SUMMARY
Bacillus and Clostridium organisms initiate the sporulation process when unfavorable conditions are detected. The sporulation process is a carefully orchestrated cascade of events at both the transcriptional and posttranslational levels involving a multitude of sigma factors, transcription factors, proteases, and phosphatases. Like Bacillus genomes, sequenced Clostridium genomes contain genes for all major sporulation-specific transcription and sigma factors (spo0A, sigH, sigF, sigE, sigG, and sigK) that orchestrate the sporulation program. However, recent studies have shown that there are substantial differences in the sporulation programs between the two genera as well as among different Clostridium species. First, in the absence of a Bacillus-like phosphorelay system, activation of Spo0A in Clostridium organisms is carried out by a number of orphan histidine kinases. Second, downstream of Spo0A, the transcriptional and posttranslational regulation of the canonical set of four sporulation-specific sigma factors (σF, σE, σG, and σK) display different patterns, not only compared to Bacillus but also among Clostridium organisms. Finally, recent studies demonstrated that σK, the last sigma factor to be activated according to the Bacillus subtilis model, is involved in the very early stages of sporulation in Clostridium acetobutylicum, C. perfringens, and C. botulinum as well as in the very late stages of spore maturation in C. acetobutylicum. Despite profound differences in initiation, propagation, and orchestration of expression of spore morphogenetic components, these findings demonstrate not only the robustness of the endospore sporulation program but also the plasticity of the program to generate different complex phenotypes, some apparently regulated at the epigenetic level.
INTRODUCTION
Clostridium organisms are anaerobic, Gram-positive, rod-shaped, endospore-forming bacteria belonging to the phylum Firmicutes, and they constitute both a class and a genus in the phylum (1). They are ancient microorganisms that existed prior to the big oxidation event (1) and thus can be viewed as evolutionary predecessors of aerobic Firmicutes such as Bacillus. The class Clostridia contains a large number of orders, families, and genera. Clostridium organisms will not grow under aerobic conditions, but several are aerotolerant. In addition, Clostridium organisms are able to differentiate into metabolically inert endospores typically, but not always, upon sensing unfavorable environmental conditions. The genus includes species of importance to human physiology and health, including organisms of importance to human and animal microbiota (2, 3) as well as human pathogens such as Clostridium botulinum, C. difficile, C. perfringens, and C. tetani (4–7). Clostridium organisms (e.g., C. leptum and C. coccoides) in the human intestinal microbiota provide a number of health benefits and immune protection (8). The genus Clostridium also includes organisms of importance to detoxification, remediation, and the carbon cycle, such as acetogens, which use the Wood-Ljungdahl pathway to fix CO2 and generate enormous quantities of acetate and which are responsible for fixing ∼20% of the CO2 on earth (9). Several Clostridium species (e.g., C. acetobutylicum, C. pasteurianum, C. thermocellum, and C. beijerinckii) have found important biotechnological applications in the production of chemicals. Indeed, the vast majority of members belonging to this class and genus are completely harmless and do not cause any known human disease. In fact, some strains, such as C. sporogenes and C. novyi, have been employed as anticancer drug delivery systems that specifically target hypoxic and necrotic areas of tumors albeit in animal models (10–12).
One of the best-studied and the first sequenced Clostridium genome is C. acetobutylicum. It has received extensive research attention in both physiological and genetic/genomic studies because of the well-known ABE (acetone-butanol-ethanol) industrial fermentation process, which remains one of the most important fermentation processes of both historical and current importance (13). C. acetobutylicum has also been extensively used as a model organism to study molecular mechanisms underlying endospore formation in Clostridium. These studies, along with similar studies of pathogenic Clostridium species, have revealed that the molecular program of sporulation in Clostridium organisms is substantially different from that which has been well established for the more widely studied organism Bacillus subtilis, whose sporulation program is viewed as the model for endospore formers.
MORPHOLOGICAL CHARACTERISTICS OF CELLS DURING ENDOSPORE DIFFERENTIATION
Nutrient deprivation has been implicated as the trigger that starts the process of sporulation in many endospore formers and notably in B. subtilis and other Bacillus organisms (14, 15) (Fig. 1). Similarly, accumulating evidence also suggests that this is the case for many Clostridium organisms (16), although this has been debated (10). An exception certainly is the situation of solventogenic Clostridium organisms, such as C. acetobutylicum, whereby accumulation of the organic acids butyrate and acetate and the associated lower pH during exponential growth are thought to trigger the sporulation process, in the presence of excess nutrients (10, 17, 18). Additional or alternative culture parameters, such as exposure to oxygen (18) or other stresses, may also play a role. These and other differences (e.g., the formation of the storage material granulose in Clostridium but not in Bacillus) have been well documented (10) and are attributed to the different physiological niches occupied by these different genera or classes of Firmicutes. Aside from these broad and general differences, there has not been much discussion on the physiological niches that might differentiate the signals that trigger sporulation in Bacillus versus Clostridium. For example, it is not clear if nutrient deprivation is responsible for triggering sporulation of Clostridium pathogens under in vivo conditions. This is certainly an area worthy of careful reexamination, especially now that the orphan histidine kinases (HKs) that initiate sporulation by directly phosphorylating Spo0A have been identified (see below). Expression of these HKs under various physiological conditions may identify more precise physiological niches that trigger sporulation. Lastly, it should be mentioned that unlike the Bacillus model, there is a relatively high, constitutive level of expression of σH and Spo0A (see below) in Clostridium organisms (10), which could reflect differences between Bacillus and Clostridium in the signaling that initiates sporulation.
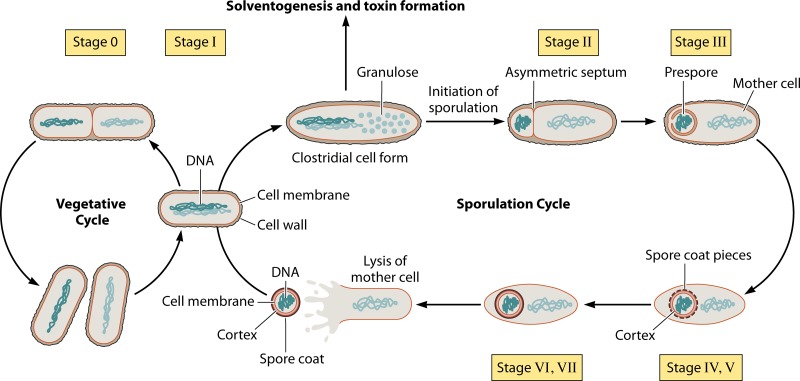
FIG 1.
Formation of spores by endospore-forming bacteria. Upon sensing unfavorable environmental conditions, the cells begin the process of differentiation and spore morphogenesis. The clostridial cell form is seen with its cigar-shaped structure, and granulose vesicles accumulate. The cells then initiate the sporulation process, and asymmetric division occurs, giving rise to the mother cell and prespore compartments. The mother cell then engulfs the prespore; subsequently, the spore cortex begins to develop around the prespore, and pieces of the spore coat form. Once the membranes of the spore are completed, the mother cell lyses, thus releasing the mature endospore. Under favorable conditions, the spore will germinate to give rise to a vegetative cell.
Since C. acetobutylicum is one of the most widely examined organisms of the genus, at both the molecular and physiological levels, we use it as a model organism to describe the sporulation program. C. difficile has also been widely examined, but it has been recently proposed that C. difficile should be reclassified as belonging to the family Peptostreptococcaceae (these Firmicutes still belong to the class Clostridium), with a new suggested name, Peptoclostridium difficile (19). This suggestion has already been implemented in the KEGG database and is thus likely to be widely adopted. Thus, this organism may not be a canonical member of the genus Clostridium, and as such, findings for C. difficile (we use the older name in this review, as it is still more widely recognized) may not necessarily be pertinent to other members of the genus Clostridium. However, we will still discuss the findings from C. difficile studies, as they demonstrate the diversity of endospore differentiation within the class Clostridia.
Under the C. acetobutylicum model, during normal vegetative growth, cells divide symmetrically by binary fission, and the cells exhibit the typical rod-shaped morphology while consuming glucose and producing organic acids. As acid concentrations increase and the pH of the medium drops, the environment becomes increasingly toxic to the cells. In response, the cells initiate two survival mechanisms: solventogenesis and sporulation (1, 20, 21). Solventogenesis provides the cells with immediate relief from the low pH of the culture and acid toxicity by reassimilating the organic acids into their respective solvents, thus raising the pH of the environment. Sporulation, which results in a highly resistant spore, is a long-term survival mechanism allowing the cells to survive until a more suitable environment is established.
During the process of sporulation, the cells undergo a number of different morphological changes as the spore develops and matures. The first distinct morphology that the cells adopt is known as the clostridial form. Clostridial-form cells (from which the name of the genus Clostridium is derived) are recognizable by their cigar-shaped and swollen phenotype, with accumulating granulose vesicles (storage vesicles made of amylopectin) (22). These clostridial-form cells were originally thought to be the ones that are responsible for solvent formation (22), but more recent studies have shown that solventogenesis is initiated before these cell types are formed (23). The clostridial form is a morphological phenotype unique to Clostridium and is not encountered among the morphological structures of B. subtilis. As sporulation continues, the cells continue to undergo many morphological changes. These changes have been detailed in the B. subtilis model, and they can also be applied to the Clostridium model, as follows (Fig. 1) (10, 24, 25). Stage 0 is where cells are undergoing normal vegetative growth by binary fission. This stage precedes the decision to sporulate. Stage I is DNA replication and the formation of an axial filament. The DNA appears to form a single axial thread. It is unclear if this stage is involved exclusively in sporulation, as the axial structure also appears during reduced rates of DNA synthesis, and the cells can revert back to vegetative growth if the environmental conditions become favorable. Stage II is asymmetric division. This is the first morphologically distinct state that indicates that the cells have initiated sporulation. Cells form an asymmetric septum at one end of the cell, with the smaller compartment destined to become the endospore. Stage III is engulfment. The membranes formed in stage II grow around the smaller compartment and engulf it in a manner similar to phagocytosis, thus creating a cell within a cell. At this stage, the developing spore is fully committed to undergo sporulation. Stage IV is cortex formation. The spore cortex now forms between the two membranes of the mother cell and the developing spore. The cortex is made of modified peptidoglycans and has very little cross-linkage between the peptide chains. Stage V is deposition of spore coat proteins. The coat proteins account for more than half of all the spore proteins, and they develop on the outer membrane of the developing forespore. They are made of cysteine-rich proteins. Stage VI is maturation of the developing spore. The spore coat and cortex proteins continue to develop and mature, and electron micrographs show a thick, whitish envelope that surrounds the forespore (21, 26). Stage VII is lysis of the mother cell and release of the mature spore. A lytic enzyme degrades the mother cell, thus releasing the mature free spore into the surrounding environment as well as other components of the mother cell. In the case of C. perfringens, the enterotoxins that cause food-borne illness are also released at this stage.
While the basic morphological changes during spore morphogenesis are conserved between Clostridium and Bacillus, the underlying genetic orchestration and regulation are considerably different.
OVERVIEW OF SPORULATION IN THE B. SUBTILIS MODEL
As a reference, we briefly discuss the sporulation process in B. subtilis before discussing the differences between this model and the Clostridium model. For B. subtilis, initiation of sporulation begins with the activation, via phosphorylation, of the master transcriptional regulator of all endospore formers, Spo0A, which is initially transcribed from a σH-dependent promoter, the first sigma factor activated at the onset of sporulation (Fig. 2A) (27, 28). Once Spo0A is phosphorylated (Spo0A∼P), it regulates the expression of upwards of 100 genes or operons (27, 29, 30). One gene that it downregulates is abrB (29, 31), a repressor protein of sigH expression. This leads to an increased expression level of sigH and subsequently increased levels of spo0A expression. Phosphorylation of Spo0A is achieved by an elaborate phosphorelay system that involves five sensory orphan HKs (KinA to KinE) (27). Orphan kinases are HKs lacking an adjoining response regulator, as is typical for most HKs in prokaryotes. Initially, it was believed that the five B. subtilis HKs (KinA, KinB, KinC, KinD, and KinE) play a role in the sporulation phosphorelay system, but recent evidence suggests that only KinA and KinB are involved in the sporulation pathway, while KinC, KinD, and KinE are unlikely to be involved (32–35). The cascade starts when either KinA or KinB autophosphorylates in response to an appropriate stimulus and subsequently transfers the phosphate to Spo0F (Fig. 2A). Spo0F∼P then phosphorylates the response regulator Spo0B, which in turn phosphorylates Spo0A (Spo0A∼P), thus initiating the sporulation cascade (1, 27). Alternatively, KinC is able to bypass (27) the phosphorelay system involving Spo0F and Spo0B to directly act on and phosphorylate Spo0A (27).
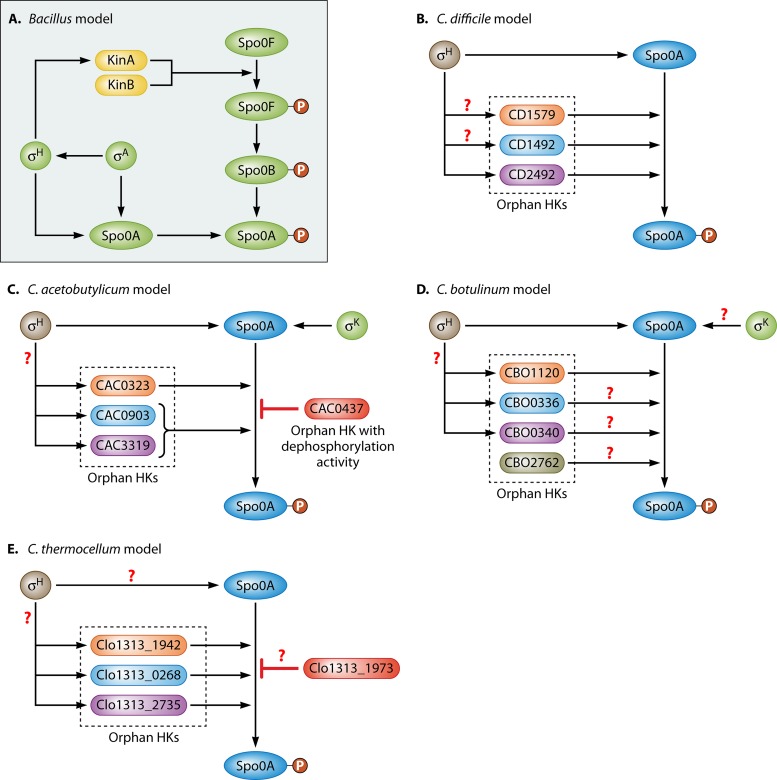
FIG 2.
Activation of Spo0A via phosphorylation in B. subtilis (A), C. difficile (B), C. acetobutylicum (C), C. botulinum (D), and C. thermocellum (E). In Bacillus, the phosphorylation process is initiated once the orphan HKs KinA and/or KinB phosphorylates Spo0F, the first component of the phosphorelay system that leads to Spo0A phosphorylation. Based on all Clostridium organisms sequenced so far, there is no evidence that they have a recognizable phosphorelay system. Instead, several orphan HKs were shown to directly transfer a phosphate group to Spo0A, thus activating it. Additionally, it was shown that the orphan HK CAC0437 in C. acetobutylicum has dephosphorylation activity and is thus able to remove the phosphate group from Spo0A, thus rendering it inactive. Clo1313_1973 in C. thermocellum may also have the ability to inactivate Spo0A, although direct evidence of dephosphorylation activity has yet to be obtained.
Spo0A∼P and σH control the expression of the spoIIA operon, which includes the gene that codes for the prespore-specific σF, the first of four sporulation-specific sigma factors (the rest being σE, σG, and σK) downstream of Spo0A (Fig. 3A). The cascade continues by the sequential activation of σE, σG, and σK until the development of the mature spore (27, 29, 36) (Fig. 3A). Interestingly, and unlike the genes coding for the other sigma factors, sigK is not expressed from a single gene but rather from two noncontiguous genes that are interrupted by the skin (sigK-intervening) element. Upon transcription, the two transcripts are joined together by the excision of the skin element, leading to the proper translation of the mature σK protein (37). The control and regulation of each of these sigma factors are discussed in detail below, as their role in B. subtilis is compared to their role in Clostridium organisms.
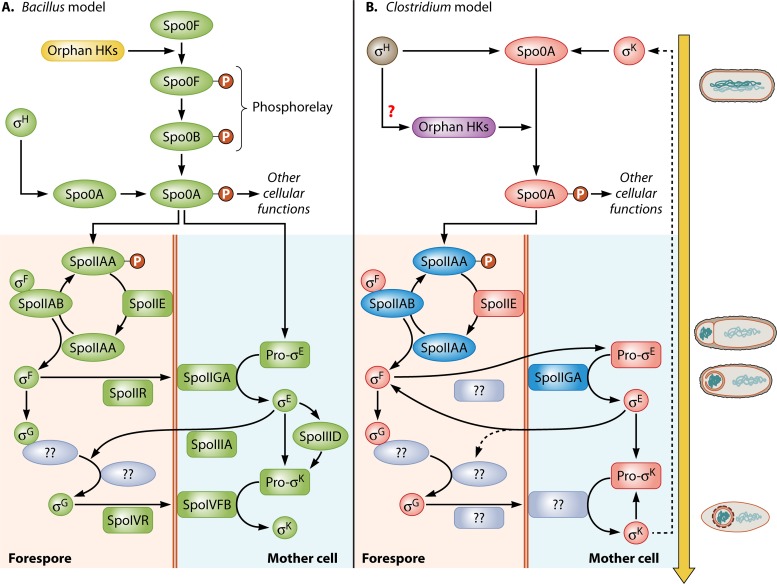
FIG 3.
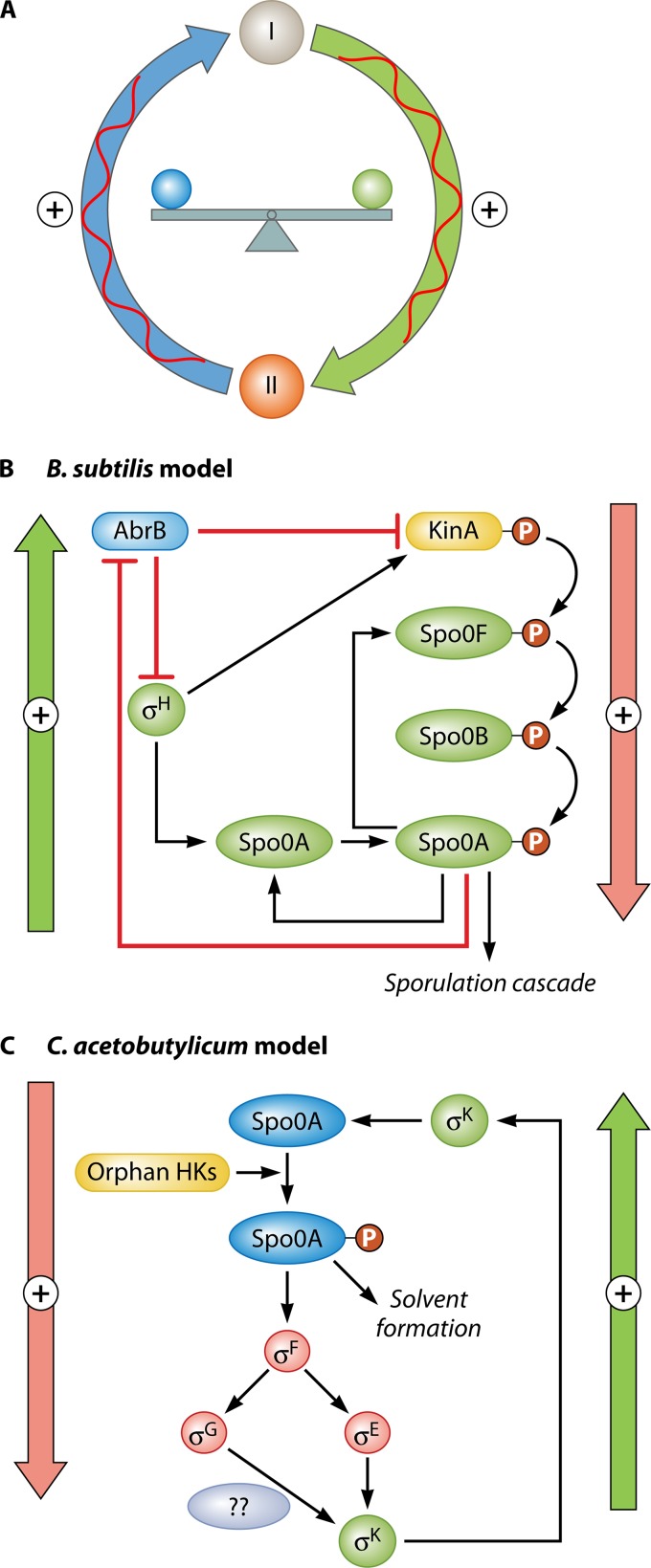
Comparative summary of the sporulation signaling cascade in Bacillus (A) versus Clostridium (B), involving the master transcriptional regulator Spo0A as well as the major sporulation-specific sigma factors up- and downstream of Spo0A. This model does not apply to C. difficile, as its sporulation program is different from those of the other studied Clostridium organisms, as discussed in the text. Approximate cellular phenotypes for the Clostridium model of differentiation are shown on the right. While similar stages take place in Bacillus, the timing is different (Fig. 5). Several notable differences in the regulation of sporulation between these species have already been discovered. First, in the Bacillus model, Spo0A is phosphorylated (Spo0A∼P) by a phosphorelay system initiated by orphan HKs, mainly KinA and KinB (Fig. 2). Once activated, Bacillus Spo0A∼P initiates the sporulation sigma factor cascade involving four downstream sigma factors (σF, σE, σG, and σK). In Clostridium, no phosphorelay system is present. Rather, orphan HKs phosphorylate Spo0A directly (Fig. 2). Second, the last sigma factor in the Bacillus model, σK, was shown to play a dual role in Clostridium, one early, upstream of Spo0A, and another late, downstream of σG, which is analogous to its role in Bacillus. As the Clostridium model is further refined, additional differences between these species are expected. Green genes indicate confirmed functional roles in B. subtilis. Red genes denote functional roles confirmed by gene inactivation in Clostridium. Blue genes indicate genes with a presumed function in Clostridium, based on homology to B. subtilis, gene organization, and consistent phenotypic evidence. Gray ovals denote suspected protein interactions. A single red question mark denotes suspected transcriptional activity. The dashed arrow for σK in the Clostridium model indicates an unknown pathway for early σK activity.
THE SPORULATION-SPECIFIC SIGMA FACTORS UPSTREAM OF Spo0A
Predivisional Stage 0 Sporulation Sigma Factor σH Is Essential for the Transition from Vegetative Growth to Sporulation
In B. subtilis, the alternative sigma factor σH is the first of five such transcriptional activators that are temporally and spatially activated as the cells prepare to undergo sporulation (29, 36, 38, 39). Because σH controls the expression of genes associated with the initiation of sporulation but also some sporulation-specific sigma factors downstream of activated Spo0A, we somewhat arbitrarily classify σH as being sporulation specific. σH was shown to direct the expression of at least 87 genes during the shift from exponential growth to the stationary phase that play a role in spore formation and genetic competence (40). It is also responsible for driving the expression of kinA, the HK that is responsible for the initiation of the Spo0A phosphorelay cascade (41, 42). In B. subtilis, sigH transcription is driven from both a σA- and a σH-dependent promoter (27, 36, 38). However, transcription of sigH is regulated through a complex positive feedback reinforcing loop involving AbrB and activated Spo0A∼P (27, 43–47). The transcription of sigH is directly suppressed by the transcriptional repressor AbrB. Once critical levels of Spo0A∼P are reached, it indirectly enhances the transcription of sigH by repressing the expression of abrB. As sigH expression is relieved from inhibition, it further drives the expression of spo0A, thus increasing Spo0A∼P levels. This further represses abrB expression and therefore increases sigH transcription.
Is σH dispensable somehow in Clostridium organisms? There has been only one reported sigH inactivation mutant that was successfully generated in Clostridium, that of the enteropathogen C. difficile (48). Global gene expression profiling of the sigH mutant of C. difficile relative to the wild-type (WT) strain revealed that 286 genes showed reduced expression, indicating that σH plays a role in their expression (48). As expected, spo0A, spoIIA, and CD2492 (an HK that takes part in Spo0A phosphorylation) (Fig. 2B) were all shown to be under the positive control of σH (48) (Fig. 4). Interestingly, the genes encoding the toxins tcdA and tcdB, as well as the sigma factor (encoded by tcdR) that transcribes these genes, showed increased expression in the sigH mutant, indicating that they are negatively regulated by σH, probably through an intermediate transcriptional regulator (48). By using the B. subtilis σH consensus binding motif (49) as a template, the promoter regions of the genes with reduced expression in the sigH mutant were examined to identify genes that were putatively directly regulated by σH. The results showed that almost 40 operons were positively regulated by σH, because they had a σH-like binding motif in their respective promoters, compared to 50 operons that were shown to be regulated by σH in B. subtilis (40). However, only 8 of those operons/genes were shown to be commonly regulated by σH between the two organisms, including the major sporulation genes/operons spo0A, spoIIA, spoVG, and spoVS. Similarly, the genes coding for an HK(s) that plays a role in the phosphorylation of Spo0A in C. difficile (CD2492) (Fig. 2B) and in B. subtilis (kinA and kinE) were also shown to be transcribed by σH. Unlike in B. subtilis, where the dnaG-sigA operon was shown to be partially dependent on σH for its expression (50, 51), the C. difficile dnaG-sigA1 operon did not show a dependence on σH (48). However, sigA2, which was presumed to be a second copy of the sigA gene on the C. difficile genome (6), showed a significant reduction in expression (by 180-fold) in the sigH mutant, thus suggesting that its expression was strongly dependent on σH. Saujet and coworkers also conducted microarray studies with the WT C. difficile strain to compare differential gene expression patterns between early exponential and early stationary phases (48). They showed that one-fifth of all C. difficile genes exhibited differential expression between the two culture phases, and of these, >400 genes were shown to be directly or indirectly regulated by σH. Therefore, it appears that σH plays a major role in the transition from vegetative, active growth to the stationary phase of C. difficile. To date, efforts by our group to isolate a sigH inactivation mutant in C. acetobutylicum have been unsuccessful (data not shown), and this might suggest that sigH inactivation might be lethal in C. acetobutylicum.
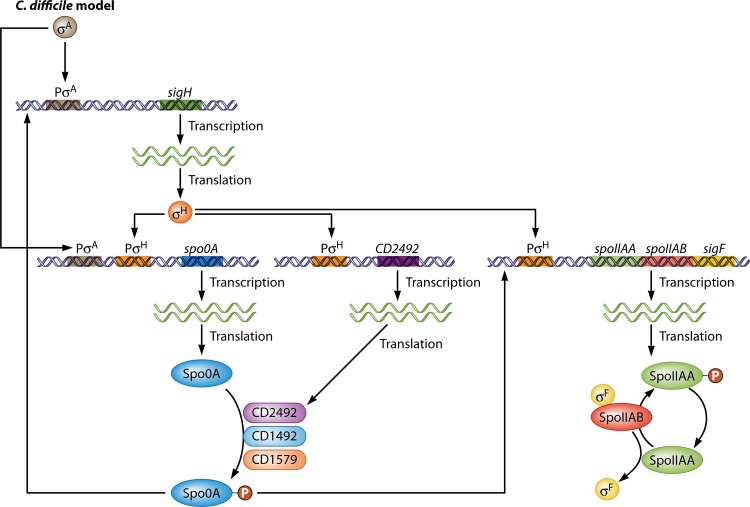
FIG 4.
Transcriptional control and activation of sigH, spo0A, and the spoIIAA operon in C. difficile. Expression of sigH is initially driven from a σA-dependent promoter. Once translated, σH regulates the expression of spo0A, the orphan HK CD2492, and the spoIIAA operon, where sigF resides. Once expressed, CD2492, along with other HKs, directly phosphorylates Spo0A, thus activating it. Subsequently, Spo0A∼P augments the expression of sigH and the spoIIAA operon, thus initiating the sigma factor sporulation cascade.
Is there a basal level of sigH expression and, if so, at what level? An extensive study investigating the transcriptional program during spore morphogenesis in C. acetobutylicum (21) by utilizing a combination of DNA microarray analysis and quantitative reverse transcription-PCR (Q-RT-PCR) showed that sigH expression appears to have a basal level of expression, as reported for B. subtilis (47). However, unlike B. subtilis, where sigH is expressed at low levels before being further stimulated at the onset of sporulation, the expression level of sigH in C. acetobutylicum appears to be significantly higher and remains constant throughout its life cycle. In fact, its expression level appeared to be consistently higher than that of σA, the presumed housekeeping sigma factor (21). In all Clostridium genomes, a σA consensus binding sequence was identified upstream of the sigH gene (10). However, unlike B. subtilis, no apparent σH-like consensus binding sequence was recognized in the promoter region of the sigH gene in many Clostridium strains (52). Thus, it appears that the control of sigH expression is different between the two genera.
σK Is Activated Late in the Bacillus Model but Also Has a Surprising Early Role in Clostridium Sporulation
In B. subtilis, σK has been established to be the last mother cell-specific sigma factor activated during sporulation (53). Its expression was shown to be driven from a promoter that is σE and SpoIIID dependent during stage III of sporulation in the mother cell (54, 55). Mutants of the sigK gene were shown to have their sporulation blocked at stage IV of the sporulation cycle (56). Expression of sigK is tightly regulated in B. subtilis, at both the transcriptional and translational levels, aiming to prevent the premature activation of σK. The 5′ end (encoded by spoIVCB) and the 3′ end (encoded by spoIIIC) of the sigK open reading frame (ORF) are disrupted by a stretch of 48 kb, known as the skin element (57, 58). The skin element was shown to encode a site-specific recombinase that is explicitly expressed in the mother cell compartment during late-stage sporulation (37, 59). Once translated, the activity of the recombinase results in the precise excision of the skin element, thus restoring the complete and functional expression of the intact sigK gene. Initially, the skin element was thought to play an important role in the precise temporal and spatial expression of sigK. However, it was shown that deletion of the skin element did not have any adverse effects on the sporulation frequency of B. subtilis (37). Like σE (see below), σK is translated in an inactive form, pro-σK. Subsequently, the proteolytic activity of a membrane-localized protein, SpoIVFB, cleaves 20 residues in the N terminus of pro-σK, thus generating the mature σK (60, 61).
The Clostridium genomes sequenced to date show that only some strains of C. difficile (6, 62) and C. tetani (4) have a skin element disrupting their sigK gene, named skinCd and skinCt, respectively. Other members of the genus do not appear to have such a skin element in their genomes. In contrast to B. subtilis, the C. difficile 630 skin element was shown to be necessary for efficient sporulation of the strain (62). Strains that did not carry the skinCd element (ATCC 9698 and CD37) were shown to be less efficient at producing heat-resistant endospores (62). Nucleotide sequence comparisons between strains lacking the skin element showed that the sigK gene sequence was identical to that of the strains carrying it. Additionally, the simultaneous introduction of both disrupted (i.e., with a skinCd element) and undisrupted copies of the sigK gene into a strain lacking the skinCd element did not result in an increase of the sporulation frequency in these strains (62). Therefore, it appears that the skinCd element is necessary for the proper spatial and temporal activation of σK in C. difficile.
The first evidence showing that σK in Clostridium organisms may play an earlier role than in the B. subtilis model came from a sigK disruption mutant (strain KM1) generated in C. perfringens (63). This sigK disruption mutant was unable to sporulate efficiently, and transmission electron microscopy (TEM) images showed that only a very small number of cells developed any kind of asymmetric septa or accumulated granulose, thus suggesting that sporulation was blocked prior to stage II (Fig. 5) (63). In the same study, a sigE disruption mutant (strain KM2) was also generated (63). The authors of this study reported that sporulation was blocked at an earlier stage in the sigK disruption mutant than in the sigE disruption mutant. TEM images of the sigE disruption mutant showed that the cells failed to enter stage III (engulfment) (Fig. 5). Additionally, gene expression profiling by semiquantitative reverse transcription-PCR (sq-RT-PCR) of sigF, sigE, and sigG in the sigK disruption mutant showed that the strain failed to efficiently transcribe these genes during the early exponential phase of culture. Furthermore, the sigK transcript was detected in the sigE disruption mutant albeit at what appears to be a slightly lower level than in the control strain (C. perfringens SM101), thus strongly suggesting that σK is activated earlier in the sporulation cascade of C. perfringens than σE. Moreover, promoter reporter assays and sq-RT-PCR data suggest that sigK expression follows a biphasic pattern during the growth of C. perfringens culture, with low expression levels during exponential phase and higher expression levels during stationary phase. These authors proposed that early expression of the sigK gene in C. perfringens is due to read-through from the transcription of an upstream gene (CPR_1739) and not from its natural promoter (63). Promoter fusion assays using the gusA gene (encoding a β-glucuronidase) from Escherichia coli in the sigE disruption mutant with the sigK promoter region (PsigK-gusA) did not detect any reporter activity from the sigK promoter. However, because sigG transcription was also affected in the sigE disruption mutant, it is unclear if the lack of reporter activity from the sigK promoter in the sigE disruption mutant is due to the absence of σE or simply an artifact of the altered expression of σG, since σG is downstream of σE in the sporulation cascade. Nonetheless, these data suggest that late expression of sigK was directly or indirectly dependent upon σE activation in C. perfringens.
FIG 5.
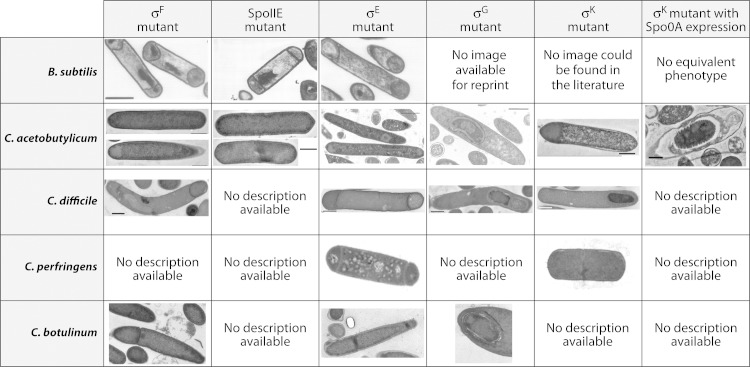
TEM images comparing the various sporulation-specific sigma factor mutants generated in B. subtilis and Clostridium organisms, including C. acetobutylicum, C. difficile, C. perfringens, and C. botulinum. In the sigK deletion mutant of C. acetobutylicum, sporulation was blocked prior to stage II, which is also true for the C. acetobutylicum sigF, spoIIE, and sigE disruption mutants as well as the sigK mutant of C. perfringens. However, the sigK mutant of C. difficile appeared to progress further in sporulation, and a developing forespore appears to be present. On the other hand, the sigF, spoIIE, and sigE disruption mutants of B. subtilis were all able to develop asymmetric septa and exhibited a disporic phenotype. The sigG disruption mutant generated in C. acetobutylicum appeared to progress further into the sporulation pathway than its counterpart in B. subtilis due to the presence of what appears to be a spore coat and cortex, which were not visible in the sigG mutant of B. subtilis. (For an image of a B. subtilis σG mutant, see Fig. 2 in reference 104.) Expression of Spo0A in the sigK deletion mutant of C. acetobutylicum showed that the cells were able to progress further in spore morphogenesis than the sigG mutant; however, they were still unable to form viable spores, and the spore coat and cortex appeared to be ill formed, thus indicating that σK is also needed during the last stages of sporulation. (Images of the B. subtilis σF mutant, SpoIIE mutant, and σE mutant reprinted from reference 91 with permission; image of the C. acetobutylicum σF mutant reprinted from reference 88; image of the C. acetobutylicum SpoIIE mutant reprinted from reference 115; images of the C. acetobutylicum σE mutant and σG mutant reprinted from reference 26; image of the C. acetobutylicum σK mutant reprinted from reference 66; images of the C. difficile σF mutant, σE mutant, σG mutant, and σK mutant reprinted from reference 25; images of the C. perfringens σE mutant and σK mutant reprinted from reference 63 with permission; images of the C. botulinum σF mutant, σE mutant, and σG mutant reprinted from reference 96 with permission.)
The involvement of σK in early sporulation was also demonstrated in C. botulinum ATCC 3052 (64). Gene expression analysis by Q-RT-PCR showed that the levels of both spo0A and sigF transcripts were significantly downregulated in the sigK disruption mutant. The lower levels of the sigF transcript can be explained by the fact that the sigF operon is known to be positively regulated by Spo0A∼P. This was the first evidence that suggested that σK may be active upstream of Spo0A and that Spo0A expression may directly or indirectly be dependent upon early σK expression/activation (Fig. 3B). Moreover, the authors of that study examined the expression profile of sigK during late exponential and early stationary phases by Q-RT-PCR and observed that the sigK transcript is present in a relatively larger amount during late exponential phase than during early stationary phase (64). However, whether sigK expression peaks during mid- to late stationary phase in C. botulinum, as seen in C. acetobutylicum (21) and B. subtilis (65), is still unknown. Spore staining and microscopy analysis of the sigK mutant generated in C. botulinum ATCC 3052 showed that the mutant did not produce spores and that the cells exhibited an elongated phenotype (64). These images suggest that sporulation was blocked prior to stage II, as observed for the C. perfringens sigK mutant (63).
A recent report (66) demonstrated unambiguously that, in C. acetobutylicum at least, σK is necessary to initiate sporulation by enabling Spo0A expression, in addition to having an indispensable role in late sporulation, as discussed below. Deletion of the sigK gene in C. acetobutylicum (66, 67) abolished the mutant's (named ΔsigK) ability to express Spo0A, as evidenced by the lack of Spo0A protein in Western blots, and hence, the ability to sporulate and produce solvents was also abolished. Moreover, protein and/or transcript levels of σF, σE, and σG were all diminished. Western blots did not detect any Spo0A or σG, while a small band was seen for σF. Q-RT-PCR showed that the transcript levels of all the sporulation-related genes (e.g., spo0A, sigF, sigE, sigG, and spoIIE) as well as the adhE1 (or aad) gene, which is an important solventogenesis gene, were all significantly downregulated. The sigK deletion mutant was unable to produce viable spores, and TEM image analysis revealed that sporulation was blocked prior to stage II (asymmetric division) (Fig. 5). The same study also demonstrated a role for σK late in the sporulation process, analogous to that in B. subtilis, and this role is discussed below.
Spo0A PHOSPHORYLATION TO ACTIVATE THE SPORULATION CASCADE IN CLOSTRIDIUM ORGANISMS
In Clostridium organisms, there is no evidence of a phosphorelay system for phosphorylating Spo0A, and thus, it was proposed that orphan HKs directly phosphorylate Spo0A (1). Indeed, in the last 5 years, several orphan HKs that directly phosphorylate Spo0A in C. difficile (Fig. 2B) (68), C. acetobutylicum (Fig. 2C) (69), C. botulinum (Fig. 2D) (70), and C. thermocellum (Fig. 2E) (71) have been identified.
In C. botulinum, the orphan HK encoded by CBO1120 was shown to be responsible for the phosphorylation of Spo0A in vivo (70) (Fig. 2D). While there are three other HKs closely related to CBO1120 (namely, CBO0336, CBO0340, and CBO2762) (Fig. 2D), Worner and coworkers were unable to clone them due to their possible lethal effect on E. coli as they were being subcloned (70). Interestingly, when C. botulinum Spo0A was cloned into a B. subtilis spo0A mutant, it was unable to complement it, although the Spo0A protein was detected by Western analysis. Thus, it appears that the native B. subtilis phosphorelay system is unable to recognize and phosphorylate the C. botulinum Spo0A protein. On the other hand, when a Spo0A chimera was created with the N-terminal domain of B. subtilis Spo0A and the C-terminal domain of C. botulinum and cloned into the B. subtilis spo0A mutant, it was able to restore sporulation (70). This indicates that the C. botulinum Spo0A N-terminal domain is not readily phosphorylated in B. subtilis. Interestingly, though, WT C. botulinum Spo0A was able to repress abrB expression in the B. subtilis spo0A mutant, indicating that phosphorylation was not required for this activity.
Similarly, in C. difficile, multiple orphan HKs encoded on its genome were implicated in the phosphorylation of Spo0A (68). Three orphan HKs were identified by using bioinformatics analyses: CD2492, CD1492, and CD1579 (Fig. 2B). Efforts to disrupt CD1492 and CD1579 by using the group II intron system (commercially known as TargeTron or ClosTron [72, 73]) were unsuccessful. On the other hand, disruption of CD2492 was successful and resulted in a reduced frequency of sporulation relative to that of the parental strain but did not completely abolish sporulation. This is similar to the phenotype observed when the kinA gene was disrupted in B. subtilis (74). Therefore, it appears that other HKs are involved in the sporulation initiation process in C. difficile. Indeed, in vivo phosphorylation of Spo0A by the HK CD1579 was demonstrated, strongly indicating that this HK is also involved in Spo0A phosphorylation (68). Thus, it appears that multiple HKs are responsible for Spo0A phosphorylation in C. difficile.
Predictions based on transcriptional data indicated that multiple orphan HKs may be responsible for the direct phosphorylation of Spo0A in C. acetobutylicum (1). Bioinformatics analysis indicted that there are five orphan HKs that are encoded by ca_c0323, ca_c0903, ca_c2730, ca_c0437, and ca_c3319 in C. acetobutylicum. Time course transcriptional profiling using microarray analysis indicated that the expression of four out of the five HKs peaked during the onset of sporulation (the exception being ca_c3319) (1, 21). This is consistent with the transcriptional pattern of the five B. subtilis phosphorelay kinases in which expression increased at the onset of sporulation. These early predictions were later confirmed by experimental analyses (69).
Steiner and coworkers were able to unravel two independent pathways for Spo0A phosphorylation that confirmed the roles of the above-mentioned HKs in C. acetobutylicum (69). The first pathway was dependent solely on the HK encoded by ca_c0323, while the second involved two HKs, encoded by ca_c0903 and ca_c3319 (Fig. 2C). Individual mutants of the above-mentioned HKs were generated, and the data showed that the sporulation frequency was reduced by 95% to 99% relative to that of the parental strain, thus indicating that the products of these genes are necessary for spore development (69). Moreover, double-disruption mutants, of either ca_c0323 and ca_c0903 or ca_c0323 and ca_c3319, resulted in the complete inability of the strains to sporulate, while a ca_c0903 ca_c3319 double mutant resulted in only a small reduction in spore formation relative to the respective single mutants. The ability of CAC0323, CAC0903, and CAC3319 to autophosphorylate and subsequently transfer the phosphoryl group to Spo0A in vitro was also tested (69). Both CAC0903 and CAC3319 successfully autophosphorylated and transferred their phosphoryl group to Spo0A, while no activity was detected for CAC0323. The authors of this study hypothesized that this was due to the truncated version of the protein that was purified and used in subsequent experiments. Thus, taken together, these data suggest that there are two pathways through which Spo0A becomes phosphorylated in C. acetobutylicum. Moreover, Steiner and coworkers showed that a ca_c0437 disruption mutant was able to produce an abnormally high number of spores (∼30-fold higher) relative to the parental strain (Fig. 2C). The introduction of a ca_c0437 mutation into either the ca_c0903 or ca_c3319 mutant resulted in the restoration of sporulation to greater-than-WT levels, and plasmid-based expression of ca_c0437 resulted in sporulation that was reduced by >99% in the WT strain (69). Additionally, it was shown that CAC0437 is unable to autophosphorylate but is still able to dephosphorylate Spo0A∼P in the presence of ATP, thus providing evidence that CAC0437 plays a role in controlling the levels of Spo0A∼P to prevent premature sporulation and to maintain cellular equilibrium and the ratios of Spo0A/Spo0A∼P.
More recently, orphan HKs have been identified and implicated in the sporulation of C. thermocellum (71). Mearls and Lynd identified three putative HKs, namely, clo1313_0286, clo1313_2735, and clo1313_1942, that positively control the sporulation process (71). A fourth one, clo1313_1973, appeared to have a negative effect on sporulation similar to that of the C. acetobutylicum ca_c0437 HK (69, 71). Deletion of clo1313_0286, clo1313_2735, and clo1313_1942 independently resulted in the inability of the strains to form heat-resistant spores, while deletion of clo1313_1973 resulted in the mutant forming an abnormally high number of heat-resistant spores (71). Thus, like C. acetobutylicum, C. thermocellum may have the ability to control the levels of Spo0A∼P by a phosphatase-like enzyme.
In summary, unlike the B. subtilis phosphorelay system, which is absent in clostridia, it has been demonstrated that the initiation of sporulation in Clostridium is carried out by orphan HKs that directly interact with and phosphorylate Spo0A (68–70).
SPORULATION-SPECIFIC SIGMA FACTORS THAT ARE ACTIVATED DOWNSTREAM OF Spo0A
σF, the First Forespore-Specific σ Factor, Is Involved in Stage II of Sporulation
In B. subtilis, σF is the first sporulation-specific sigma factor activated in the developing prespore compartment (75, 76) and is tightly regulated at both the transcriptional and posttranslational levels. The transcription of the spoIIA tricistronic operon, which contains spoIIAA, spoIIAB, and sigF, is driven from a σH-dependent RNA polymerase (RNAP), with the activated Spo0A∼P acting as an enhancer of transcription (76, 77). Once translated, σF is held inactive by the binding of the anti-sigma factor SpoIIAB. SpoIIAB also phosphorylates SpoIIAA (the anti-anti-sigma factor), rendering it inactive (29, 78, 79). To release σF from inhibition, SpoIIAA is dephosphorylated by the membrane-bound phosphatase SpoIIE, thus enabling SpoIIAA to bind to SpoIIAB and the mature σF to be subsequently released (78–82). Unbound σF then promotes the expression of 48 genes in the prespore compartment (83), including spoIIR, which is required to activate the processing of pro-σE into active σE (84, 85). Thus, σF is the first sigma factor, after σH, to become active in the sporulation cascade.
The first study that showed the involvement of σF in the sporulation process of a Clostridium organism came from a sigF mutant that was generated in C. perfringens SM101 (a transformable variant of the food poisoning strain NCTC 8798) (86). Since enterotoxin (C. perfringens enterotoxin [CPE]) production is observed only in sporulating cells of C. perfringens (86, 87), it is important to understand the underlying mechanism that controls this process and whether these sporulation-related sigma factors directly play a role in toxin production in this important pathogen. The C. perfringens sigF disruption mutant (SM101::sigF) was, as expected, unable to form any heat-resistant endospores, which indicates that σF is necessary for sporulation in C. perfringens (86). However, it is unclear at what stage sporulation was blocked, since no TEM analyses of the mutant were conducted. Western blot analysis using antibodies that were raised against the B. subtilis sigma factors (σF, σE, σG, and σK) was used to detect the levels of these proteins in the C. perfringens sigF mutant under sporulation conditions. The data showed that the sigF mutant produced sharply reduced levels of σE, σG, or σK, while no σF was detected. This is consistent with the B. subtilis model in which the expression of the sigma factors downstream of σF is dependent on the presence and activation of σF (27). In addition, the authors of this study were able to show that CPE production is directly regulated by σF.
The involvement of σF in early stages of sporulation was also demonstrated in C. acetobutylicum (88) (Fig. 6). Western analysis revealed that the sigF inactivation mutant (FKO1) was unable to accumulate any σE or σG, thus suggesting that σF was needed for the expression of these downstream sigma factors. In B. subtilis, the mother cell-specific σE is translated into an inactive pro-σE form before it matures into active σE by a σF-directed process (27). Western blot analyses of the sigF inactivation mutant did not detect any pro-σE protein, thus suggesting that σE expression is under the positive control of σF, which is in contrast to the B. subtilis model, where σA was shown to drive sigE expression (89). The lack of detectable σG protein in the sigF inactivation mutant is consistent with the B. subtilis model, where sigG expression is directly controlled by σF (27, 90). TEM image analysis of the sigF inactivation mutant revealed that the cells failed to initiate asymmetric division (Fig. 5) (88), with no identifiable clostridial forms or endospores (23). This is in contrast to the B. subtilis sigF mutant strain, which develops asymmetric septa (91). Thus, it appears that σF plays an earlier role in the process of spore morphogenesis in C. acetobutylicum and possibly other Clostridium spp. (Fig. 3). Gene expression profiling using Q-RT-PCR was also used on the sigF inactivation mutant to determine if the σF regulon in B. subtilis was similar to that in C. acetobutylicum. Six candidate genes that were shown to belong to the σF regulon of B. subtilis were chosen due to the presence of similar orthologs that are encoded in the C. acetobutylicum genome (83, 92). These genes were csfB (CAC0296), gpr (CAC1275), spoIIP (CAC1276), sigG (CAC1696), lonB (CAC2638), and spoIIR (CAC2898), and they were previously predicted to have a σF- or σG-like binding motif upstream of the start codon (93). Interestingly, of the six genes tested, only sigG exhibited a likely dependence on σF for its expression. These data suggest that the regulon of σF in C. acetobutylicum is apparently quite different from that in B. subtilis. The sigF inactivation mutant was able to produce solvents in an inoculum-dependent manner. If cultures of the sigF inactivation mutant were inoculated by using cells from mid- to late exponential phase (as is typical with WT C. acetobutylicum cultures), the final solvent levels were considerably lower than those in the WT culture. On the other hand, if the sigF inactivation mutant culture was inoculated from a stationary-phase culture, normal levels of solvents were produced (88). A potential mechanism for this bistable phenotype is discussed in a separate section, below.
FIG 6.
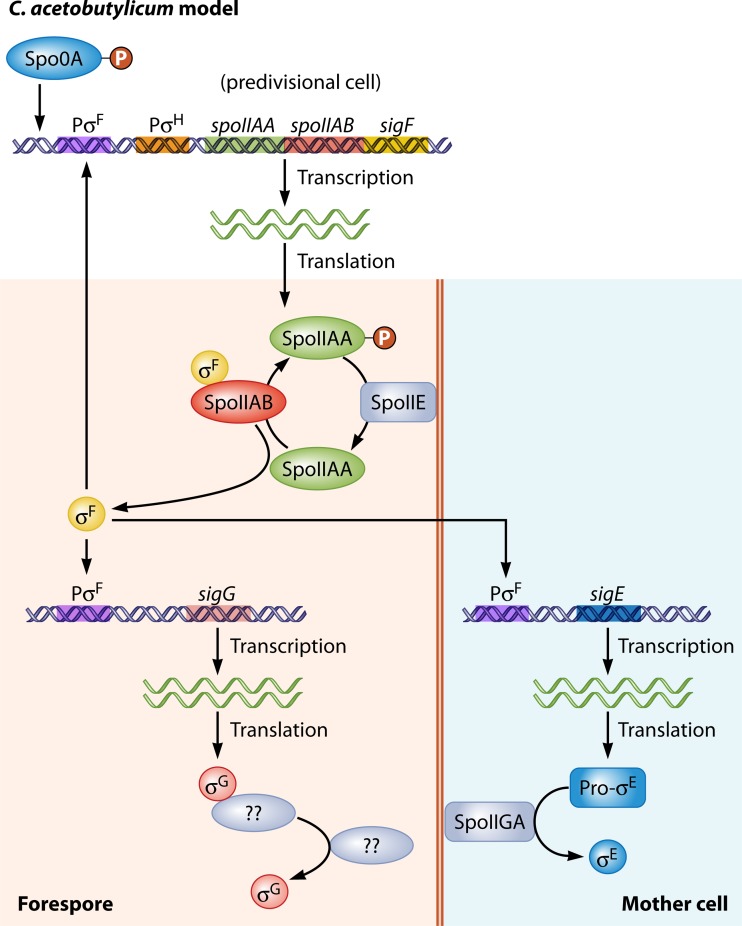
Transcriptional and posttranslational control of σF, σE, and σG in C. acetobutylicum. Initial transcriptional expression of the spoIIAA operon is regulated by Spo0A∼P and σH. Once translated, σF is held inactive by the sequestrating actions of SpoIIAB while also inhibiting SpoIIAA by phosphorylating it. To release σF from inhibition, the membrane-bound SpoIIE dephosphorylates SpoIIAA∼P, resulting in its binding to SpoIIAB, thus releasing σF from inhibition. Once activated, σF autoregulates its own expression as well as those of both sigG and sigE. Initially, σE, the mother cell-specific sigma factor, is translated in the inactive form, pro-σE, which is subsequently activated by the cleavage of the prosequence by the membrane-bound protease SpoIIGA. In the forespore compartment, σG is translated, but its actions are hypothesized to be inhibited by a yet-to-be-determined factor analogous to σF inhibitions.
The sigF gene was also disrupted in the important pathogen C. difficile (generating sigF disruption mutant strain AHCD533) (94). As expected, the C. difficile sigF mutant was unable to form any heat-resistant spores. TEM image analysis revealed that the sigF disruption mutant had its sporulation blocked at asymmetric division and was unable to progress further in spore morphogenesis, which is similar to the observations of the B. subtilis sigF mutant (25, 91, 94) (Fig. 5). However, a more electron-translucent region with some electron-dense layers was observed in some of the C. difficile sigF mutant cells, which is in contrast to the B. subtilis sigF mutant. Additionally, DNA staining showed that the DNA was strongly localized in the forespore in the C. difficile sigF mutant (25, 94). Using transcriptional SNAPCd fusions to CD2470 (the ortholog of the B. subtilis gpr gene), which is predicted to be under the control of σF, Pereira et al. demonstrated that σF is active exclusively in the forespore despite being transcribed in both the mother cell and forespore compartments (94). Genome-wide gene transcription analysis of the C. difficile sigF mutant revealed that 25 genes were downregulated in the C. difficile sigF mutant, indicating that they are potentially under σF control (95). Unlike in C. acetobutylicum, it appears that gpr, spoIIR, and spoIIP are all under the control of σF in C. difficile (95).
Recently, the sigF gene was disrupted in C. botulinum ATCC 3502 (96). As expected, the C. botulinum sigF disruption mutant was unable to form heat-resistant spores, and TEM image analysis revealed that sporulation was blocked at stage II (Fig. 5) (96). Additionally, Q-RT-PCR data showed that expression levels of spo0A in the C. botulinum sigF disruption mutant were significantly lower than those in the parent strain and that the cells were also unable to induce the expression of either sigE or sigG, while levels of sigK were also significantly lower in the mutant strain (96).
The data from sigF inactivation mutants generated in Clostridium organisms to date implicate σF in early stages of sporulation (86, 88, 94). However, there are differences between the sigF mutants generated in Clostridium and those in B. subtilis. TEM images revealed that the C. acetobutylicum sigF inactivation mutant (88) appeared to have its sporulation blocked prior to stage II, while the B. subtilis sigF mutant did not complete stage III (91) (Fig. 5). This is a significant difference between the two organisms. How σF is involved in the initiation of septum formation in C. acetobutylicum is still unclear. It is unknown at what stage of the sporulation process the sigF mutant in C. perfringens is blocked, although it appears that, like C. acetobutylicum, the mutant was impaired for the production of σE. This is also a departure from the B. subtilis model, in which the expression of sigE was shown to be dependent on σA (27, 89). Moreover, Q-RT-PCR analysis of the C. acetobutylicum sigF inactivation mutant revealed that, with the exception of sigG, the established regulon of σF in B. subtilis is considerably different in C. acetobutylicum (83, 88). This would partially explain why the sigF mutants of both Bacillus and Clostridium appeared to be blocked at different stages of sporulation (stage II versus stage III).
σE, the First Mother Cell-Specific Sigma Factor, Is Involved in Stage II of Sporulation
In B. subtilis, σE is the first mother cell-specific sigma factor to be activated. Expression of the sigE operon was shown to be dependent on σA and activated Spo0A∼P (27, 89, 97, 98). Initially, σE is translated into an inactive form, pro-σE, which requires the cleavage, by the SpoIIGA protease, of a 27-amino-acid residue from its N terminus to release the mature σE. SpoIIGA is the product of the first gene in the sigE operon (99). Disruption of sigE in B. subtilis blocks sporulation at stage II, and the mutant cells exhibited a disporic morphology (Fig. 5) that is quite similar to that of the B. subtilis sigF inactivation mutant (91).
The first reported study in which the sporulation-specific σE was disrupted in a Clostridium organism came from the pathogen C. perfringens (63). The sigE disruption mutant (sigE::pNLDE; named KM2) was unable to produce any heat-resistant endospores, and TEM image analysis revealed that the cells never developed beyond stage II and that a few cells exhibited a disporic morphology, similar to what has been previously reported for B. subtilis (91) (Fig. 5). sq-RT-PCR analysis of the sporulation-related genes sigF, sigE, sigG, and sigK in WT C. perfringens strain SM101 revealed that these genes were expressed during early exponential growth. This is in contrast to the B. subtilis and C. acetobutylicum counterparts, in which high-level expression of these genes was not observed during early exponential growth but rather during the transitional and early stationary phases of culture as the cells commit to sporulation (21). In the sigE disruption mutant, however, the sigF and sigG transcripts were expressed only during early stationary phase, and not during exponential phase, as in the WT case. In addition, the sigK transcript accumulated successfully in the sigE disruption mutant, which provided the first evidence that σK maybe activated before σE (Fig. 3) and potentially other sigma factors (discussed in more detail in the section on σK, below). In the same study (63), the authors also generated a sigK mutant (sigK::pNLDK; named KM1), and promoter reporter fusion assays of the sigK disruption mutant and the sigE disruption mutant demonstrated that induction of expression from the spoIIGA operon (i.e., the sigE operon) was dependent on the presence of both σK and σE. Western blot analyses using antibodies raised against the B. subtilis σE and σK proteins showed that no σE protein could be detected in the sigK disruption mutant, further confirming that σK is activated upstream of σE and is necessary for the successful expression of σE. Additionally, only small amounts of pro-σK protein were detected in the sigE disruption mutant, indicating that some other sigma or transcription factor was needed for transcription initiation from the sigK promoter and/or the successful processing of pro-σK to the mature σK. Moreover, the SpoIIID protein, a DNA-binding protein that aids in the transcription of the sigK gene in B. subtilis (100), was detected in the sigE mutant of C. perfringens, thus indicating that it is not under σE transcriptional control, which is the case in B. subtilis. Finally, those authors demonstrated that the production of the CPE is dependent upon σE.
A sigE disruption mutant (sigE::pKORSIGE; named EKO1) was also generated for C. acetobutylicum (26). The sigE disruption mutant was unable to form viable spores, and TEM images revealed that sporulation was blocked prior to stage II (Fig. 5). This is in contrast to sigE disruption mutants of both B. subtilis (91) and C. perfringens (63), for which sporulation appears to be blocked at early stage II, as is evident by the ability of the mutants to form disporic cells. Additionally, TEM images showed that the sigE disruption mutant failed to accumulate any detectable levels of granulose, thus implicating σE in the synthesis of granulose bodies which are intracellular structures made of amylopectin that accumulate during sporulation (101), and possibly in the morphogenesis of the cigar-shaped clostridial cell form. Western blot analysis detected the presence of σF, σG, and Spo0A proteins in the sigE disruption mutant. The presence of the σG protein in the sigE disruption mutant demonstrates that σG is not part of the sigE operon and that its expression is independent of σE. However, the protein levels of σF were up to 10 times lower in the sigE disruption mutant than in the WT, possibly indicating a role for σE in the transcriptional activation of sigF. This is consistent with the B. subtilis model in which sigF was also shown to be transcribed from a σE-dependent promoter (in addition to the σH-dependent promoter discussed above) (102, 103). Like the C. acetobutylicum sigF disruption mutant discussed above, the age of the inoculum (i.e., the stage of growth at which the inoculum was withdrawn) had a profound effect on the ability of the sigE disruption mutant to produce solvents. Like the sigF disruption mutant, if the inoculum was harvested at the mid-exponential growth phase, the ability of the subsequent batch culture to produce normal amounts of solvents was significantly impaired. However, if the inoculum came from the stationary phase, the ability of the subsequent batch culture to produce normal levels of solvents was restored. A potential explanation for this bistable phenotype is discussed below.
TEM image analysis of the sigE disruption mutant in C. difficile revealed that this mutant had its sporulation blocked at asymmetric division (Fig. 5) and, as expected, was unable to form any heat-resistant spores (25, 94). The sigE mutants formed disporic cells similar to those observed in both the B. subtilis and C. perfringens sigE mutants (63, 91). This is in contrast to the C. acetobutylicum sigE disruption mutant, in which the cells do not complete asymmetric division (26). The PspoIIIA-SNAPCd promoter fusion in C. difficile showed that σE was confined to the mother cell, similar to what is seen for B. subtilis (94). Transcriptional profiling using microarray analysis showed that there are 97 genes under the control of σE in C. difficile (95). As in B. subtilis, this makes the C. difficile σE regulon the largest regulon among the four sporulation-specific sigma factors. Genes under σE transcriptional control included those known to control engulfment, cortex formation, and initiation of coat assembly.
The sigE gene was also disrupted in C. botulinum ATCC 3502 (96). This sigE mutant was unable to form viable heat-resistant spores, and TEM image analysis showed that sporulation was blocked at stage II, similar to the C. botulinum sigF disruption mutant (Fig. 5) (96). In addition, Q-RT-PCR analysis showed that expression levels of spo0A, sigF, sigG, and sigK were all lower in the sigE mutant than in the parent strain (96).
σG, the Second Forespore-Specific Sigma Factor, Is Involved in Stage III of Sporulation
In B. subtilis, σG is the second prespore-specific sigma factor activated following the expression and activation of σF (104, 105). Once translated, σG is held inactive by an unknown factor, which was initially thought to be SpoIIAB, with SpoIIAA releasing it from inhibition in a manner analogous to the posttranslational control of σF (27, 106). However, another study showed that the actions of SpoIIIA were sufficient to release σG from the inhibitory action of the unknown factor through a pathway that was facilitated by SpoIIIJ (27, 107). Once activated, σG drives the expression of a number of forespore-specific genes, such as sspE, which is one of five genes that encode the small acid-soluble proteins (SASPs). It is also responsible for stimulating the expression of the SpoIVB signaling protein that is involved in the posttranslational activation of the mother cell-specific σK (105, 108, 109).
The sigG gene was disrupted in C. perfringens (SM101::sigG), and inactivation of σG led to abolished sporulation (86). However, the ability of the sigG disruption mutant to produce toxins was not affected, indicating that unlike σF, σG does not play a role in toxin formation. Western blot analysis indicated that the disruption of sigG had no effect on the production of either the σE or σK protein, suggesting that σG is not directly responsible for the transcription of either of these sigma factors. However, it is unclear at what stage of sporulation the C. perfringens SM101::sigG strain was arrested, as no TEM images were presented. Still, one would assume that since the σF, σE, and σK proteins were all detected by Western analysis, the sigG disruption mutant was further along in the differentiation stages than the sigF disruption mutants generated in C. acetobutylicum and C. perfringens (86, 88). Like B. subtilis and C. acetobutylicum (26, 88), production of σG was dependent on σF, as no σG protein was detected in the sigF disruption mutant (86). Bioinformatics analysis identified a σF-binding consensus sequence in the promoter region of sigG in C. perfringens (86).
The second study that confirmed the role of σG in spore development in Clostridium came from the sigG disruption mutant (GKO1) of C. acetobutylicum (26). Like C. perfringens, the C. acetobutylicum sigG disruption mutant was unable to form any viable spores. Additionally, Western analysis detected Spo0A, σF, and σE proteins, indicating that σG is further downstream in the sporulation cascade than either σF or σE. This was further confirmed by TEM analysis, in which the sigG disruption mutant strain completes engulfment (stage III) and begins development of the endospore (stages IV to VI) but aborts development at some point before full maturation (Fig. 5). The sigG disruption mutant was able to form granulose vesicles, which suggests that σG does not directly have a role in granulose biosynthesis. Additionally, sigG disruption did not have any adverse effects on the production of solvents, which indicates that sigG does not play a role in the expression of the genes that are essential for solventogenesis (26).
Disruption of sigG in C. difficile resulted in an asporogenous phenotype, as evidenced by the inability of the mutant to form heat-resistant endospores (94). TEM image analysis revealed that the C. difficile sigG mutant completed engulfment but did not proceed any further in spore morphogenesis (94) (Fig. 5). However, the forespore appeared ill formed, with multiple defects observed, which included forespore ruffling and incomplete engulfment (94). Additionally, an electron-dense region was seen around the forespore, indicating deposition of spore coat proteins (94), which is not seen in the B. subtilis sigG mutant (104). Transcriptional profiling using microarray analysis showed that there are 50 genes under the control of σG in C. difficile (95), including genes responsible for the import of dipicolinic acid, cell wall synthesis, and cortex formation (95). Expression of the promoter fusion PsigG-SNAPCd was not detected in the C. difficile sigF mutant, indicating that σF is necessary for sigG expression (94).
Recently, sigG was disrupted in C. botulinum ATCC 3502, and the mutant was unable to form viable heat-resistant spores (96). TEM image analysis revealed that the C. botulinum sigG disruption mutant, like the C. acetobutylicum sigG disruption mutant (26), was able to complete the engulfment stage of spore morphogenesis (stage III) (96). Spore staining with malachite green indicated the presence of a spore coat; however, the cells were still unable to form heat-resistant spores (96). Q-RT-PCR analysis revealed that the expression level of spo0A in the sigG disruption mutant was significantly lower than that in the parent strain (96). Additionally, induction of sigE and sigF expression was absent during late exponential and transitional phases in the sigG mutant relative to the parent strain, and the expression level of sigK was significantly lower during stationary phase (96).
σK Has a Second Role, Late in Sporulation, as the Second Mother Cell-Specific Sigma Factor and Is Involved in Stage IV of Sporulation
Evidence from C. acetobutylicum showed that not only is σK needed during the very early stages of sporulation (66) (as discussed above), it is also necessary for the terminal stages of sporulation, analogous to its role in B. subtilis. In order to demonstrate the role of σK in late sporulation, the initial involvement of σK in the sporulation cascade was bypassed by expressing spo0A from the strong and early ptb promoter (110) in the sigK deletion strain [termed ΔsigK(p94Spo0A)] (66). The ΔsigK(p94Spo0A) strain was able to produce solvents (which is controlled by activated Spo0A∼P), and Western analysis showed that protein and/or mRNA expression of Spo0A, σF, σE, and σG was restored. However, spore assays and TEM analysis revealed that the ΔsigK(p94Spo0A) strain (Fig. 5) was still unable to successfully complete the sporulation process (66). TEM image analysis (Fig. 5) showed that these cells proceeded to a more advanced stage of spore morphogenesis than did cells of the C. acetobutylicum sigG disruption mutant (26) and appeared to be releasing an immature or severely underdeveloped forespore (66). These findings demonstrate that σK plays a critical role in late sporulation as well as in very early sporulation prior to Spo0A activation, as supported by the nonsporulating phenotype of the ΔsigK(p94Spo0A) strain.
This second, late σK role is probably also valid for both C. perfringens and C. botulinum, both of which were shown to have early activity of σK. However, in C. difficile, it was shown that σK acts exclusively during the later stages of sporulation, with no early role, which is in contrast to the role of σK in C. acetobutylicum, C. botulinum, and C. perfringens but is in agreement with the B. subtilis model. Disruption of sigK in C. difficile was achieved by the insertional inactivation of the retargeted group II intron in codon 34 in the 5′ end of the split sigK gene that is interrupted by skinCd (25, 94). Surprisingly, disruption of sigK in C. difficile did not lead to the complete loss of sporulation, as 103 heat-resistant spores/ml were detected at 72 h (25, 94). This finding is in stark contrast to the sigK mutants generated in B. subtilis, C. perfringens, C. botulinum, and C. acetobutylicum, all of which resulted in an asporogenous phenotype (37, 63, 64, 66). TEM image analysis revealed that the C. difficile sigK mutant produced forespores with what appears to be a cortex layer, but no coat layer was observed (94) (Fig. 5). Additionally, phase-contrast microscopy showed the presence of some phase-bright spores, yet free spores were sparsely seen (94). Moreover, the transcription of sigK appeared to be confined to the mother cell, which is consistent with its role as a mother cell-specific sigma factor (94). Global transcription profiling revealed that there are 56 genes that are putatively under the control of σK in C. difficile (95). These included genes that play a role in spore coat assembly, spore maturation, and mother cell lysis (95).
REGULATORY FACTORS INVOLVED IN ACTIVATING SPORULATION-SPECIFIC SIGMA FACTORS
In the B. subtilis model, all sigma factors are initially held inactive after translation, through various mechanisms, until a specific signal releases the active form: σF is held inactive by an anti-sigma factor protein, both σE and σK must have a leader sequence removed from their N terminus before they become active (as discussed above), and σG has a complex regulatory structure that is still not fully understood (27). Orthologs of most of these regulatory factors have been identified in Clostridium organisms, but functional studies to confirm their role have only recently been started.
Regulation of σH
In B. subtilis, the transcriptional regulator AbrB suppresses transcription of the sigH gene, thus indirectly affecting the expression of spo0A, since σH helps promote the expression of spo0A. In this way, AbrB acts as an inhibitor of sporulation. abrB in turn is negatively controlled by Spo0A∼P. This feedback loop ensures that sporulation is delayed until a certain threshold level of Spo0A∼P is reached, at which time abrB is fully suppressed and sigH is fully relieved, leading to additional transcription of spo0A and even higher levels of Spo0A∼P (36).
In Clostridium organisms, AbrB's role in the regulation of sporulation has been investigated only in C. acetobutylicum. Three homologs of abrB are annotated in the genome of C. acetobutylicum: abrB310, abrB1941, and abrB3647 (92, 111). Of these, only abrB310 appeared to be actively transcribed based on chloramphenicol acetyltransferase (CAT) reporter analysis (111), and when expression of this gene is knocked down by using antisense RNA, both solventogenesis and differentiation are delayed (111). This result is in agreement with B. subtilis abrB-null mutants, in which sporulation is delayed in the absence of abrB (112). Thus, even though ArbB is a repressor of sporulation, its expression is still required for the proper timing of differentiation. However, it is still unclear if the role that abrB310 plays in clostridial sporulation is analogous to its role in B. subtilis.
Regulation of σF
In the B. subtilis model, the regulation of σF involves three factors: SpoIIAB (the anti-sigma factor), SpoIIAA (the anti-anti-sigma factor), and SpoIIE (a membrane-bound phosphatase). spoIIAB and spoIIAA are transcribed with sigF in a tricistronic operon (113, 114), while spoIIE is transcribed independently. Upon translation, SpoIIAB binds to and keeps σF sequestered while also phosphorylating SpoIIAA, preventing it from interacting with SpoIIAB to release σF (Fig. 6). The membrane-bound SpoIIE is localized to the newly formed asymmetric septum and dephosphorylates SpoIIAA (27). The dephosphorylated SpoIIAA then binds to SpoIIAB, resulting in the release of σF and allowing it to become active. Thus, without SpoIIE, σF would never be released from SpoIIAB and would not become active, resulting in a phenotype similar to that of a σF deletion.
The crucial role of SpoIIE in activating sporulation was confirmed in the C. acetobutylicum spoIIE disruption mutant (strain SPOIIEKO) (115). Sporulation in the spoIIE disruption mutant was blocked prior to stage II, similar to the sigF inactivation mutant phenotype (88) (Fig. 5). Importantly, σF protein was detected in the disruption mutant although at lower levels than in the WT strain, suggesting an autostimulatory role for σF or the need for σE to further stimulate σF expression, as has been suggested (26). Neither σE nor σG was detected. While confirming SpoIIE's role in activating σF, the mechanism of how it activates σF remains unclear. In B. subtilis, SpoIIE is localized to the asymmetric septum, and the larger surface area-to-volume ratio of the prespore compartment allows dephosphorylated SpoIIAA to build up to a critical level to effectively bind all of SpoIIAB, thus activating σF (103). In the larger mother cell compartment, not enough dephosphorylated SpoIIAA can be achieved to overcome the phosphorylation effect of SpoIIAB. However, in C. acetobutylicum, σF and σE are needed for asymmetric septum formation (26, 88); thus, it is not clear how SpoIIE would activate σF without the large surface area-to-volume ratio of the prespore.
PHENOTYPIC VARIATION, BISTABILITY, AND EPIGENETIC REGULATION IN RELATION TO SPORULATION
There are several experimental observations that demonstrate nontrivial phenotypic variation among isogenic Clostridium cultures, giving rise to two or more distinct cellular states. This is referred to as bistability (116). Such variations have been documented for many years in prokaryotic systems, but the systematic examination of such variations has received increased attention only in the last few years (117). Phenotypic variation can result from the structure of genetic regulatory networks that give rise to distinct phenotypes within an otherwise clonal population or can derive from epigenetic phenomena such as DNA methylation or epigenetic inheritance. The latter refers to the passage of cellular properties from one generation to the other without any changes in the DNA of the cells (117). Phenotypic variation, bistability, and epigenetic inheritance are frequently interrelated, as has been elegantly reviewed (117). Their study increasingly provides a molecular understanding of complex phenotypic phenomena, especially for sporulating organisms.
Bistability requires an appropriate feedback circuit (notably, a positive feedback loop), a nonlinearity that is part of this circuit, and a “good” balance between the two legs of the feedback loop (116). This is captured in a simple way in Fig. 7A. Bistability has been well established for B. subtilis sporulation, where the bistable phenotype (sporulate versus do not sporulate) is derived from the positive feedback of phosphorylated Spo0A on its own transcription and the nonlinear dynamics introduced by the phosphorelay system (KinA, Spo0F, and Spo0B), which is part of this positive feedback circuit (118) (Fig. 7B). This bistability is intimately associated with epigenetic inheritance, which has also been well documented for B. subtilis, where it was shown that the signal to initiate the sporulation process (the sporulation phosphorelay system) is present during exponential growth and appears to be epigenetically passed on to the next generation and for several generations (118). Although the bistability phenotype of sporulation in B. subtilis is for now ascribed to the nonlinearity of the phosphorelay system, sporulation in Clostridium organisms displays signs of bistability despite the absence of a phosphorelay system.
FIG 7.
The concept of bistable phenotypes, the basis of bistability (sporulate versus do not sporulate in B. subtilis), and the proposed basis for bistable phenotypes in C. acetobutylicum. (A) The three key elements that lead to bistable phenotypes: a positive feedback circuit with embedded nonlinearities (shown by the red twisting lines) and a good balance in the two arms of the circuit. (B) These key requirements are present in the B. subtilis model of sporulation: on the right, it is the “down” positive but highly nonlinear control of Spo0A phosphorylation through the phosphorylation relay, and on the left, there are several “up” positive feedback circuits, with two resulting from the direct transcriptional stimulation of spo0A and spo0F by activated (phosphorylated) Spo0A. Two other loops are derived from the transcriptional stimulation of kinA expression by phosphorylated Spo0A via two negative feedback loops involving AbrB and σH. Additional such positive feedback loops exist in the B. subtilis model. (C) Cartoon displaying the two feedback loops from Spo0A to σK, which we propose are the basis for the observed bistable phenotypes in C. acetobutylicum, as discussed in the text.
Phenotypic Variations of Note in Clostridium Organisms
There are four documented phenomena of phenotypic variation in Clostridium that appear to be related to bistability and epigenetic inheritance.
First, and perhaps foremost, is the so-called “strain degeneration” issue, which has been known since Pasteur's time, namely that continuous, vegetative transfers of solventogenic Clostridium organisms lead to a greatly diminished (or a loss of the) ability to produce solvents and to sporulate (119). Degeneration can be permanent, as in the case of C. acetobutylicum, whereby the genes for solvent formation are carried on the pSOL1 megaplasmid, which, when lost, leads to a permanent asporogenous strain that produces no solvents (119). However, in most other solventogenic Clostridium organisms, the solventogenic genes are carried on the chromosome, and thus, a different mechanism must account for the degeneration process, which in most cases can be at least partially restored by altering culture conditions (119). Significantly, starting cultures by “heat shocking” spores almost invariably guarantees good solvent production and reasonable sporulation (119). We propose that this degeneration phenotype is derived from the bistability of the system due to the large positive feedback circuit (Fig. 3B and 7C) that is generated by the biphasic action of σK. This circuit would satisfy the bistability requirement for a feedback circuit, a nonlinearity in the circuit, and a good balance between the two legs of the feedback loop (116). In essence, as the σK-controlled expression of Spo0A propagates down the sporulation cascade through σF, σE, and σG and then to the 2nd phase of σK action, the stochasticity of gene expression and protein activation could lead to either strong or weak σK expression the next time around the circuit (Fig. 3B and 7C), thus affecting sustained Spo0A expression and activation (see also reference 21). This can lead to either a weakening or strengthening of the signal and, thus, a bistable phenotype. One of the explanations as to why heat shocking reestablishes a strong sporulation and solvent-producing phenotype is the epigenetic inheritance of the sporulation signal (which would be preserved in spores and passed on to the next generation of cells upon germination), as in B. subtilis (118). This signal could be phosphorylated Spo0A, a protein, or a small molecule, which leads to strong Spo0A expression and activation (and thus to strong sporulation and solvent formation).
A second phenotype is the low, variable, and stochastic-like frequency of sporulation of solventogenic Clostridium organisms (23). According to the proposed model (Fig. 3B) for C. acetobutylicum, and likely most other Clostridium organisms, the positive feedback loop between Spo0A and σK appears to ensure that the signal to sporulate remains strong during the sporulation cycle. Sporulation in B. subtilis requires a high threshold level of Spo0A expression (120), and this is likely valid for C. acetobutylicum based on detailed transcriptional data (21), whereby all major sigma factors display a bimodal pattern of expression, with low early expression levels followed by strong upregulation later. Thus, the proposed model provides a mechanism by which the required threshold expression level of the sporulation-related genes is maintained at high enough levels to ensure the successful completion of sporulation. However, it is well known that sporulation is not always very strong and does not always take place at a high frequency in C. acetobutylicum (23) and other Clostridium organisms, and this suggests a weak link in the feedback loop (Fig. 3B) that results in a weakening of the signal, thus leading to weak initiation or aborted completion of sporulation.
A third phenotype relates to the mechanism by which, as discussed above, σK activity becomes available for very, very early exponential growth of C. acetobutylicum to make Spo0A expression possible (66). We argued that epigenetic inheritance may play a role here (66). We hypothesize that σK is passed down to the spore and subsequently to the vegetative cells after germination via epigenetic inheritance (117), similar to how the sporulation signal is inherited in B. subtilis (118). Moreover, it was shown that in B. subtilis, these sporulation-specific sigma factors are not always confined to the compartment in which they are predominantly needed in the developing endospore or mother cell. For example, it was shown that σG can become active in the mother cell (121), although it is a prespore-specific sigma factor. Could it be that σK behaves in a similar manner by becoming active in the prespore and subsequently inherited in its active form in the mature spore? Next, once the spore starts to germinate, σK may “jump start” the expression of spo0A, either directly or indirectly. Indeed, a σK-like consensus binding sequence was identified, albeit with a slightly larger spacer sequence, in the spo0A promoter region (66).
The fourth phenotype is the finding that the pattern of solvent formation by the C. acetobutylicum sigF and sigE inactivation mutants is dependent on the age of the inoculum: inocula from the mid- to late exponential phase result in weak solvent production, in contrast to cultures initiated with inocula drawn from early exponential or stationary phase, which produce normal levels of solvents (26, 88). This phenotype is not observed in the sigG inactivation mutant, where solvent formation is independent of the inoculum age, as is the case for the parent (WT) strain (26). This bistable phenotype should be examined in light of four key facts. Two older, well-established facts are that Spo0A expression and activation are necessary for transcribing the solventogenic genes (1, 122) and that Spo0A expression and activation appear to be bimodal and taking place in mid- to late exponential phase (21). Four recent findings are likely important in crafting a hypothesis to explain this bistable phenotype. Early expression of σK is necessary for Spo0A expression, and σE is the sigma factor that controls the late expression of σK (66). Finally, the sigF and sigE inactivation mutants express no σE (which controls late σK expression), but the sigG inactivation mutant expresses σE. These findings then suggest that the mid- to late-exponential-phase-derived inocula of the sigF and sigE mutants cannot sustain good expression or activation of Spo0A, unlike inocula from other stages of the cultures (early exponential or stationary phase). These findings also suggest that the sigF and other σ mutants do not express σK late, in contrast to the sigG mutant and the WT cells, which do. These observations suggest that the bistable solventogenic phenotype of the sigF and sigE mutants could be explained by the interplay of σK and Spo0A expression and activation in enabling solventogenesis. In this hypothesis, a strong Spo0A expression/activation signal might not be temporarily available in mid- to late-exponential-phase-cells of the sigF or sigE mutants, in contrast to early-exponential- or stationary-phase cells or WT and sigG mutant cells.
In summary, if the signaling of sporulation (Fig. 3B) is preserved in other Clostridium organisms, and notably in Clostridium pathogens, there are significant implications of bistable phenotypes that this circuit can lead to and in understanding and treating any potentially disease-associated bistable phenotypes.
CONCLUDING REMARKS
Sporulation is a fundamental feature of many Firmicutes. Most of our current knowledge regarding the mechanism of sporulation and its regulation, at both the molecular and cellular levels, stems from investigations of B. subtilis, and it was assumed that these mechanisms were conserved between the spore-forming genera of Bacillus and Clostridium. However, as discussed above, it appears that the regulation of sporulation in Clostridium is drastically different from that in Bacillus at both the molecular and cellular levels. While much has been achieved in the last few years in the understanding of key aspects of the molecular underpinning of Clostridium sporulation, much remains to be elucidated, and as a result, reliance on the B. subtilis model remains heavy. While it is clear that the regulation of sporulation in Clostridium organisms differs from that in Bacillus, it is equally clear that there is a high level of diversity in the regulation of sporulation, even among Clostridium organisms, despite the apparent similarities. Much remains to be investigated regarding the implications of this diversity, including the identification of the regulons of the key sporulation-specific sigma factors and Spo0A. With genomic tools currently becoming more accessible and accurate, the hope is that identification of these regulons will lead to a better understanding of this diversity and, by comparative analysis, identify the source of robustness of the sporulation process in these and other endospore formers. Indeed, an understanding of the mechanisms underlying the governance of sporulation in Clostridium is paramount in finding ways to combat the pathogenic members of this genus, such as C. perfringens and C. botulinum, as well as exploiting the metabolic repertoire of those of industrial and environmental relevance. These species include those that produce solvents, such as C. acetobutylicum, C. pasteurianum, and C. beijerinckii; the acetogens that grow on CO2/CO/H2, including C. ljungdahlii and C. carboxidivorans, to name a few (123); and the cellulolytic clostridia, including C. thermocellum, C. cellulolyticum, and C. phytofermentans, among others (123).
ACKNOWLEDGMENTS
This work was supported, in part, by a genomic science grant from the U.S. Department of Energy (grant number DE-SC0007092). Financial support for Mohab A. Al-Hinai was provided by the Government of Oman.
Biographies

Mohab A. Al-Hinai is currently an Assistant Professor at Sultan Qaboos University, Oman. He started his scientific career at Sultan Qaboos University, where he earned a B.Sc. in Biotechnology in 2003. He later received his M.Sc. and Ph.D. from Northwestern University in Evanston, IL, and the University of Delaware in Newark, DE, respectively, working in the research group of Professor E. T. Papoutsakis. During his graduate career, his research focused on expanding the genetic toolbox of clostridia to enable genetic and physiological studies of sporulation-deficient mutants as well as genetic engineering of superior biofuel-producing strains. His current research focuses on the engineering of microbial platforms for the production of renewable energy and biofuels.

Shawn W. Jones is currently a Research Scientist at Elcriton Inc. in New Castle, DE. He earned his B.S. in Chemical Engineering at NC State University in Raleigh, NC, and working with Prof. E. T. Papoutsakis, he then received his Ph.D. in Chemical Engineering from Northwestern University in Evanston, IL. After a postdoc with Prof. E. T. Papoutsakis, Dr. Jones joined Elcriton and has worked to develop novel strains of clostridia for various industrial applications.

Eleftherios T. Papoutsakis is currently Eugene DuPont Professor at the Department of Chemical and Biomolecular Engineering, Department of Biological Sciences, and Faculty Fellow at the Delaware Biotechnology Institute at the University of Delaware. He received his undergraduate education at the National Technical University of Athens, Greece, and his M.S. and Ph.D. from Purdue University in West Lafayette, IN. He started his academic career at Rice University in Houston, TX, where he worked with two prominent biologists, George Bennett and the late Fred Rudolph, to start developing fundamental and applied tools for Clostridium biology and biotechnology. Later, he moved to Northwestern University in Evanston, IL, where he taught and did research for almost 20 years before moving to the University of Delaware. Prof. Papoutsakis' group is active and has made important contributions in Clostridium genetics and metabolic and genome engineering. His laboratory has pioneered the development of transformation and chromosomal integration protocols, expression vectors, antisense RNA design, genome-scale metabolic models, and the identification of small noncoding RNAs of importance to Clostridium biology. His laboratory has trained over 55 Ph.D. students and 25 postdoctoral fellows.
REFERENCES
- 1.Paredes CJ, Alsaker KV, Papoutsakis ET. 2005. A comparative genomic view of clostridial sporulation and physiology. Nat Rev Microbiol 3:969–978. doi: 10.1038/nrmicro1288. [DOI] [PubMed] [Google Scholar]
- 2.Sekirov I, Russell SL, Antunes LCM, Finlay BB. 2010. Gut microbiota in health and disease. Physiol Rev 90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 3.Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, Dore J. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol 65:4799–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruggemann H, Baumer S, Fricke WF, Wiezer A, Liesegang H, Decker I, Herzberg C, Martinez-Arias R, Merkl R, Henne A, Gottschalk G. 2003. The genome sequence of Clostridium tetani, the causative agent of tetanus disease. Proc Natl Acad Sci U S A 100:1316–1321. doi: 10.1073/pnas.0335853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sebaihia M, Peck MW, Minton NP, Thomson NR, Holden MTG, Mitchell WJ, Carter AT, Bentley SD, Mason DR, Crossman L, Paul CJ, Ivens A, Wells-Bennik MHJ, Davis IJ, Cerdeno-Tarraga AM, Churcher C, Quail MA, Chillingworth T, Feltwell T, Fraser A, Goodhead I, Hance Z, Jagels K, Larke N, Maddison M, Moule S, Mungall K, Norbertczak H, Rabbinowitsch E, Sanders M, Simmonds M, White B, Whithead S, Parkhill J. 2007. Genome sequence of a proteolytic (group I) Clostridium botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res 17:1082–1092. doi: 10.1101/gr.6282807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N, Stabler R, Thomson NR, Roberts AP, Cerdeno-Tarrraga AM, Wang HW, Holden MTG, Wright A, Churcher C, Quail MA, Baker S, Bason N, Brooks K, Chillingworth T, Cronin A, Davis P, Dowd L, Fraser A, Feltwell T, Hance Z, Holroyd S, Jagels K, Moule S, Mungall K, Price C, Rabbinowitsch E, Sharp S, Simmonds M, Stevens K, Unwin L, Whithead S, Dupuy B, Dougan G, Barrell B, Parkhill J. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet 38:779–786. doi: 10.1038/ng1830. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci U S A 99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, Dore J, Corthier G, Furet JP. 2009. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ljungdahl LG. 2009. A life with acetogens, thermophiles, and cellulolytic anaerobes. Annu Rev Microbiol 63:1–25. doi: 10.1146/annurev.micro.091208.073617. [DOI] [PubMed] [Google Scholar]
- 10.Dürre P. 2005. Sporulation in clostridia (genetics), p 659–669. In D̈urre P. (ed), Handbook on clostridia. CRC Press, Boca Raton, FL. [Google Scholar]
- 11.Lambin P, Theys J, Landuyt W, Rijken P, Van der Kogel A, Van der Schueren E, Hodgkiss R, Fowler J, Nuyts S, de Bruijn E, Van Mellaert L, Anne J. 1998. Colonisation of Clostridium in the body is restricted to hypoxic and necrotic areas of tumours. Anaerobe 4:183–188. doi: 10.1006/anae.1998.0161. [DOI] [PubMed] [Google Scholar]
- 12.Minton NP, Mauchline ML, Lemmon MJ, Brehm JK, Fox M, Michael NP, Giaccia A, Brown JM. 1995. Chemotherapeutic tumor targeting using clostridial spores. FEMS Microbiol Rev 17:357–364. doi: 10.1111/j.1574-6976.1995.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 13.Green EM. 2011. Fermentative production of butanol—the industrial perspective. Curr Opin Biotechnol 22:337–343. doi: 10.1016/j.copbio.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Schultz D, Wolynes PG, Jacob EB, Onuchic JN. 2009. Deciding fate in adverse times: sporulation and competence in Bacillus subtilis. Proc Natl Acad Sci U S A 106:21027–21034. doi: 10.1073/pnas.0912185106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens C. 1998. Bacterial sporulation: a question of commitment? Curr Biol 8:R45–R48. doi: 10.1016/S0960-9822(98)70031-4. [DOI] [PubMed] [Google Scholar]
- 16.Sorg JA, Sonenshein AL. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long S, Jones DT, Woods DR. 1984. The relationship between sporulation and solvent production in Clostridium acetobutylicum P262. Biotechnol Lett 6:529–534. doi: 10.1007/BF00139997. [DOI] [Google Scholar]
- 18.Sauer U, Santangelo JD, Treuner A, Buchholz M, Dürre P. 1995. Sigma factor and sporulation genes in Clostridium. FEMS Microbiol Rev 17:331–340. doi: 10.1111/j.1574-6976.1995.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 19.Yutin N, Galperin MY. 2013. A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. Environ Microbiol 15:2631–2641. doi: 10.1111/1462-2920.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dürre P, Böhringer M, Nakotte S, Schaffer S, Thormann K, Zickner B. 2002. Transcriptional regulation of solventogenesis in Clostridium acetobutylicum. J Mol Microbiol Biotechnol 4:295–300. [PubMed] [Google Scholar]
- 21.Jones SW, Paredes CJ, Tracy B, Cheng N, Sillers R, Senger RS, Papoutsakis ET. 2008. The transcriptional program underlying the physiology of clostridial sporulation. Genome Biol 9:R114. doi: 10.1186/gb-2008-9-7-r114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones D, Van der Westhuizen A, Long S, Allcock E, Reid S, Woods D. 1982. Solvent production and morphological changes in Clostridium acetobutylicum. Appl Environ Microbiol 43:1434–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tracy BP, Gaida SM, Papoutsakis ET. 2008. Development and application of flow-cytometric techniques for analyzing and sorting endospore-forming clostridia. Appl Environ Microbiol 74:7497–7506. doi: 10.1128/AEM.01626-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Hoon MJ, Eichenberger P, Vitkup D. 2010. Hierarchical evolution of the bacterial sporulation network. Curr Biol 20:R735–R745. doi: 10.1016/j.cub.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fimlaid KA, Bond JP, Schutz KC, Putnam EE, Leung JM, Lawley TD, Shen A. 2013. Global analysis of the sporulation pathway of Clostridium difficile. PLoS Genet 9:e1003660. doi: 10.1371/journal.pgen.1003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tracy BP, Jones SW, Papoutsakis ET. 2011. Inactivation of sigma(E) and sigma(G) in Clostridium acetobutylicum illuminates their roles in clostridial-cell-form biogenesis, granulose synthesis, solventogenesis, and spore morphogenesis. J Bacteriol 193:1414–1426. doi: 10.1128/JB.01380-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piggot PJ, Hilbert DW. 2004. Sporulation of Bacillus subtilis. Curr Opin Microbiol 7:579–586. doi: 10.1016/j.mib.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Siranosian KJ, Grossman AD. 1994. Activation of spo0A transcription by sigma H is necessary for sporulation but not for competence in Bacillus subtilis. J Bacteriol 176:3812–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins D, Dworkin J. 2012. Recent progress in Bacillus subtilis sporulation. FEMS Microbiol Rev 36:131–148. doi: 10.1111/j.1574-6976.2011.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molle V, Fujita M, Jensen ST, Eichenberger P, Gonzalez-Pastor JE, Liu JS, Losick R. 2003. The Spo0A regulon of Bacillus subtilis. Mol Microbiol 50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 31.Strauch MA, Spiegelman GB, Perego M, Johnson WC, Burbulys D, Hoch JA. 1989. The transition-state transcription regulator abrB of Bacillus subtilis is a DNA-binding protein. EMBO J 8:1615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi K, Shoji K, Shimizu T, Nakano K, Sato T, Kobayashi Y. 1995. Analysis of a suppressor mutation ssb (kinC) of sur0B20 (spo0A) mutation in Bacillus subtilis reveals that kinC encodes a histidine protein kinase. J Bacteriol 177:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez D, Fischbach MA, Chu F, Losick R, Kolter R. 2009. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci U S A 106:280–285. doi: 10.1073/pnas.0810940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shemesh M, Kolter R, Losick R. 2010. The biocide chlorine dioxide stimulates biofilm formation in Bacillus subtilis by activation of the histidine kinase KinC. J Bacteriol 192:6352–6356. doi: 10.1128/JB.01025-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tojo S, Hirooka K, Fujita Y. 2013. Expression of kinA and kinB of Bacillus subtilis, necessary for sporulation initiation, is under positive stringent transcription control. J Bacteriol 195:1656–1665. doi: 10.1128/JB.02131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haldenwang WG. 1995. The sigma factors of Bacillus subtilis. Microbiol Rev 59:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunkel B, Losick R, Stragier P. 1990. The Bacillus subtilis gene for the development transcription factor sigma K is generated by excision of a dispensable DNA element containing a sporulation recombinase gene. Genes Dev 4:525–535. doi: 10.1101/gad.4.4.525. [DOI] [PubMed] [Google Scholar]
- 38.Dubnau E, Weir J, Nair G, Carter L III, Moran C Jr, Smith I. 1988. Bacillus sporulation gene spo0H codes for sigma 30 (sigma H). J Bacteriol 170:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grossman AD. 1995. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet 29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 40.Britton RA, Eichenberger P, Gonzalez-Pastor JE, Fawcett P, Monson R, Losick R, Grossman AD. 2002. Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J Bacteriol 184:4881–4890. doi: 10.1128/JB.184.17.4881-4890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoch JA. 1993. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol 47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- 42.Trach K, Burbulys D, Strauch M, Wu JJ, Dhillon N, Jonas R, Hanstein C, Kallio P, Perego M, Bird T, Spiegelman G, Fogher C, Hoch JA. 1991. Control of the initiation of sporulation in Bacillus subtilis by a phosphorelay. Res Microbiol 142:815–823. doi: 10.1016/0923-2508(91)90060-N. [DOI] [PubMed] [Google Scholar]
- 43.Cosby WM, Zuber P. 1997. Regulation of Bacillus subtilis sigmaH (spo0H) and AbrB in response to changes in external pH. J Bacteriol 179:6778–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perego M, Spiegelman GB, Hoch JA. 1988. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol Microbiol 2:689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 45.Stragier P, Losick R. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet 30:297–241. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 46.Strauch MA. 1995. Delineation of AbrB-binding sites on the Bacillus subtilis spo0H, kinB, ftsAZ, and pbpE promoters and use of a derived homology to identify a previously unsuspected binding site in the bsuB1 methylase promoter. J Bacteriol 177:6999–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weir J, Predich M, Dubnau E, Nair G, Smith I. 1991. Regulation of spo0H, a gene coding for the Bacillus subtilis sigma H factor. J Bacteriol 173:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saujet L, Monot M, Dupuy B, Soutourina O, Martin-Verstraete I. 2011. The key sigma factor of transition phase, SigH, controls sporulation, metabolism, and virulence factor expression in Clostridium difficile. J Bacteriol 193:3186–3196. doi: 10.1128/JB.00272-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Predich M, Nair G, Smith I. 1992. Bacillus subtilis early sporulation genes KinA, Spo0F, and Spo0A are transcribed by the RNA polymerase containing sigma H. J Bacteriol 174:2771–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carter HL III, Wang LF, Doi RH, Moran CP Jr. 1988. rpoD operon promoter used by sigma H-RNA polymerase in Bacillus subtilis. J Bacteriol 170:1617–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi FX, Doi RH. 1990. Localization of a second SigH promoter in the Bacillus subtilis sigA operon and regulation of dnaE expression by the promoter. J Bacteriol 172:5631–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruno D, Abraham LS, Jeralyn DH. 2005. RNA polymerase and alternative sigma factors, p 607–629, In Dürre P. (ed), Handbook on clostridia. CRC Press, Boca Raton, FL. [Google Scholar]
- 53.Kunkel B, Sandman K, Panzer S, Youngman P, Losick R. 1988. The promoter for a sporulation gene in the SpoIVC locus of Bacillus subtilis and its use in studies of temporal and spatial control of gene expression. J Bacteriol 170:3513–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Driks A, Losick R. 1991. Compartmentalized expression of a gene under the control of sporulation transcription factor sigma(E) in Bacillus subtilis. Proc Natl Acad Sci U S A 88:9934–9938. doi: 10.1073/pnas.88.22.9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halberg R, Kroos L. 1994. Sporulation regulatory protein spoIIID from Bacillus subtilis activates and represses transcription by both mother-cell-specific forms of RNA-polymerase. J Mol Biol 243:425–436. doi: 10.1006/jmbi.1994.1670. [DOI] [PubMed] [Google Scholar]
- 56.Farquhar R, Yudkin MD. 1988. Phenotypic and genetic characterization of mutations in the spoIVC locus of Bacillus subtilis. J Gen Microbiol 134:9–17. [DOI] [PubMed] [Google Scholar]
- 57.Stragier P, Kunkel B, Kroos L, Losick R. 1989. Chromosomal rearrangement generating a composite gene for a developmental transcription factor. Science 243:507–512. doi: 10.1126/science.2536191. [DOI] [PubMed] [Google Scholar]
- 58.Takemaru K, Mizuno M, Sato T, Takeuchi M, Kobayashi Y. 1995. Complete nucleotide sequence of a skin element excised by DNA rearrangement during sporulation in Bacillus subtilis. Microbiology 141:323–327. doi: 10.1099/13500872-141-2-323. [DOI] [PubMed] [Google Scholar]
- 59.Popham DL, Stragier P. 1992. Binding of the Bacillus subtilis SpoIVCA product to the recombination sites of the element interrupting the sigma K-encoding gene. Proc Natl Acad Sci U S A 89:5991–5995. doi: 10.1073/pnas.89.13.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu S, Cutting S, Kroos L. 1995. Sporulation protein SpoIVFB from Bacillus subtilis enhances processing of the sigma factor precursor pro-sigma K in the absence of other sporulation gene products. J Bacteriol 177:1082–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou R, Chen K, Xiang X, Gu L, Kroos L. 2013. Features of pro-sigmaK important for cleavage by SpoIVFB, an intramembrane metalloprotease. J Bacteriol 195:2793–2806. doi: 10.1128/JB.00229-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haraldsen JD, Sonenshein AL. 2003. Efficient sporulation in Clostridium difficile requires disruption of the sigmaK gene. Mol Microbiol 48:811–821. doi: 10.1046/j.1365-2958.2003.03471.x. [DOI] [PubMed] [Google Scholar]
- 63.Harry KH, Zhou R, Kroos L, Melville SB. 2009. Sporulation and enterotoxin (CPE) synthesis are controlled by the sporulation-specific sigma factors SigE and SigK in Clostridium perfringens. J Bacteriol 191:2728–2742. doi: 10.1128/JB.01839-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirk DG, Dahlsten E, Zhang Z, Korkeala H, Lindstrom M. 2012. Involvement of Clostridium botulinum ATCC 3502 sigma factor K in early-stage sporulation. Appl Environ Microbiol 78:4590–4596. doi: 10.1128/AEM.00304-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Errington J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev 57:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Hinai MA, Jones SW, Papoutsakis ET. 2014. SigmaK of Clostridium acetobutylicum is the first known sporulation-specific sigma factor with two developmentally separated roles, one early and one late in sporulation. J Bacteriol 196:287–299. doi: 10.1128/JB.01103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Hinai MA, Fast AG, Papoutsakis ET. 2012. Novel system for efficient isolation of Clostridium double-crossover allelic exchange mutants enabling markerless chromosomal gene deletions and DNA integration. Appl Environ Microbiol 78:8112–8121. doi: 10.1128/AEM.02214-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Underwood S, Guan S, Vijayasubhash V, Baines SD, Graham L, Lewis RJ, Wilcox MH, Stephenson K. 2009. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. J Bacteriol 191:7296–7305. doi: 10.1128/JB.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steiner E, Dago AE, Young DI, Heap JT, Minton NP, Hoch JA, Young M. 2011. Multiple orphan histidine kinases interact directly with Spo0A to control the initiation of endospore formation in Clostridium acetobutylicum. Mol Microbiol 80:641–654. doi: 10.1111/j.1365-2958.2011.07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Worner K, Szurmant H, Chiang C, Hoch JA. 2006. Phosphorylation and functional analysis of the sporulation initiation factor Spo0A from Clostridium botulinum. Mol Microbiol 59:1000–1012. doi: 10.1111/j.1365-2958.2005.04988.x. [DOI] [PubMed] [Google Scholar]
- 71.Mearls EB, Lynd LR. 2014. The identification of four histidine kinases that influence sporulation in Clostridium thermocellum. Anaerobe 28:109–119. doi: 10.1016/j.anaerobe.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70:452–464. doi: 10.1016/j.mimet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 73.Shao L, Hu S, Yang Y, Gu Y, Chen J, Yang Y, Jiang W, Yang S. 2007. Targeted gene disruption by use of a group II intron (targetron) vector in Clostridium acetobutylicum. Cell Res 17:963–965. doi: 10.1038/cr.2007.91. [DOI] [PubMed] [Google Scholar]
- 74.Perego M, Cole SP, Burbulys D, Trach K, Hoch JA. 1989. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J Bacteriol 171:6187–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Errington J, Mandelstam J. 1986. Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIA in Spo mutants of Bacillus subtilis. J Gen Microbiol 132:2967–2976. [DOI] [PubMed] [Google Scholar]
- 76.Wu JJ, Howard MG, Piggot PJ. 1989. Regulation of transcription of the Bacillus subtilis spoIIA locus. J Bacteriol 171:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burbulys D, Trach KA, Hoch JA. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64:545–552. doi: 10.1016/0092-8674(91)90238-T. [DOI] [PubMed] [Google Scholar]
- 78.King N, Dreesen O, Stragier P, Pogliano K, Losick R. 1999. Septation, dephosphorylation, and the activation of sigma(F) during sporulation in Bacillus subtilis. Genes Dev 13:1156–1167. doi: 10.1101/gad.13.9.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt R, Margolis P, Duncan L, Coppolecchia R, Moran CP Jr, Losick R. 1990. Control of developmental transcription factor sigma F by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis. Proc Natl Acad Sci U S A 87:9221–9225. doi: 10.1073/pnas.87.23.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arigoni F, Duncan L, Alper S, Losick R, Stragier P. 1996. SpoIIE governs the phosphorylation state of a protein regulating transcription factor sigma(F) during sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A 93:3238–3242. doi: 10.1073/pnas.93.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arigoni F, Guerout-Fleury AM, Barak I, Stragier P. 1999. The SpollE phosphatase, the sporulation septum and the establishment of forespore-specific transcription in Bacillus subtilis: a reassessment. Mol Microbiol 31:1407–1415. doi: 10.1046/j.1365-2958.1999.01282.x. [DOI] [PubMed] [Google Scholar]
- 82.Wu LJ, Feucht A, Errington J. 1998. Prespore-specific gene expression in Bacillus subtilis is driven by sequestration of SpoIIE phosphatase to the prespore side of the asymmetric septum. Genes Dev 12:1371–1380. doi: 10.1101/gad.12.9.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang ST, Setlow B, Conlon EM, Lyon JL, Imamura D, Sato T, Setlow P, Losick R, Eichenberger P. 2006. The forespore line of gene expression in Bacillus subtilis. J Mol Biol 358:16–37. doi: 10.1016/j.jmb.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 84.Karow ML, Glaser P, Piggot PJ. 1995. Identification of a gene, spoIIR, that links the activation of sigma E to the transcriptional activity of sigma F during sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A 92:2012–2016. doi: 10.1073/pnas.92.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Londono-Vallejo JA, Stragier P. 1995. Cell-cell signaling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev 9:503–508. doi: 10.1101/gad.9.4.503. [DOI] [PubMed] [Google Scholar]
- 86.Li J, McClane BA. 2010. Evaluating the involvement of alternative sigma factors SigF and SigG in Clostridium perfringens sporulation and enterotoxin synthesis. Infect Immun 78:4286–4293. doi: 10.1128/IAI.00528-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Czeczulin JR, Hanna PC, McClane BA. 1993. Cloning, nucleotide sequencing, and expression of the Clostridium perfringens enterotoxin gene in Escherichia coli. Infect Immun 61:3429–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones SW, Tracy BP, Gaida SM, Papoutsakis ET. 2011. Inactivation of sigma(F) in Clostridium acetobutylicum ATCC 824 blocks sporulation prior to asymmetric division and abolishes sigma(E) and sigma(G) protein expression but does not block solvent formation. J Bacteriol 193:2429–2440. doi: 10.1128/JB.00088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kenney TJ, York K, Youngman P, Moran CP. 1989. Genetic evidence that RNA polymerase associated with sigma A factor uses a sporulation-specific promoter in Bacillus subtilis. Proc Natl Acad Sci U S A 86:9109–9113. doi: 10.1073/pnas.86.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Errington J. 2003. Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol 1:117–126. doi: 10.1038/nrmicro750. [DOI] [PubMed] [Google Scholar]
- 91.Illing N, Errington J. 1991. Genetic regulation of morphogenesis in Bacillus subtilis: roles of sigma-E and sigma-F in prespore engulfment. J Bacteriol 173:3159–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nolling J, Breton G, Omelchenko MV, Makarova KS, Zeng QD, Gibson R, Lee HM, Dubois J, Qiu DY, Hitti J, GTC Center Production, Finishing, and Bioinformatics Teams, Wolf YI, Tatusov RL, Sabathe F, Doucette-Stamm L, Soucaille P, Daly MJ, Bennett GN, Koonin EV, Smith DR. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J Bacteriol 183:4823–4838. doi: 10.1128/JB.183.16.4823-4838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paredes CJ, Rigoutsos I, Papoutsakis ET. 2004. Transcriptional organization of the Clostridium acetobutylicum genome. Nucleic Acids Res 32:1973–1981. doi: 10.1093/nar/gkh509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pereira FC, Saujet L, Tome AR, Serrano M, Monot M, Couture-Tosi E, Martin-Verstraete I, Dupuy B, Henriques AO. 2013. The spore differentiation pathway in the enteric pathogen Clostridium difficile. PLoS Genet 9:e1003782. doi: 10.1371/journal.pgen.1003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saujet L, Pereira FC, Serrano M, Soutourina O, Monot M, Shelyakin PV, Gelfand MS, Dupuy B, Henriques AO, Martin-Verstraete I. 2013. Genome-wide analysis of cell type-specific gene transcription during spore formation in Clostridium difficile. PLoS Genet 9:e1003756. doi: 10.1371/journal.pgen.1003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kirk DG, Zhang Z, Korkeala H, Lindstrom M. 2014. Alternative sigma factors SigF, SigE, and SigG are essential for sporulation in Clostridium botulinum ATCC 3502. Appl Environ Microbiol 80:5141–5150. doi: 10.1128/AEM.01015-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gholamhoseinian A, Piggot PJ. 1989. Timing of spoII gene expression relative to septum formation during sporulation of Bacillus subtilis. J Bacteriol 171:5747–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Satola SW, Baldus JM, Moran CP Jr. 1992. Binding of Spo0A stimulates spoIIG promoter activity in Bacillus subtilis. J Bacteriol 174:1448–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.LaBell TL, Trempy JE, Haldenwang WG. 1987. Sporulation-specific sigma factor sigma 29 of Bacillus subtilis is synthesized from a precursor protein, P31. Proc Natl Acad Sci U S A 84:1784–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kroos L, Kunkel B, Losick R. 1989. Switch protein alters specificity of RNA polymerase containing a compartment-specific sigma factor. Science 243:526–529. doi: 10.1126/science.2492118. [DOI] [PubMed] [Google Scholar]
- 101.Robson RL, Robson RM, Morris JG. 1974. The biosynthesis of granulose by Clostridium pasteurianum. Biochem J 144:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duncan L, Alper S, Arigoni F, Losick R, Stragier P. 1995. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science 270:641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- 103.Iber D, Clarkson J, Yudkin MD, Campbell ID. 2006. The mechanism of cell differentiation in Bacillus subtilis. Nature 441:371–374. doi: 10.1038/nature04666. [DOI] [PubMed] [Google Scholar]
- 104.Karmazyn-Campelli C, Bonamy C, Savelli B, Stragier P. 1989. Tandem genes encoding sigma-factors for consecutive steps of development in Bacillus subtilis. Genes Dev 3:150–157. doi: 10.1101/gad.3.2.150. [DOI] [PubMed] [Google Scholar]
- 105.Sun DX, Stragier P, Setlow P. 1989. Identification of a new sigma-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev 3:141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- 106.Serrano M, Neves A, Soares CM, Moran CP, Henriques AO. 2004. Role of the anti-sigma factor SpoIIAB in regulation of SigG during Bacillus subtilis sporulation. J Bacteriol 186:4000–4013. doi: 10.1128/JB.186.12.4000-4013.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Serrano M, Corte L, Opdyke J, Moran CP, Henriques AO. 2003. Expression of spoIIIJ in the prespore is sufficient for activation of sigma(G) and for sporulation in Bacillus subtilis. J Bacteriol 185:3905–3917. doi: 10.1128/JB.185.13.3905-3917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cutting S, Oke V, Driks A, Losick R, Lu S, Kroos L. 1990. A forespore checkpoint for mother cell gene expression during development in B. subtilis. Cell 62:239–250. doi: 10.1016/0092-8674(90)90362-I. [DOI] [PubMed] [Google Scholar]
- 109.Nicholson WL, Sun DX, Setlow B, Setlow P. 1989. Promoter specificity of sigma G-containing RNA polymerase from sporulating cells of Bacillus subtilis: identification of a group of forespore-specific promoters. J Bacteriol 171:2708–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tummala SB, Welker NE, Papoutsakis ET. 1999. Development and characterization of a gene expression reporter system for Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 65:3793–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scotcher MC, Rudolph FB, Bennett GN. 2005. Expression of abrB310 and SinR, and effects of decreased abrB310 expression on the transition from acidogenesis to solventogenesis, in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 71:1987–1995. doi: 10.1128/AEM.71.4.1987-1995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shafikhani SH, Leighton T. 2004. AbrB and Spo0E control the proper timing of sporulation in Bacillus subtilis. Curr Microbiol 48:262–269. doi: 10.1007/s00284-003-4186-2. [DOI] [PubMed] [Google Scholar]
- 113.Fort P, Piggot PJ. 1984. Nucleotide sequence of sporulation locus spoIIA in Bacillus subtilis. J Gen Microbiol 130:2147–2153. [DOI] [PubMed] [Google Scholar]
- 114.Piggot PJ, Curtis CA, de Lencastre H. 1984. Use of integrational plasmid vectors to demonstrate the polycistronic nature of a transcriptional unit (spoIIA) required for sporulation of Bacillus subtilis. J Gen Microbiol 130:2123–2136. [DOI] [PubMed] [Google Scholar]
- 115.Bi C, Jones SW, Hess DR, Tracy BP, Papoutsakis ET. 2011. SpoIIE is necessary for asymmetric division, sporulation, and expression of sigmaF, sigmaE, and sigmaG but does not control solvent production in Clostridium acetobutylicum ATCC 824. J Bacteriol 193:5130–5137. doi: 10.1128/JB.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferrell JE. 2002. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 14:140–148. doi: 10.1016/S0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 117.Veening JW, Smits WK, Kuipers OP. 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol 62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- 118.Veening JW, Stewart EJ, Berngruber TW, Taddei F, Kuipers OP, Hamoen LW. 2008. Bet-hedging and epigenetic inheritance in bacterial cell development. Proc Natl Acad Sci U S A 105:4393–4398. doi: 10.1073/pnas.0700463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cornillot E, Nair RV, Papoutsakis ET, Soucaille P. 1997. The genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 reside on a large plasmid whose loss leads to degeneration of the strain. J Bacteriol 179:5442–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fujita M, Gonzalez-Pastor JE, Losick R. 2005. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol 187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chary VK, Meloni M, Hilbert DW, Piggot PJ. 2005. Control of the expression and compartmentalization of sigma(G) activity during sporulation of Bacillus subtilis by regulators of sigma(F) and sigma(E). J Bacteriol 187:6832–6840. doi: 10.1128/JB.187.19.6832-6840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harris LM, Welker NE, Papoutsakis ET. 2002. Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J Bacteriol 184:3586–3597. doi: 10.1128/JB.184.13.3586-3597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tracy BP, Jones SW, Fast AG, Indurthi DC, Papoutsakis ET. 2012. Clostridia: the importance of their exceptional substrate and metabolite diversity for biofuel and biorefinery applications. Curr Opin Biotechnol 23:364–381. doi: 10.1016/j.copbio.2011.10.008. [DOI] [PubMed] [Google Scholar]