, 96) | Less sensitive and less specific than other, more sophisticated techniques |
| Can detect substandard drugs even when the amt of the active ingredient is appropriate | ||
| Correlate the in vivo bioavailability of an oral medicine to its in vitro solubility vs time profile | ||
| GPHF Mini-lab (97) | Reliable, simple, and inexpensive method (98) | Less sensitive and less specific than other, more sophisticated techniques |
| Requires reagents, solvents, standards, training | ||
| TLC | In combination with colorimetric methods has been recognized by the WHO as appropriate technology for initial screening of a large no. of drug samples in developing countries (12) | Often uses toxic or flammable reagents and requires training |
| Fast, convenient, easy, specific, inexpensive | Less sensitive and less specific than HPLC | |
| Useful in settings where laboratory resources such as HPLC are not available | ||
| X-ray methods, such as X-ray fluorescence (99) | Can be used in resource-poor settings because they are portable | Only qualitative analysis of drugs |
| Costly methods | ||
| Spectroscopy methods, such as Raman (100) and infrared (95) | Can be used in resource-poor settings because they are rapid, accurate, and can be performed with a portable device that allows real-time analysis of suspicious drugs | Only qualitative analysis of drugs |
| Allows determination of a spectral print according to the drug ingredients and comparison with all spectra stored in a database | High initial investment cost | |
| Diagnostic methods to determine quality of drugs that are not widely used in developing countries | ||
| High-performance liquid chromatography | Determine the exact quantity of AI in the sample | Limited analysis speed |
| Assess drug quality according to the specifications established by international pharmacopoeia | Sophisticated, costly, labor-intensive | |
| Accurate identification of ingredients; used for nonvolatile chemicals (101) | ||
| Gas chromatography | As for HPLC but useful for detecting residual solvents, volatile constituents, and undeclared ingredients (101) | Sophisticated, costly, labor-intensive |
| MS, such as accurate-mass ESI-MS, accurate-mass MS/MS, LC-MS, NIR spectroscopy (31, 103–106) | Specific | |
| Precise: accurate identification of ingredients present in counterfeit drugs | ||
| Assess drug quality according to the specifications established by international pharmacopoeia | ||
| No sample prepn, increased sample throughput | ||
| Microbiological techniques | Antimicrobial activity assays with reference bacterial species are specific (102) | Not widely available |
| RFID system | Label meant to receive a radio signal and immediately respond by sending back a different radio signal containing data; this label is made of an electronic chip and an antenna | Not widely available |
Abbreviations: AI, active ingredient; GPHF, German Pharma Health Fund; TLC, thin-layer chromatography; HPLC, high-performance liquid chromatography; MS, mass spectrometry; ESI-MS, electrospray ionization MS; MS/MS, tandem MS; LC-MS, liquid chromatography-MS; NIR, near infrared; RFID, radio frequency identification.
CATEGORIES OF LOW-QUALITY ANTIMICROBIALS
Categories of substandard/counterfeit antimicrobials include antibiotics (2, 5, 7, 15, 17, 24, 28, 35, 38, 51, 62, 110–125), antituberculosis (anti-TB) drugs (62, 110, 112, 121, 122), antivirals (14, 26, 46, 126–150), vaccines (56, 144, 151–156), and antimalarials (2, 7, 9, 15, 24, 27, 28, 31, 34, 38, 40, 43-45, 48, 50, 52, 53, 55, 57, 58, 60, 63, 78, 79, 81, 82, 87–89, 91, 113, 117, 123, 131, 157–186) (see Tables 4 to 8). Low-quality antibiotics are reported 8- to 10-fold more often and antiparasitic drugs 2- to 3-fold more often than other counterfeits (187). Antimalarials, followed by antibiotics, are the most common targets for counterfeiting.
TABLE 4.
Studies of substandard/counterfeit antibioticsa
| Yr/country(ies) (reference) | Category(ies) of drugs studied (nb) | Method(s) of detection of counterfeit/substandard drug | Results | Characteristics of counterfeit/substandard drugs |
|---|---|---|---|---|
| 2013/Angola, Brazil, China, Democratic Republic of Congo, Egypt, Ethiopia, Ghana, India (n = 3), Kenya, Nigeria, Russia, Rwanda, Thailand, Turkey, Uganda, United Republic of Tanzania, Zambia (110) | Antituberculosis medicines, tablets: INH and RMP (713) | Stability testing, TLC | 9.1% were substandard/counterfeit;16.6% of these were reported in Africa, 10.1% in India, and 3.9% in other middle-income countries | Reduced dissolution and stability, reduced content of AI |
| 2012/Angola, Brazil, Cameroon, Central African Republic, Chad, Congo, Ethiopia, Guinea Bissau, Guinea Conakry, India, Kenya, Madagascar, Malawi, Rwanda, Uganda (111) | Antibiotics (76), tablets, capsules: amoxicillin (24) | UV-visible spectrophotometric assay | 30/76 (40%) of antibiotics were counterfeit; 58% of 24 amoxicillin samples were substandard/counterfeit | Reduced dissolution and stability, no AI |
| 2011/Armenia, Azerbaijan, Belarus, Kazakhstan, Ukraine, Uzbekistan (112) | Tablets, capsules, injections: antituberculosis medicines (291) | HPLC, dissolution, and mass uniformity test U.S. Pharmacopeia standards were used | 11.3% of antimicrobials were substandard/counterfeit | No AI, reduced content of AI, increased content of AI, wt variation outside pharmacopoeial limits, reduced dissolution and stability |
| 2011/India (113) | Antibiotics, tablets: ciprofloxacin, RMP (NR) | HPLC, mass spectrometry | Approximately 6% of ciprofloxacin and 40% of RMP tablets were substandard/counterfeit | Reduced dissolution and stability, inappropriate packaging/labeling, reduced content of AI, increased content of AI |
| 2010/Indonesia (114) | Tablets, capsules: amoxicillin, chloramphenicol, ciprofloxacin, co-trimoxazole, tetracycline (104) | HPLC | 18% of antibiotics were substandard/counterfeit | Reduced content of AI |
| 2009/Pakistan (115) | Tablets: ciprofloxacin, ofloxacin, levofloxacin (12 brands) | Disintegration and dissolution tests | 1/12 (8.3%) quinolone brands was substandard/counterfeit | Reduced dissolution and stability |
| 2009/Pakistan (116) | Antibiotics, injections: ceftriaxone (96) | HPLC, TLC, package inspection | 15.62% of ceftriaxone injection was substandard | Inappropriate packaging, reduced amt of AI |
| 2009/India (117) | Antibiotics, tablets: ciprofloxacin (103), erythromycin (117); antituberculosis drugs, tablets: INH (84), RMP (118) | TLC, disintegration and dissolution tests | 8% of ciprofloxacin, 7% of erythromycin, 11% of INH, and 9 % of RMP tablets were substandard/counterfeit; in total 38/422 (9%) of tested antibacterials were substandard | Reduced dissolution and stability, inappropriate packaging, reduced amt of AI |
| 2008/Lebanon, Jordan, Egypt, Saudi Arabia (118) | Capsules and suspensions, amoxicillin (111) | HPLC | 56% of amoxicillin capsules and 8% of suspensions were substandard/counterfeit | Reduced dissolution and stability, reduced content of AI |
| 2006/China (119) | Tablets: macrolides | FCIS based on color reactions and TLC methods | Poor-quality characteristics included no or altered active ingredients | No AI, incorrect ingredient |
| 2004/Rwanda, Tanzania (120) | Capsules: amoxicillin; tablets, metronidazole, TMP-SMX tablet (33) | U.S. Pharmacopeia 24 dissolution tests, HPLC | Three TMP-SMX and three metronidazole formulations had poor dissolution; 24% of the tested drugs had poor dissolution | Reduced dissolution and stability |
| 2004/Laos (121) | Tablets: ampicillin, tetracycline (300) | HPLC, potentiometric titration, UV, TLC, color reactions, mass uniformity analysis | 47% of the drugs of unknown origin were substandard | No AI, reduced content of AI, increased content of AI, wt variation outside pharmacopoeial limits |
| 2002/northern Myanmar (Burma) (122) | Tablets, injections: ceftriaxone, ciprofloxacin, erythromycin, tetracyclines, TMP-SMX, penicillin (21) | Titrimetry, UV, TLC | In total, 86% of the antibiotics were substandard and/or counterfeit; 1 (5%) tetracycline product had no active drug, 3 (14%) drugs were expired, 7 of 21 (33%) drugs had wrong concn of the AI | Inappropriate packaging/labeling, expired drug, no AI, reduced content of AI, increased content of AI |
| 2001/Nigeria (7) | Tablets, suspension, syrup, capsules, injection: antibacterials (such as ampicillin, cloxacillin, SP, metronidazole) and antituberculosis drugs (581) | HPLC and dissolution test; British Pharmacopeia standards were used | For all groups of drugs, more than 50% failed to comply with specifications and contained either low amt of the AI (ampicillin and amoxicillin, 24–40%) or no AI (metronidazole) | No AI, reduced content of AI, increased content of AI |
| 2001/Nigeria (120) | Capsules: ampicillin (5) | NR | 60% of samples were of low quality | Reduced bioavailability |
| 2001/Colombia, Estonia, India, Latvia, Russia, Vietnam (121) | Tablets: INH and RMP (71) | TLC | Overall, 10% of all samples, including 13% of RMP samples, contained <85% of stated content; more FDCs (5/24, 21%) than single-drug samples (2/16, 13%) were substandard; 2 RMP samples and 1 INH sample had additional ingredient | Reduced content of AI, unknown ingredient(s) |
| 1999/Republic of Botswana (62) | Tablets: FDC antituberculosis drugs (13) | TLC, UV, or LC | Four FDC samples (31%) were substandard and had either low AI (RMP) (15%) or excessive AI (RMP, pyrazinamide) (16%) | Reduced content of AI, increased content of AI |
| 1999/Myanmar (Burma), Vietnam (10) | Tablets and capsules: amoxicillin, ampicillin, metronidazole, tetracycline, chloroquine, chloramphenicol, RMP, co-trimoxazole (500) | Compendial quality testing according to British Pharmacopeia standards | 11% of drugs were substandard/counterfeit | Reduced content of AI, increased content of AI, wrong AI |
| 1999/South Africa (122) | FDC antituberculosis formulations (10) | NR | RMP had reduced bioavailability in 7 of 10 (70%) FDC formulations compared to standard formulations | Reduced bioavailability |
| 1998/Laos (24) | Tablets and capsules: ampicillin, tetracycline (366) | Identity assays (TLC, UV, titrimetry, color reactions, HPLC), measurement of wt variation | 3.3% of drugs contained no AI, 11.5% had reduced AI, and 35.0% had excessive wt variation; overall, 67% of ampicillin samples and 38% of tetracycline were substandard/counterfeit | No AI, reduced content of AI, abnormal wt |
| 1998/Zimbabwe (35) | Injectables: benzylpenicillin, ampicillin; tablets: penicillins and tetracycline (789) | NR | 20% of injectable ampicillin samples were substandard/counterfeit; an aqueous formulation of injectable procaine benzylpenicillin showed moderate instability with long-term storage | Reduced content of AI, reduced dissolution and stability |
| 1997/Nigeria, Thailand (2) | Tablets, capsules, suspension, and injection: amoxicillin, tetracycline, TMP-SMX, ampicillin-cloxacillin (96 [81 Nigeria, 15 Thailand]) | HPLC | 36% and 40% of drugs from Nigeria and Thailand were substandard, respectively; overall, 36.5% of drugs were substandard/counterfeit | No AI, reduced content of AI, increased content of AI |
| 1994/Bangladesh (5) | Tablets: ampicillin, TMP-SMX (137 brands) | NR | 27% of drug preparations were substandard/counterfeit; 10 brands of ampicillin and 2 of TMP-SMX were found to be substandard in this study | Reduced content of AI |
| 1995/Nigeria (123) | Capsules, suspensions: amoxicillin (40) | NR | 30% of amoxicillin capsules were substandard/counterfeit | Reduced content of AI, increased content of AI |
| 1992/Nigeria (124) | Capsules: ampicillin (13 brands) | Disintegration and dissolution tests | Approximately 25% of the drugs were substandard/counterfeit | Reduced content of AI, reduced dissolution and stability |
| 1992/India (125) | Capsules: tetracycline (7 brands) | Fluorometric method | 6 of 7 (86%) different marketed brands of tetracycline capsules were substandard/counterfeit | Reduced bioavailability, reduced content of AI |
Abbreviations: AI, active ingredient; FCIS, fast chemical identification test; FDC: fixed-dose combination; HPLC, high-performance liquid chromatography; INH, isoniazid; LC, liquid chromatography; NR, not reported; TLC, thin-layer chromatography; TMP-SMX, trimethoprim-sulfamethoxazole; RMP, rifampin; UV, UV spectrophotometry.
In most studies it was not clarified whether the number of tablets referred to a single lot or to multiple lots.
TABLE 8.
Studies regarding substandard/counterfeit antiparasitic agentsa
| Yr/country(ies) (reference) | Category of drugs studied (n) | Method(s) of detection of low-quality drug | Results | Characteristics of counterfeit/substandard drugs |
|---|---|---|---|---|
| 2012/Ghana (88) | Artemisinin-based antimalarial medicines, tablets: artesunate, amodiaquine, artemether-lumefantrine | Colorimetry, TLC, quality assessment tests (uniformity of mass, crushing strength, disintegration time, % content of active pharmaceutical ingredients) | All 14 (100%) of artemisinin-based antimalarial medicines were substandard/counterfeit | Reduced content of AI, increased content of AI, wt variation outside pharmacopoeial limits, reduced dissolution and stability |
| 2012/South American countries (157) | Antimalarial drugs, tablets: artesunate, chloroquine, doxycycline, primaquine and quinine, SP (1,663) | Visual and physical inspection, disintegration tests, TLC, HPLC | 193/1,663 (11.6%) of antimalarials were of low quality; most of these drugs (8.6%) did not pass inspection or had expired. | Inappropriate packaging and labeling, reduced content of AI, reduced dissolution and stability |
| 2012/Angola, Brazil, Cameroon, Central African Republic, Chad, Congo, Ethiopia, Guinea Bissau, Guinea Conakry, India, Kenya, Madagascar, Malawi, Rwanda, Uganda (111) | Antimalarial drugs (17) | UV-visible spectrophotometric assay | 6/17 (35%) of antimalarial drugs were counterfeit | Reduced dissolution and stability |
| 2011/India (113) | Antimalarial drugs, tablets: artesunate (NR) | HPLC, mass spectrometry | Approximately 80% of artesunate tablets were substandard/counterfeit | Reduced dissolution and stability, reduced content of AI, increased content of AI, inappropriate packaging/labeling |
| 2011/Cameroon, Ghana, Kenya, Nigeria, Tanzania (88) | Antimalarial drugs, tablets: SP, sulfamethoxy-pyrazinepyrimethamine, artemisinin-based combination (267) | Quality testing | 28.5% of drugs were substandard/counterfeit | Reduced content of AI, increased content of AI, no AI, wt variation outside pharmacopoeial limits, impurities, reduced dissolution and stability |
| 2009/Uganda, Madagascar, Senegal (89) | Antimalarial drugs, tablets: artemisinin-based combination, SP (188) | Quality testing | 32% of drugs were substandard/counterfeit | Reduced content of AI, increased content of AI, no AI, wt variation outside pharmacopoeial limits, impurities, reduced dissolution and stability |
| 2010/Nigeria, Ghana (161) | Antimalarial drugs, tablets: SP, amodiaquine, mefloquine, artemisinin | Mini-lab tests, TLC, disintegration tests | The overall rate of substandard antimalarials was reduced significantly by >30% within 3 years | Reduced dissolution and stability, reduced content of AI |
| 2009/India (117) | Antimalarial drugs, tablets: chloroquine (119) | TLC, disintegration and dissolution tests | 7% of chloroquine tablets were substandard/counterfeit | Reduced dissolution and stability, inappropriate packaging, reduced amt of AI |
| 2009/Nigeria (164) | Antimalarials, tablets: artesunate (15) and amodiaquine (5) | Subjective physical assessment of products, TLC, disintegration and dissolution tests | 66.7 % of the 15 studied artesunate tablets were substandard/counterfeit, 1 (20%) of the 5 studied amodiaquine tablets was substandard/counterfeit | Reduced dissolution and stability, inappropriate packaging, reduced amt of AI, increased content of AI |
| 2009/Nigeria (82) | Antimalarials, tablets: artesunate, dihydroartemisinin, SP, quinine, chloroquine (225) | HPLC and dissolution test; U.S. Pharmacopeia standards were used | 37% of drugs were substandard/counterfeit | No AI, wrong AI, reduced amt of AI |
| 2008/Burkina Faso (158) | Antimalarials, tablets: chloroquine, SP, quinine, amodiaquine, artesunate, artemether-lumefantrine (77) | HPLC and dissolution test; European pharmacopeia standards were used | 32/77 (42%) drug samples were found to be of poor quality | Inappropriate packaging, reduced amt of AI, reduced dissolution and stability, no AI |
| 2008/Kenya, Ghana, Nigeria, Rwanda, Tanzania, Uganda (162) | Antimalarials, tablets: amodiaquine, artesunate, artemether, artemether-lumefantrine, dihydroartemisinin, mefloquine, SP (210) | TLC and dissolution tests | Overall, 35% of tested drugs were substandard | Inappropriate packaging, expired, reduced dissolution and stability |
| 2008/Cambodia, Lao PDR, Myanmar (Burma), Vietnam (159) | Antimalarials, tablets: artesunate (391) | HPLC, mass spectrometry | 195/391 (49.9%) of drugs were counterfeit | Inappropriate packaging, reduced amt of AI, reduced dissolution and stability, no AI, wrong ingredient, impurities |
| 2008/Tanzania (81) | Antimalarial drugs, tablets: SP, sulfamethoxy-pyrazinepyrimethamine, amodiaquine, quinine, artemisinin derivative (304) | HPLC and dissolution test with U.S. Pharmacopeia standards | 12.2% of drugs were substandard/counterfeit | Reduced dissolution and stability, reduced content of AI |
| 2007/Kenya (163) | Antimalarial drugs, Capsules, dry powder suspensions, injections, tablets: containing either artemisinin derivatives or dihydroartemisinin (24) | HPLC, UV | 38% of drugs, 66% of dry powder suspensions, and up to 23% of tablets were substandard/counterfeit | Reduced content of AI, increased content of AI |
| 2007/East Congo (172) | Antimalarial drugs, tablets, injections, and syrups: chloroquine, proguanil, quinine, SP (6 for each drug) | HPLC, UV, TLC | 43% of antimalarial medicines being sold were substandard/counterfeit; 33% of chloroquine, 25% of quinine, and 25% of SP were substandard/counterfeit | Inappropriate packaging and labeling, reduced dissolution and stability, reduced content of AI |
| 2006/Sudan (166) | Antimalarial drugs, tablets, syrup, suspension, injections: chloroquine; tablets, injections: quinine; injections: artemether; tablets: mefloquine | NR | Up to 30% of antimalarials were substandard/counterfeit; almost 84% of failures were due to reduced stability of the drug (quinine, chloroquine), 8% due to low AI (chloroquine), 8% due to low dissolution (chloroquine) | Inappropriate packaging and labeling, reduced dissolution and stability, reduced content of AI |
| 2006/Congo, Burundi, Angola (60) | Antimalarials, tablets: chloroquine, quinine, mefloquine (NR) | LC | Reduced amt of AI (quinine tablets) | Inappropriate packaging, reduced amt of AI, impurities due to poor storage conditions |
| 2006/Cambodia (40) | Antimalarials, tablets: quinine, chloroquine, artesunate, mefloquine, tetracycline, dihydroartemisinin, artemether (451) | Packaging analysis disintegration tests, HPLC, Mini-lab kits, TLC | 79% of antimicrobials were unregistered; overall, 27.1% samples were substandard/counterfeit (50/451 substandard and 72/451 counterfeit) | Unknown origins and unregistered products, inappropriate packaging, wrong AI, no AI |
| 2005/Yemen (177) | Antimalarials, tablets, syrups: chloroquine, SP (4 for each drug) | UV, HPLC, dissolution tests | Up to 20% of chloroquine and 80% of SP were substandard/counterfeit | Reduced dissolution and stability, reduced content of AI, increased content of AI |
| 2005/Tanzania (183) | Antimalarials, tablets: 11 brands of SP | HPLC, physical methods | 54.5% of brands failed the hardness, disintegration, or friability tests | Reduced dissolution and stability |
| 2005/Kenya (43) | Antimalarials, tablets and suspensions: SP and amodiaquine (116) | Spectrophotometric assay, HPLC, dissolution tests | 40.5% of antimicrobials (45.3% of SP and 33.0% of amodiaquine) were substandard/counterfeit | Reduced content of AI, reduced dissolution and stability |
| 2004/Southeast Asia, Myanmar (Burma), Lao PDR, Vietnam, Cambodia, Thailand (58) | Antimalarials, tablets: artemisinin derivatives, mefloquine (188) | Colorimetric testing (Fast Red dye) and packaging analysis, HPLC | 53% of artesunate and 9% of mefloquine samples were substandard/counterfeit; overall, 44% of drugs were substandard/counterfeit (4/232 substandard and 99/232 counterfeit) | Inappropriate packaging/labeling, no AI |
| 2004/Cameroon (57) | Antimalarials, tablets, capsules: chloroquine, quinine, SP (284) | Color reaction test, TLC | 38% of chloroquine, 74% of quinine, and 12% of sulfadoxine-pyrimethamine were substandard/counterfeit; overall, 39.4% of drugs were substandard/counterfeit | No AI, reduced content of AI, wrong AI, unknown ingredient(s) |
| 2004/Rwanda, Tanzania (38) | Antimalarials, tablets: quinine, SP (33) | Dissolution tests, HPLC | In total, 24% of the sampled formulations were substandard/counterfeit | Reduced dissolution and stability, reduced content of AI |
| 2004/Laos (28) | Antimalarials, tablets: chloroquine (300) | HPLC, TLC, UV, color reactions, mass uniformity analysis | Overall, 47% of chloroquine samples were substandard/counterfeit | Increased content of AI, wt variation outside pharmacopoeial limits |
| 2003/Uganda (176) | Antimalarials, tablets, injection: chloroquine (92) | HPLC | 44.5 % of chloroquine samples were substandard/counterfeit | Reduced content of AI, increased content of AI |
| 2003/Tanzania (165) | Antimalarials, tablets: amodiaquine, SP (33) | HPLC, TLC | 36% of samples were substandard | Reduced dissolution and stability, reduced content of AI |
| 2003/Gabon, Ghana, Kenya, Mali, Mozambique, Sudan, Zimbabwe (87) | Antimalarial drugs, tablets, syrup: chloroquine and SP (278) | HPLC, drug-specific assays and dissolution test | 23% of drugs were substandard/counterfeit | Reduced content of AI |
| 2002/Tanzania (34) | Antimalarials: chloroquine (2) | HPLC | Plain formulation of chloroquine phosphate was significantly more bioavailable than sugar-coated formulation | Reduced bioavailability |
| 2002/Tanzania (31) | Antimalarials, tablets: SP, chloroquine (22) | HPLC, accelerated stability test | Two SP and two chloroquine formulations had reduced dissolution | Reduced dissolution and stability, reduced content of AI |
| 2001/Southeast Asia, Cambodia, Laos, Myanmar (Burma), Thailand, Vietnam (9) | Antimalarials, tablets: artesunate (104) | Fast Red TR dye technique, physical characteristics of drugs and packages determined by independent observer | 38% of artesunate samples were counterfeit | Inappropriate packaging/labeling, wt variation outside pharmacopoeial limits, no AI, wrong AI |
| 2001/Sudan (185) | Antiparasitic agents, tablets: Praziquantel (34) | NR | Three brands did not meet the quality pharmacopoeial for impurities and formulation characteristics, and one brand was counterfeit and contained no AI | Wt variation outside pharmacopoeial limits, no AI, inappropriate packaging/labeling |
| 2001/Nigeria (7) | Antimalarials, tablets: SP; tablets: proguanil; tablets, injections, syrups: quinine; anthelmintic, tablets: mebendazole (581) | HPLC and dissolution test, British Pharmacopeia standards were used | More than 50% of drugs were substandard/counterfeit | No AI, reduced content of AI, increased content of AI |
| 2001/Cambodia (48) | Antimalarials, tablets: mefloquine artesunate (NR) | NR | Most mefloquine and half of the artesunate tablets were counterfeit | Inappropriate packaging/labeling |
| 1998/Uganda (175) | Antimalarials, Chloroquine (53 tablets and 49 injections) | Pharmacopoeial assays | Up to 30% of the tablet samples and 33% of injection samples were substandard/counterfeit | Reduced content of AI, increased content of AI |
| 1998/Laos (24) | Antimalarials, tablets: chloroquine (366) | Identity assay (TLC, UV, HPLC, color reactions), measurement of wt variation | 49% of chloroquine samples were substandard/counterfeit | No AI, reduced content of AI, increased content of AI, inappropriate packaging/labeling, wt variation outside pharmacopoeial limits |
| 1997/South Africa (209) | Anthelmintics, tablets: three rafoxanide products (3 brands) | The drugs were tested against a known susceptible strain of Haemonchus in sheep | One of the three commercial formulations (of highly reputable companies) was markedly substandard in terms of efficacy | Reduced efficacy, inappropriate packaging/labeling |
| 1997/Nigeria, Thailand (2) | Antimalarials, tablets, capsules, suspension, injection: chloroquine (81 Nigeria, 15 Thailand) | HPLC | Overall, 36.5% of drugs were substandard/counterfeit | No AI, reduced content of AI, increased content of AI |
| 1995/Tanzania (174) | Antimalarials: chloroquine tablets of nine different brands) (NR) | NR | Only 39% of sugar-coated chloroquine tablets passed the dissolution test | Reduced dissolution and stability |
| 1995/Amazonian region (63) | Antimalarials, tablets: primaquine (12) | NR | 50% of the samples were substandard/counterfeit; reduced and increased concn of AI | Reduced content of AI, increased content of AI |
| 1995/Nigeria (123) | Antimalarials, tablets and syrup formulations of chloroquine (40) | Approximately 8% of chloroquine tablets were substandard/counterfeit | Reduced content of AI |
Abbreviations: AI, active ingredient; HPLC, high-performance liquid chromatography; LC, liquid chromatography; NR, not reported; SP, sulfadoxine-pyrimethamine; TLC, thin-layer chromatography; UV, UV spectrophotometry.
Antibacterial Agents
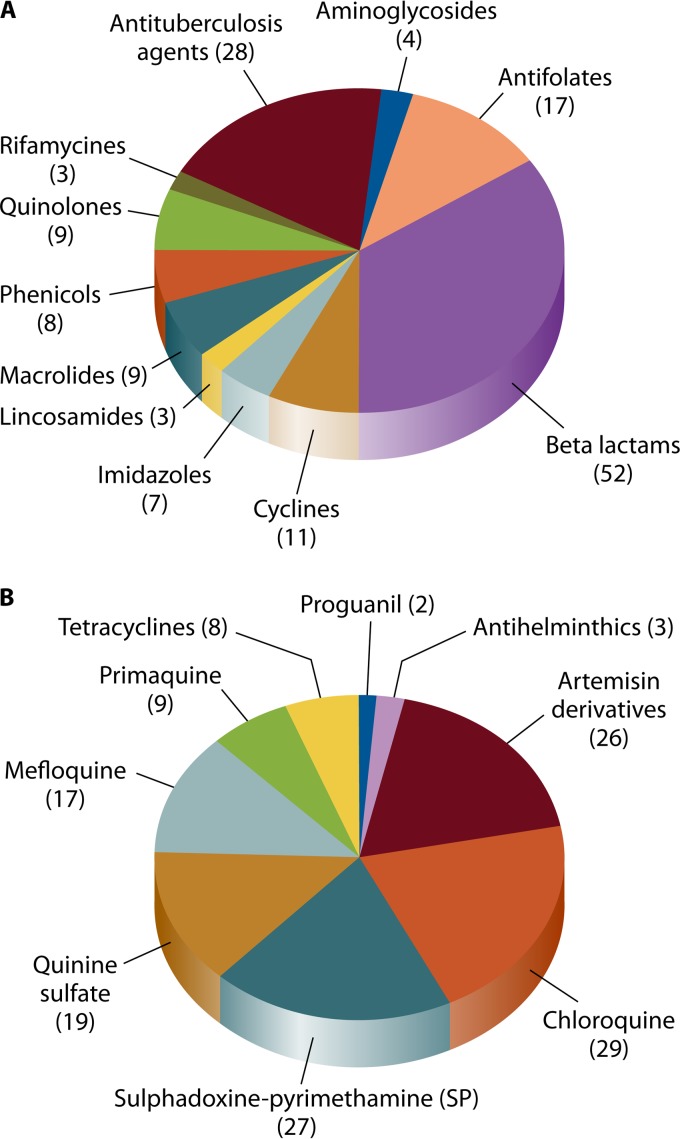
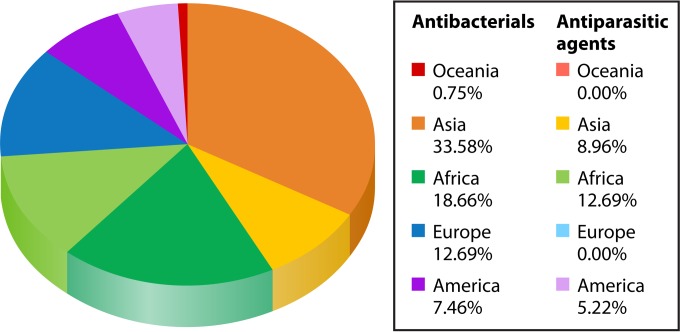
Numerous antibiotics have been reported to have low quality (76–86) (Tables 4 and 5). In a recent literature review, out of 163 counterfeit antibiotics detected in the world until 2009, 50% were beta-lactams, 12% quinolones, 11% macrolides, lincosamides, and synergistines, 7% cyclins, and 20% other antibiotics (17). In our review of the literature, beta-lactams, antifolates, and cyclins were most commonly reported as substandard/counterfeit in more than 50 countries throughout the world (Fig. 1A; Table 5). In addition, amoxicillin was the most common substandard/counterfeit antibiotic and was reported in 29 countries, followed by ampicillin (reported in 17 countries), tetracyclines, (reported in 11 countries), and trimethoprim-sulfamethoxazole (reported in 10 countries). These data are consistent with previous reports that have shown that “old” antimicrobials, such as penicillins and tetracycline, are the most common counterfeited antimicrobials (1). Counterfeited antibacterial agents are more prevalent in Asia and Africa (Fig. 2; Tables 4 and 5) (1, 15). In our review of the literature based on 54 different antimicrobials, India was the leading country, with the highest variety of reported substandard/counterfeit antibacterial agents (21 [39%] different agents), followed by Burma (15 [27%] different agents) and Nigeria (14 [25%] different agents) (Table 5). Regarding the types of poor-quality antibacterial agents, antibiotics with no active ingredient were the most common type, followed by antibiotics with reduced active ingredient and antibiotics with reduced dissolution and stability (Table 4).
TABLE 5.
Categories of reported substandard/counterfeit antibacterial and antituberculosis agents
| Agent | Country(ies) reported (references) |
|---|---|
| Aminoglycosides | |
| Amikacin | India (15, 17) |
| Gentamicin | USA, Europe, India, Nigeria, Tanzania (15, 17) |
| Netilmicin | India (15, 17) |
| Neomycin | Nigeria (15, 17) |
| Antifolates | |
| Trimethoprim | Ukraine (15, 17) |
| Trimethoprim + sulfamethoxazole | Bangladesh, Burma, Cambodia, Cameroon, Indonesia, Nigeria, Rwanda, Tanzania, Thailand, Vietnam (2, 5, 15, 17, 38, 51, 114) |
| Sulfamethizole | Australia, Ivory Coast, Nigeria, Russia, Senegal, Sierra Leone, Ukraine (15, 17) |
| Beta-lactams | |
| Penicillins | |
| Amoxicillin | Angola, Belgium, Brazil, Burma, Cameroon, Central African Republic, Chad, Congo, Egypt, Ethiopia, Guinea, India, Indonesia, Jordan, Kenya, Lebanon, Madagascar, Nepal, Nigeria, Malawi, Rwanda, Saudi Arabia, Sierra Leone, Tanzania, Thailand, Uganda, USA, Vietnam, Zimbabwe (2, 15, 17, 35, 38, 111, 114, 118, 123) |
| Amoxicillin + clavulanic acid | Germany, India, Nigeria, Philippines (15, 17) |
| Ampicillin | Bangladesh, Burma, Bolivia, Cameroon, Ghana, India, Ivory Coast, Laos, Lebanon, Nigeria, Russia, Senegal, Sierra Leone, Tanzania, USA, Vietnam, Zimbabwe (5, 7, 15, 17, 24, 28, 35, 120, 124) |
| Ampicillin + amoxicillin | India (15, 17) |
| Ampicillin + cloxacillin | Belgium, Burma, Lebanon, Nigeria, Thailand (2, 15, 17) |
| Benzathin benzylpenicillin | Burma, Zimbabwe (15, 17, 35, 51) |
| Cloxacillin | Burma, Nigeria, Uganda (7, 15, 17) |
| Penicillin V | Cambodia, Indonesia, Madagascar, Thailand (15, 17) |
| Phenylmethoxypenicillin | Zimbabwe (15, 17, 35) |
| Cephalosporins | |
| Cefaclor | USA (15, 17) |
| Cefepime | Pakistan (15, 17) |
| Cephalexin | Brazil, China, Sierra Leone (15, 17) |
| Cephazolin | Russia, Ukraine (15, 17) |
| Cephradine | USA (15, 17) |
| Cefotaxime | Russia (15, 17) |
| Ceftazidime | India, Mexico, Philippines, Russia, Vietnam (15, 17) |
| Ceftriaxone | Burma, Pakistan (15, 17, 51, 116) |
| Cefuroxime | China, India, Nigeria, Vietnam (15, 17) |
| Chlortetracycline | Burma (15, 17) |
| Cyclines | |
| Chlortetracycline | Burma (15, 17, 51) |
| Doxycycline | Burma, Zimbabwe (15, 17, 35, 51) |
| Oxytetracycline | Senegal, Sierra Leone (15, 17) |
| Oxytetracycline + polymyxin | India (15, 17) |
| Tetracycline | Burma, Cambodia, Cambodia, India, Indonesia, Laos, Nigeria, Thailand, Vietnam, Zimbabwe (2, 15, 17, 24, 28, 35, 114, 125) |
| Imidazole | |
| Metronidazole | Burma, Cambodia, Cameroon, Nigeria, Rwanda, Tanzania, Vietnam (7, 15, 17, 38) |
| Lincosamides | |
| Clindamycin | Germany (15, 17) |
| Lincomycin | Sierra Leone, Mexico (15, 17) |
| Macrolides | |
| Acetyl-spiramycin | China (15, 17, 119) |
| Acetyl-kitasamycin | China (15, 17, 119) |
| Azithromycin | China, Jordan, Russia, Ukraine (15, 17, 119) |
| Clarithromycin | China (15, 17, 120) |
| Erythromycin | Burma, China, India, Nigeria (15, 17, 51, 117, 119) |
| Kitasamycin | China (15, 17, 119) |
| Luecomycin | China (15, 17, 119) |
| Meleumycin | China (15, 17, 119) |
| Midecamycin | China (15, 17, 119) |
| Roxithromycin | Germany, China, India, Russia (15, 17, 119) |
| Phenicol | |
| Chloramphenicol | Burma, Cambodia, Cameroon, India, Indonesia, Nigeria, Thailand, Vietnam (17, 114) |
| Quinolones | |
| Ciprofloxacin | Burma, India, Indonesia, Germany, Nigeria, Pakistan, Tanzania (17, 51, 113–115, 117) |
| Levofloxacin | Pakistan (17, 115) |
| Nalidixic acid | India (15, 17) |
| Ofloxacin | China, Germany, Pakistan, Russia (15, 17, 115) |
| Rifamycin | |
| Rifampin | Burma, India, Vietnam (15, 17, 113) |
| Antituberculosis agents | |
| Ethambutol | Armenia, Azerbaijan, Belarus, India, Nigeria, Kazakhstan, Ukraine, Uzbekistan (7, 15, 17, 112, 117) |
| Rifampin | Armenia, Azerbaijan, Belarus, Burma, India, Kazakhstan, Nigeria, Republic of Botswana, South Africa, Ukraine, Uzbekistan (7, 15, 17, 112, 117, 122) |
| Rifampin + isoniazid | Angola, Armenia, Azerbaijan, Belarus, Brazil, China, Colombia, Democratic Republic of Congo, Egypt, Estonia, Ethiopia, Ghana, India, Kazakhstan, Kenya, Latvia, Nigeria, Republic of Botswana, Russia, Rwanda, South Africa, Thailand, Turkey, Uganda, Ukraine, United Republic of Tanzania, Uzbekistan, Vietnam, Zambia (7, 15, 17, 62, 110, 112, 117, 121, 122) |
| Rifampin, pyrazinamide, ethambutol, isoniazid | Armenia, Azerbaijan, Belarus, India, Kazakhstan, Nigeria, Republic of Botswana, South Africa, Ukraine, Uzbekistan (7, 15, 17, 62, 112, 122) |
FIG 1.
Number of countries, per category of antimicrobials, where poor-quality antimicrobials have been reported. (A) Antibiotics. (B) Antiparasitic agents.
FIG 2.
Relative geographic distribution of substandard/counterfeit antimicrobials. Darker colors indicate substandard/counterfeit antibiotics. Lighter colors indicate substandard/counterfeit antiparasitic agents.
Antituberculosis Drugs
Pharmaceutical agents of poor quality against tuberculosis have been reported in 28 different countries, mostly in Asia and Africa (Table 5) (1, 15). These drugs (isoniazid, rifampin, ethambutol, and pyrazinamide) contain either no (7, 62), low (62), or excessive (62) amounts of the active ingredient, contain the wrong ingredient (119), or have impurities (121), reduced bioavailability (121), or wrong labels (1). Instability is not a cause of reduced bioavailability in drugs used for the treatment of tuberculosis, contrary to the case for other antibiotics such as tetracyclines (179, 188). Drugs with a reduced amount of the active ingredient were the most commonly reported type of substandard/counterfeit antituberculosis (anti-TB) drugs, and more fixed-dose combinations than single-drug samples were substandard (62, 121, 122). However, not all studies have found low-quality antituberculosis medicines (179, 189). Fake antituberculosis drugs can promote drug resistance and lead to treatment failure (121, 190).
Antivirals
Counterfeit antiretrovirals.
In view of the increasing pandemic of AIDS in underdeveloped countries, the report of substandard/counterfeit antiretroviral agents is alarming (Table 6). The high cost and need for long-term therapy make antiretrovirals a target for counterfeiting (191). Considering the high prevalence of HIV infection worldwide, counterfeiting of antiretrovirals may bring enormous profits to the counterfeiters without easily being detected (192, 193). In addition, the fear of stigma often makes HIV-infected patients seek antiretrovirals through unauthorized retailers (191). In 2011, 3,000 people with HIV were given counterfeit antiretroviral drugs which seriously affected their treatment (194). The absence of any research studies on counterfeit antiretrovirals limits any efforts to estimate the magnitude of this problem. In 2003 the WHO reported that in Cote d'Ivoire a triple antiretroviral combination (zidovudine, lamivudine, and indinavir) contained only zidovudine and a wrong drug (stavudine) (195). In 2004, counterfeit antiretrovirals containing antidepressants were found in Congo (14). The most frequently reported counterfeit antiretrovirals are zidovudine (14, 148) and lamivudine (131, 144), either alone or in combination (26, 149), whereas in many cases the nature of counterfeit antiretrovirals is not specified (131, 132). These agents contain either wrong (14), little (142, 196), too much (145), or no (26, 149) active ingredient. Falsified packaging and the presence of impurities have also been reported (150). In one study in Tanzania, out of five samples of stavudine and indinavir, all indinavir samples had an excessive amount of active ingredient, whereas one sample of stavudine capsules failed the dissolution test (145). Most of the above reports, however, are anecdotal. In some studies no substandard preparations of antiretrovirals were identified (197). The tropical conditions may significantly affect the properties of antiretrovirals (198). Thus, counterfeit antiretrovirals may promote antiviral resistance in areas such as Africa, and since second-line therapies are more toxic, all of these factors limit access of patients to antiretroviral therapy in developing countries (192, 193, 199). However, it is unknown whether the absence of any more recent reports of counterfeit antiretrovirals is due to the increased availability of these drugs, the lack of close monitoring, or other factors.
TABLE 6.
Categories of reported substandard/counterfeit antiviral agents and vaccinesa
| Agent or vaccine | Country(ies) reported (reference[s]) |
|---|---|
| Antivirals | |
| Oseltamivir | USA (126), UK, Netherlands (127–129), Russia (TeraFlu) (130) |
| Interferon | Brazil (46) |
| Antiretrovirals | |
| Not specified | Colombia (131), Ethiopia (132, 133), Kenya (131, 134), Tanzania (135, 136), Uganda (131), Zimbabwe (137–139) |
| NRTIs | |
| Zidovudine | Vietnam (140), USA (141), Zimbabwe (142) |
| Abacavir | India, Nigeria (143) |
| Lamivudine | Hong Kong (131, 144) |
| Stavudine | Tanzania (145) |
| NNRTI | |
| Nevirapine | Nigeria (146) |
| PI | |
| Indinavir sulfate | USA (147), Tanzania (145) |
| Combination antiretroviral regimens | |
| Stavudine-lamivudine-nevirapine | DR Congo (14), USA (148) |
| Lamivudine-zidovudine | DR Congo (14), USA (26, 141, 149) |
| Zidovudine-lamivudine-indinavir | Cote d'Ivoire (131), Cameroon (131) |
| Zidovudine + lamivudine + nevirapine | Nigeria (146), Kenya (150) |
| Vaccines | |
| Meningitis | Niger (151) |
| Cholera | Europe (56) |
| Avian influenza | China (152) |
| Influenza | USA (153) |
| Hepatitis B | Pakistan (144) |
| Rabies | Thailand (154) |
| Polio | USA (155) |
| Measles | Nigeria (156) |
Abbreviations: DR Congo, Democratic Republic of Congo; NRTIs, nucleoside reverse transcriptase inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitor.
Other counterfeit antiviral agents.
Fake oseltamivir (126–130) and interferon (46) have also been reported. Technologies such as solid-phase extraction and spectrometry have been used for the determination of oseltamivir in plasma (200). Counterfeit antivirals such as oseltamivir may be a substantial threat to public health in the setting of a global avian flu pandemic (201). The risk is that low-quality antivirals have low concentrations of the active drug that may promote antiviral resistance, and thus antivirals may not be used in the setting of an influenza pandemic (126).
Vaccines
Substandard/counterfeit vaccines have been reported, such as vaccines for influenza (153), avian influenza (152), hepatitis B (144), meningitis (151), cholera (56), rabies (154), poliovirus (155), and measles (156) (Table 6). In one study only 30% of countries that produce vaccines in Asia had internationally acceptable quality control standards (202). Low-quality vaccines may lead to increased mortality due to failure to prevent life-threatening diseases such as meningitis (203) and rabies (154).
Antifungal Agents
There is limited information regarding substandard/counterfeit antifungals. Low-quality antifungals such as fluconazole, ketoconazole, nystatin, and griseofulvin have been described in the United States (141), Nigeria (7), Ukraine (204), and Sierra Leone (55), respectively. Taylor et al. assessed the quality of ketoconazole preparations (5 creams and 18 tablets) in Nigeria and found that 4/5 (80%) ketoconazole tablets were substandard/counterfeit (7). In another study in Africa and Asia, 9/13 (69%) antifungals were counterfeit (111). In one study in Nigeria, two different batches of six antimycotic drugs (nystatin, clotrimazole, iconazole, itraconazole, doxycycline, and metronidazole) had different activity against Candida spp., suggesting that the quality of certain antifungals may affect treatment of patients with fungal infections (205). Further studies that will determine the prevalence of substandard/counterfeit antifungals are needed.
Antiparasitic Agents
Antimalarials.
Based on the worldwide prevalence of malaria (206), antimalarial drugs are commonly targeted by counterfeiters. Studies have shown that substandard/counterfeit antimalarials have widely been reported in Africa (2, 7, 174, 175) and Southeast Asia (9, 48, 94). Up to 90% of antimalarial drugs were found to be of low quality in a WHO study in Africa (207). In our review of the literature, chloroquine, artemisin derivatives, sulfadoxine-pyrimethamine (SP), quinine sulfate, mefloquine, primaquine, tetracyclines, and proguanil have been reported as substandard/counterfeit in more than 30 countries throughout the world (Fig. 1B and 2; Tables 7 and 8). Chloroquine was the most common substandard/counterfeit antimalarial agent and was reported in 29 countries, followed by artemisin derivatives (reported in 26 countries), SP (reported in 24 countries), and quinine sulfate (reported in 19 countries) (Table 7). Regarding the types of poor-quality antibacterial agents, antimalarials with reduced active ingredient and reduced dissolution were the most common type (Table 8). Given that substandard/counterfeit antimalarials promote antimalarial drug resistance (91, 92) and that malaria is endemic in many developing countries and has significant morbidity and mortality, these low-quality antimalarials are a threat to the life of millions of people.
TABLE 7.
Categories and characteristics of counterfeit/substandard antiparasitic agents
| Agent | Country(ies) (reference[s]) |
|---|---|
| Antimalarial | |
| Artemisin derivatives | |
| Artesunate | Bolivia (157), Brazil (157), Burkina Faso (158), Burma (9, 58, 159), Cameroon (45, 88), Cambodia (9, 40, 48, 52, 53, 58, 91, 131, 159, 160), China (131), Colombia (157), Ecuador (157), Ghana (88, 161, 162), Guyana (157), India (113), Kenya (88, 162, 163), Laos (9, 58, 91, 131, 159), Madagascar (89), Nigeria (82, 88, 161, 162, 164), Peru (157), Rwanda (162), Senegal (89), Suriname (157) Ghana (15), Tanzania (81, 88, 162), Thailand (9, 58, 131, 159), Uganda (89, 162), Vietnam (9, 57, 131, 159) |
| Amodiaquine | Ghana (15, 161, 162), Kenya (43, 162), Nigeria (161, 162, 164), Rwanda (162), Tanzania (81, 162, 165), Uganda (162) |
| Artemether-lumefantrine | Burkina Faso (158), Cambodia (40), Ghana (15, 162), Kenya (162, 163), Nigeria (161, 162, 164), Rwanda (162), Sudan (166), Tanzania (162), Uganda (162) |
| Arteether | Kenya (163) |
| Dihydroartemisinin | Cameroon (45, 167), Cambodia (40), Kenya (163), Nigeria (82,162), Rwanda (162), Tanzania (162), Uganda (162) |
| Halofantrine | Nigeria, Ghana, Sierra Leone (168–171) |
| Other | |
| Chloroquine | Angola (60), Brazil (157), Bolivia (157), Burkina Faso (158), Burundi (60), Cameroon (57), Cambodia (40), Colombia (157), Congo (60, 172), Cote d'Ivoire (50), Ecuador (157), Gabon (87), Guinea (173), Guyana (157), India (117), Kenya (87), Laos (24, 28), Mali (87), Mozambique (87), Nigeria (2, 82, 123), Peru (157), Sierra Leone (55), Sudan (87,166,174), Suriname (157), Tanzania (31, 34, 175), Thailand (2), Uganda (175, 176), Yemen (177), Zimbabwe (87) |
| Doxycycline | Bolivia (157), Brazil (157), Colombia (157), Ecuador (157), Guyana (157), Peru (157), Suriname (157) |
| Mefloquine | Angola (60), Bolivia (157), Brazil (157), Burma (52, 58), Burundi (60), Cambodia (40, 48, 52, 58), Ghana (161, 162), Kenya (162), Laos (58), Nigeria (161, 162), Rwanda (162), Senegal (27), Sudan (166), Tanzania (162), Thailand (58), Uganda (162), Vietnam (58) |
| Primaquine | Amazonian region (63), Brazil (157), Bolivia (157), Colombia (157), Ecuador (157), Guyana (157), Namibia (78) Peru (157), Suriname (157) |
| Proguanil | Congo (172), Nigeria (7) |
| Sulfadoxine-pyrimethamine | Burkina Faso (158), Cameroon (57, 88, 173), Cote d'Ivoire (50), Ghana (88), Kenya (43, 88), Colombia (157), Congo (172), Ecuador (157), Gabon (87), Ghana (87, 161, 162), Guyana (157), Nigeria (44, 88, 162, 178), Peru (157), Sierra Leone (55), Suriname (157), Zambia (208) Kenya (43, 87, 162), Madagascar (89), Mali (87), Mozambique (87), Nigeria (7, 82, 161, 178), Rwanda (38, 162), Senegal (89), Sudan (87), Tanzania (31, 38, 81, 88, 162, 165, 183), Uganda (89, 162, 176), Zimbabwe (87) |
| Tetracycline | Cambodia (40) |
| Quinine sulfate | Angola (60), Bolivia (157), Brazil (157), Burundi (60), Burkina Faso (158), Cambodia (40, 52, 53, 79, 131), Cameroon (57, 173), Congo (172, 184), Ecuador (157), Guyana (157), Nigeria (7, 82, 184), Peru (157), Rwanda (38), Sudan (166), Suriname (157), Tanzania (38, 81), USA (182), Vietnam (131), Colombia (157) |
| Antiparasitic agents | |
| Albendazole | Cote d'Ivoire (50) |
| Lindane | Lebanon (181) |
| Mebendazole | Cambodia (79), Cote d'Ivoire (50), Nigeria (7) |
| Pentavalent antimonials | India (186), Sudan (180) |
| Piperazine | Guinea (173) |
| Praziquantel | Sudan (185), Zambia (208) |
| Pyrazinamide | Nigeria (7) |
| Rafoxanide products | South Africa (209) |
Other antiparasitic drugs.
Substandard anthelmintics such as mebendazole (50, 79), albendazole (50), praziquantel (185, 208), piperazine (173), rafoxanide (209), and pyrazinamide (7) have been reported (Tables 7 and 8). Other substandard antiparasitic drugs, such as pentavalent antimonials and lindane, have been reported in India (186), Sudan (180), and Lebanon (181). Characteristics of substandard antiparasitic drugs include no active ingredient (50), increased impurities (185) or osmolality (186), altered labeling (50) and formulation characteristics (185), expired and diluted products (180), and reduced efficacy (209). These substandard/counterfeit antiparasitic agents lead to therapeutic failure and promote antimicrobial resistance (180).
CONSEQUENCES OF COUNTERFEIT AND SUBSTANDARD ANTI-INFECTIVES
Substandard/counterfeit antimicrobials may have serious consequences for both patients and global health, such as increased antimicrobial resistance, treatment failure, and side effects (9, 28, 190, 210).
Consequences for Patients
Counterfeit and substandard antibiotics per se can directly cause deaths in humans.
An excessive dose of the active ingredient in low-quality antimicrobials may be toxic to humans, especially in children or with antimicrobials with a narrow therapeutic range, such as quinine (7, 46, 211, 212). Counterfeit antimicrobials such as antimalarials (168, 213, 214) may have dangerous components or infectious contaminants, which can cause side effects. For example, injectable counterfeit antibiotics may contain methanol, a potentially lethal product for humans which may be responsible for pancreatitis, blindness, coma, cardio-circulatory failure, and death (17). In the late 1990s in the United States, counterfeit gentamicin that had harmful impurities caused close to 2,000 adverse reactions such as eosinophilia-myalgia syndrome and 66 deaths (17, 72). Moreover, the presence of contaminating agents of microbial origin may increase the risk for serious infections, as has been reported for gentamicin eye drops (215).
Counterfeit and substandard antibiotics can cause treatment failure that leads to increased mortality in humans.
Most antibiotics have wide therapeutic ranges, and modestly substandard antibiotics may not lead to treatment failure (15). However, low-quality antimicrobials may lead to therapeutic failure (2, 4, 8, 24) and increased morbidity or mortality (6, 26, 45–48, 211, 212, 216). The number of deaths due to counterfeit drugs can often be very high. In Niger in 1995, approximately 60,000 patients received counterfeit meningitis vaccine that lacked the active ingredient, and as a result thousands of patients died or were permanently handicapped (196). Substandard/counterfeit antimicrobials may also lead to side effects that may reduce patient compliance with use of the antimicrobial agent and lead to treatment failure and increased morbidity and mortality (15, 17). For example unknown or wrong ingredients such as chloroquine have been detected in many counterfeit quinine tablets, which can lead to side effects such as pruritus, poor compliance, and failure of treatment (57). In addition, the plasma concentration of the low-quality antibiotic may be substandard, especially in undernourished subjects (125), and this combination with poor dissolution may lead to reduced bioavailability of the substandard antibiotic and treatment failure. For example, treatment failure and mortality have been reported in patients with malaria who received substandard chloroquine (174).
Consequences for the Community
Counterfeit and/or substandard antimicrobial medicines may promote antimicrobial resistance.
Emergence of antimicrobial resistance as a result of low-quality antimicrobials has been reported with antimicrobials that are often used in combination therapy, such as antimalarials (45, 45, 123, 217–220) and antituberculosis agents (1, 121, 221). The use of substandard products may lead to underdosing of antibiotics, which can increase antimicrobial resistance (2, 4, 8, 24, 222, 223). As a result, in some developing countries multidrug-resistant bacteria may emerge, and the development of travel may further promote the spread of drug-resistant bacteria worldwide (15, 17, 51). Furthermore, therapeutic failure prolongs the period of contagiousness and increases the prevalence of infections from multidrug-resistant pathogens in the community. With regard to malaria, WHO has recommended that if 10% of patients fail treatment, the malaria treatment guidelines should change (224). However, the contribution of substandard/counterfeit medicines to treatment failure for malaria needs to taken into account and addressed in future research studies.
Low-quality antimicrobials may significantly decrease confidence in the efficacy of certain antibiotics.
Poor-quality antimicrobials may lead physicians to lose confidence in specific antibiotics and thus to use broad-spectrum antibiotics as the drugs of choice for infections (215, 225). According to the WHO, this may lead to loss of efficacy of relatively inexpensive drugs and will promote the use of more expensive antibiotics that patients in developing countries are not able to afford. The public confidence in health care systems and in governments may decline significantly. If patients with infectious diseases do not take antimicrobials due to lack of trust in their efficacy, they remain infectious and pose risks for global public health.
Financial consequences of low-quality antimicrobial drugs.
The use of low-quality antimicrobial drugs may lead to antimicrobial resistance and increased morbidity that may further adversely affect the economies of governments (77). The financial burden on consumers who have to pay for more expensive drugs and on companies producing the original products can be significant (44, 56, 170, 226).
INTERVENTIONS
Several international and national strategies are required to efficiently detect counterfeits and combat this problem (107).
International Strategies To Combat Counterfeit Drugs
In an effort to eliminate counterfeiting, WHO has issued guidelines (227) and created the International Medical Products Anti-Counterfeiting Taskforce (228), which require collaboration between patients, health care providers, industry, and local and government organizations (12, 56, 196, 229). The European Council set up the international convention Medicrime, which is open to member countries and prosecutes those involved with counterfeit medicines (230). The Council of Europe launched a project named “Track & Trace,” which traces counterfeits as part of its strategy against counterfeiting of drugs (230). The World Customs Organization, WHO, and INTERPOL collaborate with police to combat counterfeiting (231, 232). The United Nations Office on Drugs and Crime established the Container Control Programme, which has improved the detection of counterfeit drugs (233). Recently, WHO has established a new strategy to combat counterfeiting (74, 234).
National Initiatives To Combat Counterfeit Drugs
The Counterfeit Drug Task Force was established by FDA to counteract counterfeiting in the United States and worldwide. Task force strategies against counterfeit drugs include using technology and regulatory security practices to create rapid response systems and public awareness programs (136, 229–233). However, most of the developing countries lack the resources to apply published guidelines (7, 49). Other important measurements from governments to combat counterfeits include reducing taxes applied on drugs and promoting the use of affordable generic drugs (235). For example, Nigeria, Thailand, and Cambodia developed information sites on counterfeit drugs, and Cambodia initiated various sensitization campaigns using mass media to educate patients to buy drugs only in authorized pharmacies (170). However, better coordination between governments and international organizations is necessary to eradicate this problem.
LIMITATIONS OF THE REVIEW
In this review we identified only few studies with sufficient methodology to determine the prevalence of substandard/counterfeit antimicrobials. In many studies there was no clear distinction between counterfeit and substandard drugs, and sampling and data analysis were not standardized. In most of the studies the source of the drugs (for example, manufacturer, pharmacies, and patients) was not identified. It was unclear if the drugs tested represented lots of drugs or individual tablets and whether patient drug-handling practices may also affect the performance of substandard/counterfeit antimicrobials. The data regarding counterfeit/substandard drugs from developed countries remain limited. It is not known whether drug efficacy studies may have been impacted by substandard/counterfeit drugs, and no links between patient outcomes and substandard/counterfeit drug sources have been identified. Finally, a significant part of the published evidence regarding counterfeit antimicrobials derived from journalism rather than the biomedical literature.
CONCLUSIONS
Substandard/counterfeit antimicrobial drugs represent an expanding problem throughout developing countries with considerable consequences for global public health. Well-designed studies are needed to determine the magnitude of this problem. A substandard concentration of the active ingredient was the main reason for low quality, which can lead to increased morbidity and mortality and emergence of antimicrobial resistance. Initiatives have been developed to counteract counterfeiting, including collaboration between patients, health workers, local, national, and international organizations, and industry. However, the lack of resources in developing countries limits the implementation of many of these measures. A multidisciplinary approach is required to combat the menace of counterfeits, which affects the health of millions of people.
Biographies

Theodoros Kelesidis, M.D., Ph.D., is an Assistant Professor of Medicine and Infectious Diseases at the David Geffen School of Medicine at UCLA. Dr. Kelesidis is a graduate of the University of Athens Medical School and the University of California, Los Angeles (Ph.D.). He is board certified in internal medicine and infectious diseases. His research focuses on infectious diseases, antimicrobial resistance, immunology, HIV infection, and metabolic complications of chronic infectious diseases.

Matthew E. Falagas, M.D., M.Sc., D.Sc., is founder and director of the Alfa Institute of Biomedical Sciences (AIBS), Athens, Greece, Adjunct Associate Professor of Medicine at Tufts University School of Medicine, Boston, MA, and Director of the Department of Internal Medicine-Infectious Diseases, Iaso General Hospital, Iaso Group, Athens, Greece. His research interests include antimicrobial resistance and nosocomial infections.
REFERENCES
- 1.WHO. 1999. Counterfeit and sub-standard drugs in Myanmar and Vietnam. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Shakoor O, Taylor RB, Behrens RH. 1997. Assessment of the incidence of substandard drugs in developing countries. Trop Med Int Health 2:839–845. doi: 10.1046/j.1365-3156.1997.d01-403.x. [DOI] [PubMed] [Google Scholar]
- 3.Pecoul B, Chirac P, Trouiller P, Pinel J. 1999. Access to essential drugs in poor countries: a lost battle? JAMA 281:361–367. doi: 10.1001/jama.281.4.361. [DOI] [PubMed] [Google Scholar]
- 4.Menkes DB. 1997. Hazardous drugs in developing countries. BMJ 315:1557–1558. doi: 10.1136/bmj.315.7122.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy J. 1994. The menace of substandard drugs. World Health Forum 15:406–407. [PubMed] [Google Scholar]
- 6.Hanif M, Mobarak MR, Ronan A, Rahman D, Donovan JJ Jr., Bennish ML. 1995. Fatal renal failure caused by diethylene glycol in paracetamol elixir: the Bangladesh epidemic. BMJ 311:88–91. doi: 10.1136/bmj.311.6997.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor RB, Shakoor O, Behrens RH, Everard M, Low AS, Wangboonskul J, Reid RG, Kolawole JA. 2001. Pharmacopoeial quality of drugs supplied by Nigerian pharmacies. Lancet 357:1933–1936. doi: 10.1016/S0140-6736(00)05065-0. [DOI] [PubMed] [Google Scholar]
- 8.Wan Po AL. 2001. Too much, too little, or none at all: dealing with substandard and fake drugs. Lancet 357:1904. doi: 10.1016/S0140-6736(00)05092-3. [DOI] [PubMed] [Google Scholar]
- 9.Newton P, Proux S, Green M, Smithuis F, Rozendaal J, Prakongpan S, Chotivanich K, Mayxay M, Looareesuwan S, Farrar J, Nosten F, White NJ. 2001. Fake artesunate in southeast Asia. Lancet 357:1948–1950. doi: 10.1016/S0140-6736(00)05085-6. [DOI] [PubMed] [Google Scholar]
- 10.Wondemagegnehu E. 1995. Counterfeit and substandard drugs in Myanmar and Vietnam. WHO/EDM/QSM/99.3 WHO, Geneva, Switzerland. [Google Scholar]
- 11.Frankish H. 2003. WHO steps up campaign on counterfeit drugs. Lancet 362:1730. doi: 10.1016/S0140-6736(03)14891-X. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. 1999. Counterfeit drugs: guidelines for the development of measures to combat counterfeit drugs. WHO, Geneva, Switzerland. [Google Scholar]
- 13.Pincock S. 2003. WHO tries to tackle problem of counterfeit medicines in Asia. BMJ 327:1126. doi: 10.1136/bmj.327.7424.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad K. 2004. Antidepressants are sold as antiretrovirals in DR Congo. Lancet 363:713. doi: 10.1016/S0140-6736(04)15670-5. [DOI] [PubMed] [Google Scholar]
- 15.Kelesidis T, Kelesidis I, Rafailidis PI, Falagas ME. 2007. Counterfeit or substandard antimicrobial drugs: a review of the scientific evidence. J Antimicrob Chemother 60:214–236. doi: 10.1093/jac/dkm109. [DOI] [PubMed] [Google Scholar]
- 16.Newton PN, Green MD, Fernandez FM, Day NP, White NJ. 2006. Counterfeit anti-infective drugs. Lancet Infect Dis 6:602–613. doi: 10.1016/S1473-3099(06)70581-3. [DOI] [PubMed] [Google Scholar]
- 17.Delepierre A, Gayot A, Carpentier A. 2012. Update on counterfeit antibiotics worldwide; public health risks. Med Mal Infect 42:247–255. doi: 10.1016/j.medmal.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Attaran A, Barry D, Basheer S, Bate R, Benton D, Chauvin J, Garrett L, Kickbusch I, Kohler JC, Midha K, Newton PN, Nishtar S, Orhii P, McKee M. 2012. How to achieve international action on falsified and substandard medicines. BMJ 345:e7381. doi: 10.1136/bmj.e7381. [DOI] [PubMed] [Google Scholar]
- 19.Videau JV, Fundafunda B. 2000. Generic drugs: the hidden issues of quality and cost. WHO drug information, p 77–81. WHO, Geneva, Switzerland. [Google Scholar]
- 20.World Health Organization. 1997. Quality assurance of pharmaceuticals, vol 1 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 21.World Health Organization. 1999. Quality assurance of pharmaceuticals, vol 2 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 22.World Health Organization. 2014. What encourages counterfeiting of drugs? http://www.who.int/medicines/services/counterfeit/faqs/15/en/ Accessed 16 January 2014.
- 23.World Health Organization. 2014. What are substandard medicines? http://www.who.int/medicines/services/counterfeit/faqs/06/en/ Accessed 26 January 2014.
- 24.Stenson B, Lindgren BH, Synhakhang L, Tomson G. 1998. The quality of drugs in private pharmacies in the Lao People's Democratic Republic. Int J Risk Safety Med 11:243–249. [Google Scholar]
- 25.Masland T, Marshall R. 1990. “A really nasty business.” Fake pharmaceuticals look like the real thing but they can be lethal. Newsweek, 5 November 1990. [Google Scholar]
- 26.Moken MC. 2003. Fake pharmaceuticals: how they and relevant legislation or lack thereof contribute to consistently high and increasing drug prices. Am J Law Med 29:525–542. [PubMed] [Google Scholar]
- 27.Reidenberg MM, Conner BA. 2001. Counterfeit and substandard drugs. Clin Pharmacol Ther 69:189–193. doi: 10.1067/mcp.2001.114672. [DOI] [PubMed] [Google Scholar]
- 28.Syhakhang L, Lundborg CS, Lindgren B, Tomson G. 2004. The quality of drugs in private pharmacies in Lao PDR: a repeat study in 1997 and 1999. Pharm World Sci 26:333–338. doi: 10.1007/s11096-004-0558-3. [DOI] [PubMed] [Google Scholar]
- 29.Hogerzeil HV, de Goeje MJ, Abu-Reid IO. 1991. Stability of essential drugs in Sudan. Lancet 338:754–755. doi: 10.1016/0140-6736(91)91470-F. [DOI] [PubMed] [Google Scholar]
- 30.Ballereau F, Prazuck T, Schrive I, Lafleuriel MT, Rozec D, Fisch A, Lafaix C. 1997. Stability of essential drugs in the field: results of a study conducted over a two-year period in Burkina Faso. Am J Trop Med Hyg 57:31–36. [DOI] [PubMed] [Google Scholar]
- 31.Risha PG, Shewiyo D, Msami A, Masuki G, Vergote G, Vervaet C, Remon JP. 2002. In vitro evaluation of the quality of essential drugs on the Tanzanian market. Trop Med Int Health 7:701–707. doi: 10.1046/j.1365-3156.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- 32.Murthy KS, Ghebre-Sellassie I. 1993. Current perspectives on the dissolution stability of solid oral dosage forms. J Pharm Sci 82:113–126. doi: 10.1002/jps.2600820202. [DOI] [PubMed] [Google Scholar]
- 33.Pandit JK, Tripathi MK, Babu RJ. 1997. Effect of tablet disintegrants on the dissolution stability of nalidixic acid tablets. Pharmazie 52:538–540. [Google Scholar]
- 34.Rimoy GH, Moshi MJ, Massele AY. 2002. Comparative bioavailability of oral sugar-coated and plain formulation of chloroquine phosphate marketed in Tanzania. Trop Doct 32:15–17. [DOI] [PubMed] [Google Scholar]
- 35.Nazerali H, Hogerzeil HV. 1998. The quality and stability of essential drugs in rural Zimbabwe: controlled longitudinal study. BMJ 317:512–513. doi: 10.1136/bmj.317.7157.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saville DJ. 2001. Influence of storage on in-vitro release of ibuprofen from sugar coated tablets. Int J Pharm 224:39–49. doi: 10.1016/S0378-5173(01)00734-7. [DOI] [PubMed] [Google Scholar]
- 37.Okeke IN, Lamikanra A. 1995. Quality and bioavailability of tetracycline capsules in a Nigerian semi-urban community. Int J Antimicrob Agents 5:250. [DOI] [PubMed] [Google Scholar]
- 38.Kayumba PC, Risha PG, Shewiyo D, Msami A, Masuki G, Ameye D, Vergote G, Ntawukuliryayo JD, Remon JP, Vervaet C. 2004. The quality of essential antimicrobial and antimalarial drugs marketed in Rwanda and Tanzania: influence of tropical storage conditions on in vitro dissolution. J Clin Pharm Ther 29:331–338. doi: 10.1111/j.1365-2710.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- 39.Barbereau S. 2006. Guinea: fake medicines. E-Med 2006:January 12. [Google Scholar]
- 40.Lon CT, Tsuyuoka R, Phanouvong S, Nivanna N, Socheat D, Sokhan C, Blum N, Christophel EM, Smine A. 2006. Counterfeit and substandard antimalarial drugs in Cambodia. Trans R Soc Trop Med Hyg 100:1019–1024. doi: 10.1016/j.trstmh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Kibwage IO, Ngugi JK. 2000. Sulphadoxine/pyrimethamine tablet products on the Kenyan market: quality concerns. East Central Afr J Pharm Sci 3:14–19. [Google Scholar]
- 42.World Health Organization. 2003. The quality of antimalarials. A study in selected African countries. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 43.Amin AA, Snow RW, Kokwaro GO. 2005. The quality of sulphadoxine-pyrimethamine and amodiaquine products in the Kenyan retail sector. J Clin Pharm Ther 30:559–565. doi: 10.1111/j.1365-2710.2005.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ten Ham M. 1992. Counterfeit drugs: implications for health. Adverse Drug React Toxicol Rev 11:59–65. [PubMed] [Google Scholar]
- 45.Newton PN, McGready R, Fernandez F, Green MD, Sunjio M, Bruneton C, Phanouvong S, Millet P, Whitty CJ, Talisuna AO, Proux S, Christophel EM, Malenga G, Singhasivanon P, Bojang K, Kaur H, Palmer K, Day NP, Greenwood BM, Nosten F, White NJ. 2006. Manslaughter by fake artesunate in Asia—will Africa be next? PLoS Med 3:e197. doi: 10.1371/journal.pmed.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman M, Lydecker M, Lee PR. 1990. The drug swindlers. Int J Health Serv 20:561–572. doi: 10.2190/P32D-0141-M86B-F7AT. [DOI] [PubMed] [Google Scholar]
- 47.Goodman PS. 2002. China's killer headache: fake pharmaceuticals. Washington Post, 30 August 2002. [Google Scholar]
- 48.Rozendaal J. 2001. Fake antimalaria drugs in Cambodia. Lancet 357:890. doi: 10.1016/S0140-6736(05)71830-4. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. 1999. Counterfeit drugs guidelines for the development of measures to combat counterfeit drugs. WHO, Geneva, Switzerland. [Google Scholar]
- 50.Legris C. 2005. Detection of counterfeit drugs by investigating their authenticity. Pilot study on the illicit drug market in Côte d'Ivoire. Thesis. University of Nancy, Nancy, France. http://www.remed.org/html/theses.html Accessed 16 January 2014.
- 51.Prazuck T, Falconi I, Morineau G, Bricard-Pacaud V, Lecomte A, Ballereau F. 2002. Quality control of antibiotics before the implementation of an STD program in northern Myanmar. Sex Transm Dis 29:624–627. doi: 10.1097/00007435-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Smine A, Phanouvong S, Chanthap L, Tsuyuoka R, Nivanna N, Blum N. 2014. Antimalarial drug quality in Mekong countries. http://www.uspdqi.org/pubs/other/AntimalarialPoster.pdf Accessed 16 January 2014.
- 53.Anonymous. 2005. Fake anti malaria drugs in Cambodia: a danger to public health. Final Report, NCM—Cambodia, USP, WHO. National Center for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia. [Google Scholar]
- 54.Center for Combating Counterfeit Drugs. 2014. The situation of counterfeit drugs. www.fda.moph.go.th/fda-net/html/product/drug/eng/zone_counterfeit/fak001.asp Accessed 16 January 2014.
- 55.Sesay M. 1988. Fake drugs—a new threat of health care delivery. Africa Health June/July 1988:13–15. [Google Scholar]
- 56.Harper J. 2006. Counterfeit medicines survey report, Council for Europe. Council of Europe Publishing, Strasbourg, France. [Google Scholar]
- 57.Basco LK. 2004. Molecular epidemiology of malaria in Cameroon. XIX. Quality of antimalarial drugs used for self-medication. Am J Trop Med Hyg 70:245–250. [PubMed] [Google Scholar]
- 58.Dondorp AM, Newton PN, Mayxay M, Van Damme W, Smithuis FM, Yeung S, Petit A, Lynam AJ, Johnson A, Hien TT, McGready R, Farrar JJ, Looareesuwan S, Day NP, Green MD, White NJ. 2004. Fake antimalarials in Southeast Asia are a major impediment to malaria control: multinational cross-sectional survey on the prevalence of fake antimalarials. Trop Med Int Health 9:1241–1246. doi: 10.1111/j.1365-3156.2004.01342.x. [DOI] [PubMed] [Google Scholar]
- 59.Almuzaini T, Choonara I, Sammons H. 2013. Substandard and counterfeit medicines: a systematic review of the literature. BMJ Open 3:e002923. doi: 10.1136/bmjopen-2013-002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaudiano MC, Antoniella E, Bertocchi P, Valvo L. 2010. Development and validation of a reversed-phase LC method for analysing potentially counterfeit antimalarial medicines. J Pharm Biomed Anal 53:158–164. doi: 10.1016/j.jpba.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization. 2014. http://www.who.int/medicines/areas/quality_safety/quality_assurance/QualityAssurancePharmVol2.pdf?ua=1 Accessed 16 January 2014.
- 62.Kenyon TA, Kenyon AS, Kgarebe BV, Mothibedi D, Binkin NJ, Layloff TP. 1999. Detection of substandard fixed-dose combination tuberculosis drugs using thin-layer chromatography. Int J Tuberc Lung Dis 3:S347–S350. [PubMed] [Google Scholar]
- 63.Petralanda I. 1995. Quality of antimalarial drugs and resistance to Plasmodium vivax in Amazonian region. Lancet 345:1433. doi: 10.1016/S0140-6736(95)92620-8. [DOI] [PubMed] [Google Scholar]
- 64.Gibson L. 2004. Drug regulators study global treaty to tackle counterfeit drugs. BMJ 328:486. doi: 10.1136/bmj.328.7438.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization. 1999. Summary of WHO counterfeit drug database as of April 1999, unpublished paper of the WHO Division of Drug Management and Policies. WHO, Geneva, Switzerland. [Google Scholar]
- 66.World Health Organization. 2000. World Health Organisation counterfeit drug reports: 1999-October 2000. www.who.int/medicines/services/counterfeit/overview/en/1 Accessed 16 January 2014.
- 67.Anonymous. 2002. Counterfeit drugs: threat to public health. Global Forum on Pharmaceutical Anticounterfeiting, Geneva, Switzerland, 22 to 25 September 2002. [Google Scholar]
- 68.Chakravarty S, Unnithan S, Ram A. 2001. Deadly doses. India Today, 29 January 2001, p 58–61. [Google Scholar]
- 69.Olori T. 1996. Nigeria-Health: bogus drugs—a national headache. Inter Press Service, 5 December 1996. [Google Scholar]
- 70.Fackler M. 2002. China's fake drugs kill thousands. San Francisco Examiner, 29 July 2002. [Google Scholar]
- 71.Rudolf PM, Bernstein IB. 2004. Counterfeit drugs. N Engl J Med 350:1384–1386. doi: 10.1056/NEJMp038231. [DOI] [PubMed] [Google Scholar]
- 72.Pharmaceutical Security Institute. 2014. Counterfeit situation. http://www.psi-inc.org/incidentTrends.cfm Accessed 24 January 2014.
- 73.Institute of Medicine. 2013. Countering the problem of falsified and substandard drugs. http://www.iom.edu/Reports/2013/Countering-the-Problem-of-Falsified-and-Substandard-Drugsaspx Accessed 24 January 2014. [PubMed]
- 74.World Health Organization. 2012. Substandard/spurious/falsely-labelled/falsified/counterfeit medical products: report of the Working Group of Member States. http://apps.who.int/gb/ebwha/pdf_files/WHA65/A65_23-en.pdf Accessed 24 January 2014.
- 75.United Nations Office on Drugs and Crime. 2010. The globalization of crime. A transnational organized crime threat assessment. Counterfeit products 2010, p 183–189. https://www.unodc.org/documents/data-and-analysis/tocta/TOCTA_Report_2010_low_res.pdf Accessed 24 January 2014.
- 76.United Nations Interregional Crime and Justice Research Institute. 14 December 2007. Counterfeiting. A global spread, a global threat. 4. The counterfeiting medicines, p 29, 63–72 http://www.unicri.it/news/article/0712-3_counterfeiting_crt_foundation Accessed 23 January 2014.
- 77.Behrens RH, Awad AI, Taylor RB. 2002. Substandard and counterfeit drugs in developing countries. Trop Doct 32:1–2. [DOI] [PubMed] [Google Scholar]
- 78.Kron MA. 1996. Substandard primaquine phosphate for US Peace Corps personnel. Lancet 348:1453–1454. doi: 10.1016/S0140-6736(04)70100-2. [DOI] [PubMed] [Google Scholar]
- 79.Giminez F, Bruneton C, Narong Rith DY. 1997. Etude de la qualite des medicaments vendus et dispenses au Cambodge. Med Mal Infect 271:541–544. [Google Scholar]
- 80.Pole D, Ransome-Kuti O, Institute for Medical Informatics. 1989. Drug distribution & fake drugs in Nigeria: international workshop: proceedings of the workshop held on 12 April, Lagos, Nigeria. Institute for Medical Informatics, Basel Switzerland. [Google Scholar]
- 81.Kaur H, Goodman C, Thompson E, Thompson KA, Masanja I, Kachur SP, Abdulla S. 2008. A nationwide survey of the quality of antimalarials in retail outlets in Tanzania. PLoS One 3:e3403. doi: 10.1371/journal.pone.0003403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Onwujekwe O, Kaur H, Dike N, Shu E, Uzochukwu B, Hanson K, Okoye V, Okonkwo P. 2009. Quality of anti-malarial drugs provided by public and private healthcare providers in south-east Nigeria. Malar J 8:22. doi: 10.1186/1475-2875-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arya SC. 1995. Inadvertent supply of substandard drugs. World Health Forum 16:269. [PubMed] [Google Scholar]
- 84.Saywell T, McManus J. 2002. What's in that pill? Far Eastern Economic Review, 21 February 2002, p 34–40. [Google Scholar]
- 85.Primo-Carpenter J. 2004. A review of drug quality in Asia with focus on anti-infectives. Global initiative for quality medicines and their appropriate use. Drug Quality and Information Program, U.S. Pharmacopeia, Rockville, MD. [Google Scholar]
- 86.Chattopadhyay S. 1999. Access to essential drugs in poor countries. JAMA 282:631. [PubMed] [Google Scholar]
- 87.Maponga C, Ondari C. 2003. The quality of antimalarials. A study in selected African countries. WHO/EDM/PAR/2003.4. World Health Organization, Geneva, Switzerland: http://apps.who.int/medicinedocs/pdf/s4901e/s4901e.pdf Accessed 24 January 2014. [Google Scholar]
- 88.Sabartova J, Toumi A, Ondari C. 2011. Survey of the quality of selected antimalarial medicines circulating in six countries of sub-Saharan Africa. WHO/EMP/QSM/2011.1. World Health Organization, Geneva, Switzerland: http://www.who.int/medicines/publications/WHO_QAMSA_report.pdf Accessed 24 January 2014. [Google Scholar]
- 89.Anonymous. 2009. A collaborative study by the WHO and DQI Survey of the quality of selected antimalarial medicines circulating in Madagascar, Senegal, and Uganda. http://apps.who.int/medicinedocs/documents/s17069e/s17069e.pdf Accessed 24 January 2014.
- 90.Abdoulaye I, Chastanier H, Azondekon A, Dansou A, Bruneton C. 2006. Survey on the illicit drug market in Cotonou, Benin in March 2003. Med Trop (Mars) 66:573–576. [PubMed] [Google Scholar]
- 91.Newton PN, Dondorp A, Green M, Mayxay M, White NJ. 2003. Counterfeit artesunate antimalarials in southeast Asia. Lancet 362:169 doi: 10.1016/S0140-6736(03)13872-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Green MD. 2006. Antimalarial drug resistance and the importance of drug quality monitoring. J Postgrad Med 52:288–290. [PubMed] [Google Scholar]
- 93.Green MD, Nettey H, Wirtz RA. 2008. Determination of oseltamivir quality by colorimetric and liquid chromatographic methods. Emerg Infect Dis 14:552–556. doi: 10.3201/eid1404.061199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Green MD, Mount DL, Wirtz RA, White NJ. 2000. A colorimetric field method to assess the authenticity of drugs sold as the antimalarial artesunate. J Pharm Biomed Anal 24:65–70. doi: 10.1016/S0731-7085(00)00360-5. [DOI] [PubMed] [Google Scholar]
- 95.Weaver AA, Reiser H, Barstis T, Benvenuti M, Ghosh D, Hunckler M, Joy B, Koenig L, Raddell K, Lieberman M. 2013. Paper analytical devices for fast field screening of beta lactam antibiotics and antituberculosis pharmaceuticals. Anal Chem 85:6453–6460. doi: 10.1021/ac400989p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kibwage IO, Thuranira J, Migosi D. 1991. Quality performance of metronidazole tablet products on the Kenyan market. East Afr Med J 68:365–371. [PubMed] [Google Scholar]
- 97.Risha PG, Msuya Z, Clark M, Johnson K, Ndomondo-Sigonda M, Layloff T. 2008. The use of Minilabs to improve the testing capacity of regulatory authorities in resource limited settings: Tanzanian experience. Health Policy 87:217–222. doi: 10.1016/j.healthpol.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 98.Jahnke RW, Kusters G. 2001. Low-cost quality assurance of medicines using the GPHF-Minilab. Drug Information J 35:941–945. [Google Scholar]
- 99.Ida H, Kawai J. 2005. Analysis of wrapped or cased object by a hand-held X-ray fluorescence spectrometer. Forensic Sci Int 151:267–272. doi: 10.1016/j.forsciint.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 100.Ricci C, Nyadong L, Yang F, Fernandez FM, Brown CD, Newton PN, Kazarian SG. 2008. Assessment of hand-held Raman instrumentation for in situ screening for potentially counterfeit artesunate antimalarial tablets by FT-Raman spectroscopy and direct ionization mass spectrometry. Anal Chim Acta 623:178–186. doi: 10.1016/j.aca.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 101.Phillips G. 2003. World Congress of Pharmacy and Pharmaceutical Sciences: anticounterfeiting measures. Pharm J 271:465. doi.org/10.1016/S1473-3099(06)70581-3. [Google Scholar]
- 102.Iqbal M, Hakimm S, Hussain A, Mirza Z, Qureshi F, Abdulla E. 2004. Ofloxacin: laboratory evaluation of the antibacterial activity of 34 brands representing 31 manufacturers available in Pakistan. Pak J Med Sci 20:349–356. [Google Scholar]
- 103.Takats Z, Wiseman JM, Gologan B, Cooks RG. 2004. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 104.Cody RB, Laramee JA, Durst HD. 2005. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem 77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 105.Mulligan KJ, Brueggemeyer TW, Crockett DF, Schepman JB. 1996. Analysis of organic volatile impurities as a forensic tool for the examination of bulk pharmaceuticals. J Chromatogr B Biomed Appl 686:85–95. doi: 10.1016/S0378-4347(96)00109-0. [DOI] [PubMed] [Google Scholar]
- 106.Peng SX, Borah B, Dobson RL, Liu YD, Pikul S. 1999. Application of LC-NMR and LC-MS to the identification of degradation products of a protease inhibitor in dosage formulations. J Pharm Biomed Anal 20:75–89. doi: 10.1016/S0731-7085(98)00311-2. [DOI] [PubMed] [Google Scholar]
- 107.Newton PN, Lee SJ, Goodman C, Fernandez FM, Yeung S, Phanouvong S, Kaur H, Amin AA, Whitty CJ, Kokwaro GO, Lindegardh N, Lukulay P, White LJ, Day NP, Green MD, White NJ. 2009. Guidelines for field surveys of the quality of medicines: a proposal. PLoS Med 6:e52. doi: 10.1371/journal.pmed.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scafi SH, Pasquini C. 2001. Identification of counterfeit drugs using near-infrared spectroscopy. Analyst 126:2218–2224. doi: 10.1039/b106744n. [DOI] [PubMed] [Google Scholar]
- 109.Maurin JK, Plucinski F, Mazurek AP, Fijalek Z. 2007. The usefulness of simple X-ray powder diffraction analysis for counterfeit control—the Viagra((R)) example. J Pharm Biomed Anal 43:1514–1518. doi: 10.1016/j.jpba.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 110.Bate R, Jensen P, Hess K, Mooney L, Milligan J. 2013. Substandard and falsified anti-tuberculosis drugs: a preliminary field analysis. Int J Tuberc Lung Dis 17:308–311. doi: 10.5588/ijtld.12.0355. [DOI] [PubMed] [Google Scholar]
- 111.Baratta F, Germano A, Brusa P. 2012. Diffusion of counterfeit drugs in developing countries and stability of galenics stored for months under different conditions of temperature and relative humidity. Croat Med J 53:173–184. doi: 10.3325/cmj.2012.53.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sabartova J, Nathanson E, Polishchuk O. 2011. Survey of the quality of anti-tuberculosis medicines circulating in selected newly independent states of the former Soviet Union. WHO/EMP/QSM/2011.2 World Health Organization, Geneva, Switzerland: http://apps.who.int/medicinedocs/documents/s19053en/s19053en.pdf Accessed 24 January 2014. [Google Scholar]
- 113.Seear M, Gandhi D, Carr R, Dayal A, Raghavan D, Sharma N. 2011. The need for better data about counterfeit drugs in developing countries: a proposed standard research methodology tested in Chennai, India. J Clin Pharm Ther 36:488–495. doi: 10.1111/j.1365-2710.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 114.Hadi U, van den Broek P, Kolopaking EP, Zairina N, Gardjito W, Gyssens IC. 2010. Cross-sectional study of availability and pharmaceutical quality of antibiotics requested with or without prescription (over the counter) in Surabaya, Indonesia. BMC Infect Dis 10:203. doi: 10.1186/1471-2334-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zaheer M, Rahman S, Mahmood S, Saleem M. 2009. In vitro analysis and data comparison of market brands of ciprofloxacin, ofloxacin and levofloxacin. Pak J Sci Ind Res 52:186–190. [Google Scholar]
- 116.Obaid A. 2009. Quality of ceftriaxone in Pakistan: reality and resonance. Pak J Pharm Sci 22:220–229. [PubMed] [Google Scholar]
- 117.Bate R, Tren R, Mooney L, Hess K, Mitra B, Debroy B, Attaran A. 2009. Pilot study of essential drug quality in two major cities in India. PLoS One 4:e6003. doi: 10.1371/journal.pone.0006003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kyriacos S, Mroueh M, Chahine RP, Khouzam O. 2008. Quality of amoxicillin formulations in some Arab countries. J Clin Pharm Ther 33:375–379. doi: 10.1111/j.1365-2710.2008.00926.x. [DOI] [PubMed] [Google Scholar]
- 119.Hu CQ, Zou WB, Hu WS, Ma XK, Yang MZ, Zhou SL, Sheng JF, Li Y, Cheng SH, Xue J. 2006. Establishment of a fast chemical identification system for screening of counterfeit drugs of macrolide antibiotics. J Pharm Biomed Anal 40:68–74. doi: 10.1016/j.jpba.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 120.Okeke IN, Lamikanra A. 2001. Quality and bioavailability of ampicillin capsules dispensed in a Nigerian semi-urban community. Afr J Med Med Sci 30:47–51. [PubMed] [Google Scholar]
- 121.Laserson KF, Kenyon AS, Kenyon TA, Layloff T, Binkin NJ. 2001. Substandard tuberculosis drugs on the global market and their simple detection. Int J Tuberc Lung Dis 5:448–454. [PubMed] [Google Scholar]
- 122.Pillai G, Fourie PB, Padayatchi N, Onyebujoh PC, McIlleron H, Smith PJ, Gabriels G. 1999. Recent bioequivalence studies on fixed-dose combination anti-tuberculosis drug formulations available on the global market. Int J Tuberc Lung Dis 3:S309–S316. [PubMed] [Google Scholar]
- 123.Taylor RB, Shakoor O, Behrens RH. 1995. Drug quality, a contributor to drug resistance? Lancet 346:122. doi: 10.1016/S0140-6736(95)92145-1. [DOI] [PubMed] [Google Scholar]
- 124.Iwuagwu MA, Onyeonwu N. 1992. In vitro assessment of ampicillin capsules marketed in Nigeria. Int J Pharm Pract 1:167–171. doi: 10.1111/j.2042-7174.1992.tb00561.x. [DOI] [Google Scholar]
- 125.Santosh KK, Raghuram TC, Krishnaswamy K. 1992. Bioavailability of different brands of tetracycline in undernourished subjects. Int J Clin Pharmacol Ther Toxicol 30:13–17. [PubMed] [Google Scholar]
- 126.Anonymous. 2014. Counterfeit Tamiflu. http://www.roche.com/identifying_counterfeit_product.pdf Accessed January 16 2014.
- 127.Lindegardh N, Hien TT, Farrar J, Singhasivanon P, White NJ, Day NP. 2006. A simple and rapid liquid chromatographic assay for evaluation of potentially counterfeit Tamiflu((R)). J Pharm Biomed Anal 42:430–433. doi: 10.1016/j.jpba.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 128.World Health Organization. 2014. Medicines: spurious/falsely-labelled/falsified/counterfeit (SFFC) medicines. http://www.who.int/mediacentre/factsheets/fs275/en/ Accessed 16 January 2014.
- 129.Food and Drug Administration. 2006. Cracking down on health fraud. http://www.fda.gov/drugs/emergencypreparedness/bioterrorismanddrugpreparedness/ucm137261.htm Accessed 16 January 2014.
- 130.Parfitt T. 2006. Russia cracks down on counterfeit drugs. Lancet 368:1481–1482. doi: 10.1016/S0140-6736(06)69619-0. [DOI] [PubMed] [Google Scholar]
- 131.Phanouvong S, Reiss S, Smine A. 2005. Why be concerned about the quality of antimalarial and ARV drugs?, poster 7th Int Congr AIDS Asia Pacific, Kobe, Japan, 1 to 5 July 2005. [Google Scholar]
- 132.Anonymous. 2003. Drug access. Ethiopian health officials warn public against counterfeit antiretroviral drug. http://www.kaiserhealthnews.org/daily-reports/2003/october/09/dr00020261.aspx?referrer=search Accessed 16 January 2014.
- 133.Anonymous. 2003. Black market bogus AIDS drugs. Addis Tribune, 10 October 2003 http://allafrica.com/stories/200310100530.html Accessed 16 January 2014.
- 134.Anonymous. 2004. AAGM: AIDS drugs selling in city streets. The Nation (Kenya), 22 January 2004 http://www.nation.co.ke/ Accessed 16 January 2014.
- 135.Taylor P. 2012. Tanzanian drugmaker asserts innocence in fake ARV case. http://www.securingindustry.com/pharmaceuticals/tanzanian-drugmaker-asserts-innocence-in-fake-arv-case/s40/a1457/ Accessed 16 January 2014.
- 136.McLaughlin K. 2013. Fake drugs flood East African markets. http://www.washingtonpost.com/world/africa/fake-drugs-flood-east-african-markets/2013/01/23/a430289c-5f6e-11e2-9940-6fc488f3fecd_story.html Accessed January 2014.
- 137.Anonymous. 2007. Zimbabwe: fake ART flood country. Financial Gazette, 26 July 2007 http://allafrica.com/stories/200707260858.html Accessed 16 January 2014.
- 138.Anonymous. 2007. Counterfeit AIDS drugs plague Zimbabwe. The Real Truth, 21 September 2007 http://realtruth.org/news/070921-001-africa.html Accessed 16 January 2014.
- 139.Anonymous. 2013. Counterfeit drugs flood Zim market. The Zimbabwe Independent, 25 October 2013 http://www.theindependent.co.zw/2013/10/25/counterfeit-drugs-flood-zim-market/ Accessed 16 January 2014.
- 140.Pharmaceutical Security Institute. 2014. Media reports on pharmaceutical counterfeiting. http://www.psi-inc.org/index.cfm Accessed 16 January 2014.
- 141.Appleby J. 2003. Fake drugs show up in US pharmacies. USA Today, 14 May 2003. http://www.usatoday.com/money/industries/health/drugs/2003-05-14-fakedrug-cover_x.htm Accessed 16 January 2014.
- 142.Apoola A, Sriskandabalan PS, Wade AA. 2001. Self-medication with zidovudine that was not. Lancet 357:1370. doi: 10.1016/S0140-6736(00)04499-8. [DOI] [PubMed] [Google Scholar]
- 143.The Partnership for Safe Medicines. 2008. Nigeria-bound HIV/AIDS drugs seized in Netherlands. http://www.safemedicines.org/nigeriabound-hivaids-drugs-seized-in-netherlands.html Accessed 16 January 2014.
- 144.Anonymous. 2002. Counterfeit pharmaceuticals. First Global Forum on Pharmaceutical Anticounterfeiting, Geneva, Switzerland, 22 to 25 September 2002. [Google Scholar]
- 145.Chambuso MH, Ngassapa OD, Sayi JG, Jande MB, Mohamed Z. 2006. Quality of antiretroviral drugs, stavudine and indinavir capsules available in the Tanzanian market. Tanzania Med J 21:8–12. doi: 10.4314/tmj.v21i1.39202. [DOI] [Google Scholar]
- 146.Nkengasong J. 2003. Counterfeit antiretroviral drugs. Medscape Infectious Diseases, 12 December 2003 http://www.medscape.com/viewarticle/465033 Accessed 16 January 2014.
- 147.Spake A. 2004. Fake drugs, real worries: high prices and the Internet are making U.S. patients easy prey. US News World Rep 137:46, 48, 50. [PubMed] [Google Scholar]
- 148.James JS. 2002. Counterfeit drugs: check combivir, serostim, epogen. AIDS Treat News 380:3–4. [PubMed] [Google Scholar]
- 149.Aoki N. 2002. Real fears over phony drugs: US health officials view spate of recent cases as evidence of rising trend. Boston Globe, 29 May 2002 http://www.bostonglobe.com/ Accessed 16 January 2014.
- 150.Anonymous. 2011. Kenya: government grapples with counterfeit ARVs. http://www.irinnews.org/report/94012/kenya-government-grapples-with-counterfeit-arvs Accessed January 2014.
- 151.Broussard P. 1996. From Nigeria to Niger, strange vaccines against meningitis. Le Monde, 26 October 1996 http://www.lemonde.fr/ Accessed 16 January 2014.
- 152.Anonymous. 2005. Thirteen fake bird flu vaccine-makers punished. China View, 7 November 2005 http://news.xinhuanet.com/english/2005-11/07/content_3745572.htm Accessed 16 January 2014.
- 153.Anonymous. 2006. Charge filed in fake flu vaccine investigation. US Newswire. http://www.prnewswire.com/ Accessed 16 January 2014.
- 154.Hemachudha T, Mitrabhakdi E, Wilde H, Vejabhuti A, Siripataravanit S, Kingnate D. 1999. Additional reports of failure to respond to treatment after rabies exposure in Thailand. Clin Infect Dis 28:143–144. doi: 10.1086/517179. [DOI] [PubMed] [Google Scholar]
- 155.Rasmussen CM, Thomas CW, Mulrooney RJ, Morrissey RA. 1973. Inadequate poliovirus immunity levels in immunized Illinois children. Am J Dis Child 126:465–469. [DOI] [PubMed] [Google Scholar]
- 156.Onoja AL, Adu FD, Tomori O. 1992. Evaluation of measles vaccination programme conducted in two separate health centres. Vaccine 10:49–52. doi: 10.1016/0264-410X(92)90419-K. [DOI] [PubMed] [Google Scholar]
- 157.Pribluda VS, Barojas A, Anez A, Lopez CG, Figueroa R, Herrera R, Nakao G, Nogueira FH, Pianetti GA, Povoa MM, Viana GM, Gomes MS, Escobar JP, Sierra OL, Norena SP, Veloz R, Bravo MS, Aldas MR, Hindssemple A, Collins M, Ceron N, Krishnalall K, Adhin M, Bretas G, Hernandez N, Mendoza M, Smine A, Chibwe K, Lukulay P, Evans L III. 2012. Implementation of basic quality control tests for malaria medicines in Amazon Basin countries: results for the 2005-2010 period. Malar J 11:202. doi: 10.1186/1475-2875-11-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tipke M, Diallo S, Coulibaly B, Storzinger D, Hoppe-Tichy T, Sie A, Muller O. 2008. Substandard anti-malarial drugs in Burkina Faso. Malar J 7:95. doi: 10.1186/1475-2875-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Newton PN, Fernandez FM, Plancon A, Mildenhall DC, Green MD, Ziyong L, Christophel EM, Phanouvong S, Howells S, McIntosh E, Laurin P, Blum N, Hampton CY, Faure K, Nyadong L, Soong CW, Santoso B, Zhiguang W, Newton J, Palmer K. 2008. A collaborative epidemiological investigation into the criminal fake artesunate trade in South East Asia. PLoS Med 5:e32. doi: 10.1371/journal.pmed.0050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Crampton T. 2003. Fake malaria pills haunt Asians. International Herald Tribune, 25 August 2003 http://www.iht.com/ Accessed 16 January 2014.
- 161.Bate R, Hess K. 2010. Anti-malarial drug quality in Lagos and Accra—a comparison of various quality assessments. Malar J 9:157. doi: 10.1186/1475-2875-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bate R, Coticelli P, Tren R, Attaran A. 2008. Antimalarial drug quality in the most severely malarious parts of Africa—a six country study. PLoS One 3:e2132. doi: 10.1371/journal.pone.0002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Atemnkeng MA, De CK, Plaizier-Vercammen J. 2007. Quality control of active ingredients in artemisinin-derivative antimalarials within Kenya and DR Congo. Trop Med Int Health 12:68–74. doi: 10.1111/j.1365-3156.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- 164.Odunfa O, Adegoke OA, Onaga IC. 2009. Pharmaceutical equivalence of some commercial samples of artesunate and amodiaquine tablets sold in southwestern Nigeria. Trop J Pharm Res 8:491–499. [Google Scholar]
- 165.Minzi OM, Moshi MJ, Hipolite D, Massele AY, Tomson G, Ericsson O, Gustafsson LL. 2003. Evaluation of the quality of amodiaquine and sulphadoxine/pyrimethamine tablets sold by private wholesale pharmacies in Dar Es Salaam Tanzania. J Clin Pharm Ther 28:117–122. doi: 10.1046/j.1365-2710.2003.00470.x. [DOI] [PubMed] [Google Scholar]
- 166.Alfadl AA, Abdoon S, Elamin M, Elnabi NG. 2006. Quality of antimalarial drugs in Sudan: results of post-marketing surveillance. Sudanese J Public Health 1:108–111. [Google Scholar]
- 167.Anonymous. 2001. Fake malaria drugs in Tanzania. The Pharmaletter, 20 February 2001. http://www.thepharmaletter.com/article/fake-malaria-drugs-in-tanzania Accessed 16 January 2014.
- 168.Wolff JC, Thomson LA, Eckers C. 2003. Identification of the ‘wrong’ active pharmaceutical ingredient in a counterfeit Halfan drug product using accurate mass electrospray ionisation mass spectrometry, accurate mass tandem mass spectrometry and liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom 17:215–221. doi: 10.1002/rcm.893. [DOI] [PubMed] [Google Scholar]
- 169.Chryss J. 2004. Transcript of “File on 4”: counterfeit drugs. Program number 04VY3040LHO. British Broadcasting Corporation, London, United Kingdom: http://news.bbc.co.uk/ Accessed 16 January 2014. (Transcript of radio program.) [Google Scholar]
- 170.Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. 2005. The global threat of counterfeit drugs: why industry and governments must communicate the dangers. PLoS Med 2:e100. doi: 10.1371/journal.pmed.0020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nigerian National Agency for Food and Drug Administration and Control. 2002. List of identified fake products (June 2002). NAFDAC reference 20-12. Nigerian National Agency for Food and Drug Administration and Control, Abuja, Nigeria: http://www.nafdac.gov.ng/ Accessed 16 January 2014. [Google Scholar]
- 172.Atemnkeng MA, Chimanuka B, Plaizier-Vercammen J. 2007. Quality evaluation of chloroquine, quinine, sulfadoxine-pyrimethamine and proguanil formulations sold on the market in East Congo DR. J Clin Pharm Ther 32:123–132. doi: 10.1111/j.1365-2710.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 173.World Health Organization. 1995. The quality of pharmaceutical drugs on the African walking. Analytical study in three countries: Cameroon, Madagascar, Chad. WHO/DAP/95.3. WHO, Geneva, Switzerland: http://apps.who.int/medicinedocs/en/d/Js2212f/ Accessed 16 January 2014. [Google Scholar]
- 174.Abdi YA, Rimoy G, Ericsson O, Alm C, Massele AY. 1995. Quality of chloroquine preparations marketed in Dar es Salaam, Tanzania. Lancet 346:1161. doi: 10.1016/S0140-6736(95)91834-5. [DOI] [PubMed] [Google Scholar]
- 175.Ogwal-Okeng JW, Okello DO, Odyek O. 1998. Quality of oral and parenteral chloroquine in Kampala. East Afr Med J 75:692–694. [PubMed] [Google Scholar]
- 176.Ogwal-Okeng JW, Owino E, Obua C. 2003. Chloroquine in the Ugandan market fails quality test: a pharmacovigilance study. Afr Health Sci 3:2–6. [PMC free article] [PubMed] [Google Scholar]
- 177.Abdo-Rabbo A, Bassili A, Atta H. 2005. The quality of antimalarials available in Yemen. Malar J 4:28. doi: 10.1186/1475-2875-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Anonymous. 2003. Counterfeiting abroad and home: British Pharmaceutical Conference 2003. J Pharm Pharmacol 55(Suppl):S1–S106. [PubMed] [Google Scholar]
- 179.Ashokraj Y, Kohli G, Kaul CL, Panchagnula R. 2005. Quality control of anti-tuberculosis FDC formulations in the global market. IIAccelerated stability studies. Int J Tuberc Lung Dis 9:1266–1272. [PubMed] [Google Scholar]
- 180.Boelaert M, Le Ray D, Van Der SP. 2002. How better drugs could change kala-azar control. Lessons from a cost-effectiveness analysis. Trop Med Int Health 7:955–959. doi: 10.1046/j.1365-3156.2002.00959.x. [DOI] [PubMed] [Google Scholar]
- 181.Cockburn R. 1982. Counterfeit drugs—the other killer in Lebanon. The Guardian, 10 December 1982. http://www.theguardian.com/uk-news Accessed 16 January 2014.
- 182.Heath WJ. 2004. America's first drug regulation regime: the rise and fall of the Import Drug Act of 1848. Food Drug Law J 59:169–200. [PubMed] [Google Scholar]
- 183.Hebron Y, Tettey JN, Pournamdari M, Watson DG. 2005. The chemical and pharmaceutical equivalence of sulphadoxine/pyrimethamine tablets sold on the Tanzanian market. J Clin Pharm Ther 30:575–581. doi: 10.1111/j.1365-2710.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 184.Sowunmi A, Salako LA, Ogunbona FA. 1994. Bioavailability of sulphate and dihydrochloride salts of quinine. Afr J Med Med Sci 23:275–278. [PubMed] [Google Scholar]
- 185.Sulaiman SM, Traore M, Engels D, Hagan P, Cioli D. 2001. Counterfeit praziquantel. Lancet 358:666–667. doi: 10.1016/S0140-6736(01)05796-8. [DOI] [PubMed] [Google Scholar]
- 186.Sundar S, Sinha PR, Agrawal NK, Srivastava R, Rainey PM, Berman JD, Murray HW, Singh VP. 1998. A cluster of cases of severe cardiotoxicity among kala-azar patients treated with a high-osmolarity lot of sodium antimony gluconate. Am J Trop Med Hyg 59:139–143. [DOI] [PubMed] [Google Scholar]
- 187.ten Ham M. 2003. Health risks of counterfeit pharmaceuticals. Drug Saf 26:991–997. doi: 10.2165/00002018-200326140-00001. [DOI] [PubMed] [Google Scholar]
- 188.Agrawal S, Panchagnula R. 2004. In vitro evaluation of fixed dose combination tablets of anti-tuberculosis drugs after real time storage at ambient conditions. Pharmazie 59:782–785. [PubMed] [Google Scholar]
- 189.Ellard GA. 1999. The colorimetric analysis of anti-tuberculosis fixed-dose combination tablets and capsules. Int J Tuberc Lung Dis 3:S343–S346. [PubMed] [Google Scholar]
- 190.Okeke IN, Lamikanra A, Edelman R. 1999. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis 5:18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Amon JJ. 2008. Dangerous medicines: unproven AIDS cures and counterfeit antiretroviral drugs. Global Health 4:5. doi: 10.1186/1744-8603-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nsimba SE. 2008. Problems associated with substandard and counterfeit drugs in developing countries: a review article on global implications of counterfeit drugs in the era of antiretroviral (ARVs) drugs in a free market economy. East Afr J Public Health 5:205–210. [DOI] [PubMed] [Google Scholar]
- 193.Nsimba SE. 2009. Anti-retroviral (ARV) rationing schemes in developing countries: a review article on strategies and ethical issues related to the successes and failures of ARV programmes. East Afr J Public Health 6:219–222. [DOI] [PubMed] [Google Scholar]
- 194.Anonymous. 2014. Risking your life: designer drugs and counterfeit medicines. http://www.kwikmed.org/risking-life-designer-drugs-counterfeit-medicines/ Accessed 16 January 2014.
- 195.World Health Organization. 2003. Counterfeit triple antiretroviral combination product (Ginovir 3D) detected in Cote d'Ivoire: Information Exchange System alert no 110. QSM/MC/IEA. WHO, Geneva, Switzerland: http://www.who.int/medicines/publications/drugalerts/DrugAlert110.pdf. [Google Scholar]
- 196.Newton PN, White NJ, Rozendaal JA, Green MD. 2002. Murder by fake drugs. BMJ 324:800–801. doi: 10.1136/bmj.324.7341.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Penzak SR, Acosta EP, Turner M, Tavel JA, Masur H. 2003. Analysis of generic nevirapine products in developing countries. JAMA 289:2648–2649. doi: 10.1001/jama.289.20.2648-c. [DOI] [PubMed] [Google Scholar]
- 198.Houston S. 2002. Justice and HIV care in Africa–antiretrovirals in perspective. J Int Assoc Physicians AIDS Care 1:46–50. doi: 10.1177/154510970200100202. [DOI] [PubMed] [Google Scholar]
- 199.Hamers RL, Kityo C, Lange JM, de Wit TF, Mugyenyi P. 2012. Global threat from drug resistant HIV in sub-Saharan Africa. BMJ 344:e4159. doi: 10.1136/bmj.e4159. [DOI] [PubMed] [Google Scholar]
- 200.Wiltshire H, Wiltshire B, Citron A, Clarke T, Serpe C, Gray D, Herron W. 2000. Development of a high-performance liquid chromatographic-mass spectrometric assay for the specific and sensitive quantification of Ro 64-0802, an anti-influenza drug, and its pro-drug, oseltamivir, in human and animal plasma and urine. J Chromatogr B Biomed Sci Appl 745:373–388. doi: 10.1016/S0378-4347(00)00300-5. [DOI] [PubMed] [Google Scholar]
- 201.U.S. Food and Drug Administration. 2005. Counterfeit drugs:questions and answers. http://www.fda.gov/drugs/drugsafety/ucm169898.htm Accessed 16 January 2014.
- 202.Children's Vaccine Initiative. 1993. Working Group Meeting on Asia Initiative, 28 to 30 July 1993. Document CVI/MAC-5/93.12 Rockefeller Archives Center, New York, NY. [Google Scholar]
- 203.Banerjee A. 1995. Fake polio vaccine kills 11 children in Nadia. The Asian Age, 5 April 1995. http://www.asianage.com/ Accessed 16 January 2014.
- 204.Pakharenko-Anderson A. 2002. Building legislation and regulatory implementation environments: the Ukraine experience. First Global Forum on Pharmaceutical Anticounterfeiting, Geneva, Switzerland, 22 to 25 September 2002. [Google Scholar]
- 205.Ogunshe AA, Adepoju AA, Oladimeji ME. 2011. Clinical efficacy and health implications of inconsistency in different production batches of antimycotic drugs in a developing country. J Pharm Bioallied Sci 3:158–164. doi: 10.4103/0975-7406.76501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Surendran A. 2004. World agencies try to stem flood of fake drugs. Nat Med 10:111. doi: 10.1038/nm0204-111a. [DOI] [PubMed] [Google Scholar]
- 208.Koch E, Simmm M, Wech M. 1994. Mafia fake drugs. Courrier International 204:36 http://www.courrierinternational.com/ Accessed 16 January 2014. [Google Scholar]
- 209.van Wyk JA, Malan FS, van Rensburg LJ, Oberem PT, Allan MJ. 1997. Quality control in generic anthelmintics: is it adequate? Vet Parasitol 72:157–165. doi: 10.1016/S0304-4017(97)00022-8. [DOI] [PubMed] [Google Scholar]
- 210.Anonymous. 1997. Quality control and essential drugs. Lancet 350:601. doi: 10.1016/S0140-6736(97)21035-4. [DOI] [PubMed] [Google Scholar]
- 211.Pandya SK. 1988. Letter from Bombay. An unmitigated tragedy. BMJ 297:117–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Okuonghae HO, Ighogboja IS, Lawson JO, Nwana EJ. 1992. Diethylene glycol poisoning in Nigerian children. Ann Trop Paediatr 12:235–238. [DOI] [PubMed] [Google Scholar]
- 213.Alter K, Fernandez F, Green M, Newton P. 2004. Analysis of counterfeit antimalarial drugs. Eur Pharm Rev 3:1–5. [Google Scholar]
- 214.Fernandez FM, Cody RB, Green MD, Hampton CY, McGready R, Sengaloundeth S, White NJ, Newton PN. 2006. Characterization of solid counterfeit drug samples by desorption electrospray ionization and direct-analysis-in-real-time coupled to time-of-flight mass spectrometry. Chem Med Chem 1:702–705. doi: 10.1002/cmdc.200600041. [DOI] [PubMed] [Google Scholar]
- 215.Issack MI. 2001. Substandard drugs. Lancet 358:1463. doi: 10.1016/S0140-6736(01)06516-3. [DOI] [PubMed] [Google Scholar]
- 216.Alubo SO. 1994. Death for sale: a study of drug poisoning and deaths in Nigeria. Soc Sci Med 38:97–103. doi: 10.1016/0277-9536(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 217.Ringwald P, Same EA, Keundjian A, Kedy MD, Basco LK. 2000. Chemoresistance of P. falciparum in urban areas of Yaounde, Cameroon. 1. Surveillance of in vitro and in vivo resistance of Plasmodium falciparum to chloroquine from 1994 to 1999 in Yaounde, Cameroon. Trop Med Int Health 5:612–619. [DOI] [PubMed] [Google Scholar]
- 218.Ringwald P, Bickii J, Basco L. 1996. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet 347:24–28. doi: 10.1016/S0140-6736(96)91558-5. [DOI] [PubMed] [Google Scholar]
- 219.White NJ. 1999. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia 41:301–308. [PubMed] [Google Scholar]
- 220.Wernsdorfer WH. 1994. Epidemiology of drug resistance in malaria. Acta Trop 56:143–156. doi: 10.1016/0001-706X(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 221.Laing R, Vrakking H, Fourie B. 2004. Quality and stability of TB medicines: let the buyer beware! Int J Tuberc Lung Dis 8:1043–1044. [PubMed] [Google Scholar]
- 222.Terlouw DJ, Nahlen BL, Courval JM, Kariuki SK, Rosenberg OS, Oloo AJ, Kolczak MS, Hawley WA, Lal AA, Kuile FO. 2003. Sulfadoxine-pyrimethamine in treatment of malaria in Western Kenya: increasing resistance and underdosing. Antimicrob Agents Chemother 47:2929–2932. doi: 10.1128/AAC.47.9.2929-2932.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Barnes KI, Watkins WM, White NJ. 2008. Antimalarial dosing regimens and drug resistance. Trends Parasitol 24:127–134. doi: 10.1016/j.pt.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 224.World Health Organization. 2010. Guidelines for the treatment of malaria, 2nd ed http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf Accessed 25 January 2014.
- 225.Basco LK, Ringwald P, Manene AB, Chandenier J. 1997. False chloroquine resistance in Africa. Lancet 350:224. doi: 10.1016/S0140-6736(05)62397-5. [DOI] [PubMed] [Google Scholar]
- 226.Saywell T, McManus J. 2002. What's in that pill? Far East Economic Review, 21 February 2002, p 34–40. [Google Scholar]
- 227.Anonymous. 2004. Protecting consumers from counterfeit drugs. FDA Consum 38:12–13. [PubMed] [Google Scholar]
- 228.Burns W. 2006. WHO launches taskforce to fight counterfeit drugs. Bull World Health Organ 084: 689–690. doi: 10.1590/S0042-96862006000900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.World Health Organization. 2014. Combating counterfeit drugs: a concept paper for effective international collaboration. http://www.who.int/medicines/services/counterfeit/CombatingCounterfeitDrugs_Conceptpaper.pdf Accessed 16 January 2014.
- 230.Council of Europe. 2014. Counterfeiting of medical products (MEDICRIME). http://www.coe.int/t/dghl/standardsetting/medicrime/default_EN.asp/ Accessed 24 January 2014.
- 231.Interpol. 2014. Pharmaceutical crime. COM/FS/2012-01/DCO-04. http://www.interpol.int/ Accessed 24 January 2014.
- 232.World Customs Organization. 2009. International operation combats the online supply of counterfeit and illegal medicines. http://www.wcoomd.org/en/media/newsroom/2009/november/international-operation-combats-the-online-supply-of-counterfeit-and-illegal-medicines.aspx Accessed 24 January 2014.
- 233.United Nations Office on Drugs and Crime. 2012. World Customs Organization make a dent in counterfeit goods and drug shipments. http://www.unodc.org/unodc/en/press/releases/2012/June/un-drugs-and-crime-office-world-customs-organization-make-a-dent-on-counterfeit-goods-and-drug-shipments.html Accessed 24 January 2014.
- 234.World Health Organization. 2012. New global mechanism to combat substandard/spurious/falselylabelled/falsified/counterfeit medical products. http://www.who.int/medicines/news/TRA-SE_EMP.pdf Accessed 24 January 2014.
- 235.Wertheimer AI, Norris J. 2009. Safeguarding against substandard/counterfeit drugs: mitigating a macroeconomic pandemic. Res Social Adm Pharm 5:4–16. doi: 10.1016/j.sapharm.2008.05.002. [DOI] [PubMed] [Google Scholar]