SUMMARY
Autoinduction (AI), the response to self-produced chemical signals, is widespread in the bacterial world. This process controls vastly different target functions, such as luminescence, nutrient acquisition, and biofilm formation, in different ways and integrates additional environmental and physiological cues. This diversity raises questions about unifying principles that underlie all AI systems. Here, we suggest that such core principles exist. We argue that the general purpose of AI systems is the homeostatic control of costly cooperative behaviors, including, but not limited to, secreted public goods. First, costly behaviors require preassessment of their efficiency by cheaper AI signals, which we encapsulate in a hybrid “push-pull” model. The “push” factors cell density, diffusion, and spatial clustering determine when a behavior becomes effective. The relative importance of each factor depends on each species' individual ecological context and life history. In turn, “pull” factors, often stress cues that reduce the activation threshold, determine the cellular demand for the target behavior. Second, control is homeostatic because AI systems, either themselves or through accessory mechanisms, not only initiate but also maintain the efficiency of target behaviors. Third, AI-controlled behaviors, even seemingly noncooperative ones, are generally cooperative in nature, when interpreted in the appropriate ecological context. The escape of individual cells from biofilms, for example, may be viewed as an altruistic behavior that increases the fitness of the resident population by reducing starvation stress. The framework proposed here helps appropriately categorize AI-controlled behaviors and allows for a deeper understanding of their ecological and evolutionary functions.
INTRODUCTION
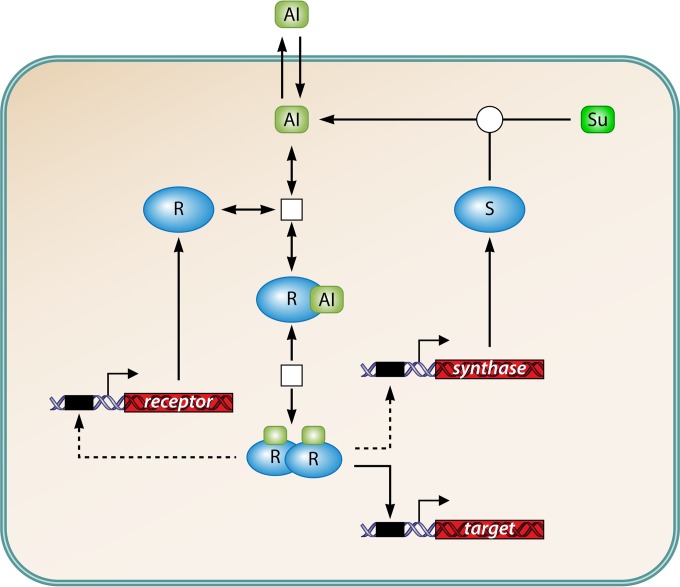
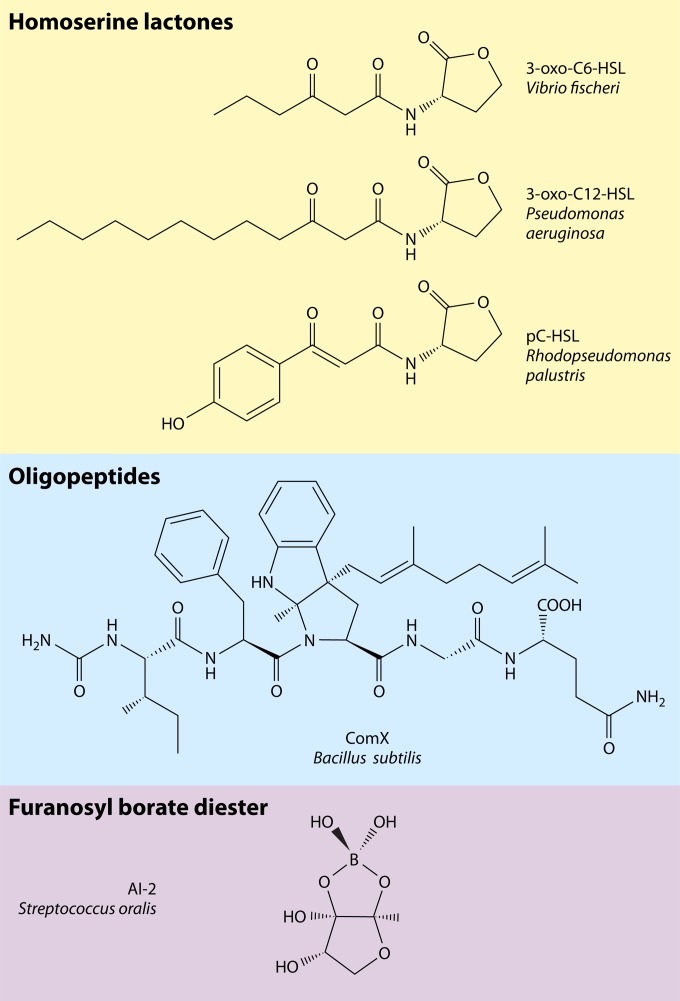
Intercellular signaling via small diffusible molecules, usually termed quorum sensing (QS), represents a common behavior in bacteria, often of high relevance from a human perspective. QS regulates a vast array of different target functions. In many symbiotic bacteria, these functions constitute life-style switches that are beneficial or pathogenic for their eukaryotic hosts (1, 2). An example for pathogenic bacteria is the switch from low to high virulence. QS is also crucial in environmental processes such as biofouling, degradation processes in sewage plants or environmental pollutions, and nitrogen cycling (3–6). Biochemically, the core of a generic system comprises a cytoplasmic signal synthase (or several involved enzymes), a small, diffusible signal that is released into the environment, and a signal receptor located in the cell membrane or in the cytoplasm. The signal-receptor complex directly or indirectly controls the expression of target genes (Fig. 1). As the same cells produce and respond to the signal molecules, the signal was originally termed autoinducer (AI). There are various chemical realizations of this core design (for an overview, see, e.g., references 7 and 8). The AI may passively diffuse through the cell membrane or be secreted by the cell and extracellularly modified or packaged into vesicles for trafficking between cells (9). Originally, three main types of AI molecules were described: (i) acyl-homoserine lactones (AHLs), primarily in Gram-negative proteobacteria but also in some bacteriodetes, cyanobacteria, and archaea (10–12); (ii) oligopeptide AIs in Gram-positive bacteria; and (iii) autoinducer-2 (AI-2), a furanosyl borate diester, as a universal signal for interspecies communication (Fig. 2). Still, an increasing number of AIs belonging to various chemical classes are being discovered (see, e.g., reference 13).
FIG 1.
Minimal AI signaling system in a cell, based on AHL signaling in proteobacteria. AI, autoinducer; R, receptor; S, AI synthase; Su, substrate. Metabolites are in green, proteins are in blue, and genes are in red. Black boxes indicate promoter regions, open squares indicate noncovalent interactions, and open circles indicate enzymatic conversions. The dashed arrow indicates optional feedback in some species, which may be positive (often with respect to synthase expression) or negative. There are variations of this basic scheme. In Gram-positive bacteria, oligopeptide AIs are secreted by a permease and sensed by a membrane receptor. A cognate transcriptional regulator is activated by phosphorylation.
FIG 2.
Examples of AI signals. The classes, structures, corresponding names, and bacterial species are shown.
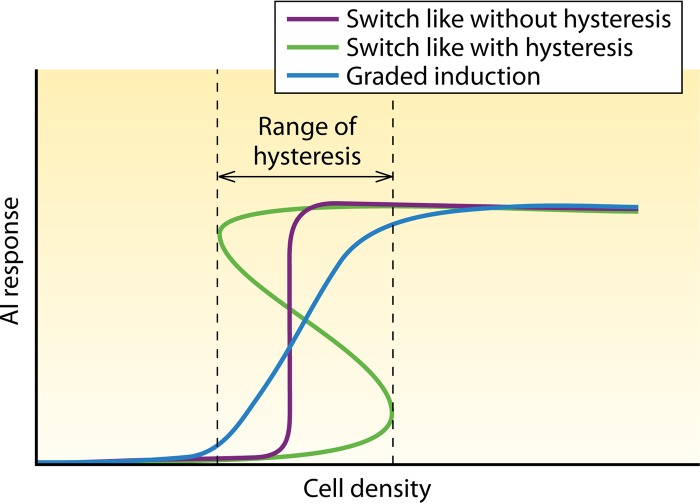
The generic systems can be adapted in various ways. For example, several oligopeptide AIs are posttranslationally modified (14), and AI transfer through the cytoplasmic membrane can be passive (by diffusion) or active (15). Two properties relevant for ecological function have been described for most, but not all, AI systems: (i) autoregulation, i.e., AIs positively regulate their own activity via expression of their synthase, and (ii) cooperativity of the AI effect (Hill coefficient of >1), e.g., via multimerization of receptor-AI complexes. As a consequence, for an appropriate parameter range, mathematical modeling usually predicts bistability (i.e., two different phenotypes associated with a locally stable on-state and off-state) and hysteresis (i.e., dependence of the actual AI production rate on the past state), which introduces a kind of memory and a switch-like transition between the on- and off-states (Fig. 3). This property can support a synchronous all-or-none behavior of cells in a population. However, the existence of bistability was reported to depend on the stability of receptor-AI complexes and has only rarely been confirmed experimentally (16–18). For other AI systems, a graded response at the population level has been reported (19). Of note, induction dynamics has almost exclusively been studied at the population level rather than in individual cells.
FIG 3.
Dynamics of AI system activation. AI systems show different induction behaviors. Whether an AI system can display graded or switch-like behavior with or without hysteresis depends on the architecture of the AI system (160). Interestingly, it was recently suggested that the same AI-based control circuit can display both switch-like and graded responses at the population level, dependent on values of specific parameters (161). As these could change in response to environmental conditions, the same species could display both behaviors. Mathematically, bistability is defined as the existence of two stable states at the same cell density (an “off”-state and an “on”-state), which is often associated with hysteresis. Although for many AI systems, the existence of bistability and hysteresis has been predicted by mathematical models, experimental evidence for the presence or absence of hysteresis is poor. Note that, deviating from the mathematical definition, the dominance of two stable states at different cell densities without hysteresis is sometimes called “bistable” in biological papers.
The expression of target genes at high density may be brought about by direct activation or by derepression. For example, among the family of LuxR-type regulators that recognize AHL signals, most members function as transcriptional activators in the presence of AI, while some function as transcriptional repressors in the absence of AI, and AI relieves repression (20).
Another aspect that is poorly understood is the dependence of the AI circuit on other processes. AI activity often depends on the metabolic state of the cell. Ulitzur (21) showed, for example, that starvation may enhance QS. The function of these interdependencies of different regulation systems with respect to the homoeostasis of cells and ecological function deserves more attention. The situation is even more complex if there are several feedback loops or if several interconnected AI systems coexist in one species. An appropriate description of the interactions between the AI system and interconnected regulatory pathways is central to our understanding of the ecological function of AIs.
The classical interpretation regards AI regulation as a mechanism to control target gene expression by cell density, limiting the cooperative behavior of the entire population in a synchronous all-or-none response to sufficiently high cell densities that generate a group benefit. This seemingly uniform functional principle behind all AI systems was challenged subsequently. The integration of different cellular and environmental factors into AI signaling intensity in different species broadened the interpretation of QS as a type of regulation system with greatly different ecological target functions that vary according to the species and its specific situation. Following this view, there seem to be no principles that unify all QS systems, possibly with the exception of an influence of cell density. However, both perspectives may be too extreme.
Here, we try to give an overview of the extent of information integration in QS, the purpose of the information exchange, and the participants in the communication process (who is communicating with whom). This is done comprehensively, based on different projections, including biochemical and physical aspects, ecology, mathematical modeling, and social evolution theory. Our analysis is guided by the notion that there are principles common to all AI systems. Before we begin with our analysis, we define terms that have not always been used consistently in the field.
DEFINITION OF TERMS
First of all, we want to clarify the uses of the terms “signaling,” “signal,” and “communication,” in the context of QS in this review, as we feel that there is some degree of confusion and disagreement in scientific publications. In agreement with, for example, Winzer et al. (22) and Diggle et al. (23), we apply a rather strict definition of the terms “signal” and “signaling.” We limit them to systems in which there are direct or indirect evolutionary benefits for the receiver to respond and the response of the receiver is beneficial for the sender. Thus, there is a coevolution between signal and response. Consequently, following the definition of Diggle et al. (23), we exclude interactions based on coercion (only the sender benefits) and cues (only the receiver benefits). In eavesdropping, for example, a “cue” provides important information to a recipient, but the cue did not evolve owing to this effect.
The distinction of processes representing coercion and cues from signaling also makes sense with respect to the consequences for the development of adequate AI-targeting treatment strategies, which aim to promote or suppress beneficial or adverse (e.g., pathogenic) bacteria, respectively. Inhibiting “true” signaling will impede both the sender and the receiver species, whereas in the case of coercion or cues, only one bacterial partner will be negatively affected, whereas the other may even benefit. We point out that the “benefit” can depend on the specific habitat conditions. Exemplarily, an AI-regulated virulence factor may be beneficial for bacteria in infections only under certain conditions in some host organs, whereas it might be redundant in others (24). This obviously also has consequences for the effect of inhibitors. Evolutionary benefit can thus be interpreted only in long-term analyses including potential habitat switches.
If being released and evoking any kind of effect in a receiver would be sufficient to be called a signal, i.e., ignoring the coevolutionary aspect, then, principally, all released molecules, including products toxic for the sender (e.g., waste) or the receiver (e.g., antibiotics), would be “signals,” and all these processes would be “signaling,” which would dilute the meaning of these terms to a level that renders them useless, at least for the discussion of QS and related aspects. We are aware that an unambiguous decision, whether a molecule is actually a signal in this sense or not, is often difficult and may depend on the specific situation. For example, molecules like AI-2 might be intraspecies signals for some species, including Vibrio species, but waste products for others (22, 25, 26).
For the term “communication,” the situation is even more ambiguous, as Diggle et al. (23), among others, use it in a broad sense, i.e., including cues and coercion, whereas others, such as Winzer et al. (22), tend to use it in a more strict way. An in-depth discussion of the semantics is beyond the scope of this paper; however, “communication” in this text is used synonymously with “signaling” as defined above.
In cases where the sender and receiver are genetically identical, i.e., for intraspecies interactions via AIs, receiver or sender cells may not need to directly benefit from the signaling molecule, as long as the signal-controlled target activity increases the inclusive fitness, i.e., the sum of direct and indirect fitness. An example is the AI-triggered death of a subpopulation of cells that enables other cells to benefit from competence or released nutrients.
According to the original definition, AIs are intercellular signals that are identical for sender cells and receiver cells (27, 28). Note that this does not require the presence of an autoregulation loop, i.e., the upregulation of AI production induced by AIs. However, it requires that an AI has to carry a certain degree of self-information. Unidirectional intraspecies communication, where sender and receiver cells belong to different subpopulations of differentiated cells in an isogenic population, would be called paracrine signaling (29, 30). In reality, to which degree sender and receiver cells belong to the same subpopulation remains unclear for most QS systems due to the lack of experimental data. An alternative term covering both aspects would be “pheromone.”
Finally, for reasons of clarity, we use the term “quorum sensing” in is original, strict definition, i.e., as a system restricted to measuring cell density, although it is now often used in a broader sense, including aspects of, e.g., cell distribution patterns and diffusion limitation (31). We term the broader concept “autoinducer sensing.” We are aware that the broader interpretation of QS has gained acceptance in the scientific community (31), although it is prone to misunderstanding.
ECOLOGICAL FUNCTIONALITY OF AI SYSTEMS
A Combination of Factors Determines Spatial AI Patterns
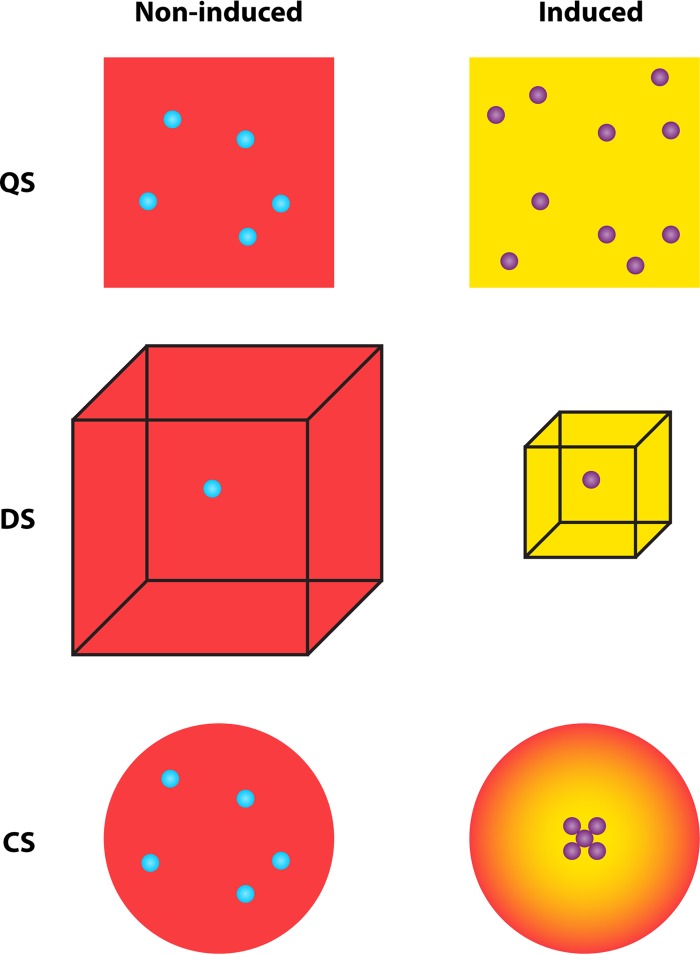
The original QS concept focused on the relevance of cell density (or quorum) for the regulation of gene expression via AIs. The later realization that other factors such as spatial cell organization (including aspects of clustering) and diffusion rates (or, more generally, mass transfer properties, including flow conditions) also affect the AI concentration led to the development of other concepts with names related to these factors, e.g., diffusion sensing (DS), positional sensing, cluster sensing, compartment sensing, or cumulative gradient sensing (for an overview, see reference 31) (Fig. 4). Most of these concepts were initially based on theoretical considerations and partly on modeling, but the relevance of spatial distribution and mass transfer properties has been confirmed experimentally. Most bacterial populations do not show spatially homogeneous cell distributions but rather show a more or less strong clustering of cells, e.g., in attached microcolonies, floating flocks, or bacterial cell assemblages in hosts. Clustering, which may be quantified by using, e.g., a radial distribution function (32), promotes the induction of the clustered cells, potentially resulting in an induction of rather small colonies consisting of only a small number of cells (33–37). Flow delays induction and may affect the spatial induction pattern in biofilms or microcolonies (34, 38, 39). Limited diffusion due to enclosure by solid material or gas promotes AI accumulation and thus induction (33, 40–42). The same concept applies to mass transfer limitation by matrices with decreased diffusion rates, such as mucus (43).
FIG 4.
Designation of AI signaling according to the factors that determine AI accumulation. QS, quorum sensing based on cell density; DS, diffusion sensing based on mass transfer properties; CS, cluster sensing based on the spatial distribution of cells. Red and yellow backgrounds indicate low and high AI concentrations, respectively; cyan and purple dots indicate noninduced and induced cells, respectively. (Modified from reference 47.)
As Platt and Fuqua pointed out (31), the multitude of concepts and concept names led to unnecessary confusion in the field. In nature, usually a combination of factors, namely, cell density, mass transfer, and clustering, determines the local AI concentration, and there is experimental evidence for this notion (40, 44, 45). Which factor(s) dominates varies depending on the specific conditions and can hardly be estimated by the cells, although an independent estimation of environmental (e.g., mass transfer) properties via multiple signals with different physical characteristics or via other measurement strategies is possible under certain conditions (46). Note that cell density and cell distribution (or degree of cell aggregation) are independent physical traits (Fig. 4).
The effects of various influencing factors are incorporated in the efficiency-sensing (ES) concept, which views the regulation system from the perspective of its ecological or evolutionary purpose (47). The ES concept assumes that the ecologically relevant function of AI sensing is to preassess the efficiency of producing diffusible extracellular effectors or “public goods,” whose concentration is influenced by the same combination of factors that influence the concentration of diffusible extracellular AIs. AI systems integrate the net influence of these factors, called measurement factors, into one information signal. Relatively cheap AIs can therefore function as a proxy for the production of more expensive effectors such as exoenzymes, siderophores, or antibiotics. AIs can act as significant cost savers by limiting costly target activities to when they are cost-effective (efficient). ES unifies the concepts of what cells sense and why, including hypotheses about the fitness benefits derived from AI sensing.
As in all measurement systems, disturbing environmental factors may falsify the intended measurements of cell density or diffusion limitation or the prediction of efficiency. Examples are the absorption of AI to components of the environmental matrix, AI solubility, and abiotic or biotic degradation dependent on the biotic and abiotic environment. All of these factors may additionally vary in time and space. Note that these environmental factors also affect released effectors. Differences in the strength of the influence of the factors between AIs and effectors further limit the predictive power of AIs for QS, DS, or ES purposes. However, as long as these disturbing factors are predictable, they may at least partly be compensated for.
The idea behind the ES concept, which is largely integrated into a recently broader interpretation of the term “QS” (48), was to shift perspectives from the question, “What does the cell want to know (e.g., cell density or mass transfer limitation)?” to “What information does the cell get, and how can it benefit?” This is in accordance with the hypothesis that many AIs or their evolutionary precursors may have originally been released for other purposes, e.g., as siderophores, as antibiotics, or simply as waste products (22, 49–51). Only in a secondary evolutionary process would cells have realized the additional value of information contained in the local concentration of these substances: it allows prediction of the efficiency (i.e., cost-effectivity) of the release of other effectors (52). Additionally, existing AI systems that evolved in other bacterial species may have been acquired by horizontal gene transfer and brought the expression of these effectors under their transcriptional control (53). Interestingly, some AIs, including certain AHLs and bacteriocins, still also function as antibiotics or siderophores (49, 50).
Showing that a factor influences the local distribution pattern of released AIs does not necessarily bear ecological significance. Some factors may rather be ecologically irrelevant or confounding. If cells live under constant, predictable mass transfer conditions, cell densities, or distribution patterns throughout their life history, one or several of these factors may be negligible. The purpose of AI systems would then be to measure only the variable influencing factor(s) and to control cellular activities whose efficiency correlates with them. For example, the evolutionary benefit of light emission by bacteria living in light organs may be connected with the number of emitting cells, determining visibility, as cell distribution patterns and diffusion properties of the environment in the light organ are predictable (54). Depending on how critical an AI-regulated decision is for fitness, a relatively small impact of influencing factors not correlating with efficiency may be tolerable. However, predictable, constant conditions are probably quite rare in nature and have to be proven before assuming that the system's purpose is to measure only one or two dominating factors. Generally, we propose that broader explanations should be considered for the interpretation of the ecological role of a specific AI system. These explanations assume that the AI is useful for efficiency prediction under a broader set of influencing factors.
Additional environmental factors affect the local concentration pattern of released AIs. Temperature generally influences the stability of biological molecules, and pH affects the stability of AHL (55, 56). AHL signals are more rapidly degraded at alkaline pH (57). Sorption or even binding to surfaces can decrease the soluble AI concentration. Hydrophobic AIs such as quinolones or long-chain AHLs could partition to biotic or abiotic structures such as membranes or lipophilic surfaces. Other bacterial or eukaryotic species influence AI systems by, e.g., the degradation or production of the same or similar AIs, AI-mimicking substances, or AI-blocking activities. Usually, these effects are interpreted as secondary effects disturbing the actual purpose of the AI system. However, if the efficiency of the regulated target activities is affected by pH, the sorbing matrix, or the interfering species in a correlated way, it cannot be excluded that these factors are part of the measurement objective. Exemplarily, it has been proposed that in some cases, AHLs may be used for pH sensing (58). Critical questions in this context are as follows. Is the interpretation of the ecological purpose of AIs meaningful in the context of actual, natural conditions? Is the use of AIs for this purpose reasonably cost-effective compared to other possible regulatory strategies? Does it present an evolutionarily stable strategy (ESS)? Or does the influencing factor indeed just perturb what should be measured? The question of what cells actually measure by AIs and for what benefit is still not completely satisfactorily answered and probably varies within and between species (some of which harbor multiple AI systems) and in relation to environmental conditions.
In an evolutionary context, the fitness consequences of AI-regulated phenotypes that can benefit individual cells as well as groups of cooperating cells are important to consider. Would we expect them to always work in the same direction, and why? Let us consider AI-controlled bioluminescence in some marine Vibrio species as an example. Costly luminescence is visible and thus beneficial only if produced by many cells but presents a waste of energy if produced by a single cell in a diffusion-limited environment. As the cells probably cannot distinguish between both scenarios when measuring the AI concentration, the question of the evolutionary stability of such behavior arises. Whether AI-controlled bioluminescence represents an ESS depends on how often single cells experience a situation dominated by mass transfer versus one dominated by changes in cell density. However, if we alternatively assume that oxygen detoxification is the main or at least the former purpose of bioluminescence, direct benefits of spatially isolated (and thus noncooperating) cells and inclusive fitness benefits of cooperating cells in a population would work in the same direction (59). Cooperation can provide a direct fitness benefit to the reproductive success of the individual performing the behavior that outweighs the cost of performing the behavior. Cooperation also provides an indirect benefit because it is directed toward other individuals who carry the cooperative gene (60).
AIs as Proxies for Environmental Changes beyond Effector Release
The original definition of the ES concept, although thought to explain most cases of QS, was relatively narrow. For example, it focused on the regulation of released effectors; i.e., it argued that AIs are suitable to control genes that are directly or indirectly involved in secretion. However, several AI systems control intracellular enzymes that are not involved in secretion but that affect the concentration of environmental substances. These intracellular enzymes, for example, reduce oxidative stress or acidification (61–64). In these cases, mathematical modeling predicts that AIs can also be used as reliable predictors of costs and benefits (B. A. Hense and J. Müller, unpublished data). The efficiency of a population of cells in reducing the concentration of harmful environmental substances in their neighborhood via intracellular enzymatic reactions increases if more cells contribute. Aggregation of cells and mass transfer restriction of the environmental matrix further contribute to this reduction. Thus, changes in the concentration of AI and harmful environmental substances are inversely correlated (i.e., an increase of the cell number will result in a decrease in the amount of the environmental substance). In this sense, the ES concept is applicable for situations without effector release.
However, prediction of the efficiency of changing the environment by the cells via intracellular enzymes is not necessarily restricted to degradation processes. Butanediol fermentation in Serratia plymuthica RVH1 is repressed by an AI system before acidification caused by fermentation impedes population growth (65). Alternatively, in some Burkholderia spp., AI activates the production of oxalate to avoid toxic alkalinization by ammonia (66). Here, the AI allows the cells to predict the environmental pH if the population continues to produce waste products and thereby to avoid negative fitness effects by changing the environment. In these cases, concentrations of AI and the environmental substance (acid or base) are positively correlated.
All these considerations fit into the concept of AIs as predictors of the efficiency of regulated target activities to modify the cell's environment, i.e., ES. In many bacteria, AI circuits function as life-style switches in which master regulators govern the expression of up to several hundred genes rather than individual phenotypes (67–72). Here, efficiency refers to the whole regulon rather than an individual gene or operon; i.e., not every single, regulated gene may be directly connected with changes in the environment. However, to our knowledge, at least some genes of each AI regulon code for activities that directly influence the environment. A forthcoming meta-analysis of bacterial QS regulons supports this notion (L. McNally and S. Brown, personal communication). A simple example is AI-dependent toxin-antitoxin production in E. coli (73). Here, AI coregulation of an intracellular product, antitoxin, makes sense because it is tightly coupled with AI regulation of an extracellular product, secreted toxin.
Another example is the QS regulon of Pseudomonas aeruginosa. Microarray studies revealed that AHL QS directly and indirectly controls the expression of >300 genes (68, 74, 75). Only a fraction of these genes encode secreted factors; however, this fraction is primarily directly regulated by QS (76). Among the many genes indirectly controlled by QS are central metabolic genes. It is conceivable that many of these genes help shift cellular physiology from a growth state to a secretion state (76). This shift may include the induction of metabolic pathways that produce the precursors of secreted products as well as those that process nutrients made accessible by the secreted product.
Taken together, AIs regulate life-style switches whose evolutionary benefit is strongly coupled with the efficiency of controlling environmental changes. This is reasonable for all scenarios where AIs can act as cost-saving proxies.
AIs as Triggers of Noncooperative Behavior?
The evidence that we have provided thus far supports the original idea that AI regulation is associated with the induction of cooperative behavior. Once a critical AI concentration (or sometimes cell density) is reached, all cells in a population induce a coordinated, phenotypically homogeneous, and synchronous joint activity, which is not efficient if done by a single cell or a small number of cells (77). This transition may be accompanied by a concomitant repression of noncooperative traits. For example, in the pathogen Staphylococcus aureus, the Agr QS system activates secreted toxins and represses surface adhesins at high cell density (78). As discussed above, a direct connection to cooperative behavior does not have to exist for each single gene in the AI regulon, which often may rather assist switches to cooperative life-styles. However, there are examples where AIs terminate joint activity and even seem to trigger solely noncooperative behavior.
In several Pseudomonas putida strains, an AI system (called Ppu) triggers the release of the cell-associated surfactant putisolvin (79). Putisolvin promotes the removal of cells from the colony/biofilm. Fluorescence labeling showed that in smaller colonies, only single cells detach from the colony once their AI system reaches the induced state (34). Although the degree to which induction is dominated by AI accumulation or by stochastic upregulation is not completely clear, AI-controlled putisolvin release can hardly be interpreted in terms of public goods, as only the induced cell is able to detach. The response does not occur synchronously within the population.
In several other species, AIs trigger the detachment of cells from biofilms (80). In P. aeruginosa and S. aureus, subpopulations are released from individual microcolonies, resulting in cycles of detachment and regrowth (81–84). This seemingly noncooperative behavior (the cells leave their aggregate) has been connected with escape from nutrient limitation (85) due to overcrowding. Triggering removal directly by starvation would be a response to the prevailing environmental conditions. In contrast, coupling with AI introduces a predictive element to the decision process, i.e., information on the degree of nutrition depletion if the present population continues consumption under the actual conditions. The same applies to the accumulation of waste products.
Colonies or biofilms present a certain degree of protection, e.g., against antibiotics and grazing. Leaving of colonies by single cells may thus be accompanied by an increase in the risk of death (decreased fitness). However, the population can benefit by, e.g., decreased starvation pressure or by the potential colonization of new localities. Such behavior could be considered altruistic and can be beneficial even to the detaching cell in light of the inclusive fitness theory, if detaching and resident cells share the same cooperative trait (86). By leaving, the individual cell increases the fitness of resident cells in the colony and, due to their high relatedness, indirectly increases its own fitness (87). Consequently, although not connected with the production of public goods, we can consider behaviors such as detachment from colonies “cooperative.” In fact, it is hard to imagine how cell-cell communication, itself representing a cooperative regulation strategy within a species or even a clone, could control noncooperative, selfish cell behavior of single cells in an evolutionarily stable way. Thus, we assume that AIs generally regulate cooperative behavior. Note that this does not mean that the specific circumstances always allow for cooperation. A borderline case would be diffusion sensing of an individual, isolated cell (discussed in more detail below), such as that residing in the phagosomal compartment of a macrophage (88, 89). AI induction promotes escape from the endosome and apoptosis of the host cell. Such a behavior is simply nonsocial, neither cooperative nor selfish, just because the situation of the isolated cell is nonsocial. However, as soon as the compartment contains more than one cell, the AI-controlled behavior turns into cooperation; that is, it is the situation that determines whether such a behavior is actually cooperative and not the behavior itself.
AI-mediated cooperative behavior by no means requires synchronous, uniform responses of all cells in a population. Studies with single-cell resolution show the absence of population-wide synchronicity of AI responses in isogenic populations grown planktonically or as a biofilm (19, 81, 84, 90). It is possible that emerging heterogeneities, caused by chemical gradients or stochastic variations, are sometimes a purposeless side effect. However, there is increasing evidence for the potential benefits of a division of work within a population, i.e., the emergence of subpopulations with different tasks. In the well-studied case of Bacillus subtilis differentiation, multiple AI systems are centrally involved in triggering the development, maintenance, and functionality of a number of subpopulations with different, complementary phenotypes (29, 30). In this species, AI systems control the stochastic emergence of phenotypic heterogeneity, i.e., when and to which degree cells of a certain subpopulation develop, but also control activities within subtypes; that is, they control a nonsynchronous, nonhomogenic response network, which is nevertheless cooperative and coordinated in the way that it benefits the whole population by a controlled division of labor.
At the level of the individual gene product, a relation to cooperativity is often difficult to determine. For example, AI control of intracellular nucleoside hydrolase (Nuh) in P. aeruginosa has received considerable attention in the context of social evolution (91–94). Nuh is a periplasmic enzyme that hydrolyzes adenosine to yield adenine and ribose, allowing the cell to grow on adenosine as the sole carbon or nitrogen source. This is, at first sight, a strictly noncooperative, cell-associated behavior. However, there are several, not necessarily mutually exclusive, scenarios that link AI control of Nuh to cooperation. First, AI control of Nuh may help stabilize other cooperative behaviors that are regulated by the same AI system. This regulatory linkage, termed pleiotropic constraint, can incur a cost to AI receptor-deficient cheater mutants whenever adenosine is a substrate (94, 95). Alternatively, Nuh may have a role in optimizing AI production by degrading by-products during AHL synthesis, as proposed by Heurlier et al. (91). Finally, the periplasmic location of the Nuh enzyme may render it or its metabolites prone to leakage into the extracellular space. Such periplasmic leakage is common and has been reported for Escherichia coli, for example (96). In this case, AI signaling would directly control a (partially) shared, cooperative behavior.
Role of AI Systems and Accessory Mechanisms in Homeostasis of Cooperative Behavior
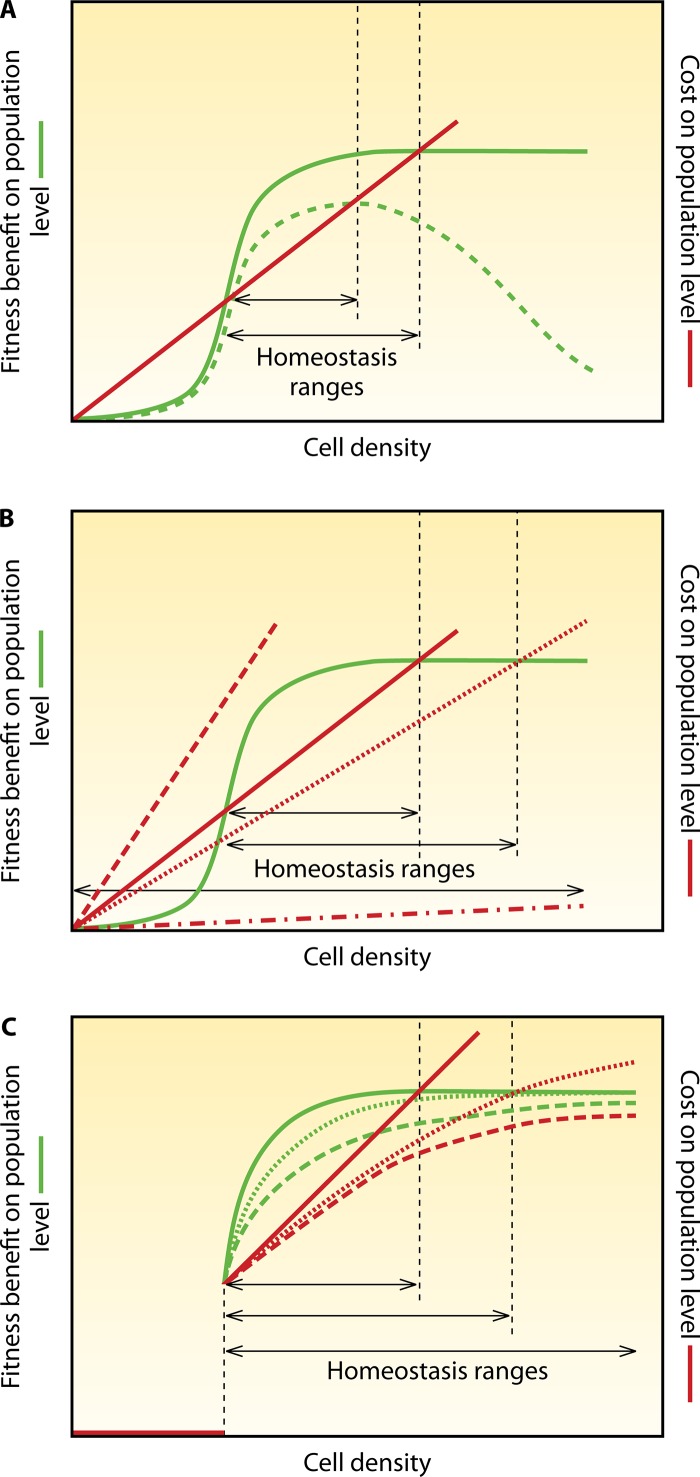
Ecological interpretations of AI systems focus on the idea that a certain minimum threshold (i.e., a certain cell density) is required to render a joint activity effective. Examples are the release of antibiotics or digestive exoenzymes. When released by a single cell in an open ocean, both will dilute, and the benefit for the cell will be minimal; i.e., the net benefit due to the production cost will be negative. With increasing cell density, the production costs for the population increase roughly linearly (the costs per cell remain the same, of course), whereas the fitness benefit of the population increases disproportionally strongly (Fig. 5). Above a certain cell density, cooperative production pays off. Ideally, in such a scenario of synergistic benefits, AIs control the production of target functions (92, 97, 98).
FIG 5.
Costs, benefits, and homeostasis in cooperative activities. (A) Variation in benefits. Shown are sketches of fitness benefit and cost, calculated as the sum of all individuals in a population over cell density (or cell number in the case of colonies). For simplicity, costs per cells are assumed to be constant; i.e., each cell contributes equally to cooperation. The benefit curve eventually approaches saturation, e.g., if all substrate is degraded by exoenzymes (green continuous line), or declines, e.g., if high concentrations of released toxins harm the producers (green dashed line). Consequently, the cell density range in which cooperation pays off changes (homeostasis range [indicated by arrows]); here, AI induction of cooperative activity can be expected. (B) Variation in costs. Different costs of cooperative behaviors (e.g., production of exoenzymes or antibiotics) influence the homeostasis range. The dashed line indicates cooperation that never pays off, and the dashed dotted line indicates cooperation that always pays off; in both cases, no AI regulation is required. The continuous and dotted lines indicate cooperation that pays off only in a certain cell density range (shown by the arrows). Here, AI control of cooperative activity can be expected. According to the metabolic prudence concept, the cost of cooperation can also vary for the same activity (162). In fact, variation in AI control in accordance with the metabolic prudency concept has been observed (125). (C) AI control of costs and benefits. AI control avoids the costs of cooperative behavior for low cell densities (continuous lines). Once induced by AIs, populations can subsequently reduce costs (dashed and dotted lines) and thus widen the homeostasis range, e.g., by downregulating the production of public goods by AIs in a density-dependent manner. If this is realized by individual cells switching from the producing to the nonproducing subpopulation, in principle, the homeostasis range can be infinite (dashed lines). However, if it is realized by decreasing the average production of public goods per cell, the cost curve will at some point cross the benefit curve, because the costs for the effector production machinery cannot be below a certain minimum value, and the regulatory machinery is costly (dotted lines). Note that these idealized graphs are meant to visualize principles. The same applies to Fig. 6 and 9. These graphs do not consider additional, interfering effects such as negative feedback via AI-regulated public goods or nutrient depletion.
However, there are counteracting effects that appear with increasing cell density. The increasing relevance of resource (nutrient) limitation is discussed above. Additionally, in almost all cases, the increase of the benefit gained by joint cooperative activity approaches saturation or may even decline with a further increase in cell density. If antibiotics reach a concentration that kills all competitors, a further increase of production does not pay off. Similarly, if all protein substrate is effectively degraded by released exoproteases, additional release by further cells is not beneficial. An excess of antibiotics may even cause harm to the producers themselves, and other, competitive species may exploit excess exoprotein production. Thus, there is a population density range, bounded by a lower and an upper threshold, at which cooperation pays off and benefit exceeds cost. Here, cooperation can be regarded as stable, i.e., in homeostasis (defined as the stability of systems, homeostasis in our case refers to a cooperating population). Beyond keeping the AI system within this homeostatic range, the system can be expected to be further tuned to the point where the net benefit is optimal (Fig. 6). For reasons of simplicity, we ignored additional layers of complexity such as those caused by feedback between nutrients and cell density. These factors would complicate the regulation of homeostasis but would not qualitatively change the outcome.
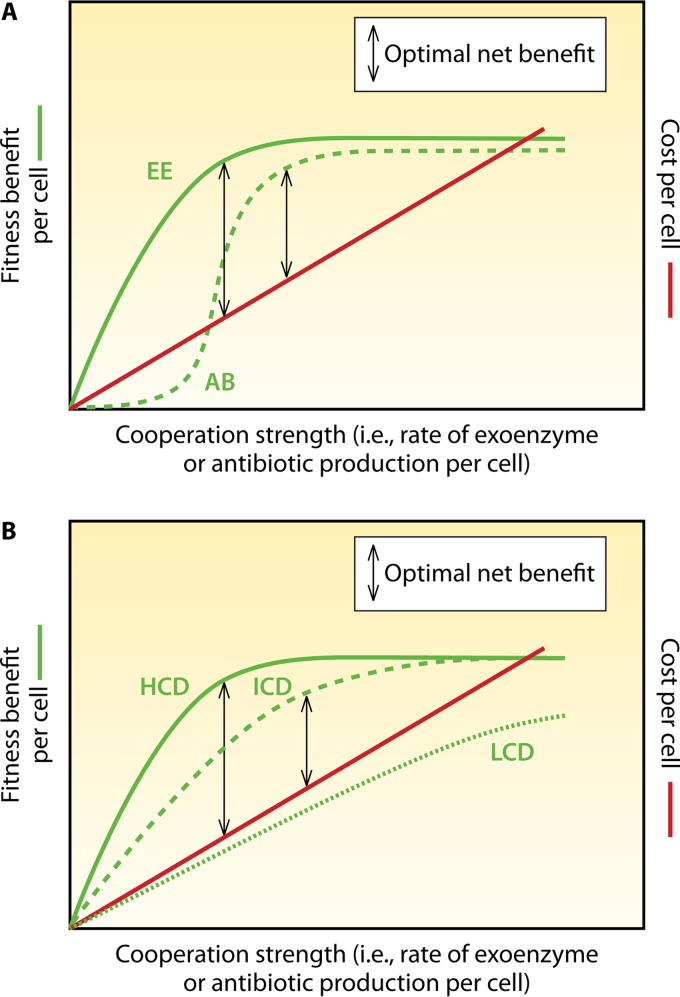
FIG 6.
Optimality as a function of costs and benefits at various strengths of cooperation. (A) Production of two different public goods. The shape of the curves of fitness benefit over cooperation strength varies for different cooperative behaviors. Whereas exoenzyme (EE) production, e.g., for nutritional purposes, may simply follow a hyperbolic curve, antibiotic (AB) production is best reflected by a sigmoid curve (the antibiotic simply has little to no effect on susceptible species at concentrations far below the MIC). Fitness benefits may even decrease at very high antibiotic concentrations due to possible self-inhibition. As described in the text, AI control and accessory mechanisms fine-tune cooperation strength such that the net benefit for a given cooperative behavior is optimal. Note that the use of the difference between fitness benefit and cost as an indicator for optimization oversimplifies the situations, for reasons of presentability. In some cases, the cost/benefit ratio may be more suitable. (B) Exoenzyme production at different cell densities. HCD, high cell density; ICD, intermediate cell density; LCD, low cell density. For high cell densities and intermediate cell densities, different levels of cooperation strength are optimal. For the scenario presented here, at a low cell density, cooperation never pays off; thus, AI-controlled induction should not occur.
Consequently, in a growing population, there should be regulatory systems to dampen the contribution of individual cells to cooperative behavior once activated. In fact, there is ample evidence that AIs either control this process directly or are intimately tied to accessory feedback mechanisms. In principle, excessive cooperation can be avoided by limiting the number of cooperating cells or by decreasing the contribution from each cooperating cell.
There are various molecular mechanisms of how AIs systems can tune the cooperative activity of each participating cell. Negative feedback loops introduced into the molecular architecture of AI production and/or sensing are often employed. For example, in P. aeruginosa, AI expression is repressed by the negative regulator RsaL (99, 100), while receptor activity is inhibited by the antiactivators QteE and QscR (101–103). AI induces the transcription of RsaL and QteE (76) and activates QscR as a ligand (104). Furthermore, AI levels may be controlled by self-produced AI-degrading enzymes (105). In Vibrio fischeri, the expression of the AI receptor LuxR is upregulated at intermediary but downregulated at high AI concentrations, which was assumed to maintain light production within a certain strength range (106). Similar combinations of positive and negative feedbacks at intermediary and high AI concentrations, respectively, have been described for other species (107, 108). In a basic version, such a combination can be realized by a single receptor-AI system, binding with different affinities to two (or, theoretically, more) counteracting DNA target sites such that an AI response may be activated at low but repressed at high AI concentrations (107, 109). Such regulatory principles have been described for many AI systems, although their function in the context of homeostasis has been interpreted only rarely (108, 110–112).
An alternative strategy is to limit the proportion of cooperating cells within a population. The release of individual cells or groups of cells from biofilms, as mentioned above, can be interpreted in this context, as it represents a method for how the number of locally cooperating cells can be restricted. Periasamy et al. (84) reported that the agr AI system in S. aureus regulates surfactant-mediated periodic detachment of cells from biofilms and structural development-like channels within the biofilms, achieving biofilm homeostasis by maintaining a maximum thickness and a nutrient supply to deeper biofilm layers, and also the spread of infection.
A similar effect can be achieved if cells remain physically associated with a population but cease to cooperate. This can occur if intercellular signals control both the induction and termination of the cooperative behavior. In B. subtilis populations, a complex signaling network governs the differentiation of cells into at least seven subtypes. Under certain conditions, some cells develop into matrix producers that release exopolysaccharide and the matrix protein TasA as public goods (29, 30, 113). A subfraction of the matrix producers may eventually sporulate. The level of phosphorylated Spo0A (Spo0A-P), a major transcriptional regulator, controls both developmental steps. A certain albeit low level of Spo0A-P triggers matrix producers, whereas a high level of Spo0A-P triggers sporulation. External regulation is complex and still not fully understood. Besides environmental factors such as starvation, AIs are involved in the control of Spo0A phosphorylation. Matrix producer development is promoted by an intercellular signal called surfactin. Spores, which halt the production of public goods, were reported to be triggered by another AI, PhrA, a small signal peptide of the Phr family. In this sense, the beginning and termination of the release of public goods in a specific subpopulation are AI controlled.
In the case of B. subtilis development, entry and exit decisions are based on different signals. However, in principle, the same effect is possible with one signal that binds different receptors with different affinities. The more sensitive receptor might induce a cooperative behavior, and a second, less sensitive receptor might terminate it by, for example, acting as a transcriptional repressor. Although the details are not clear, the two AI receptors in Legionella pneumophila, which have opposite effects on the majority of the regulated target genes, may be interpreted in this way (114). Vibrio harveyi in turn limits AI-dependent activation in a proportion of participants: the number of AI-induced bioluminescing cells in an isogenic population never exceeds 69%. Only the addition of exogenous AI results in almost 100% bioluminescing cells (19). Although the underlying mechanism remains unclear, here, AIs control the onset of bioluminescence in the population but also control the maximum contribution.
Homeostasis in cooperativity is not limited to individual behavioral traits. If different cooperative behaviors have different cost/benefit ratios and are expressed in one and the same organism, then there must be regulatory mechanisms to properly tune each behavior and minimize interferences. An example is again cell differentiation in B. subtilis, specifically the release of public goods on one side and the induction of detachment on the other side. The former behavior usually favors spatial stationarity (i.e., the cells should stay in the area where the public goods are released to maximize the net benefit), whereas the latter is connected with spread (29, 30). At the single-cell level, the benefit of one behavior is diminished by the other if simultaneously expressed; thus, both phenotypes appear to be mutually exclusive. Consequently, a division of labor with respect to mobility versus the release of public goods is indeed observed in B. subtilis populations. As indicated above, the underlying circuitry involves different AI thresholds for different phenotypes and the use of multiple AI systems and reciprocal suppression mechanisms on gene expression (29, 30). Unfortunately, to our knowledge, a rigorous, comprehensive analysis of such sophisticated systems with respect to cost/benefit optimization has not yet been undertaken.
From another perspective, AI control of cooperative behavior represents an optimized manifestation of the Allee effect, a well-known phenomenon in population ecology. The Allee effect describes the fact that within a certain population density range, the growth rate (or, more generally, fitness) is sometimes positively correlated with the density, or number, of individuals in a population (115). At very high densities, resource competition interferes with the positive effect; i.e., the positive correlation may turn into a negative one. AI regulation ensures that behavioral properties that enable the Allee effect are expressed only in the density range where they pay off. The role of the Allee effect in the trade-off between population spread and survival was recently investigated with an engineered bacterial system, using V. fischeri QS components (116).
Beyond microbiology, recent studies of mammalian systems also suggest that QS-like strategies are involved in the homeostatic control of the immune response as well as cell differentiation processes in cancer development (117–121).
Variability of the AI Production Rate: the Hybrid Push-Pull Model
It was generally assumed that prequorate cells produce AIs at a constant low rate and that upon reaching a quorum, positive feedback results in an increased (constant) production rate. This is also the foundation for most mathematical models. For more pragmatic reasons, some later models include a term for the nutrient dependency of AI production, i.e., a lower level of AI production under nutrient-depleted conditions (38, 122). As an alternative, the AI concentration has been suggested to reflect the change in cell density rather than the actual cell density, at least in cases where AI production is linked to central metabolic pathways (123). The idea behind this is that, generally, a high growth rate is connected with high metabolic activity. In fact, the level of AI-2 accumulation in V. harveyi is highest during the exponential growth phase, and the AI-2 concentration declines when the growth rate decreases upon entry into stationary phase (124). AI-2 is an unavoidable waste product of an essential metabolic pathway in many bacteria. Interestingly, a similar AI dynamic is observed during the growth of P. putida IsoF, although its AI (mainly 3-oxo-decanoyl-homoserine lactone) is not known to be a waste product (105). Due to different stabilities of AIs and some AI-receptor complexes, actual AI concentrations may either reflect more the actual population (for unstable AIs) or integrate information over several generations (for stable AIs).
A number of cellular and extracellular factors directly and indirectly affect either the AI production rate or receptor expression levels. Both have the potential to modulate the quorum threshold. For example, AI systems often regulate responses to stress such as starvation, and the scarce quantitative data suggest nonlinear or even nonmonotonous relationships between the activity of AI systems and the degree of starvation (21, 125). In V. fischeri, at least in a certain range, nutrient deficiency promotes AI activity. At very low nutrient levels, AI production finally ceases (21). (Note that the latter indicates, again, that AI systems serve to control not just the onset of cooperative activities but also their termination.)
Coupling AI release with information about starvation allows the cell to integrate information about its individual demand into the communication signal (126). The increased AI production in the case of nutrient depletion can be interpreted as a kind of emergency call. Starving cells may have an increased demand for a coordinated behavior improving the supply of a limiting nutrient. The demand is communicated by increased AI release. This reduces the number of cells required to reach the induction threshold. In the case of V. fischeri, demand stops once starvation becomes too severe. This could be interpreted as a reaction to a scenario where cells can no longer afford to engage in cooperative activities but concentrate on maintaining essential cellular metabolism.
For an increasing number of species, the influence of various environmental substances or, connected with this, the cellular (growth) state on AI activity is known (see, e.g., references 125 and 127). This includes signals from hosts (for example, about their health state) in mutual or parasitic relationships (see, e.g., references 128–130). The demand might often be coupled with a willingness or ability to contribute. In a stressful situation, lower reproductive potential may favor altruistic behavior, which at its extreme may cause some cells to commit suicide to the benefit of others (131). From an efficiency point of view, all these factors affect the actual need of the bacterial cells for the regulated target behavior, their potential or willingness to contribute to it, or the opportunity for the regulated behavior to be effective. Unfortunately, in batch culture experiments, it is difficult to discriminate between the influence of changing AI production rates per cell and the influence of changing cell densities on the actual AI concentration as nutrient availability changes over time. Attributing experimental results purely to changes in cell density may have led to misinterpretations.
In spatially structured populations as colonies or biofilms, physicochemical gradients can require different cellular responses. Each cell can convey information about its individual demands for cooperative activities by producing different amounts of AIs (126). The specific demands of each cell at its specific location can thus be integrated into spatially structured communication, resulting in optimized regulation of the target behavior at the population level (Fig. 7).
FIG 7.
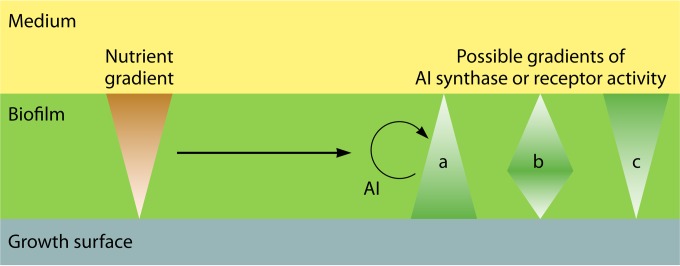
Asymmetric communication in a biofilm. Gradients of nutrients (or other environmental factors) can affect AI production and perception. Emerging AI gradients can themselves affect AI synthase or receptor activities, generating feedback. Often, these impacts are nonmonotonous. For example, nutrients may upregulate AI at low concentrations and downregulate AI at high concentrations. Depending on the environmental conditions and the intracellular regulation architecture, this can result in different spatial patterns of components involved in AI production (AI synthase) and/or AI sensing (receptor). These impacts might be strongest at the upper or lower biofilm surface or somewhere in between, which is depicted by scenarios a to c (126). In other words, different layers in the biofilm talk or listen with different intensities. Asymmetric communication can be further complicated by stochastic effects in communication, the development of subpopulations, heterogeneity of biofilm morphology, or the presence of other species.
Referring to concepts from economics, the term “hybrid push-pull control” for this kind of regulation design has been proposed (126), where “pull” (the actual demand of the buyers for a product) reflects the demand of a cell for the target behavior, transported by a change in AI production (Fig. 8). “Push” (the potential strength of a company to produce the product) reflects the possible reached strength of the target behavior. As the pull and push factors both influence the cost-effectiveness of the regulated activity, the authors of that study hypothesize that the core purpose of this regulation system is to promote gene control based on a preassessment of the efficiency of the target behavior. Beside nutrients, other intra- and extracellular factors affect efficiency. Examples are other environmental factors (such as pH and temperature), the presence of competing or beneficial microbes, and the developmental state of the cell (such as sporulation and mobility).
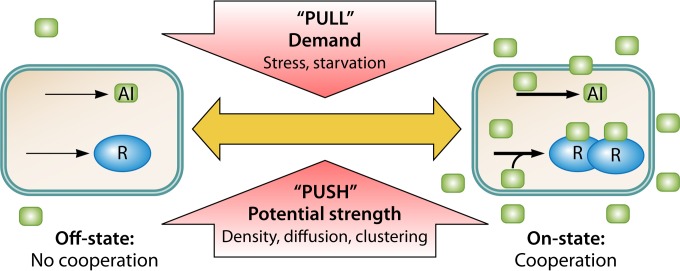
FIG 8.
Hybrid push-pull model of AI-controlled cooperation. Push factors impact the strength and potential for beneficial cooperation. These factors are environmental factors that influence AI accumulation and perception by an individual cell, namely, cell density, diffusion, and cell clustering. Pull factors impact the cellular demand for cooperation. These factors are often stress factors, such as nutrient starvation, that affect the physiological state of the cell. Pull factors often positively regulate AI production or reception. AI and R denote AI signals and receptors, respectively. Thin and thick black arrows denote the activity of production in the off- and on-states, respectively.
Interlinking the cell's need, its internal ability, and/or the opportunity for efficient target behavior with the AI production rate adds an internal demand or pull aspect to the AI system (“Do it when you want/need it—and ask others to give you a hand”), reflecting the demand of a cell for the target behavior, transported by a changing AI production rate. The external factors cell density, cell distribution, and mass transfer limitation determine the potential strength of the coordinated target activity and could be regarded as push factors (“Do it when your activity has an impact” or “Do it when it is effective”). AI regulation systems integrate information about both aspects into the local AI concentration. Sensing the combined pull-push information carried by the AIs allows each cell a contextual interpretation of the state of the neighboring cells relative to its own under actual push conditions (132). This results in an adaptive behavior of cells within the growing colony, highly dynamic in space and time. Cooperativity and division of labor can emerge. Their spatiotemporal flexibility goes beyond those of real multicellular organisms, e.g., because cell differentiation with respect to the division of work can be reversible. This remarkable phenotypic plasticity probably enables an adaptive life-style optimization of the colony under the prevailing conditions.
Signaling systems that employ aryl-homoserine lactone signals can serve as an example of how pull information is integrated. These types of signals contain a cinnamoyl or p-coumaroyl group. Cinnamate and coumaric acid are both predegradation and degradation products of lignin (133). The soil bacterium Rhodopseudomonas palustris, which can grow on p-coumarate as the sole C source, relies on the uptake of external p-coumarate to produce its AI p-coumarate–homoserine lactone. It has been suggested that genes regulated by this compound promote the approach of R. palustris to host plant roots, i.e., to a potential nutrient source (134). This represents an elegant, simple way of integrating information about pull aspects (here, the presence of nutrient sources) into the signal concentration.
The control architecture of the cadAB operon in E. coli may be interpreted as an interesting example for a hybrid push-pull strategy. The cad module is a conditional pH stress response system (135, 136). Induced by acidification, the sensor CadC, a one-component receptor, promotes the expression of cadAB. The enzyme CadA converts lysine under the consumption of a cytoplasmic proton to cadaverine and CO2; the lysine/cadaverine antiporter CadB subsequently exports cadaverine. The expression of cadAB is promoted by low pH, high substrate concentrations (lysine), and the absence of the reaction product cadaverine, all measured externally. Due to the negative feedback via cadaverine, the system displays only a transient response, even if the conditions for its induction (e.g., low pH) persist. The purpose of this regulation design is unclear. However, cadaverine fulfills the basic requirements for an AI, as it is released and sensed by the same cells and controls gene expression. A high extracellular cadaverine concentration in combination with a low pH indicates high but ineffective antiacidification activity by the cell and potential neighbors. Thus, from an economical point of view, the Cad response should be stopped. Here, pull information about the demand side of the response (lysine as an indicator that it can be done and low pH as an indicator that it should be done) in combination with AI-mediated push information (how effective it would it be to continue) control the antistress response and the “AI” itself.
The demand of cells within a population for cooperation directly influences its benefit and thus the homeostatic range (Fig. 9), rendering the control of AI systems by pull aspects useful with respect to the maintenance of efficiency.
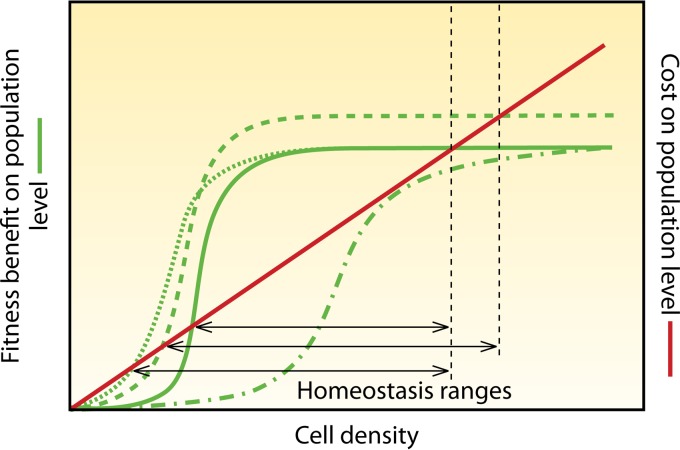
FIG 9.
Costs and benefits of exoenzyme production under different pull conditions. The red line indicates costs, and the green continuous line indicates fitness benefit at the population level as a baseline for comparison with the various pull conditions. Under stronger nutrient-limited albeit still growth-permitting conditions (starvation pressure), a population at lower cell densities will benefit from exoenzyme secretion involved in nutrient acquisition, and the maximal benefit from the exoenzyme will be greater (dashed line). In other instances, the maximum benefit of cooperative activities may be constant, for example, if competitive species can be killed by the cooperative release of toxins, because more than killing of all competitors is not possible (dotted lines). This influences the homeostatic range, i.e., the cell density range where cooperation pays off, and thus, AI regulation can be expected. In extreme cases, decreased demand might reduce the potential benefit to a level where cooperation never pays off (dashed dotted line). For clarity, the schematic shows equilibrium conditions. Differences in the growth rate are neglected, and the physicochemical environmental conditions are assumed to be constant. The depicted scenarios do not include AI control per se, but they suggest where AI regulation would be beneficial and how it should vary under different pull conditions.
From an ecological perspective, changing environments can shift push and pull aspects in opposite directions. Dilution events such as rainfall decrease the cell density of a microbial population but increase starvation. Depending on the quantitative connections in the regulation network, such an event could induce the system through increased AI production, although the cell density declines. This notion is supported by recent experimental data. Nutrient dilution in minimal medium batch cultures decreases the growth yield but increases QS gene expression per cell (125).
The existence of a pull aspect in AI production may also shed light on the poorly understood existence of multiple AI systems in a number of species. Originally, a constant, constitutive, noninduced AI production rate, almost identical for all cells, was assumed to allow for cell density estimation, requiring usually only one AI system. The efficiencies of different target behaviors depend on different, often independent pull aspects, varying over time and, in spatially structured environments, over space. The relevance of pull aspects can furthermore vary between different subpopulations (phenotypes). Multiple AI systems allow for the independent transportation of such different pull information. Consequently, AI production rates should vary over time, independently for each AI produced by a cell, as a function of different environmental and/or cellular conditions. In fact, there are only a few studies that have analyzed the production rates of multiple AIs over time (e.g., see references 124 and 137) under batch culture conditions. In V. harveyi, the growth phase-dependent production of different AIs was reflected by the corresponding expression of different AI-regulated genes (124). The authors of that study and others suggested that multiple AI systems enable the cells to appropriately time target gene expression (68, 138). However, the sequential gene expression observed in batch cultures, where conditions monotonously change over time, may not be the desired purpose in the natural environment. Here, adaptation to more time-constant or oscillating conditions, such as nutrient delivery and waste removal by flow, may be more desirable. Therefore, under these conditions, adaptation to a particular condition and achievement of a specific steady state are more important than regulating the timing of expression. Additionally, the temporal pattern of target gene expression in planktonic batch culture experiments, which depend on gradients of AIs over time and of factors influencing the AI system as nutrients, can be expected to be partly reflected by the corresponding spatial patterns in natural biofilms.
As noted above, the integration of the cell's demand for a regulated activity within the AI system can be realized on the signal-sending side (e.g., by regulating AI synthases) or on the receiver side (e.g., by regulating the AI receptor). Usually, both are coupled via the autoregulation of the AI gene, but there are exceptions without such feedback. Especially in the latter case, regulation on the receiver side allows the cell to adapt its response on the population's call for cooperation, as transferred by the environmental AI concentration, to its own specific “willingness” to contribute, which also belongs to the pull factors. This is of specific interest when several cellular activities are regulated by an AI. Depending on its specific conditions, the willingness of a cell to contribute may vary independently between these activities. The existence of “orphan” AI receptors, i.e., the presence of several receptors for a single AI, can (partly) be explained by this. In fact, in the case of L. pneumophila mentioned above, the two receptors for the lqs system, LqsS and LqsT, display differential expression patterns during exponential growth (114). Interestingly, 90% of the 105 genes downregulated in lqsT mutants are upregulated in lqsS mutants. Thus, LqsS and LqsT seem to regulate a set of genes related to growth phase reciprocally, but both receptors depend on the same AI, 3-hydroxypentadecane-4-one.
Different AI-regulated target behaviors may even exclude each other. In B. subtilis, both competence and sporulation are AI mediated, although they cannot work simultaneously in the same cell (29, 30). Moreover, each sporulating cell decreases the benefit of other cells that develop competence. Thus, there is a conflict of both activities in the cell and the population.
In summary, due to the pull aspect, multiple AIs enable the use of different “words” by bacteria, which can be understood individually or in combination. Theoretically, the content of push information could also vary between different AIs; e.g., if dependent on molecule size or hydrophilic properties, information about different aspects of mass transfer properties of the surrounding environment are transported, which may be relevant for different public goods. Very recent experimental evidence supports this notion, suggesting that under certain conditions, a combination of multiple AI systems in P. aeruginosa could be employed to discriminate between different push aspects (46). However, the differential regulation of these AI systems by nutrient conditions indicates the additional relevance of pull factors (125).
WHO COMMUNICATES WITH WHOM?
It has been assumed that AIs are employed for communication in a given population; i.e., all cells within it “talk” and “listen” simultaneously in the same way. Note that we focus on intraspecies communication here; the multitude of ecologically relevant interspecies interactions via extracellular substances is beyond the scope of this article, as they differ significantly from intraspecies communication with respect to evolutionary and ecological consequences. Rigorous investigations of whether interspecies communication fits the definition of “signaling” as discussed above are often missing and in fact difficult to conduct (22). Strictly speaking, even the concept of “autoinduction” is ill suited here. The reader is referred to, e.g., work by Atkinson and Williams (7) for further reading on this subject.
The original concept has now been refined significantly. As discussed above, analysis of gene expression in AI systems at the single-cell level, primarily by using fluorescence markers, demonstrates the existence of heterogeneity for genes of AI systems as well as AI-regulated genes in planktonic studies with a mono- or multimodal distribution (e.g., see references 17 and 139 to 141). Typically, in induced populations, there is also a fraction of noncontributing (nonfluorescing) cells, which obviously does not or only weakly participates in the coordinated behavior. Furthermore, spatially structured populations in microcolonies or biofilms have physicochemical gradients, which cause spatially asymmetric communication. Until now, there have been no comprehensive studies simultaneously investigating single-cell activities for AI production, AI sensing, and target gene expression; thus, we do not understand the mechanisms and functions behind the development of heterogeneity in depth. However, in many cases it is evident that not all cells within a population communicate with and/or listen to each other in a symmetric way. Division of labor may thus be an underestimated phenomenon, although we cannot exclude the possibility that molecular noise and stochasticity also have a role.
In the context of social evolution, one process that stabilizes cooperation is assortment, the preferential interaction of identical individuals to avoid the displacement of cooperative cells by noncooperative cheater mutants (142–144). In microbes, colony growth is a form of assortment, because a single cell or a few founding cells lead to the formation of clonal groups. For the long-term maintenance of cooperation, repeated cycles of colony dispersal, mixing, and regrowth constitute an important mechanism (145). As a consequence, communication and regulated cooperation should primarily occur within rather than between colonies. This bias indeed received some experimental support (34). Unfortunately, in-depth data specifically designed to analyze the question of who talks to whom at the colony level are still missing.
We suggest that communication occurs locally rather than globally. Measurement of AI concentrations in relatively large samples (milliliter volumes) of spatially structured populations in spatially structured environments (e.g., soil, cystic fibrosis [CF] lung, and gut) averages spatially heterogeneous concentrations and thus underestimates the local concentration to which cells might be exposed (see, e.g., reference 146). This has consequences for the estimation of the relevance of signaling within the population but also for the impact of AIs on other bacteria or host cells. This may (partly) explain the conflicting findings that AIs are central regulators of virulence in most pathogens and that some AIs directly affect host cells in vitro but that most studies detect little or no AI in samples of lung, sputum, gut, and others (146). Mixing events before or during sampling may further destroy spatial structure. Spatially and temporally resolved measurement of AI concentrations and activity patterns under less disturbed, close-to-in situ conditions is therefore highly desirable but requires new technologies (147).
The assortment concept mentioned above challenges the evolutionary stability of intercolony communication, i.e., the cooperation between subgroups of the same species but of uncertain genetic kinship. This raises interesting questions about the role of extracellular AI-degrading (quorum-quenching [QQ]) enzymes in AI-producing species, which are able to degrade their own AI (148, 149). QQ enzymes have been interpreted mainly in terms of disturbing AI systems of other species, limiting or terminating cooperation activities in certain growth states, or using AIs just as nutrients (149, 150). However, the efficiency of extracellular QQ enzymes for these purposes has yet to be proven in situ. Alternatively (or additionally), extracellular QQ enzymes could serve to suppress intercolony communication or to suppress the AI-regulated target genes in neighboring colonies of the same species. Such a behavior is contrary to the originally assumed population-wide communication and cooperation. Thus, a neighboring colony would not necessarily be a partner but would primarily be a competitor for resources.
CONCLUSIONS
It is clear that the original idea of AI systems as cell density-dependent triggers that enable uniform, synchronous behavior requires refinement. Based on our considerations in the sections above, we can define properties common to all AI systems, allowing for a deeper understanding of the ecological and evolutionary functions.
Mechanistically, AI systems govern the release of diffusible substances into the environment, the measurement of their concentration by the releasing cells, and a targeted response via changes in gene expression. This behavior is cooperative in nature and is clearly separate from eavesdropping, coercion, and paracrine signaling. The latter may exploit the same molecules, but the sender and receiver are different. Furthermore, these behaviors have completely different ecological functionalities and evolutionary properties (23). Although the specific ecological purpose of AIs varies depending on the specific system, AI systems generally allow for an estimation of the efficiency of a regulated target activity in the context of the specific conditions to which the cells are exposed. The AI system integrates push and pull information relevant to the regulated activities, thereby enabling robust decision-making.
AI systems acquire information about the physical environment, specifically mass transfer properties, enabling isolated cells as well as groups of cells to optimize their behavior with respect to this environment. Simultaneously, AI systems collect information about the presence, distribution, and demand of cells in a population, enabling a coordinated, cooperative behavior within cell (sub)populations but not necessarily connected with synchronicity and uniformity of the reaction. We suggest that AI systems at least predominantly regulate cooperative behaviors (or, more generally, life-styles) and that the fitness benefit of these behaviors is affected by all aspects integrated into the signal information in a correlated way. This, for example, applies to the release of public goods (47) but also to a number of other regulation targets, as discussed above. Taken together, AI regulation is generally connected with cooperativity and coordination within (sub)populations but may also regulate the behavior of isolated cells.
According to the definition of AI systems by such common principles, their spread over a wide taxonomic range, including fungi, plants (algae), animals, and possibly even viruses, where host cell lysis may depend on the virus concentration, is not surprising (151–159). AI systems evolved several times independently. This is plausible given that species release a plethora of substances into the environment. In its most basic form, just the measurement (sensing) of these released substances is sufficient to be considered a rudimentary “AI system.” Thus, it is not so much the wide distribution of AI systems but rather the lack of their description in some taxa such as archaea that seems surprising and may be the result of biased research (12).
Taken together, we have made an attempt in this review to identify principles common to bacterial AI systems, primarily from an ecological point of view. We have argued that such core principles include (i) the regulation of behaviors that are largely cooperative in nature, (ii) assessment of their efficiency by integrating push and pull factors, and (iii) mechanisms of homeostatic control.
ACKNOWLEDGMENTS
We have no financial interests or support from institutions or companies mentioned in the manuscript.
Work in this review was supported by a grant from the National Science Foundation (MCB1158553 to M.S.), by a fellowship from the Alexander von Humboldt Foundation (to M.S.), and by the GSF-National Research Center for Environment and Health supporting the project network Molecular Interactions in the Rhizosphere (to B.A.H.).
Biographies

Burkhard A. Hense was trained as an experimental biologist (microbiology) at the Ruhr University Bochum (Germany) and moved for his Ph.D. thesis to the Institute of Ecological Chemistry at the Technical University Munich and to the GSF-National Research Center for Environment and Health, both in Munich, Germany. Subsequently, his focus changed to mathematical modeling of biological processes, working in close cooperation with experimentalists. He is now head of the Research Group on Interactions in Biological Systems at the Institute for Computational Biology in Helmholtz Zentrum München, Germany. The research group is interested in analyzing the functional and evolutionary principles of interacting biological systems. Control of bacterial behavior, especially via cell-cell communication (quorum sensing), has been his major scientific field for 9 years.

Martin Schuster studied biology and chemistry at the University of Göttingen in Germany before coming to the United States as an exchange student. His dissertation work was in bacterial chemotaxis at the University of North Carolina (with Bob Bourret). His postdoctoral training was in quorum sensing at the University of Iowa and the University of Washington (with Pete Greenberg). In 2006, he joined the faculty at Oregon State University, where he currently is an Associate Professor in the Department of Microbiology. His lab is interested in the mechanisms and evolution of bacterial communication and cooperation. During the 2013-2014 academic year, he was an Alexander von Humboldt Sabbatical Fellow at the Max-Planck Institute Magdeburg and the Helmholtz Zentrum München (Germany), conducting collaborative research in systems biology.
REFERENCES
- 1.Antunes LCM, Ferreira RBR, Buckner MMC, Finlay BB. 2010. Quorum sensing in bacterial virulence. Microbiology 156:2271–2282. doi: 10.1099/mic.0.038794-0. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford ST, Bassler BL. 2012. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Clippeleir H, Defoirdt T, Vanhaecke L, Vlaeminck SE, Carballa M, Verstraete W, Bonn N. 2011. Long-chain acylhomoserine lactones increase the annoxic ammonium oxidation rate in an OLAND biofilm. Appl Microbiol Biotechnol 90:1511–1519. doi: 10.1007/s00253-011-3177-7. [DOI] [PubMed] [Google Scholar]
- 4.Shrout JD, Nerenberg R. 2012. Monitoring bacterial twitter: does quorum sensing determine the behaviour of water and wastewater treatment biofilms? Environ Sci Technol 46:1995–2005. doi: 10.1021/es203933h. [DOI] [PubMed] [Google Scholar]
- 5.Valle A, Bailey MJ, Whiteley AS, Manefield M. 2004. N-Acyl-L-homoserine lactones (AHLs) affect microbial community composition and function in activated sludge. Environ Microbiol 6:424–433. doi: 10.1111/j.1462-2920.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 6.Dobretsov S, Teplitski M, Paul V. 2009. Mini-review: quorum sensing in the marine environment and its relationship to biofouling. Biofouling 25:413–427. doi: 10.1080/08927010902853516. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson S, Williams P. 2009. Quorum sensing and social networking in the microbial world. J R Soc Interface 6:959–978. doi: 10.1098/rsif.2009.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng WL, Bassler BL. 2009. Quorum sensing network architectures. Annu Rev Genet 43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mashburn LM, Whiteley M. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 10.Sharif DI, Gallon J, Smith CJ, Dudley E. 2008. Quorum sensing in Cyanobacteria: N-octanoyl-homoserine lactone release and response, by the epilithic colonial cyanobacterium Gloeothece PCC6909. ISME J 2:1171–1182. doi: 10.1038/ismej.2008.68. [DOI] [PubMed] [Google Scholar]
- 11.Huang YL, Ki JS, Case RJ, Quian PY. 2008. Diversity and acyl-homoserine lactone production among subtidal biofilm-forming bacteria. Aquat Microb Ecol 52:185–193. doi: 10.3354/ame01215. [DOI] [Google Scholar]
- 12.Zhang G, Zhang F, Ding G, Li J, Guo X, Zhu J, Zhou L, Cai S, Liu X, Luo Y, Zhang Y, Shi W, Dong X. 2012. Acyl homoserine lactone-based quorum sensing in a methanogenic archaeon. ISME J 6:1336–1344. doi: 10.1038/ismej.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Kolodkin-Gal I, Engelberg-Kulka H. 2013. Novel quorum-sensing peptides mediating interspecies bacterial cell death. mBio 4(3):e00314-13. doi: 10.1128/mBio.00314-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thoendel M, Horswill AR. 2010. Biosynthesis of peptide signals in gram-positive bacteria. Adv Appl Microbiol 71:91–112. doi: 10.1016/S0065-2164(10)71004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson JP, Van Delden C, Iglewski BH. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol 181:1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goryachev AB. 2009. Design principles of the bacterial quorum sensing network. WIREs Syst Biol Med 1:45–60. doi: 10.1002/wsbm.27. [DOI] [PubMed] [Google Scholar]
- 17.Williams JW, Cui X, Levchenko A, Stevens AM. 2008. Robust and sensitive control of a quorum-sensing circuit by two interlocked feedback loops. Mol Syst Biol 4:234. doi: 10.1038/msb.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haseltine EL, Arnold FH. 2008. Implications of rewiring bacterial quorum sensing. Appl Environ Microbiol 74:437–445. doi: 10.1128/AEM.01688-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anetzberger C, Pirch T, Jung K. 2009. Heterogeneity in quorum sensing-regulated bioluminescence in Vibrio harveyi. Mol Microbiol 73:267–277. doi: 10.1111/j.1365-2958.2009.06768.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsai CS, Winans SC. 2010. LuxR-type quorum-sensing regulators that are detached from common scents. Mol Microbiol 77:1072–1082. doi: 10.1111/j.1365-2958.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulitzur S. 1989. The regulatory control of the bacterial luminescence system—a new view. J Biolumin Chemilumin 4:317–325. doi: 10.1002/bio.1170040144. [DOI] [PubMed] [Google Scholar]
- 22.Winzer K, Hardie KR, Williams P. 2002. Bacterial cell-to-cell communication: sorry, can't talk now—gone to lunch! Curr Opin Microbiol 5:216–222. doi: 10.1016/S1369-5274(02)00304-1. [DOI] [PubMed] [Google Scholar]
- 23.Diggle SP, Gardner A, West S, Griffin AS. 2007. Evolutionary theory about bacterial quorum sensing: when is a signal not a signal? Philos Trans R Soc Lond B Biol Sci 362:1241–1249. doi: 10.1098/rstb.2007.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen RC, Popat R, Diggle SP, Brown SP. 2014. Targeting virulence: can we make evolution-proof drugs? Nat Rev Microbiol 12:300–308. doi: 10.1038/nrmicro3232. [DOI] [PubMed] [Google Scholar]
- 25.Rezzonico F, Duffy B. 2008. Lack of genomic evidence of AI-2 receptors suggests a non-quorum sensing role for luxS in most bacteria. BMC Microbiol 8:154. doi: 10.1186/1471-2180-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezzonico F, Smits TH, Duffy B. 2012. Detection of AI-2 receptors in genomes of Enterobacteriaceae suggests a role of type-2 quorum sensing in closed ecosystems. Sensors 12:6645–6665. doi: 10.3390/s120506645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nealson KH, Platt T, Hastings JW. 1970. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol 104:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nealson KH, Hastings JW. 1979. Bacterial bioluminescence: its control and ecological significance. Microbiol Rev 43:496–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez D, Vlamakis H, Losick R, Kolter R. 2009. Paracrine signaling in a bacterium. Genes Dev 23:1631–1638. doi: 10.1101/gad.1813709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López D, Kolter R. 2010. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol Rev 34:134–149. doi: 10.1111/j.1574-6976.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 31.Platt TG, Fuqua C. 2010. What's in a name? The semantics of quorum sensing. Trends Microbiol 18:383–387. doi: 10.1016/j.tim.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass L, Tobler WR. 1971. Uniform distribution of objects in a homogeneous field: cities on a plain. Nature 233:67–68. doi: 10.1038/233067a0. [DOI] [PubMed] [Google Scholar]
- 33.Dulla G, Lindow SE. 2008. Quorum size of Pseudomonas syringae is small and dictated by water availability on the leaf surface. Proc Natl Acad Sci U S A 105:3082–3087. doi: 10.1073/pnas.0711723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer A, Megerle JA, Kuttler C, Müller J, Aguilar C, Eberl L, Hense BA, Rädler JO. 2012. Dynamics of AHL mediated quorum sensing under flow and non-flow conditions. Phys Biol 9:026007. doi: 10.1088/1478-3975/9/2/026007. [DOI] [PubMed] [Google Scholar]
- 35.Alberghini S, Polone E, Corich V, Carlot M, Seno F, Trovato A, Squartini A. 2009. Consequences of relative cellular positioning on quorum sensing and bacterial cell-to-cell communication. FEMS Microbiol Lett 292:149–161. doi: 10.1111/j.1574-6968.2008.01478.x. [DOI] [PubMed] [Google Scholar]
- 36.Barak M, Ulitzur S. 1981. The induction of bacterial bioluminescence system on solid medium. Curr Microbiol 5:299–301. doi: 10.1007/BF01567922. [DOI] [Google Scholar]
- 37.Lui LT, Xue X, Sui C, Brown A, Pritchard DI, Halliday N, Winzer K, Howdle SM, Fernandez-Trillo F, Krasnogor N, Alexander C. 2013. Bacteria clustering by polymers induces the expression of quorum-sensing-controlled phenotypes. Nat Chem 5:1058–1065. doi: 10.1038/nchem.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirisits MJ, Margolis JJ, Purevdorj-Gage BL, Vaughan B, Chopp DL, Stoodley P, Parsek MR. 2007. Influence of the hydrodynamic environment on quorum sensing in Pseudomonas aeruginosa biofilms. J Bacteriol 189:8357–8360. doi: 10.1128/JB.01040-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughan BL Jr, Smith BG, Chopp DL. 2010. The influence of fluid flow on modeling quorum sensing in bacterial biofilms. Bull Math Biol 72:1143–1165. doi: 10.1007/s11538-009-9485-8. [DOI] [PubMed] [Google Scholar]
- 40.Timp W, Mirsaidov U, Matsudaira P, Timp G. 2009. Jamming prokaryotic cell-to-cell communications in a model biofilm. Lab Chip 9:925–934. doi: 10.1039/b810157d. [DOI] [PubMed] [Google Scholar]
- 41.Carnes EC, Lopez DM, Donegan NP, Cheung A, Gresham H, Timmins GS, Brinker CJ. 2010. Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat Chem Biol 6:41–45. doi: 10.1038/nchembio.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boedicker JQ, Vincent ME, Ismagilov RF. 2009. Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angew Chem Int Ed Engl 48:5908–5911. doi: 10.1002/anie.200901550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, Taylor RM, Superfine R, Rubinstein M, Iglewski BH, Boucher RC. 2006. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A 103:18131–18136. doi: 10.1073/pnas.0606428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flickinger ST, Copeland MF, Downes EM, Braasch AT, Tuson HH, Eun YJ, Weibel DB. 2011. Quorum sensing between Pseudomonas aeruginosa biofilms accelerates cell growth. J Am Chem Soc 133:5966–5975. doi: 10.1021/ja111131f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connell JL, Wessel AK, Parsek MR, Ellington AD, Whiteley M, Shear JB. 2010. Probing prokaryotic social behaviors with bacterial “lobster traps.” mBio 1(4):e00202-10. doi: 10.1128/mBio.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cornforth DM, Popat R, McNally L, Gurney J, Scott-Phillips TC, Ivens A, Diggle SP, Brown SP. 2014. Combinatorial quorum sensing allows bacteria to resolve their social and physical environment. Proc Natl Acad Sci U S A 111:4280–4284. doi: 10.1073/pnas.1319175111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hense BA, Kuttler C, Müller J, Rothballer M, Hartmann A, Kreft J-U. 2007. Does efficiency sensing unify diffusion and quorum sensing? Nat Rev Microbiol 5:230–239. doi: 10.1038/nrmicro1600. [DOI] [PubMed] [Google Scholar]
- 48.West SA, Winzer K, Gardner A, Diggle SP. 2012. Quorum sensing and the confusion about diffusion. Trends Microbiol 20:586–594. doi: 10.1016/j.tim.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogan AP, Dickerson TJ, Janda KD. 2005. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci U S A 102:309–314. doi: 10.1073/pnas.0408639102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dufour A, Hindré T, Haras D, Le Pennec JP. 2007. The biology of lantibiotics from the lacticin 481 group is coming of age. FEMS Microbiol Rev 31:134–167. doi: 10.1111/j.1574-6976.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 51.Turovskiy Y, Kashtanov D, Paskhover B, Chikindas ML. 2007. Quorum sensing: fact, fiction, and everything in between. Adv Appl Microbiol 62:191–234. doi: 10.1016/S0065-2164(07)62007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KM. 2005. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol 3:383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 53.Lerat E, Moran NA. 2004. The evolutionary history of quorum-sensing systems in bacteria. Mol Biol Evol 21:903–913. doi: 10.1093/molbev/msh097. [DOI] [PubMed] [Google Scholar]
- 54.Ruby EG, Lee KH. 1998. The Vibrio fischeri-Euprymna scolopes light organ association: current ecological paradigms. Appl Environ Microbiol 64:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Englmann M, Fekete A, Kuttler C, Frommberger M, Li X, Gebefügi I, Schmitt-Kopplin P. 2007. The hydrolysis of unsubstituted N-acylhomoserine lactones to their homoserine metabolites; analytical approaches using ultra performance liquid chromatography. J Chromotogr A 1160:184–193. doi: 10.1016/j.chroma.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 56.Schaefer AL, Hanzelka BL, Parsek R, Greenberg EP. 2000. Detection, purification, and structural elucidation of the acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol 305:288–301. doi: 10.1016/S0076-6879(00)05495-1. [DOI] [PubMed] [Google Scholar]
- 57.Yates EA, Philipp B, Buckley C, Atkinson S, Chhabra SR, Sockett RE, Goldner M, Dessaux Y, Cámara M, Smith H, Williams P. 2002. N-Acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect Immun 70:5635–5646. doi: 10.1128/IAI.70.10.5635-5646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Decho AW, Visscher PT, Ferry J, Kawaguchi T, He L, Przekop KM, Norma RS, Reid RP. 2009. Autoinducers extracted from microbial mats reveal a surprising diversity of N-acylhomoserine lactones (AHLs) and abundance changes that may relate to diel pH. Environ Microbiol 11:409–420. doi: 10.1111/j.1462-2920.2008.01780.x. [DOI] [PubMed] [Google Scholar]
- 59.Timmins GS, Jackson SK, Swartz HM. 2001. The evolution of bioluminescent oxygen consumption as an ancient oxygen detoxification mechanism. J Mol Evol 52:321–332. [DOI] [PubMed] [Google Scholar]
- 60.West SA, Griffin AS, Gardner A. 2008. Social semantics: how useful has group selection been? J Evol Biol 21:374–385. doi: 10.1111/j.1420-9101.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 61.Hassett DJ, Ma JF, Elkins JG, Ochsner UA, West SEH, Huang C-T. 1999. Control of catalase and superoxide dismutase by quorum sensing in Pseudomonas aeruginosa: catalase is important in resistance of biofilm organisms to hydrogen peroxide. Mol Microbiol 34:1082–1093. doi: 10.1046/j.1365-2958.1999.01672.x. [DOI] [PubMed] [Google Scholar]
- 62.Pontes MH, Babst MH, Lochhead R, Oakeson K, Smith K, Dale C. 2008. Quorum sensing primes the oxidative stress response in the insect endosymbiont, Sodalis glossinidius. PLoS One 3:e3541. doi: 10.1371/journal.pone.0003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pierson LS III, Pierson EA. 2010. Metabolism and function of phenazines in bacteria: impacts on the behaviour of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol 86:1659–1670. doi: 10.1007/s00253-010-2509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Studer SV, Mandel MJ, Ruby EG. 2008. AinS quorum sensing regulates the Vibrio fischeri acetate switch. J Bacteriol 190:5915–5923. doi: 10.1128/JB.00148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Houdt R, Moons P, Buj MH, Michiels CW. 2006. N-Acyl-L-homoserine lactone quorum sensing controls butanediol fermentation in Serratia plymuthica RVH1 and Serratia marcenses MG1. J Bacteriol 188:4570–4572. doi: 10.1128/JB.00144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goo E, Majerczyk CD, An JH, Chandler JR, Seo YS, Ham H, Lim JY, Kim H, Lee B, Jang MS, Greenberg EP, Hwang I. 2012. Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc Natl Acad Sci U S A 109:19775–19780. doi: 10.1073/pnas.1218092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suppiger A, Schmid N, Aguilar C, Pessi G, Eberl L. 2013. Two quorum sensing systems control biofilm formation and virulence in members of the Burkholderia cepacia complex. Virulence 4:400–409. doi: 10.4161/viru.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specifity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willcox MDP, Zhu H, Conibear TCR, Hume EBH, Givskov M, Kjelleberg S, Rice SA. 2008. Role of quorum sensing by Pseudomonas aeruginosa in microbial keratitis and cystic fibrosis. Microbiology 154:2184–2194. doi: 10.1099/mic.0.2008/019281-0. [DOI] [PubMed] [Google Scholar]
- 70.Coggan KA, Wolfgang MC. 2012. Global regulatory pathways and cross-talk control Pseudomonas aeruginosa environmental lifestyle and virulence phenotype. Curr Issues Mol Biol 14:47–69. [PubMed] [Google Scholar]
- 71.Ramachandran R, Burke AK, Cormier G, Jensen RV, Stevens AM. 2014. Transcriptome-based analysis of the Pantoea stewartii quorum-sensing regulon and identification of EsaR direct targets. Appl Environ Microbiol 80:5790–5800. doi: 10.1128/AEM.01489-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Majerczyk C, Brittnacher M, Jacobs M, Armour CD, Radey M, Schneider E, Phattarasokul S, Bunt R, Greenberg EP. 2014. Global analysis of the Burkholderia thailandensis quorum sensing-controlled regulon. J Bacteriol 196:1412–1424. doi: 10.1128/JB.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kolodkin-Gal I, Sat B, Keshet A, Engelberg-Kulka H. 2008. The communication factor EDF and the toxin-antitoxin module mazEF determine the mode of action of antibiotics. PLoS Biol 6:e319. doi: 10.1371/journal.pbio.0060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song ZJ, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Hoiby N, Givskov M. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gilbert KG, Kim TH, Gupta R, Greenberg EP, Schuster M. 2009. Global position analysis of the Pseudomonas aeruginosa quorum-sensing transcription factor LasR. Mol Microbiol 73:1072–1085. doi: 10.1111/j.1365-2958.2009.06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 79.Dubern JF, Lugtenberg BJJ, Bloemberg GV. 2006. The ppuI-rsaL-ppuR quorum-sensing system regulates biofilm formation of Pseudomonas putida PCL1445 by controlling biosynthesis of the cyclic lipopeptides putisolvins I and II. J Bacteriol 188:2898–2906. doi: 10.1128/JB.188.8.2898-2906.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaplan JB. 2010. Biofilm dispersal: mechanisms, clinical implications, potential therapeutic uses. J Dent Res 89:205–218. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yarwood JM, Bartels DJ, Volper EM, Greenberg EP. 2004. Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol 186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boles BR, Thoendel M, Singh PK. 2005. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol Microbiol 57:1210–1223. doi: 10.1111/j.1365-2958.2005.04743.x. [DOI] [PubMed] [Google Scholar]
- 83.Karatan E, Watnick P. 2009. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73:310–347. doi: 10.1128/MMBR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Periasamy S, Joo HS, Duong AC, Bach THL, Tan VY, Chatterjee SS, Cheung GYC, Otto M. 2012. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A 109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gjermansen M, Nilsson M, Yang L, Tolker-Nielsen T. 2010. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms: genetic elements and molecular mechanisms. Mol Microbiol 75:815–826. doi: 10.1111/j.1365-2958.2009.06793.x. [DOI] [PubMed] [Google Scholar]
- 86.Hamilton WD. 1964. The genetical evolution of social behaviour. I and II. J Theor Biol 7:1–52. [DOI] [PubMed] [Google Scholar]
- 87.Hamilton WD, May RM. 1977. Dispersal in stable habitats. Nature 269:578–581. doi: 10.1038/269578a0. [DOI] [Google Scholar]
- 88.Shompole S, Henon KT, Liou LE, Dziewanowska K, Bohach GA, Bayles KW. 2003. Biphasic intracellular expression of Staphylococcus aureus virulence factors and evidence for Agr-mediated diffusion sensing. Mol Microbiol 49:919–927. doi: 10.1046/j.1365-2958.2003.03618.x. [DOI] [PubMed] [Google Scholar]
- 89.Redfield R. 2002. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol 10:365–370. doi: 10.1016/S0966-842X(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 90.Lenz A, Williamson K, Pitts B, Stewart P, Franklin M. 2008. Localized gene expression in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 74:4463–4471. doi: 10.1128/AEM.00710-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heurlier K, Denervaud V, Haas D. 2006. Impact of quorum sensing on fitness of Pseudomonas aeruginosa. Int J Med Microbiol 296:93–102. doi: 10.1016/j.ijmm.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 92.Darch SE, West SA, Winzer K, Diggle SP. 2012. Density-dependent fitness benefits in quorum-sensing bacterial populations. Proc Natl Acad Sci U S A 109:8259–8263. doi: 10.1073/pnas.1118131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mellbye B, Schuster M. 2011. The sociomicrobiology of antivirulence drug resistance: a proof of concept. mBio 2(5):e00131-11. doi: 10.1128/mBio.00131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dandekar AA, Chugani S, Greenberg EP. 2012. Bacterial quorum sensing and metabolic incentives to cooperate. Science 338:264–266. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foster KR, Shaulsky G, Strassmann JE, Queller DC, Thompson CRL. 2004. Pleiotropy as a mechanism to stabilize cooperation. Nature 431:693–696. doi: 10.1038/nature02894. [DOI] [PubMed] [Google Scholar]
- 96.Rinas U, Hoffmann F. 2004. Selective leakage of host-cell proteins during high-cell-density cultivation of recombinant and non-recombinant Escherichia coli. Biotechnol Prog 20:679–687. doi: 10.1021/bp034348k. [DOI] [PubMed] [Google Scholar]
- 97.Cornforth DM, Sumpter DJ, Brown SP, Brannstrom A. 2012. Synergy and group size in microbial cooperation. Am Nat 180:296–305. doi: 10.1086/667193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pai A, Tanouchi Y, You L. 2012. Optimality and robustness in quorum sensing (QS)-mediated regulation of a costly public good enzyme. Proc Natl Acad Sci U S A 109:19810–19815. doi: 10.1073/pnas.1211072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Kievit T, Seed PC, Nezezon J, Passador L, Iglewski BH. 1999. RsaL, a novel repressor of virulence gene expression in Pseudomonas aeruginosa. J Bacteriol 181:2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rampioni G, Bertani I, Zennaro E, Polticelli F, Venturi V, Leoni L. 2006. The quorum-sensing negative regulator RsaL of Pseudomonas aeruginosa binds to the lasI promoter. J Bacteriol 188:815–819. doi: 10.1128/JB.188.2.815-819.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chugani SA, Whiteley M, Lee KM, D'Argenio D, Manoil C, Greenberg EP. 2001. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 98:2752–2757. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siehnel R, Traxler B, An DD, Parsek MR, Schaefer AL, Singh PK. 2010. A unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 107:7916–7921. doi: 10.1073/pnas.0908511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupta R, Schuster M. 2013. Negative regulation of bacterial quorum sensing tunes public goods cooperation. ISME J 7:2159–2168. doi: 10.1038/ismej.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee JH, Lequette Y, Greenberg EP. 2006. Activity of purified QscR, a Pseudomonas aeruginosa orphan quorum-sensing transcription factor. Mol Microbiol 59:602–609. doi: 10.1111/j.1365-2958.2005.04960.x. [DOI] [PubMed] [Google Scholar]
- 105.Fekete A, Kuttler C, Rothballer M, Hense BA, Fischer D, Buddrus-Schiemann K, Lucio M, Müller J, Schmitt-Kopplin P, Hartmann A. 2010. Dynamic regulation of N-acyl-homoserine lactone production and degradation in Pseudomonas putida IsoF. FEMS Microbiol Ecol 72:22–34. doi: 10.1111/j.1574-6941.2009.00828.x. [DOI] [PubMed] [Google Scholar]
- 106.Shadel GS, Baldwin TO. 1991. The Vibrio fischeri LuxR protein is capable of bidirectional stimulation of transcription and both positive and negative regulation of the luxR gene. J Bacteriol 173:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McIntosh M, Meyer S, Becker A. 2009. Novel Sinorhizobium meliloti quorum sensing positive and negative regulatory feedback mechanisms respond to phosphate availability. Mol Microbiol 74:1238–1256. doi: 10.1111/j.1365-2958.2009.06930.x. [DOI] [PubMed] [Google Scholar]
- 108.Hazan R, He J, Xiao G, Dekimpe V, Apidianakis Y, Lesic B, Astrakas C, Deziel E, Lepine F, Rahme LG. 2010. Homeostatic interplay between bacterial cell-cell signaling and iron in virulence. PLoS Pathog 6:e1000810. doi: 10.1371/journal.ppat.1000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shadel GS, Baldwin TO. 1992. Identification of a distantly located regulatory element in the luxD gene required for negative autoregulation of the Vibrio fischeri luxR gene. J Biol Chem 267:7690–7695. [PubMed] [Google Scholar]
- 110.Rampioni G, Schuster M, Greenberg EP, Bertani I, Grasso M, Venturi V, Zennaro E, Leoni L. 2007. RsaL provides quorum sensing homeostasis and functions as a global regulator of gene expression in Pseudomonas aeruginosa. Mol Microbiol 66:1557–1565. doi: 10.1111/j.1365-2958.2007.06029.x. [DOI] [PubMed] [Google Scholar]
- 111.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. 2012. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jimenez PN, Koch G, Papaioannou E, Wahjudi M, Krzeslak J, Coenye T, Cool RH, Quax WJ. 2010. Role of PvdQ in Pseudomonas aeruginosa virulence under iron-limiting conditions. Microbiology 156:49–59. doi: 10.1099/mic.0.030973-0. [DOI] [PubMed] [Google Scholar]
- 113.Lopez D, Vlamakis H, Kolter R. 2009. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol Rev 33:152–163. doi: 10.1111/j.1574-6976.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 114.Kessler A, Schell U, Sahr T, Tiaden A, Harrison C, Buchrieser C, Hilbi H. 2013. The Legionella pneumophila orphan sensor kinase LqsT regulates competence and pathogen-host interactions as a component of the LAI-1 circuit. Environ Microbiol 15:646–662. doi: 10.1111/j.1462-2920.2012.02889.x. [DOI] [PubMed] [Google Scholar]
- 115.Courchamp F, Clutton-Brock T, Grenfell B. 1999. Inverse density dependence and the Allee effect. Trends Ecol Evol 14:405–410. doi: 10.1016/S0169-5347(99)01683-3. [DOI] [PubMed] [Google Scholar]
- 116.Smith R, Tan C, Srimani JK, Pai A, Riccione KA, Song H, You L. 2014. Programmed Allee effect in bacteria causes a tradeoff between population spread and survival. Proc Natl Acad Sci U S A 111:1969–1974. doi: 10.1073/pnas.1315954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Montaudouin C, Anson M, Hao Y, Duncker SV, Fernandez T, Gaudin E, Ehrenstein M, Kerr WG, Colle JH, Bruhns P, Daëron M, Freitas AA. 2013. Quorum sensing contributes to activated IgM-secreting B cell homeostasis. J Immunol 190:106–114. doi: 10.4049/jimmunol.1200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Almeida ARM, Amado IF, Reynolds J, Berges J, Lythe G, Molina-París C, Freitas AA. 2012. Quorum-sensing in CD4+ T cell homeostasis: a hypothesis and a model. Front Immunol 3:125. doi: 10.3389/fimmu.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burroughs NJ, Oliveira BMPM, Pinto AA, Ferreira M. 2011. Immune response dynamics. Math Comput Model 53:1410–1419. doi: 10.1016/j.mcm.2010.02.040. [DOI] [Google Scholar]
- 120.Amado IF, Berges J, Luther RJ, Mailhé MP, Garcia S, Bandeira A, Weaver C, Liston A, Freitas AA. 2013. IL-2 coordinates IL-2-producing and regulatory T cell interplay. J Exp Med 210:2707–2720. doi: 10.1084/jem.20122759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Agur Z, Kirnasovsky OU, Vasserman G, Tencer-Hershkowicz L, Kogan Y, Harrison H, Lamb R, Clarke RB. 2011. Dickkopf1 regulates fate decision and drives breast cancer stem cells to differentiation: an experimentally supported mathematical model. PLoS One 6:e24225. doi: 10.1371/journal.pone.0024225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goryachev A, Toh D, Wee K, Lee T, Zhang H, Zhang L. 2005. Transition to quorum sensing in an Agrobacterium population: a stochastic model. PLoS Comput Biol 1:e37. doi: 10.1371/journal.pcbi.0010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xavier KB, Bassler BL. 2003. LuxS quorum sensing: more than just a numbers game. Curr Opin Microbiol 6:191–197. doi: 10.1016/S1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 124.Anetzberger C, Reiger M, Fekete A, Schell U, Stambrau N, Plener L, Kopka J, Schmitt-Kopplin P, Hilbi H, Jung K. 2012. Autoinducers act as biological timers in Vibrio harveyi. PLoS One 7:e48310. doi: 10.1371/journal.pone.0048310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mellbye B, Schuster M. 2014. A physiological framework for the regulation of quorum-sensing-dependent public goods in Pseudomonas aeruginosa. J Bacteriol 196:1155–1164. doi: 10.1128/JB.01223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hense BA, Müller J, Kuttler C, Hartmann A. 2012. Spatial heterogeneity of autoinducer regulation systems. Sensors 12:4156–4171. doi: 10.3390/s120404156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van Delden C, Comte R, Bally M. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J Bacteriol 183:5376–6384. doi: 10.1128/JB.183.18.5376-5384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Newton J, Fray R. 2004. Integration of environmental and host-derived signals with quorum sensing during plant-microbe interactions. Cell Microbiol 6:213–224. doi: 10.1111/j.1462-5822.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 129.Hegde M, Wood T, Jayaraman A. 2009. The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway. Appl Microbiol Biotechnol 84:763–776. doi: 10.1007/s00253-009-2045-1. [DOI] [PubMed] [Google Scholar]
- 130.Hassan S, Mathesius U. 2012. The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J Exp Bot 63:3429–3444. doi: 10.1093/jxb/err430. [DOI] [PubMed] [Google Scholar]
- 131.Perry JA, Cvitkovitch DG, Levesque CM. 2009. Cell death in Streptococcus mutans biofilms: a link between CSP and extracellular DNA. FEMS Microbiol Lett 299:261–266. doi: 10.1111/j.1574-6968.2009.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jacob E, Shapira Y, Tauber A. 2006. Seeking the foundations of cognition in bacteria: from Schroedinger's negative entropy to latent information. Physica A 369:495–524. doi: 10.1016/j.physa.2005.05.096. [DOI] [Google Scholar]
- 133.Schaefer AL, Greenberg PE, Oliver CM, Oda Y, Huang JJ, Bittan-Banin G, Peres CM, Schmidt S, Juhaszova K, Sufrin JR, Harwood CS. 2008. A new class of homoserine lactone quorum-sensing signals. Nature 454:595–599. doi: 10.1038/nature07088. [DOI] [PubMed] [Google Scholar]
- 134.Cooley M, Chhabra SR, Williams P. 2008. N-Acylhomoserine lactone-mediated quorum sensing: a twist in the tail and a blow for host immunity. Chem Biol 15:1141–1147. doi: 10.1016/j.chembiol.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 135.Fritz G, Koller C, Burdack K, Tetsch L, Haneburger I, Jung K, Gerland U. 2009. Induction kinetics of a conditional pH stress response system in Escherichia coli. J Mol Biol 393:272–286. doi: 10.1016/j.jmb.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 136.Haneburger I, Fritz G, Jurkschat N, Tetsch L, Eichinger A, Skerra A, Gerland U, Jung K. 2012. Deactivation of the E. coli pH stress sensor CadC by cadaverine. J Mol Biol 424:15–27. doi: 10.1016/j.jmb.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 137.Blosser-Middleton R, Gray KM. 2001. Multiple N-acyl homoserine lactone signals of Rhizobium leguminosarum are synthesized in a distinct temporal pattern. J Bacteriol 183:6771–6777. doi: 10.1128/JB.183.23.6771-6777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lupp C, Ruby EG. 2005. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J Bacteriol 187:3620–3629. doi: 10.1128/JB.187.11.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garmyn D, Gal L, Briandet R, Guilbaud M, Lemaitre JP, Hartmann A, Piveteau P. 2011. Evidence of autoinduction heterogeneity via expression of the Agr system of Listeria monocytogenes at the single-cell level. Appl Environ Microbiol 77:6286–6289. doi: 10.1128/AEM.02891-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Perez PD, Hagen SJ. 2010. Heterogeneous response to a quorum-sensing signal in the luminescence of individual Vibrio fischeri. PLoS One 5:e15473. doi: 10.1371/journal.pone.0015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anetzberger C, Schell U, Jung K. 2012. Single cell analysis of Vibrio harveyi uncovers functional heterogeneity in response to quorum sensing signals. BMC Microbiol 12:209. doi: 10.1186/1471-2180-12-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chuang JS, Rivoire O, Leibler S. 2009. Simpson's paradox in a synthetic microbial system. Science 323:272–275. doi: 10.1126/science.1166739. [DOI] [PubMed] [Google Scholar]
- 143.Damore JA, Gore J. 2012. Understanding microbial cooperation. J Theor Biol 299:31–41. doi: 10.1016/j.jtbi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.West SA, Griffin AS, Gardner A, Diggle SP. 2006. Social evolution theory for microorganisms. Nat Rev Microbiol 4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 145.Cremer J, Melbinger A, Frey E. 2012. Growth dynamics and the evolution of cooperation in microbial populations. Sci Rep 2:281. doi: 10.1038/srep00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Swearingen MC, Sabag-Daigle A, Ahmer BMM. 2013. Are there acyl-homoserine lactones within mammalian intestines? J Bacteriol 195:173–179. doi: 10.1128/JB.01341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Musat N, Foster R, Vagner T, Adam B, Kuypers MMM. 2012. Detecting metabolic activities in single cells, with emphasis on nanoSIMS. FEMS Microbiol Rev 36:486–511. doi: 10.1111/j.1574-6976.2011.00303.x. [DOI] [PubMed] [Google Scholar]
- 148.Krysciak D, Schmeisser C, Preuß S, Riethausen J, Quitschau M, Grond S, Streit WR. 2011. Involvement of multiple loci in quorum quenching of autoinducer I molecules in the nitrogen-fixing symbiont Rhizobium (Sinorhizobium) sp. strain NGR234. Appl Environ Microbiol 77:5089–5099. doi: 10.1128/AEM.00112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Terwagne M, Mirabella A, Lemaire J, Deschamps C, De Bolle X, Letesson JJ. 2013. Quorum sensing and self-quorum quenching in the intracellular pathogen Brucella melitensis. PLoS One 8:e82514. doi: 10.1371/journal.pone.0082514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boyer M, Wisniewski-Dy F. 2009. Cell-cell signalling in bacteria: not simply a matter of quorum. FEMS Microbiol Ecol 70:1–19. doi: 10.1111/j.1574-6941.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 151.Sprague GF Jr, Winans SC. 2006. Eukaryotes learn to count: quorum sensing by yeast. Genes Dev 20:1045–1049. doi: 10.1101/gad.1432906. [DOI] [PubMed] [Google Scholar]
- 152.Hogan DA. 2006. Talking to themselves: autoregulation and quorum sensing in fungi. Eukaryot Cell 5:613–619. doi: 10.1128/EC.5.4.613-619.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burnard D, Gozlan RE, Griffiths SW. 2008. The role of pheromones in freshwater fishes. J Fish Biol 73:1–16. doi: 10.1111/j.1095-8649.2008.01872.x. [DOI] [Google Scholar]
- 154.Sorensen PW, Stacey NE. 2004. Brief review of fish pheromones and discussion of their possible uses in the control of non-indigenous teleost fishes, New Zealand. J Mar Freshw Res 38:399–417. doi: 10.1080/00288330.2004.9517248. [DOI] [Google Scholar]
- 155.Hallmann A, Godl K, Wenzl S, Sumper M. 1998. The highly efficient sex-inducing pheromone system of Volvox. Trends Microbiol 6:185–189. doi: 10.1016/S0966-842X(98)01234-7. [DOI] [PubMed] [Google Scholar]
- 156.Hallmann A. 2011. Evolution of reproductive development in the volvocine algae. Sex Plant Reprod 24:97–112. doi: 10.1007/s00497-010-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Timmermeyer N, Stelzer CP. 2006. Chemical induction of mixis in the rotifer Synchaeta tremula. J Plankton Res 28:1233–1239. doi: 10.1093/plankt/fbl052. [DOI] [Google Scholar]
- 158.Moussa SH, Kuznetsov V, Tran TA, Sacchettini JC, Young R. 2012. Protein determinants of phage T4 lysis inhibition. Protein Sci 21:571–582. doi: 10.1002/pro.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weitz JS, Mileyko Y, Joh RI, Voit EO. 2008. Collective decision making in bacterial viruses. Biophys J 95:2673–2680. doi: 10.1529/biophysj.108.133694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gustafsson E, Nilsson P, Karlsson S, Arvidson S. 2004. Characterizing the dynamics of the quorum-sensing system in Staphylococcus aureus. J Mol Microbiol Biotechnol 8:232–242. doi: 10.1159/000086704. [DOI] [PubMed] [Google Scholar]
- 161.Fujimoto K, Sawai S. 2013. A design principle of group-level decision making in cell populations. PLoS Comp Biol 9:e1003110. doi: 10.1371/journal.pcbi.1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xavier JB, Kim W, Foster KR. 2011. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Mol Microbiol 79:166–179. doi: 10.1111/j.1365-2958.2010.07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]