Abstract
Liver transplant allocation policy does not give model for end-stage liver disease (MELD) exception points for patients with a single hepatocellular carcinoma (HCC) <2 cm in size, but does give points to patients with multiple small nodules. Because standard-of-care imaging for HCC struggles to differentiate HCC from other nodules, it is possible that a subset of patients receiving liver transplant for multiple nodules <2 cm in size does not have HCC.
We evaluate risk of post-transplant HCC recurrence and wait-list dropout for patients with multiple small nodules using competing risks regression based on the Fine and Gray model.
We identified 5002 adult HCC patients in the OPTN/UNOS dataset diagnosed and transplanted between January 2006 and September 2010. Compared to patients with >1 tumor <2 cm, risk of developing recurrence was significantly higher in patients with one or more tumors with only one tumor ≥2 cm (SHR 1.63, p = 0.009), as well as in patients with 2–3 tumors ≥2 cm (SHR 1.84, p = 0.02). Dropout risk was not significantly different among size categories.
HCC recurrence risk was significantly lower in patients with multiple nodules <2 cm in size than in those with larger tumors, supporting the possibility that some patients received unnecessary transplants. The priority given to these patients must be re-examined.
Keywords: hepatocellular carcinoma, liver transplant, recurrence, small nodules
The development of hepatocellular carcinoma (HCC) in cirrhotic liver is understood to be either a multistep progression from low-grade dysplastic nodule to overt carcinoma, or as developing from a single dysplastic cell (1–3). While high-grade dysplastic nodules are most likely to give rise to HCC, the gradual malignant progression makes it difficult for current imaging techniques to differentiate between high-grade nodules and small HCCs (3). This is complicated by the frequent occurrence of small nodules in cirrhotic livers, many of which may disappear at follow-up with only a small percentage progressing to HCC (4). For this reason, patients with a single tumor smaller than 2 cm are no longer eligible for model for end-stage liver disease (MELD) exception points, as it was found that one-third of patients with arterially enhancing nodules <2 cm had no tumor at explant pathology (5). While patients with single nodules <2 cm are not given HCC exception priority, patients with more than one nodule <2 cm are given priority, a distinction of uncertain benefit.
It is not clear that multiple small nodules are more accurately imaged than single small lesions. Non-invasive imaging modalities have struggled to accurately diagnose these small tumors: A comparison of imaging diagnosis to explant pathology reports in the United Network for Organ Sharing (UNOS) database demonstrated that radiologic and pathologic tumor stage were identical in only 44.1% of cases, with considerable and unexplained variation in accuracy among centers (6). Radiologic diagnostic accuracy also decreases with size (7): Lesions <2 cm in size have an 82% lower chance of an accurate diagnosis. Whether multiple nodules affect the accuracy of diagnosis was not determined. Single-center state-of-the-art studies show more optimistic numbers, with MR specificity as high as 100% (8), but this is not indicative of the national average. There is hope that the recent change in policy using better-defined radiologic criteria (LIRADS) will improve the accuracy of diagnosis (9).
While it has been shown that larger HCCs are more likely to have multiple accompanying lesions, there is not yet evidence to demonstrate that multiple small lesions increase the likelihood of one of them being HCC. Coupled with the slow progression of liver nodules, the present lack of specificity in small-nodule imaging lays open the possibility that some patients with small tumors transplanted for HCC do not actually have cancer. In this study, we examine the recurrence rate of HCC after liver transplant for patients with multiple tumors <2 cm in size.
Methods
Adults listed for primary liver transplant with an initial exception for HCC diagnosis meeting Stage T2 criteria (single lesion 2–5 cm or 2–3 lesions none >3 cm) granted between January 1, 2006 and September 30, 2010 were included in the wait-list cohort, and those candidates transplanted in the same time period were included in the transplant cohort. All patients were identified from the UNOS Standard Transplant Analysis and Research (STAR) files. Patients with a post-transplant cause of death from cholangiocarcinoma (n = 11) or with laboratory MELD ≥22 (n = 25) were excluded from the analysis.
Hepatoma was designated as the primary diagnosis for 34% of patients. To identify the underlying liver disease in patients with a primary diagnosis of hepatoma, secondary diagnosis at listing and diagnosis at transplant (when secondary diagnosis was unavailable or also hepatoma) were evaluated. Patients with only a diagnosis of hepatoma and evidence of viral hepatitis (hepatitis C virus seropositive or hepatitis B virus surface antigen positive) were categorized to their respective viral hepatitis diagnosis.
Frequency distributions and medians (interquartile ranges [IQR]) for recipient, donor, and tumor characteristics were described for the transplant cohort and by tumor burden (>1 tumor, all <2 cm; 1 tumor ≥2 cm or multiple tumors with only 1 tumor ≥2 cm; 2–3 tumors ≥2 cm). We evaluated statistical differences in recipient, donor, and tumor characteristics using the chi-square and Wilcoxon rank sum tests, as appropriate. We calculated tumor volume in cm3 as the volume of a sphere ( * pi * tumor radius3) where the tumor radius was half of the reported tumor size and cumulated the tumor volumes for patients with multiple tumors. An alpha-fetoprotein (AFP) cut-off of 500 ng/µL was used in accordance with studies showing AFP of around 500 to be predictive of poor post-transplant survival (10) and increased waiting-list dropout (11). Donor risk index (DRI) was calculated per Feng et al (12). Regions were categorized based on the median wait time from exception to transplant for HCC liver transplant recipients. Short (regions 3, 6, 10, and 11), mid (regions 2, 4, and 8), and long (regions 1, 5, 6, and 9) wait regions had median regional wait times ranging from 30 to 39, 83 to 108, and 137 to 191 d, respectively.
Transplant cohort
Risk of post-transplant HCC recurrence was evaluated in the transplant cohort using competing risks regression with the Fine and Gray model (13). Recurrence was defined as either a diagnosis of HCC recurrence or a post-transplant HCC-related death: determined by physician review (JPR) of indication of recurrence in malignancy follow-up data, or primary and contributory causes of post-transplant death, respectively. Post-transplant follow-up terminated in HCC recurrence (event) or death due to other causes (competing risk). Time to event was measured in years from liver transplant to (a) date of diagnosis for HCC recurrence (if reported) or HCC-related death for patients with HCC recurrence, (b) date of death from non-HCC causes for patients with a competing event, or (c) date of last follow-up for patients alive or lost to follow-up (censored). For patients subsequently receiving a second or third liver transplant, follow-up time was evaluated from the date of first transplant to death, recurrence, or last follow-up after retransplant. Post-transplant follow-up status and date were updated when valid Social Security death certificate master file data were available. In the transplant cohort, observed cumulative incidence and 95% confidence intervals (95% CI) of post-transplant HCC recurrence were calculated while accounting for competing risks and evaluated by tumor load. Single predictor estimates for risk of post-transplant HCC recurrence (subdistribution hazard ratios [SHR]) were first estimated by modeling the cumulative incidence function with competing risks regression for tumor, recipient, and donor characteristics. Characteristics with p < 0.1 were further evaluated in the multivariable model. The final model included tumor load, factors where multivariable p values were <0.05, and accounted for center-level clustering of outcomes. We evaluated the assumption of proportional subdistribution hazards and modeled covariates violating the assumption as time-varying covariates. We also evaluated potential interactions between tumor load and AFP and ablative therapy (p > 0.05, data not shown).
Wait-list cohort
In the wait-list cohort, we evaluated risk of wait-list dropout. Dropout (event) was defined as death on the wait-list or removal from the wait-list for worsening condition, with transplant as the competing event. Patients who were removed from the wait-list for refusal of LT, center transfers, improvement in condition, were removed in error, or who were lost to follow-up were censored at wait-list removal. Time to event was measured in months from assignment of HCC exception to (a) date of drop out, (b) date of liver transplant for patients with the competing event, or (c) last date on the wait-list (censored). Observed cumulative incidences within three, six, and 12 months of HCC exception and 95% confidence intervals were estimated by tumor load. Single predictor estimates for risk of wait-list dropout were evaluated by Fine and Gray competing risks regression for patient and tumor characteristics, and characteristics with p < 0.1 were further evaluated in the multivariate model (13).
Data manipulation and analysis were completed with SAS version 9.3 (Cary, NC, USA). Competing risks regression was completed with Stata/IC 11.1 (College Station, TX, USA). This study received approval from the UCSF Committee on Human Research.
Results
HCC liver wait-list candidates (n = 7266) and transplant recipients (n = 5002) were primarily male, white, and non-diabetic. HCV was the most common diagnosis. At transplant, patients were a median 57 yr of age (IQR 53–62) with a laboratory MELD of 12 (IQR 9–16) (Table 1). At the time of initial HCC exception, 12.9% and 12.6% of waitlist candidates and transplant recipients, respectively, had >1 tumor < 2 cm in size, 81.5% and 81.5% had 1 tumor ≥2 cm or multiple tumors with only one ≥2 cm, and 5.5% and 5.9% had 2–3 tumors ≥2 cm. Median tumor volume for all tumors combined was 9.2 cm3. Wait-list candidates and transplant recipients frequently had ablative therapy (43.4% and 43.3%, respectively), and AFP was >500 at exception for 7.0% and 5.9%, respectively (Table 2).
Table 1.
Recipient and donor characteristics for hepatocellular carcinoma liver transplant candidates and recipients by tumor load
| Transplant cohort | |||||
|---|---|---|---|---|---|
| Wait-list cohort (n = 7266) |
Transplant cohort (n = 5002) |
>1 tumor, all <2 cm (n = 630) |
1 tumor ≥2 cm or multiple tumors with only 1 ≥ 2 cm (n = 4077) |
2–3 tumors ≥2 cm (n = 295) | |
| Recipient characteristics | N (%) | N (%) | N (%) | N (%) | N (%) |
| Male sex | 5577 (76.8) | 3872 (77.4) | 468 (74.3) | 3165 (77.6) | 239 (81.0) |
| Ethnicity | |||||
| White | 4766 (65.6) | 3373 (67.4) | 429 (68.1) | 2755 (67.6) | 189 (64.1) |
| Black | 645 (8.9) | 447 (8.9) | 60 (9.5) | 354 (8.7) | 33 (11.2) |
| Hispanic/Latino | 1078 (14.8) | 684 (13.7) | 79 (12.5) | 563 (13.8) | 42 (14.2) |
| Asian | 689 (9.5) | 435 (8.7) | 57 (9.0) | 352 (8.6) | 26 (8.8) |
| Other/multirace | 88 (1.2) | 63 (1.3) | 5 (0.8) | 53 (1.3) | 5 (1.7) |
| Model for end-stage liver disease exception points at transplant | |||||
| 22 | NA | 2666 (53.3) | 328 (52.1) | 2176 (53.4) | 162 (54.9) |
| 23–24 | NA | 44 (0.9) | 4 (0.6) | 35 (0.9) | 5 (1.7) |
| 25 | NA | 1199 (24.0) | 145 (23.0) | 984 (24.1) | 70 (23.7) |
| 26–27 | NA | 110 (2.2) | 18 (2.9) | 85 (2.1) | 7 (2.4) |
| 28 | NA | 471 (9.4) | 54 (8.6) | 396 (9.7) | 21 (7.1) |
| 29–30 | NA | 245 (4.9) | 40 (6.3) | 192 (4.7) | 13 (4.4) |
| 31 | NA | 117 (2.3) | 19 (3.0) | 92 (2.3) | 6 (2.0) |
| 32–40 | NA | 150 (3.0) | 22 (3.5) | 117 (2.9) | 11 (3.7) |
| ICU at transplant | NA | 72 (1.4) | 10 (1.6) | 57 (1.4) | 5 (1.7) |
| Diagnosis | |||||
| HCV | 4361 (60.0) | 3108 (62.1) | 399 (63.3) | 2518 (61.8) | 191 (64.7) |
| Alcoholic cirrhosis | 639 (8.8) | 422 (8.4) | 62 (9.8) | 334 (8.2) | 26 (8.8) |
| Non-cholestatic cirrhosis | 395 (5.4) | 287 (5.7) | 28 (4.4) | 244 (6.0) | 15 (5.1) |
| HBV | 408 (5.6) | 290 (5.8) | 39 (6.2) | 236 (5.8) | 15 (5.1) |
| NASH | 301 (4.1) | 215 (4.3) | 17 (2.7) | 186 (4.6) | 12 (4.1) |
| Other | 1162 (16.0) | 680 (13.6) | 85 (13.5) | 559 (13.7) | 36 (12.2) |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| Age at transplant | NA | 57 (53–62) | 57 (53–61) | 57 (53–62)* | 57 (53–62) |
| Donor risk index | NA | 1.37 (1.13–1.68) | 1.40 (1.16–1.72) | 1.37 (1.12–1.67)** | 1.42 (1.17–1.72) |
p < 0.05, compared to >1 tumor all <2 cm category.
p < 0.01, compared to >1 tumor all <2 cm category
Table 2.
Hepatocellular carcinoma (HCC) tumor characteristics for HCC liver transplant candidates and recipients by tumor load
| Transplant cohort | |||||
|---|---|---|---|---|---|
| Wait-list cohort (n = 7266) |
Transplant cohort (n = 5002) |
>1 tumor, all <2 cm (n = 630) |
1 tumor ≥2 cm or multiple tumors with only 1 ≥ 2 cm (n = 4077) |
2–3 tumors ≥2 cm (n = 295) | |
| Tumor characteristics | N (%) | N (%) | N (%) | N (%) | N (%) |
| Milan criteria at exception | 7177 (98.8) | 4946 (98.9) | 630 (100.0) | 4021 (98.6)** | 295 (100.0) |
| Ablative therapy at exception | 3150 (43.4) | 2167 (43.3) | 216 (34.3) | 1813 (44.5)** | 138 (46.8)** |
| Alpha-fetoprotein >500 ng/mL at exception | 507 (7.0) | 293 (5.9) | 28 (4.4) | 251 (6.2) | 14 (4.7) |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| Total tumor volume at exception (cm3) | 9.2 (5.4–17.2) | 9.2 (5.6–17.2) | 2.9 (1.8–4.1) | 10.3 (6.4–18.8)** | 15.2 (12.1–18.5)** |
| Time from exception to transplant (days) | NA | 77 (27–158) | 86 (33–178) | 75 (26–157)* | 69 (26–124)* |
| Post-transplant follow-up time (yr) | NA | 2.1 (1.0–3.6) | 2.0 (1.1–3.8) | 2.1 (1.0–3.6) | 2.0 (1.0–3.7) |
p < 0.005, compared to >1 tumor all <2 cm category.
p < 0.001, compared to >1 tumor all <2 cm category.
Transplant cohort
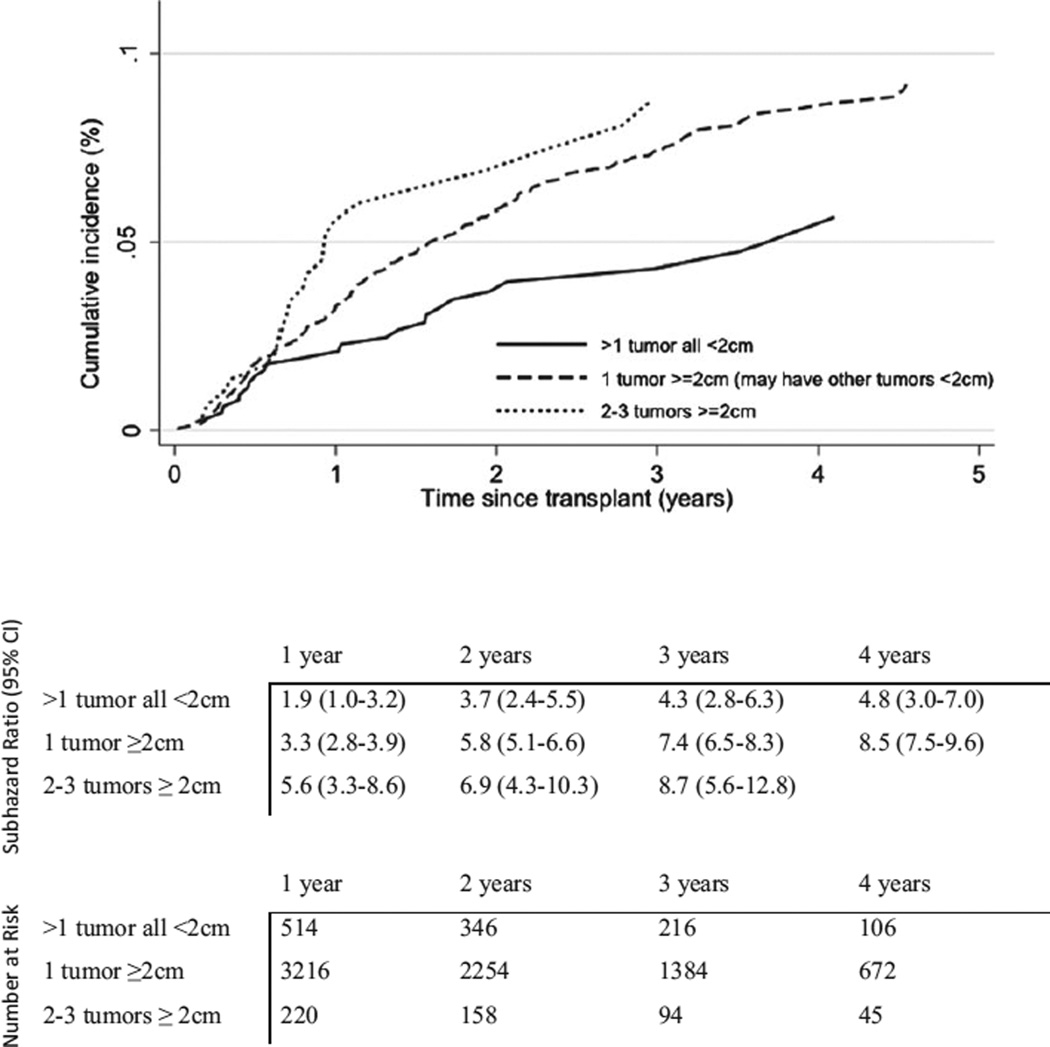
Cumulative incidence of HCC recurrence was 3.3% (95% CI 2.8–3.8) and 5.6% (95% CI 5.0–6.3) within one and two yr of transplant, respectively. Recurrence increased with tumor load: HCC recurrence within one yr of transplant was 1.9% for patients with >1 tumor all <2 cm compared to 3.3% among patients with one tumor ≥2 cm (p = 0.07) and 5.6% among patients with 2–3 tumors ≥2 cm (p = 0.005). Similarly, within two yr of transplant, patients with multiple small tumors observed significantly lower recurrence (3.7%) than patients with one (5.8%, p = 0.04) or multiple tumors (6.9%, p = 0.03) ≥2 cm (Fig. 1).
Figure 1.
Cumulative incidence, 95% confidence intervals, and number at risk for hepatocellular carcinoma recurrence by tumor load.
After adjusting for covariates, the risk of HCC recurrence was increased for liver transplant recipients with one tumor ≥2 cm (SHR 1.62 95% CI 1.13–2.35, p = 0.009) and 2–3 tumors ≥2 cm (SHR 1.84, 95% CI 1.08–3.12, p = 0.02) compared to patients with >1 tumor all <2 cm. HCC recurrence risk was also elevated among patients if they had received ablative therapy vs. none (SHR 1.39, 95% CI 1.10–1.75, p = 0.005), AFP >500 vs. ≤500 ng/mL (SHR 4.68, 95% CI 2.75–7.95, p < 0.001), and with increasing DRI (SHR 1.91, 95% CI 1.43–2.55, p < 0.001). HCC recurrence subhazard ratios were decreased for alcoholic cirrhosis compared to HCV (SHR 0.24, 95% CI 0.10–0.60, p = 0.002) (Table 3).
Table 3.
Risk of post-transplant hepatocellular carcinoma recurrence estimated with the Fine and Gray multivariable competing risks regression model, accounting for center clustering and adjusted for time on the wait-list
| Characteristic | SHR (95% CI) | p-value |
|---|---|---|
| Tumor characteristics | ||
| >1 tumor all <2 cm | 1.00 | |
| 1 tumor ≥2 cm or multiple tumors with only 1 ≥ 2 cm | 1.62 (1.13–2.35) | 0.009 |
| 2–3 tumors ≥ 2 cm | 1.84 (1.08–3.12) | 0.02 |
| Ablative therapy at exception | 1.39 (1.10–1.75) | 0.005 |
| Alpha-fetoprotein >500 ng/mL at exception (vs. ≤500)* | 4.68 (2.75–7.95) | <0.001 |
| Diagnosis* | ||
| HCV | 1.00 | |
| Alcoholic cirrhosis | 0.24 (0.10–0.60) | 0.002 |
| Non-cholestatic cirrhosis | 0.9 (0.34–2.41) | 0.84 |
| HBV | 1.12 (0.61–2.07) | 0.71 |
| NASH | 0.41 (0.14–1.21) | 0.10 |
| Other | 0.84 (0.51–1.40) | 0.51 |
| Donor risk index | 1.91 (1.43–2.55) | <0.001 |
SHR, subdistribution hazard ratio.
Significant interaction with time post-transplant.
Although we failed to detect a significant interaction between tumor load and AFP, we further evaluated the predictive value of AFP in the tumor load subgroups. For patients with multiple tumors <2 cm, the SHR for HCC recurrence for AFP >500 ng/mL vs. ≤500 was 6.47 (95% CI 1.84–22.7, p = 0.004) as compared to 4.67 (95% CI 2.72–8.02, p < 0.001) for patients with one tumor >2 cm and 0.85 (95% CI 0.11–6.55, p = 0.88) for patients with 2–3 tumor ≥2 cm. Of the patients with multiple small tumors, 17.9% of those with an AFP >500 ng/mL experienced recurrence compared to 3.3% of those with AFP ≤500 ng/mL. However, our analysis of recurrence by AFP >500 ng/mL among patients with multiple large tumors was limited by few recurrences (n = 22) in this smaller subgroup (n = 295).
Wait-list cohort
Cumulative incidence of wait-list dropout was 5.0% (95% CI 4.5–5.5) within three months, 8.9% (8.3–9.6) within six months, and 13.2% (12.4–14.9) within 12 months of listing for transplant. No significant differences in wait-list dropout were detected when comparing patients with multiple small nodules to one tumor ≥ 2 cm or 2–3 tumors ≥2 cm in size (p > 0.05, data not shown). Dropout risk was increased in longer waiting regions, with increasing AFP, but not significantly higher with increasing tumor load (Table 4).
Table 4.
Risk of wait-list dropout estimated with the Fine and Gray multivariable competing risks regression model
| Characteristic | SHR (95% CI) | p-value |
|---|---|---|
| OPTN region | ||
| Short wait regions: 3, 6, 10, 11 | 1.00 | |
| Mid wait regions: 2, 4, 8 | 2.01 (1.62–2.48) | <0.001 |
| Long wait regions: 1, 5, 7, 9 | 3.54 (2.92–4.31) | <0.001 |
| Tumor characteristics | ||
| >1 tumor all <2 cm | 1.00 | |
| 1 tumor ≥2 cm (may have other tumors <2 cm) | 1.06 (0.89–1.26) | 0.59 |
| 2–3 tumors ≥2 cm | 1.13 (0.84–1.51) | 0.49 |
| Ablate (any ablation while listed) | 0.81 (0.72–0.92) | 0.001 |
| Laboratory model for end-stage liver disease at listing | 1.07 (1.06–1.08) | <0.001 |
| Alpha-fetoprotein >500 ng/mL (vs. ≤500) | 2.59 (2.18–3.08) | <0.001 |
| Age at listing (per one yr increase) | 1.01 (1.00–1.02) | 0.001 |
| Diagnosis | ||
| HCV | 1.00 | |
| Alcoholic cirrhosis | 1.13 (0.92–1.39) | 0.24 |
| Non-cholestatic cirrhosis | 1.04 (0.79–1.36) | 0.78 |
| HBV | 0.67 (0.49–0.91) | 0.01 |
| NASH | 0.90 (0.66–1.24) | 0.52 |
| Other | 1.57 (1.35–1.81) | <0.001 |
SHR, subdistribution hazard ratio.
Discussion
In this study, we found that patients within conventional transplant criteria with multiple HCC tumors <2 cm in diameter had significantly lower HCC recurrence rates than any other size category transplanted. We also found that among the few who did recur, AFP level >500 ng/mL was a significant predictor of recurrence. Because of the limitations of staging imaging practices, we speculate that some patients with multiple small nodules do not actually have HCC and are getting unnecessary transplants. Alternatively, these small tumors may have a low risk of recurrence when transplanted early.
Our study is limited by the characteristics of the UNOS/OPTN database. While systematic differences in reporting HCC recurrence were not identified by center (14), reporting HCC recurrence is not mandated within UNOS/OPTN, and therefore some cases of recurrence may be misclassified. As a result, our study underestimates HCC recurrence rates relative to reported averages (15). However, this is unlikely to alter our conclusions regarding tumor size category: unless under-reporting occurs systematically by tumor size category, this limitation only attenuates our results. As imaging practices vary widely, we were also not in the position to evaluate the specificity of imaging used for diagnosis. Additionally, patient selection for ablative therapy is not detailed, thus associations between recurrence and ablation may reflect un-captured differences in disease severity rather than ablative therapy itself. Finally, despite using nearly five yr of data, our analysis is limited by the small numbers of recurrences among some subgroups queried.
There is some variety in the reported quality of small HCC imaging, with specificity ranges for multidetector CT and MRI varying between 79% and 100% (8, 16–18). A systematic review of the accuracy of spiral CT and MRI in diagnosing HCC reported pooled estimates of 93% and 85% specificity for CT and MRI, respectively (19). While the smaller single-center studies of HCC imaging show specificities up to 100%, they are biased towards high-volume centers more likely to use state-of-the-art practices not necessarily representative of the national average (20). Additionally, one-third of patients with arterially enhancing nodules smaller than 2 cm, presumed to be HCC at imaging, had no tumor at explant pathology (5). These results suggest that in patients with small tumors, there might be a tendency for over-reporting the radiologic stage of questionable lesions in the effort to match T2 criteria for wait-list priority (6). This is a strategy perhaps advantageous at the individual level, but not optimal for allocation of a scarce resource.
The difficulties in imaging one small lesion apply to imaging several in that there are no data to support that imaging specificity increases for multiple small nodules vs. one, or that having multiple small nodules makes it more likely for one of them to be HCC. This has led to more stringent diagnostic guidelines for multiple small tumors, in line with the LI-RADS classification of tumor characteristics (9). In the past, 2–3 lesions <2 cm with late arterial enhancement and washout were automatically eligible for transplant. Recent guidelines, however, require that these lesions demonstrate peripheral rim enhancement, be confirmed by biopsy, or grow by 50% in six months to be eligible for HCC exception points (21). Given the recent implementation of this policy, we were unable to assess the impact of these enhanced criteria on HCC recurrence in patients with multiple small tumors—although they would presumably reduce the number of transplants allocated to patients with questionable disease.
Additionally, small HCC is associated with relatively benign behavior: Smaller lesions are less likely to show microvascular invasion (22), and nodules <3 cm were found not to be significantly associated with hepatic seeding of HCC (23). Single-center longitudinal studies from Japan (24) and San Francisco (25) as well as a study modeling growth rates (26) reported that most patients with early-stage HCC do not progress rapidly for approximately six months after being placed on the transplant wait-list. A more recent analysis of patients with one untreated lesion <2 cm found the risk of progression beyond T2 to be 4.4%, with a one-yr survival of 94.5% (27). The San Francisco study reported a dropout rate of 0% at six months and 10% at 12 months for patients with lesions <3 cm. Mehta et al. showed that patients with 2–3 cm lesions have significantly lower dropout risk than larger tumor burden categories (28). Our analysis did not show decreasing tumor load to lower dropout risk. While the former study was able to precisely evaluate the etiology of wait-list dropout such as tumor progression or liver-related death, the data in the current study did not allow us to differentiate these etiologies as closely, which may have limited our ability to detect a difference in dropout based on tumor burden.
The use of waiting time as a selection criterion, as is implemented by the most recent guidelines, is in keeping with several studies suggesting waiting times ranging from three months to one yr (29–34). These studies, however, do not address disease presenting with multiple small tumors. Our own recent investigation on the use of waiting time to reduce post-LT HCC recurrence found the benefit of waiting >120 d to be smaller for patients with multiple small tumors than for the other size categories (35), suggesting that these small tumors tolerate a lag time without metastasizing. Other groups, however, suggest that waiting for transplant may not be the best treatment option for patients with small HCCs, having found that immediate ablation or resection is more cost-effective (36) and potentially more beneficial (37).
Finally, we found that among patients with multiple small tumors, those with AFP>500 ng/mL were at significantly higher risk of experiencing HCC recurrence and that the hazard ratio decreased with larger tumor load categories. This suggests that AFP level may be particularly informative for patients with multiple tumors <2 cm in size, when imaging specificity is lacking. However, the use of AFP for treatment decisions is still controversial, as a range of different cutoff values have been shown to predict post-transplant outcomes (38). At our center, elevated AFP is not required for HCC diagnosis, although patients with AFP > 1000 ng/mL are required to show a decrease in AFP level to <500 ng/mL before they are transplanted (39). The diagnostic and prognostic accuracy of AFP in patients with multiple small tumors bears further inquiry.
With the continuing increase in HCC cases in the US (40), care must be taken to ensure maximum utility in liver allocation. Our study suggests that a subset of patients with multiple small tumors may be getting unnecessary transplants. Because of the limitations of diagnostic imaging modalities when it comes to small nodules, we believe that the low recurrence rate observed is due in part to the transplantation of patients who do not have HCC. While this is impossible to verify without a detailed histologic analysis of explanted tissue, it is consistent with previous analyses of radiologic staging (1, 5, 6). Further exploration of this theme will require analysis of explant pathology. Based on our findings, we suggest that patients with multiple tumors <2 cm in size not be given HCC MELD exception points for transplant priority unless they have definitively been shown to be HCC by LI-RADS criteria, and either wait or undergo ablation/resection therapy instead.
Acknowledgments
Grants and financial support
This work was supported by the Biostatistics Core of the UCSF Liver Center (P30 DK026743) and by the Dean’s Office Medical Student Research Program at UCSF.
Footnotes
Conflict of interest: None reported.
Authors’ contributions
Mariya Samoylova: Contributed to design, drafting the article, data interpretation, and approval of the final version; Jennifer Dodge: Contributed to statistical analysis, data interpretation, critical revision, and approval of the final version; Mehta and Yao: Contributed to data interpretation, critical revision, and approval of the final version; Roberts: Contributed to concept/design, critical revision, funding, and approval of the final version.
References
- 1.Libbrecht L, Bielen D, Verslype C, et al. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749. doi: 10.1053/jlts.2002.34922. [DOI] [PubMed] [Google Scholar]
- 2.Coleman WB. Mechanisms of human hepatocarcinogenesis. Curr Mol Med. 2003;3:573. doi: 10.2174/1566524033479546. [DOI] [PubMed] [Google Scholar]
- 3.Efremidis SC, Hytiroglou P. The multistep process of hepatocarcinogenesis in cirrhosis with imaging correlation. Eur Radiol. 2002;12:753. doi: 10.1007/s00330-001-1142-z. [DOI] [PubMed] [Google Scholar]
- 4.Seki S, Sakaguchi H, Kitada T, et al. Outcomes of dysplastic nodules in human cirrhotic liver: a clinicopathological study. Clin Cancer Res. 2000;6:3469. [PubMed] [Google Scholar]
- 5.Hayashi PH, Trotter JF, Forman L, et al. Impact of pre-transplant diagnosis of hepatocellular carcinoma on cadaveric liver allocation in the era of MELD. Liver Transpl. 2004;10:42. doi: 10.1002/lt.20020. [DOI] [PubMed] [Google Scholar]
- 6.Freeman RB, Mithoefer A, Ruthazer R, et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: a retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504. doi: 10.1002/lt.20847. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 8.Bhartia B, Ward J, Guthrie JA, Robinson PJ. Hepatocellular carcinoma in cirrhotic livers: double-contrast thin-section MR imaging with pathologic correlation of explanted tissue. AJR Am J Roentgenol. 2003;180:577. doi: 10.2214/ajr.180.3.1800577. [DOI] [PubMed] [Google Scholar]
- 9.American College of Radiology. Liver Imaging Reporting and Data System version 2013.1. [Accessed February 2014]; from http://www.acr.org/Quality-Safety/Resources/LIRADS/ [Google Scholar]
- 10.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10:1643. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 12.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496. [Google Scholar]
- 14.Samoylova ML, Dodge JL, Vittinghoff E, Yao FY, Roberts JP. Validating posttransplant hepatocellular carcinoma recurrence data in the United Network for Organ Sharing Database. Liver Transpl. 2013;19:1318. doi: 10.1002/lt.23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 16.Burrel M, Llovet JM, Ayuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 17.Rode A, Bancel B, Douek P, et al. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327. doi: 10.1097/00004728-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Choi SH, Lee JM, Yu NC, et al. Hepatocellular carcinoma in liver transplantation candidates: detection with gadobenate dimeglumine–enhanced MRI. Am J Roentgenol. 2008;191:529. doi: 10.2214/AJR.07.2565. [DOI] [PubMed] [Google Scholar]
- 19.Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 20.Rimola J, Forner A, Tremosini S, et al. Non-invasive diagnosis of hepatocellular carcinoma ≤ 2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J Hepatol. 2012;56:1317. doi: 10.1016/j.jhep.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 21.OPTN Policies and Bylaws [Internet] OPTN. [cited 2014 Jan 1];2013 Available from: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_8.pdf.
- 22.Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intra-hepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26:142. doi: 10.1016/s1386-6346(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 23.Ohnishi H, Sakaguchi K, Nouso K, et al. Outcome of small liver nodules detected by computed tomographic angiography in patients with hepatocellular carcinoma. Hepatol Int. 2010;4:562. doi: 10.1007/s12072-010-9190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuno S, Yokoi H, Shiraki K, et al. Prospective study on the outcome of patients with hepatocellular carcinoma registered for living donor liver transplantation: how long can they wait? Transplantation. 2010;89:650. doi: 10.1097/TP.0b013e3181cd4ae9. [DOI] [PubMed] [Google Scholar]
- 25.Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waitlist for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9:684. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 26.Cheng SJ, Freeman RB, Jr, Wong JB. Predicting the probability of progression-free survival in patients with small hepatocellular carcinoma. Liver Transpl. 2002;8:323. doi: 10.1053/jlts.2002.31749. [DOI] [PubMed] [Google Scholar]
- 27.Mehta N, Dodge J, Fidelman N, Roberts JP, Yao FY. Intention-to-treat Outcome of T1 Hepatocellular Carcinoma Using the Approach of “Wait and not Ablate” until Meeting T2 Criteria for Liver Transplant Listing. 2013 doi: 10.1002/lt.24360. from http://liverlearning.aasld.org/aasld/2013/thelivermeeting/34965/neil.mehta.intention-to-treat.outcome.of.t1.hepatocellular.carcinoma.using.the.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl. 2013;19:1343. doi: 10.1002/lt.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taouli B, Goh JS, Lu Y, et al. Growth rate of hepatocellular carcinoma: evaluation with serial computed tomography or magnetic resonance imaging. J Comput Assist Tomogr. 2005;29:425. doi: 10.1097/01.rct.0000164036.85327.05. [DOI] [PubMed] [Google Scholar]
- 30.Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48:581. doi: 10.1023/a:1022505203786. [DOI] [PubMed] [Google Scholar]
- 31.Ebara M, Hatano R, Fukuda H, Yoshikawa M, Sugiura N, Saisho H. Natural course of small hepatocellular carcinoma with underlying cirrhosis. A study of 30 patients. Hepatogastroenterology. 1998;45(Suppl. 3):1214. [PubMed] [Google Scholar]
- 32.Choi D, Mitchell DG, Verma SK, et al. Hepatocellular carcinoma with indeterminate or false-negative findings at initial MR imaging: effect on eligibility for curative treatment initial observations. Radiology. 2007;244:776. doi: 10.1148/radiol.2443061355. [DOI] [PubMed] [Google Scholar]
- 33.Furlan A, Marin D, Agnello F, et al. Hepatocellular carcinoma presenting at contrast-enhanced multi-detector-row computed tomography or gadolinium-enhanced magnetic resonance imaging as a small (≤2 cm), indeterminate nodule: growth rate and optimal interval time for imaging follow-up. J Comput Assist Tomogr. 2012;36:20. doi: 10.1097/RCT.0b013e31823ed462. [DOI] [PubMed] [Google Scholar]
- 34.O’Malley ME, Takayama Y, Sherman M. Outcome of small (10–20 mm) arterial phase-enhancing nodules seen on triphasic liver CT in patients with cirrhosis or chronic liver disease. Am J Gastroenterol. 2005;100:1523. doi: 10.1111/j.1572-0241.2005.41814.x. [DOI] [PubMed] [Google Scholar]
- 35.Samoylova ML, Dodge JL, Yao FY, Roberts JP. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after transplant. Liver Transpl. 2014;20:937. doi: 10.1002/lt.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naugler WE, Sonnenberg A. Survival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinoma. Liver Transpl. 2010;16:1186. doi: 10.1002/lt.22129. [DOI] [PubMed] [Google Scholar]
- 37.Bremner KE, Bayoumi AM, Sherman M, Krahn MD. Management of solitary 1 cm to 2 cm liver nodules in patients with compensated cirrhosis: a decision analysis. Can J Gastroenterol. 2007;21:491. doi: 10.1155/2007/182383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplant for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 39.Hameed B, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein >1000 ng/mL as an exclusion criterion for liver transplant in patients with hepatocellular carcinoma meeting Milan criteria [Abstract] Hepatology. 2011;54(Suppl1. 1):414A. doi: 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]