Abstract
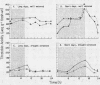
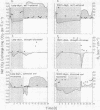
Crassulacean acid metabolism (CAM) was investigated in leaves and stems of the succulent C4 dicot Portulaca oleracea L. Diurnal acid fluctuations, CO2 gas exchange, and leaf resistance were monitored under various photoperiod and watering regimes. No CAM activity was seen in well watered plants grown under 16-hour days. Under 8-hour days, however, well watered plants showed a CAM-like pattern of acid fluctuation with amplitudes of 102 and 90 microequivalents per gram fresh weight for leaves and stems, respectively. Similar patterns were also observed in detached leaves and defoliated stems. Leaf resistance values indicated that stomata were open during part of the dark period, but night acidification most likely resulted from refixation of respiratory CO2. In water-stressed plants maximum acid accumulations were reduced under both long and short photoperiods. At night, these plants showed short periods of net CO2 uptake and stomatal opening which continued all night long during preliminary studies under natural environmental conditions. Greatest acid fluctuations, in P. oleracea, with amplitudes of 128 microequivalents per gram fresh weight, were observed in water-stressed plants which had been rewatered, especially when grown under short days. No net CO2 uptake took place, but stomata remained open throughout the night under these conditions. These results indicate that under certain conditions, such as water stress or short photoperiods, P. oleracea is capable of developing an acid metabolism with many similarities to CAM.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Osmond C. B. Salt responses of carboxylation enzymes from species differing in salt tolerance. Plant Physiol. 1972 Feb;49(2):260–263. doi: 10.1104/pp.49.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander P. F., Bradstreet E. D., Hemmingsen E. A., Hammel H. T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science. 1965 Apr 16;148(3668):339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Szarek S. R., Johnson H. B., Ting I. P. Drought Adaptation in Opuntia basilaris: Significance of Recycling Carbon through Crassulacean Acid Metabolism. Plant Physiol. 1973 Dec;52(6):539–541. doi: 10.1104/pp.52.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek S. R., Ting I. P. Respiration and Gas Exchange in Stem Tissue of Opuntia basilaris. Plant Physiol. 1974 Dec;54(6):829–834. doi: 10.1104/pp.54.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek S. R., Ting I. P. Seasonal Patterns of Acid Metabolism and Gas Exchange in Opuntia basilaris. Plant Physiol. 1974 Jul;54(1):76–81. doi: 10.1104/pp.54.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Hanscom Z. Induction of Acid Metabolism in Portulacaria afra. Plant Physiol. 1977 Mar;59(3):511–514. doi: 10.1104/pp.59.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]