Abstract
The transmembrane glycoprotein CD98 is a potential regulator of multiple functions, including integrin signaling and amino acid transport. Abnormal expression or function of CD98 and disruption of the interactions between CD98 and its binding partners result in defects in cell homeostasis and immune responses. Indeed, expression of CD98 has been correlated with diseases such as inflammation and tumor metastasis. Modulation of CD98 expression and/or function therefore represents a promising therapeutic strategy for the treatment and prevention of such pathologies. Herein, we review the role of CD98 with focus on its functional importance in homeostasis and immune responses, which could help to better understand the pathogenesis of CD98-associated diseases.
Keywords: CD98, Integrin signaling, Amino acid transport, Cell homeostasis, Immune response
Introduction
CD98 (also termed CD98 heavy chain; encoded by SLC3A2 gene) is a type II transmembrane protein that covalently links to one of several L-type amino acid transporters (light chains) to form large, functionally heterodimeric neutral amino acid transport systems [1, 2]. Following its discovery as an antigen of lymphocyte activation [3, 4], CD98 has since been identified in all cell types, except platelets, with the highest levels of expression found in the gastrointestinal tract and the tubules of kidney [1]. In addition to playing a role in amino acid transport, CD98 has been shown to associate with β1 and β3 integrins and regulate integrin signaling, which is in turn involved in cell adhesion, fusion, proliferation, and growth [1, 2]. Evidence has shown a correlation between CD98 expression and human diseases such as intestinal disorders [5, 6] and tumor metastasis [7–10]. Recently, mouse lines featuring tissue-specific CD98 overexpression/deletion generated by our group and others have provided a powerful tool facilitating investigations of the role of CD98, and the underlying mechanism of action, under pathophysiological conditions [11–14]. In this review, we will focus on the role of CD98 and its functional importance in homeostasis, immune responses, and disease development.
Regulation of CD98-encoding gene expression and molecular structure
CD98 is encoded by the solute carrier family 3 member 2 (SLC3A2) gene mapped to chromosome 11, which is highly conserved throughout mammalian evolution [15, 16]. CD98-encoding cDNA was originally cloned in 1987 using its ability to react with a monoclonal antibody raised against a lymphoblastic cell surface antigen [16, 17].
The CD98 transcript is composed of 1,863 base pairs (bp) along with an open reading frame of 1,590 bp. It encodes the glycoprotein CD98 of 529 amino acids, with a predicted size of 80 kDa. The hydropathy plot of CD98 indicates that the protein contains a cytoplasmic domain (amino acids 1–81), a single-pass transmembrane domain (amino acids 82–104) and an extracellular domain (amino acids 105–529) [16–20]. The protein has been classified as a type II membrane glycoprotein, with an extracellular carboxyl terminus and a cytoplasmic amino terminus (Fig. 1). Interestingly, the C-terminus of human CD98 contains a potential class II PDZ-binding domain (amino acids 520–529; GLLLRFPYAA), suggesting that CD98 may associate with extracellular PDZ domain-containing proteins. The extracellular C-terminal half of the protein also contains four potentially glycosylated asparagine residues (amino acids 264, 280, 323, and 405) and Cys-109, which has been shown to participate in formation of disulfide-bond between CD98 and amino-acid transporters (CD98 light chains) (Fig. 1). Several vertebrate “glycoprotein-associated” amino-acid transporters LAT-1, LAT-2, y+LAT-1, y+LAT-2, xCT, or asc-1 have been shown to associate with CD98 (Table 1) [21–28]. In intestinal epithelial cells (IECs), CD98 is linked to the L-type amino-acid transporters LAT-2 and y+LAT-1, which appear to be exclusively expressed in such cells [26, 29].
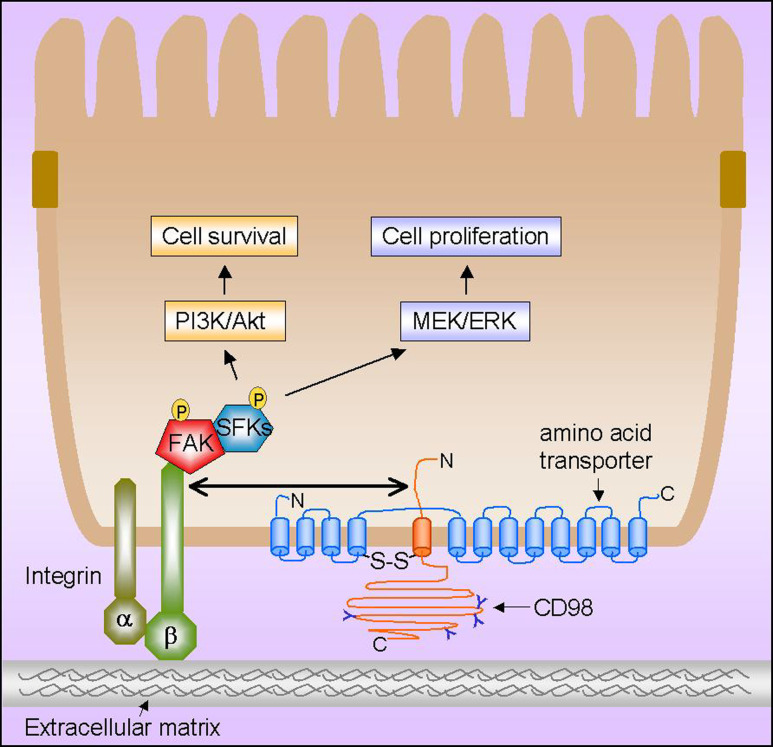
Fig. 1.
Schematic representation of the CD98-regulated cellular functions in intestinal epithelial cells mediated by interactions of CD98 with integrin and amino acid transporter. CD98 (heavy chain) with potential N-glycosylation sites (indicated as Y) is associated with an amino acid transporter (light chain) to form a functional heterodimer through a conserved disulphide bridge (S–S). The cytoplasmic tail of CD98 can interact with β1 and β3 integrin, thereby regulating integrin signaling. CD98-mediated integrin activation results in phosphorylation of the focal adhesion kinase (FAK), which functions as a phosphorylation-regulated scaffold to recruit Src-family kinases (SFKs) to focal adhesions. This consequently leads to activation of the phosphatidylinositol 3-kinase (PI3K)/Akt and mitogen-activated protein (MAP) kinase kinase (called MEK)/extracellular signal-regulated kinase (ERK) signaling pathways, promoting cell survival, proliferation, and migration
Table 1.
CD98-linked amino acid transporters
| Name | Transport system | Tissue localization | Not detected (a) or lowest expression |
|---|---|---|---|
| LAT-1 | Large neutral amino acids; Na+-independent | Ubiquitous: ovary, placenta, skeletal muscle, brain, spleen, testis and fetal liver, activated lymphocytes, some tumor cells | Livera, intestinea |
| LAT-2 | Small and large neutral amino acids;Na+-independent | Kidney, small intestine, ovary, placenta, brain | Lung |
| y+LAT-1 | Cationic amino acids: Na+-independent; large neutral amino acids: Na+-dependent | Small intestine, kidney cortex, peripheral blood, leukocytes, placenta, lung and many other tissues | |
| y+LAT-2 | Cationic amino acids: Na+-independent; large neutral amino acids: Na+-dependent | Brain, small intestine, testis, heart, kidney, lung, liver | |
| xCT | Cystine/glutamate exchange; mainly Na+-independent | Activated macrophages, brain | Lung, liver, kidneya |
| asc-1 | Small neutral amino acids; Na+-independent | Brain, kidney, heart, liver, lung, small intestine, pancreas, placenta |
CD98 is expressed in all cell types with the exception of platelets, and is expressed at the highest levels in the tubules of kidney and in the gastrointestinal tract [1, 29, 30]. A number of distinct molecular mechanisms, which are triggered via activation of discrete intracellular pathways, have been described to be involved in the regulation of human CD98 gene expression. Early pioneering studies showed that activation of resting human peripheral T lymphocytes with phytohemagglutinin (PHA), a combination of 4 beta-phorbol 12-myristate 13-acetate (PMA) and ionomycin, or anti-CD3 antibodies, resulted in a dramatic rise in CD98 mRNA level [31–33]. Both an enhancement of transcription initiation by ionomycin and a removal of a block in elongation by PMA were initially proposed [32]. However, CD98 transcription was only moderately changed upon stimulation with anti-CD3 antibody [33], and postulated attenuating sequences have yet been identified [34]. In addition, co-stimulation with phorbol ester TPA (a protein kinase C activator) and A23187 (calcium ionophore) strongly induced CD98 expression in rat and human lymphocytes [35]; the effects of TPA and the calcium ionophore were synergistic. Addition of EGTA to the culture completely blocked the stimulatory effect, suggesting that influx of extracellular calcium is required for the induction of CD98 [35]. In agreement with these studies, other investigators observed a very low level of CD98 and transferrin receptor on the surface of resting lymphocytes, which was markedly increased after growth stimulation [3, 36–40]. Nii and colleagues investigated the molecular mechanism underlying the regulation of CD98 during T-cell activation and showed that the increase in CD98 expression levels in activated T cells compared with quiescent T cells was due to elevated levels of CD98 mRNA as analyzed by Northern blotting [41]. The low levels of CD98 transcripts were shown to be the result of a block to transcription elongation within the exon 1-intron 1 regions [41], suggesting that a removal of the block to CD98 mRNA elongation stimulates the induction of CD98 expression in activated T cells.
Apart from the transcriptional regulatory mechanism, a post-transcriptional mechanism has been reported to account for the strong accumulation of CD98 in activated lymphocytes [42]. Teixeira et al. [42] showed that CD98 mRNA levels were barely detectable in mouse resting spleen cells, but increased 60-fold within 4 h of stimulation with PMA, the calcium ionophore, ionomycin and recombinant interleukin-2, and the protein became measurable at the cell surface after 6 h, prior to the S phase. However, run-on assays using nuclei isolated from resting lymphocytes and growth-stimulated cell populations showed only 5-fold induction of CD98 gene transcription [42]. This suggests that post-transcriptional mechanisms are mainly responsible for the accumulation of CD98 mRNA at the onset of cell proliferation.
Recently, we explored the involvement of microRNAs, a new group of gene regulators with pivotal roles in various biological processes [43, 44], in the regulation of CD98 expression during IEC differentiation [5]. We showed that microRNA-7 post-transcriptionally down-regulated CD98 expression in IECs by binding to the 3′-untranslated region of the CD98 mRNA. In addition, our study suggested that microRNAs were responsible for the decrease of CD98 expression levels during differentiation of IECs in culture or along the crypt-villus axis [5].
In the context of intestinal inflammation, our studies and others have shown that pro-inflammatory cytokines up-regulate CD98 expression in IECs [5, 45, 46]. CD98 expression is highly up-regulated in colonic tissues from mice with active colitis [47]. Increased expression levels of CD98 have also been found in colonic biopsies from patients with Crohn’s disease [5], or at the surface of intestinal B cells, CD4+ T cells and CD8+ T cells isolated from patients with Crohn’s disease and ulcerative colitis [6]. Crohn’s disease and ulcerative colitis are the two primary forms of inflammatory bowel disease (IBD), which is chronic, relapsing, immunologically mediated disorders of the gastrointestinal tract with the etiology is largely unknown [48]. In addition, 5-aminosalicylic acid, which is used for treatment of intestinal inflammation in IBD, inhibited, in a dose-dependent manner, expression of cell-surface activation antigens including CD98 in mitogen-activated peripheral blood lymphocytes [6]. We have investigated the mechanism underlying the regulation of CD98 expression by interferon-gamma (IFN-γ) in IECs by cloning and functionally characterizing the 5′-flanking region of the CD98 gene from intestinal epithelial Caco2-BBE cell line [45]. Sequence analysis revealed four GC/GT boxes potentially capable for the binding of Sp1 transcription factor, and a binding site for the nuclear factor-kappaB (NF-κB) transcription factor [45]. These results are in agreement with the previous sequence analysis of the cloned CD98 cDNA from the HPB-MLT human T-cell tumor line, which revealed that the 5′-flanking region of the CD98 gene contains a hypomethylated CpG island and four potential binding sites for the Spl transcription factor, but does not contain TATA or CCAAT boxes [34]. Both competition electrophoretic mobility shift assay (EMSA) and supershift experiments confirmed the binding of Sp1 [Sp1(-874), Sp1(-386), Sp1(-187), and Sp1(-177)] and NF-κB [NF-κB(-213)] to the CD98 promoter. Furthermore, we found that different Sp1-binding sites exhibited different DNA–protein interaction profiles, indicating that each of these binding sites has distinct functional properties. Importantly, chromatin immunoprecipitation (ChiP) studies showed that the CD98 promoter interacts with Sp1 and NF-κB under both basal and IFN-γ-stimulated conditions [45]. Primer extension and rapid amplification of cDNA ends (RACE) assays revealed that a major transcriptional initiation site is located at 129 bases upstream of the first ATG codon [45]. Transfection of Caco2-BBE cells with luciferase reporter constructs fused to the full-length CD98 promoter or its serially truncated and site-mutated CD98 promoters revealed that the Sp1(-187), Sp1(-177), and NF-κB binding sites were essential for the basal and the IFN-γ-stimulated CD98 promoter activities, whereas the Sp1(-874) and Sp1(-386) binding sites were not. This study highlighted the important role of Sp1 and NF-κB transcription factors in the regulation of CD98 during physiological and pathological states. Interestingly, in another study, we showed that microRNA-7 is directly involved in IL1-β-mediated activation of CD98 expression in Caco2-BBE cells, and that the down-regulation of microRNA-7 in actively inflamed Crohn’s disease colonic tissues is probably responsible for the up-regulation of CD98 [5]. To our knowledge, this was the first report showing the role of microRNAs in the regulation of CD98 expression during intestinal inflammation. It would be of interest to further study the signaling cascades activated by immune responses during intestinal inflammation, which regulate CD98-targeting microRNAs and consequently CD98, to explore a complete mechanism underlying the regulation of CD98 during pathological state.
A high level of CD98 expression has been observed in proliferative normal tissues [49] and in almost all human melanoma cell lines, regardless of the tissue of origin [50]. CD98 is also highly expressed in several carcinomas, such as squamous cell carcinoma of the larynx [51], lung adenocarcinomas [52], breast cancer [53], renal cell cancer (where its expression level correlates with the extent of progression, metastasis and malignancy; [54]), and various human neoplasms [10]. The study by Parmacek et al. [49], designed to elucidate the pattern of CD98 gene regulation in neonatal and adult mouse tissues, failed to show a consistent result, i.e., while CD98 expression was higher in neonatal liver than in adult liver, the opposite was found for lung. This suggests that the regulation of CD98 could be tissue-dependent. Studies on the regulation of CD98 during carcinogenesis need further investigations. Since accumulating evidence has shown an important role for microRNAs in cell cycle, cell proliferation, and cancer development [3, 18, 55, 56], we hypothesize that this group of gene regulators could be a potential candidate for such studies.
CD98 and amino acid transport
An elegant study by Estevez and colleagues provided the first evidence that the minimal functional unit for amino acid transport is a heterodimeric complex [57]. The authors mutated two cysteine residues of CD98 (C109 and C330) to serine, singly or in combination, and found that the C109S mutant exhibited only 30–50% of y+L amino acid transport activity compared to the wild-type, whereas the C330S mutation had no effect. Hg++ and the impermeant reagent p-(chloromercuri)benzenesulfonate (pCMBS) inhibited the transport activity of the wild-type and all the mutants. Such inhibition was reversed by mercaptoethanol, but notably, the C109S mutant showed a much higher sensitivity towards the sulfhydryl reagents. Increased sensitivity could also be induced in the wild-type with mercaptoethanol pretreatment. It was concluded that for the expression of system y+L transport activity, CD98 must associate with an endogenous protein of the oocyte, and that such association involved a disulfide bond with C109. Immunoprecipitation of CD98 under reducing or non-reducing conditions showed that the endogenous protein was similar in size to the light chain of the CD98 surface antigen. CD98 has since been shown to associate with different light chains including LAT-1 [24], LAT-2 [26, 28], y+LAT-1, y+LAT-2 [58], xCT [27], and asc-1 [59, 60] to form heterodimers that are the minimal functional units for amino acid transport activity (Table 1). All the CD98-linked amino acid transporters (except rat LAT-2, which is able to perform net transport) appear to function as highly coupled amino acid exchangers, but the specificity depends on the associated light chain. Asc-1 may have some unidirectional flux of substrates in addition to the exchange transport [59].
The extracellular domain of CD98 is necessary for its interaction with the light chains to support amino acid transport [61]. The mechanisms by which CD98 renders the light chain functional remain largely unknown, but evidence has suggested its role in guiding the light chains to the membrane surface. Nakamura et al. [62] demonstrated that CD98 is required for the proper sorting of the light chain LAT-1 to the plasma membrane of COS cells as in its absence, LAT-1 was mostly remained in the Golgi. It was also shown that CD98 is capable of guiding LAT-1 to the plasma membrane independently of disulfide linkage between the two proteins, suggesting a presence of noncovalent steric interaction. In another study, Broer et al. [63] showed that a deletion of 68 amino acids from the C-terminus of human CD98 was sufficient to disrupt proper translocation of LATs to the plasma membrane, causing severe impairment of the transporter activity.
We and other groups have shown that in polarized IECs, CD98 is targeted to the basolateral membrane, where it is covalently linked to LAT-2, and presumably also y+LAT-1, to form functional heterodimers [20, 29, 30]. Studies have demonstrated that basolaterally expressed CD98/LAT-2 in IECs represents the minimal functional unit for a Na+-independent transporter of zwitterionic amino acids of any size [26, 28] (Fig. 1). We have shown that cross-linking of CD98 affects the intrinsic activity of the LAT-2 transporter by increasing the affinity and reducing the capacity of LAT-2-mediated leucine uptake in Caco2-BBE monolayers [64]. In addition, we have reported that cross-linking of CD98 regulates LAT-2-dependent leucine efflux [64]. Other studies have shown that serial C-terminal truncated constructs of CD98 (ranging from 15 to 404 residues) caused a complete loss of light chain function, although all heterodimers were expressed at the cell surface. This indicates that the 15 C-terminal residues of CD98 are required for the transport function of the heterodimer. Mutation of the conserved residue leucine 523 to glutamine in the carboxyl terminus reduced the V max of arginine and leucine uptake without affecting the affinity of the transporter for both arginine and leucine residues [65]. Interestingly, we have demonstrated that in the intestinal epithelial Caco2-BBE cell line, the heterodimer CD98/LAT-2 basolaterally interacts with the intracellular adhesion molecule I (ICAM-1), modulating LAT-2-mediated amino acid transport activity [64]. ICAM-1 has been shown to be important in cell–cell, cell-extracellular matrix interactions and cellular interactions such as the immune response [66, 67]. Therefore, CD98 could regulate amino acid transport either on its own or in combination with other molecules. The mechanisms by which CD98 controls amino acid transport activity, and the possible interactions involved, remain largely unknown and need further investigations.
CD98 and integrin activation
CD98 has been shown to associate with integrins and to regulate integrin functions [68] (Fig. 1). Integrins are a major family of cell-surface receptors that mediate cell adhesive and signaling events during interactions with the extracellular matrix or other cells [69]. They are heterodimeric transmembrane glycoproteins composed of noncovalently associated α and β subunits. Integrins are involved in various biological functions including cell growth, migration, development, metastasis, and immunologic and inflammatory responses [69, 70]. The first evidence indicating that CD98 interacts with integrins was provided by a genetic screen for genes that complement the suppression of β integrin activation that occurs in the presence of free β1 tails [68]. In this pioneer study, a Chinese hamster ovary cell line stably expressed a constitutively activated chimeric integrin complex was used. Overexpression of a β integrin cytoplasmic tail located at the plasma membrane leads to dominant suppression of integrin activation, most likely by titration of intracellular effector molecules. CD98 was isolated as one of these possible effectors since its overexpression led to the complementation of the dominant suppression (CODS) caused by the β integrin cytoplasmic tail [68]. These investigators further investigated that CD98 binds specifically to β1A and β3 integrins, but not β1D or β7 [71]. In a following study, by generating chimeric constructs of CD98 and CD69, another type-II transmembrane protein, this group showed that the cytoplasmic and transmembrane domains of CD98 are necessary and sufficient for integrin binding and complementation of dominant suppression, whereas the extracellular domain of this protein is required for amino acid transport function of the heterodimer [61]. Such interaction of CD98 with integrins has been shown to be important in integrin signaling that regulates a wide variety of biological processes, including cell fusion, differentiation, proliferation, aggregation, adhesion, migration, and malignant transformation [20, 43, 44, 61, 68, 71–77], which will be described more profoundly in the following sections (Table 2).
Table 2.
Summary of the functions of CD98 mentioned in this review
| Function | CD98 domain involved | Reference |
|---|---|---|
| Amino acid transport-associated functions | ||
| Interact with amino acid transporters and support amino acid transport | Extracellular domain | [61–65] |
| Integrin signaling-associated functions | ||
| Regulate cell homeostasis: cell growth, proliferation, migration, adhesion development | Cytoplasmic and transmembrane domains | [11, 14, 20, 43, 44, 61, 68–77, 81, 82, 86, 87] |
| Regulate immune responses | [11–13, 90–96] | |
CD98 and cell homeostasis
As discussed above, CD98 is a multifunctional protein that regulates integrin signals and amino acid transport. In addition to a direct physical regulation of integrin function, CD98 may also regulate other downstream signalings via its effects on transmembrane signaling pathways [78]. We recently showed that CD98 basolaterally expressed in IECs can be extracellularly phosphorylated by protein kinases released by T lymphocytes [79]. Extracellular (ecto-) phosphorylation has been implicated in many physiological processes, such as cell–cell interactions, cellular differentiation and proliferation, ion fluxes, and cellular activation [80]. In our system, we demonstrated that the ecto-phosphorylation of CD98 directly affected its multiple functions, including its role in the attachment and spreading of IECs, and homotypic and heterotypic cell–cell interactions [79].
Because of its crucial role, abnormal expression or function of CD98 or disruption of its interactions with its binding partners can lead to defects in cell homeostasis. Loss of CD98 resulted in early embryonic lethal in mice [81]. To investigate the potential stage-specific roles of CD98 in murine embryonic development, Sato et al. [82] recently generated a mouse model from embryonic stem (ES) cell line harboring a mutant CD98 allele that encodes a truncated form of CD98 protein (ΔCD98) fused to β galactosidase and neomycin phosphotransferase II (β geo). The authors showed that the mutant protein CD98-β geo was able to interact with β1 integrin, although it was mainly remained in the cytoplasm and thus may be unable to support amino acid transport [82]. Further analysis showed that the homozygous mutant (CD98Δ/Δ) embryos died between E7.5 and E9.5 [82]. The authors therefore proposed that CD98 may be essential for early post-implantation development by regulating integrin signaling, while the other function of CD98 as a component of amino acid transporters may be required for embryonic development at later stages [82].
In other studies, ES cell lines that lack CD98 gene (CD98−/−), although have normal β integrin levels, exhibit an impaired ability to spread on fibronectin or laminin and are susceptible to detachment-induced cell death, which were restored by a chimera that couples to integrins but not amino acid transporters [73]. Conversely, mutations of integrin β tails that block CD98 binding impair cell spreading, an important biological response to integrin signaling [75]. CD98 has been shown to stimulate adhesion of breast cancer cells to laminin via the integrin α3β1 [83]. CD98 interacts with β1 integrin in transfected CHO cells and mouse embryonic fibroblasts, and such interaction is required for integrin signaling including cell spreading, migration, proliferation, and survival [74, 75]. Failure of the interaction between these two proteins leads to a defect in focal adhesion, contributing to cell transformation [72]. Indeed, overexpression of the transmembrane portion of CD98 can lead to cellular transformation in vitro [72], and this suggests that the high levels of CD98 found in several human cancers can contribute to tumorigenesis. CD98 was also shown to be involved in fibronectin matrix assembly in vitro and in vivo, a process important in development, wound healing, and tumorigenesis, by mediating integrin signaling [74]. It was noted that CD98 expression was not required for fibronectin biosynthesis or affinity of cellular fibronectin binding integrins, which are important contributors for fibronectin assembly [74]. Recently, it was shown that CD98 is involved in integrin trafficking, thereby regulating keratinocyte adhesion and differentiation [76]. Interestingly, by profiling of adhesion molecules expressed in tumor endometrial-derived cell lines, used as models to identify markers of cellular receptivity, Dominguez et al. [43] identified CD98 as a novel molecule selectively and significantly associated with the receptive phenotype. Endometrial overexpression of CD98 markedly enhanced mouse blastocyst adhesion, and its silencing by siRNA reduced the blastocyst adhesion rate [43]. The authors therefore suggested that CD98 is an important determinant of endometrial receptivity for embryonic implantation in humans [43]. Our group has shown that expression of human CD98 in a CD98-deficient Madin Darby Canine Kidney cell line disrupts intercellular adhesion, leading to cytoskeletal disorder [20]. This phenotypic conversion, which probably depends on the interaction of human CD98 with respective “ligands”, is accompanied by reorganization of the actin cytoskeleton. Interactions between CD98 and amino acid transporters are unlikely to be involved in this process, as overexpression of the CD98 mutated at residue 109 (C109S) preventing disulfide linkage between CD98 and amino acid transporter did not inhibit the process. This observation is in agreement with the finding that CD98-amino acid transporter interaction is not required for the association of CD98 and integrins [61].
Recently, by generating tissue-specific gain- and loss-of-function mouse models with genetically manipulated CD98 expression specifically in IECs, we showed that CD98 has a crucial role in controlling homeostatic and innate immune responses in the gut [11]. We showed that IEC-specific CD98 overexpression in mice resulted in abnormal integrin signaling, leading to dysregulated cell proliferation and survival [11]. A balance between cell death and survival is key for the maintenance of intestinal homeostasis, and its dysregulation has been linked to intestinal pathologies. IEC-specific CD98 overexpression also caused barrier dysfunction [11] and a burdened increase of cultivable bacteria in the gastrointestinal tract and in feces from mice (unpublished data). It has been known that commensal bacteria in the intestinal lumen while provide benefits to the mammalian host such as increased metabolic/digestive capabilities and exclusion of harmful microbes, may behave as a pathogen and elicit an immune inflammatory response in the context of a dysfunctional intestinal epithelial barrier [84]. Indeed, a comparative analysis of gene expression profiles in the intestine of transgenic mice with IEC-specific CD98 overexpression showed a huge number of dysregulated genes, including those involved in inflammatory responses [11]. A correlation between the levels of CD98 overexpression and the abnormality of some phenotype indexes was observed in transgenic mice. Importantly, we provided direct evidence that IEC-specific CD98 overexpression aggravates intestinal inflammation in a mouse model of dextran-sulphate sodium (DSS)-induced colitis by inducing barrier dysfunction and perturbing inflammatory responses, both factors known to contribute to the pathophysiology of IBD [11]. It should be noted that our analysis of SLC3A2 polymorphisms in IBD patients showed that no polymorphism in the SLC3A2 gene was significantly associated with IBD susceptibility [11]. In addition to induction of gut homeostatic defects and increased inflammatory responses to DSS-induced colitis, IEC-specific CD98 overexpression promotes colitis-associated tumorigenesis in mice. Further analysis indicated that the ability of IEC-specific CD98 overexpression to induce tumorigenesis was linked to its capacity to induce barrier dysfunction and to stimulate cell proliferation and production of pro-inflammatory mediators [11]. Conversely, CD98 downregulation specifically in IECs resulted in attenuated inflammatory responses and resistance to both DSS-induced colitis and colitis-associated tumorigenesis [11]. Our study was in agreement with previous reports that loss of CD98 impairs the tumorigenicity of ES cells, which can be restored by re-expression of the integrin-interacting domains of CD98 [73]. Indeed, the oncogenic activity of CD98 has been suggested for years as it is highly expressed in proliferative normal tissue [85] and almost all human melanoma cell lines, regardless of the tissue of origin [50], as well as in several carcinomas, such as squamous cell carcinoma of the larynx [51], lung adenocarcinomas [52], breast cancer [53], renal cell cancer [54], and various human neoplasms [10]. Furthermore, overexpression of CD98 has been shown to result in malignant transformation [72, 86, 87]. However, to our knowledge, our study is the first to provide direct evidence that IEC-specific CD98 overexpression aggravates intestinal inflammation and consequently promotes colitis-associated tumorigenesis in mice [11]. Our current unpublished data showed that CD98 expression was increased in intestinal adenomas developed in the ApcMin/+ mouse model, which harbors a germ-line mutation in adenomatous polyposis coli (APC) gene and spontaneously develop numerous adenomas in the small intestine [88, 89]. In addition, IEC-specific CD98 overexpression increased intestinal tumor incidence and tumor size in ApcMin/+ mice, which correlated with enhanced proliferation and decreased apoptosis of IECs, as well as increased the production of pro-inflammatory cytokines and chemokines (unpublished data). We also validated these results using mice exhibiting IEC-specific CD98 downregulation. IEC-specific CD98 down-regulation efficiently attenuated IEC proliferation and cytokine/chemokine production, consequently reducing tumor incidence and tumor growth in ApcMin/+ mice (unpublished data). Studies from other groups using CD98 conditional null mouse showed that smooth muscle cell-specific deletion of CD98 although did not affect normal vessel morphology, suppressed proliferation and induced apoptosis in vascular smooth muscle cells, indicating its requirement for neointimal formation after arterial injury [14]. Together, these findings demonstrate clearly a crucial role for CD98 in the regulation of cell homeostasis, and that abnormal in its expression/function may contribute to human diseases.
CD98 in immune responses
CD98 was discovered since 1981 as a lymphocyte-activating antigen [3, 4], however, the mechanisms of CD98 activity in the immune system is largely unknown. It has been shown that anti-CD98 antibodies can co-stimulate T lymphocytes, whereas others can inhibit them [90, 91]. Treatment of T cells with immobilized CD98 antibodies resulted in proliferation when used in conjunction with submitogenic anti-CD3 stimulation [92]. In agreement with this study, screening of antibodies for their ability to co-stimulate T cells together with anti-CD3 antibodies revealed a new CD98-specific antibody, indicating a co-stimulatory role for CD98 in T-cell activation and function [93]. It should be noted that the CD98 antibody-mediated co-stimulation required a functional interaction between integrins and CD98 as it was inhibited by β1 integrin-blocking antibodies [93]. The same group later showed the induction of homotypic aggregation among human T cells through CD98, which was inhibited by β1 integrin monoclonal antibody [94]. This study also provided evidence for the specific association of α4β1 integrin and CD98 on human T lymphocytes [94]. Therefore, CD98-dependent lymphocyte proliferation and adhesion may involve integrins. In other studies, CD98 antibodies were shown to block antigen-specific responses of T cells. Because antigen-presenting cells were included in these experiments, it was concluded that monocytes regulated T cell activation through CD98 [90]. There are also conflicting reports of the involvement of IL-2 or the IL-2 receptor in CD98 antibody-augmented proliferation [90, 95]. However, it was not known in these experiments whether the anti-CD98 antibodies were indeed crosslinking/activating, or blocking, with one notable exception, where intracellular phosphorylation was observed after treatment with anti-CD98 antibody [96]. In addition to regulation of T cell functions by interacting with certain integrin β-subunits, CD98 is involved in the transport of leucine and isoleucine, which are important regulators of the mammalian target of rapamycin (mTOR) pathway governing nutrient-regulated lymphocyte function [97, 98].
In an elegant study by Cantor et al. [13], by using conditional knockout mice with B cell-specific CD98 deletion, it was shown that CD98 facilitates adaptive humoral immunity by supporting integrin-dependent rapid proliferation of B cells that is necessary for clonal expansion and subsequent differentiation into plasma cells. Reconstitution with CD98 mutants that mediates integrin signaling, but not amino acid transport, supported the proliferation of CD98-deficient B cells, indicating that the integrin-binding domain of CD98 was required for B cell proliferation. This study importantly establishes a connection of CD98-dependent integrin and immunoreceptor signaling pathways that regulate lymphocyte proliferation [13]. Recently, this group investigated the role of CD98 in T cell-mediated immunity and autoimmune disease pathogenesis. They showed that in contrast to B cells, CD98-deficient T cells showed only modestly impaired antigen-driven proliferation and nearly normal homeostatic proliferation. However, T cell-specific CD98 deletion resulted in dramatically reduced T cell clonal expansion and consequently prevented experimental autoimmune diabetes [12]. The integrin-binding domain of CD98 was shown to be required for antigen-driven T cell clonal expansion in the pathogenesis of an autoimmune disease [12]. These findings suggested that targeting CD98–integrin interaction might selectively ablate clonal expansion and preserve other integrin-dependent T cell functions, such as formation of immune synapses and homing to peripheral tissues [12].
As described in the previous section, we showed that IEC-specific CD98 overexpression resulted in barrier dysfunction, among the earliest events that precede evident inflammation or mucosal damage [48] and spontaneous upregulation of pro-inflammatory genes such as tumor necrosis factor-alpha (TNF-α), consequently increased inflammatory responses to DSS-induced colitis [11]. In a recent review of interest, Turner [99] has discussed about the regulation of intestinal mucosal barrier in response to physiological and immunological stimuli, and provided evidence that this regulation shapes mucosal immune responses in the gut and may contribute to disease when dysfunctional. It was also emphasized in this review that defects in barrier function alone are insufficient to cause disease, and that there are immune mechanisms that maintain mucosal homeostasis despite barrier dysfunction [99]. Therefore, in our model, both barrier dysfunction and dysregulated innate immune responses, with the consequent defects in controlling gut microbiota, contribute to the robust DSS-induced intestinal inflammation observed in mice having IEC-specific CD98 overexpression. IEC-specific CD98 downregulation while did not cause any defects in intestinal homeostasis, attenuated inflammatory responses and resistance to both DSS-induced colitis and colitis-associated tumorigenesis [11]. Our findings indicate that intestinal CD98 expression has a crucial role in controlling homeostatic and innate immune responses in the gut.
Apart from the findings for CD98 functions via its interactions with either integrin or amino acid transporters, as discussed in the previous section, we demonstrated that CD98 expressed in the basolateral membrane of IECs could be ecto-phosphorylated by ecto-protein kinases released from T lymphocytes, and that its ecto-phosphorylation could modulate epithelial cell–T lymphocyte interactions [79]. Since IEC–lymphocyte interactions have been shown to play a key role in immune responses within the intestinal tract [100], it is interesting to speculate that CD98 ecto-phosphorylation could be effective under pathological conditions, where extracellular ATP required for ecto-phosphorylation can reach high concentration [80], thereby regulating T cell-mediated immune responses. In this study, the molecular mechanism by which ecto-phosphorylation of CD98 modulates cell–cell interactions remains unrevealed, giving an exciting area for future investigation. We speculate that CD98 ecto-phosphorylation may have substantial effects on the binding affinity of CD98 for surface proteins expressed on other cells, or it may functionally affect other proteins that interact with CD98 and are involved in cell–cell interactions.
Perspective
The complicated molecular structure and multifunctional nature of CD98, while raise challenging questions, make works in this field intriguing. How the amino acid transport and integrin function are combined in one protein is still mysterious. It is not clear whether association with the light chain is necessary for CD98-mediated integrin activation. Linking these two functions might be important for polarized cells, such as in intestinal epithelium [44]. Another important issue is that external stimuli via CD98 can generate intracellular phosphorylation-mediated signals, making the biology of such interactions likely to be a particularly interesting area of future study. Indeed, CD98 has been shown to bind galectin-3 [101, 102], a lectin that is involved in a broad diversity of biological functions including immunomodulation, host–pathogen interactions, embryogenesis, angiogenesis, cell migration, wound healing, and apoptosis [103]. More studies are needed to identify other possible ligands of the CD98 complex and to investigate the mechanism of down-stream intracellular signaling pathways activated by recognition of such ligands by CD98. Finally, since CD98 has an important role in the regulation of cellular homeostasis and immunity, modulation of CD98 expression and/or function represents a promising therapeutic strategy for the treatment and prevention of immunological diseases and malignancies. Future studies targeting CD98 expression or CD98–integrin interaction in genetically modified mice or in experimental disease models using advanced biotechnologies, such as nanotechnology that we have been developing in the laboratory, could address this issue and promise to offer exciting results.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs and the National Institutes of Health of Diabetes and Digestive and Kidney by the grant RO1-DK-071594 (to D.M). We dedicate this article to the memory of Prof. Shanthi V. Sitaraman, a brilliant scientist, dedicated physician, passionate humanitarian and dearest friend.
Contributor Information
Hang Thi Thu Nguyen, Email: hang.nguyen@u-clermont1.fr.
Didier Merlin, Email: dmerlin@gsu.edu.
References
- 1.Yan Y, Vasudevan S, Nguyen HT, Merlin D. Intestinal epithelial CD98: an oligomeric and multifunctional protein. Biochim Biophys Acta. 2008;1780(10):1087–1092. doi: 10.1016/j.bbagen.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deves R, Boyd CA. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J Membr Biol. 2000;173(3):165–177. doi: 10.1007/s002320001017. [DOI] [PubMed] [Google Scholar]
- 3.Haynes BF, Hemler ME, Mann DL, Eisenbarth GS, Shelhamer J, Mostowski HS, Thomas CA, Strominger JL, Fauci AS. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981;126(4):1409–1414. [PubMed] [Google Scholar]
- 4.Moretta A, Mingari MC, Haynes BF, Sekaly RP, Moretta L, Fauci AS. Phenotypic characterization of human cytolytic T lymphocytes in mixed lymphocyte culture. J Exp Med. 1981;153(1):213–218. doi: 10.1084/jem.153.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen HT, Dalmasso G, Yan Y, Laroui H, Dahan S, Mayer L, Sitaraman SV, Merlin D. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J Biol Chem. 2010;285(2):1479–1489. doi: 10.1074/jbc.M109.057141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber S, MacDermott RP, Raedler A, Pinnau R, Bertovich MJ, Nash GS. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991;101(4):1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- 7.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Kanai Y, Endou H, et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in early stage squamous cell carcinoma of the lung. Cancer Sci. 2009;100(2):248–254. doi: 10.1111/j.1349-7006.2008.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Kanai Y, Nakajima T, et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in stage I pulmonary adenocarcinoma. Lung Cancer. 2009;66(1):120–126. doi: 10.1016/j.lungcan.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Kawashima O, Kamide Y, Ishizuka T, et al. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann Surg Oncol. 2009;16:3473–3481. doi: 10.1245/s10434-009-0685-0. [DOI] [PubMed] [Google Scholar]
- 10.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, et al. L-type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 2008;99(12):2380–2386. doi: 10.1111/j.1349-7006.2008.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen HT, Dalmasso G, Torkvist L, Halfvarson J, Yan Y, Laroui H, Shmerling D, Tallone T, D’Amato M, Sitaraman SV, et al. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J Clin Invest. 2011;121(5):1733–1747. doi: 10.1172/JCI44631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantor J, Slepak M, Ege N, Chang JT, Ginsberg MH. Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity. J Immunol. 2011;187(2):851–860. doi: 10.4049/jimmunol.1100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantor J, Browne CD, Ruppert R, Feral CC, Fassler R, Rickert RC, Ginsberg MH. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat Immunol. 2009;10(4):412–419. doi: 10.1038/ni.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogelstrand P, Feral CC, Zargham R, Ginsberg MH. Dependence of proliferative vascular smooth muscle cells on CD98hc (4F2hc, SLC3A2) J Exp Med. 2009;206(11):2397–2406. doi: 10.1084/jem.20082845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francke U, Foellmer BE, Haynes BF. Chromosome mapping of human cell surface molecules: monoclonal anti-human lymphocyte antibodies 4F2, A3D8, and A1G3 define antigens controlled by different regions of chromosome 11. Somatic Cell Genet. 1983;9(3):333–344. doi: 10.1007/BF01539142. [DOI] [PubMed] [Google Scholar]
- 16.Quackenbush E, Clabby M, Gottesdiener KM, Barbosa J, Jones NH, Strominger JL, Speck S, Leiden JM. Molecular cloning of complementary DNAs encoding the heavy chain of the human 4F2 cell-surface antigen: a type II membrane glycoprotein involved in normal and neoplastic cell growth. Proc Natl Acad Sci USA. 1987;84(18):6526–6530. doi: 10.1073/pnas.84.18.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira S, Di Grandi S, Kuhn LC. Primary structure of the human 4F2 antigen heavy chain predicts a transmembrane protein with a cytoplasmic NH2 terminus. J Biol Chem. 1987;262(20):9574–9580. [PubMed] [Google Scholar]
- 18.Lumadue JA, Glick AB, Ruddle FH. Cloning, sequence analysis, and expression of the large subunit of the human lymphocyte activation antigen 4F2. Proc Natl Acad Sci USA. 1987;84(24):9204–9208. doi: 10.1073/pnas.84.24.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quackenbush EJ, Gougos A, Baumal R, Letarte M. Differential localization within human kidney of five membrane proteins expressed on acute lymphoblastic leukemia cells. J Immunol. 1986;136(1):118–124. [PubMed] [Google Scholar]
- 20.Merlin D, Sitaraman S, Liu X, Eastburn K, Sun J, Kucharzik T, Lewis B, Madara JL. CD98-mediated links between amino acid transport and beta 1 integrin distribution in polarized columnar epithelia. J Biol Chem. 2001;276(42):39282–39289. doi: 10.1074/jbc.M105077200. [DOI] [PubMed] [Google Scholar]
- 21.Palacin M, Bertran J, Zorzano A. Heteromeric amino acid transporters explain inherited aminoacidurias. Curr Opin Nephrol Hypertens. 2000;9(5):547–553. doi: 10.1097/00041552-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Verrey F, Jack DL, Paulsen IT, Saier MH, Jr, Pfeiffer R. New glycoprotein-associated amino acid transporters. J Membr Biol. 1999;172(3):181–192. doi: 10.1007/s002329900595. [DOI] [PubMed] [Google Scholar]
- 23.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273(37):23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 24.Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395(6699):288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer R, Rossier G, Spindler B, Meier C, Kuhn L, Verrey F. Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J. 1999;18(1):49–57. doi: 10.1093/emboj/18.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274(28):19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- 27.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274(17):11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 28.Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274(28):19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 29.Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, Kuhn LC. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem. 1999;274(49):34948–34954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]
- 30.Dave MH, Schulz N, Zecevic M, Wagner CA, Verrey F. Expression of heteromeric amino acid transporters along the murine intestine. J Physiol. 2004;558(Pt 2):597–610. doi: 10.1113/jphysiol.2004.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottesdiener KM, Karpinski BA, Lindsten T, Strominger JL, Jones NH, Thompson CB, Leiden JM. Isolation and structural characterization of the human 4F2 heavy-chain gene, an inducible gene involved in T-lymphocyte activation. Mol Cell Biol. 1988;8(9):3809–3819. doi: 10.1128/mcb.8.9.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsten T, June CH, Thompson CB, Leiden JM. Regulation of 4F2 heavy-chain gene expression during normal human T-cell activation can be mediated by multiple distinct molecular mechanisms. Mol Cell Biol. 1988;8(9):3820–3826. doi: 10.1128/mcb.8.9.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 34.Karpinski BA, Yang LH, Cacheris P, Morle GD, Leiden JM. The first intron of the 4F2 heavy-chain gene contains a transcriptional enhancer element that binds multiple nuclear proteins. Mol Cell Biol. 1989;9(6):2588–2597. doi: 10.1128/mcb.9.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka T, Masuko T, Hashimoto Y. Appearance of a proliferation-associated antigen, gp125, on rat and human lymphocytes by co-stimulation with phorbol ester and calcium ionophore. J Biochem. 1988;103(4):644–649. doi: 10.1093/oxfordjournals.jbchem.a122322. [DOI] [PubMed] [Google Scholar]
- 36.Neckers LM, Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci USA. 1983;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumagai N, Benedict SH, Mills GB, Gelfand EW. Requirements for the simultaneous presence of phorbol esters and calcium ionophores in the expression of human T lymphocyte proliferation-related genes. J Immunol. 1987;139(5):1393–1399. [PubMed] [Google Scholar]
- 38.Kumagai N, Benedict SH, Mills GB, Gelfand EW. Comparison of phorbol ester/calcium ionophore and phytohemagglutinin-induced signaling in human T lymphocytes. Demonstration of interleukin 2-independent transferrin receptor gene expression. J Immunol. 1988;140(1):37–43. [PubMed] [Google Scholar]
- 39.Reed JC, Alpers JD, Nowell PC, Hoover RG. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci USA. 1986;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cotner T, Williams JM, Christenson L, Shapiro HM, Strom TB, Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nii T, Segawa H, Taketani Y, Tani Y, Ohkido M, Kishida S, Ito M, Endou H, Kanai Y, Takeda E, et al. Molecular events involved in up-regulating human Na+-independent neutral amino acid transporter LAT1 during T-cell activation. Biochem J. 2001;358(Pt 3):693–704. doi: 10.1042/0264-6021:3580693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teixeira S, Kuhn LC. Post-transcriptional regulation of the transferrin receptor and 4F2 antigen heavy chain mRNA during growth activation of spleen cells. Eur J Biochem. 1991;202(3):819–826. doi: 10.1111/j.1432-1033.1991.tb16438.x. [DOI] [PubMed] [Google Scholar]
- 43.Dominguez F, Simon C, Quinonero A, Ramirez MA, Gonzalez-Munoz E, Burghardt H, Cervero A, Martinez S, Pellicer A, Palacin M, et al. Human endometrial CD98 is essential for blastocyst adhesion. PLoS One. 2010;5(10):e13380. doi: 10.1371/journal.pone.0013380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai S, Bulus N, Fonseca-Siesser PM, Chen D, Hanks SK, Pozzi A, Zent R. CD98 modulates integrin beta1 function in polarized epithelial cells. J Cell Sci. 2005;118(Pt 5):889–899. doi: 10.1242/jcs.01674. [DOI] [PubMed] [Google Scholar]
- 45.Yan Y, Dalmasso G, Sitaraman S, Merlin D. Characterization of the human intestinal CD98 promoter and its regulation by interferon-gamma. Am J Physiol Gastrointest Liver Physiol. 2007;292(2):G535–G545. doi: 10.1152/ajpgi.00385.2006. [DOI] [PubMed] [Google Scholar]
- 46.Fais S, Pallone F. Ability of human colonic epithelium to express the 4F2 antigen, the common acute lymphoblastic leukemia antigen, and the transferrin receptor. Studies in inflammatory bowel disease and after in vitro exposure to different stimuli. Gastroenterology. 1989;97(6):1435–1441. doi: 10.1016/0016-5085(89)90387-9. [DOI] [PubMed] [Google Scholar]
- 47.Kucharzik T, Lugering A, Yan Y, Driss A, Charrier L, Sitaraman S, Merlin D. Activation of epithelial CD98 glycoprotein perpetuates colonic inflammation. Lab Invest. 2005;85(7):932–941. doi: 10.1038/labinvest.3700289. [DOI] [PubMed] [Google Scholar]
- 48.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(7):390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 49.Parmacek MS, Karpinski BA, Gottesdiener KM, Thompson CB, Leiden JM. Structure, expression and regulation of the murine 4F2 heavy chain. Nucleic Acids Res. 1989;17(5):1915–1931. doi: 10.1093/nar/17.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dixon WT, Sikora LK, Demetrick DJ, Jerry LM. Isolation and characterization of a heterodimeric surface antigen on human melanoma cells and evidence that it is the 4F2 cell activation/proliferation molecule. Int J Cancer. 1990;45(1):59–68. doi: 10.1002/ijc.2910450113. [DOI] [PubMed] [Google Scholar]
- 51.Esteban F, Ruiz-Cabello F, Concha A. Perez Ayala M, Delgado M, Garrido F: Relationship of 4F2 antigen with local growth and metastatic potential of squamous cell carcinoma of the larynx. Cancer. 1990;66(7):1493–1498. doi: 10.1002/1097-0142(19901001)66:7<1493::AID-CNCR2820660710>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 52.Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen GD, Perou CM, Whyte RI, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA. 2001;98(24):13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esseghir S, Reis-Filho JS, Kennedy A, James M, O’Hare MJ, Jeffery R, Poulsom R, Isacke CM. Identification of transmembrane proteins as potential prognostic markers and therapeutic targets in breast cancer by a screen for signal sequence encoding transcripts. J Pathol. 2006;210(4):420–430. doi: 10.1002/path.2071. [DOI] [PubMed] [Google Scholar]
- 54.Prager GW, Poettler M, Schmidinger M, Mazal PR, Susani M, Zielinski CC, Haitel A. CD98hc (SLC3A2), a novel marker in renal cell cancer. Eur J Clin Invest. 2009;39(4):304–310. doi: 10.1111/j.1365-2362.2009.02096.x. [DOI] [PubMed] [Google Scholar]
- 55.Osada H, Takahashi T. MicroRNAs in biological processes and carcinogenesis. Carcinogenesis. 2007;28(1):2–12. doi: 10.1093/carcin/bgl185. [DOI] [PubMed] [Google Scholar]
- 56.Kumagai N, Benedict SH, Mills GB, Gelfand EW. Induction of competence and progression signals in human T lymphocytes by phorbol esters and calcium ionophores. J Cell Physiol. 1988;137(2):329–336. doi: 10.1002/jcp.1041370217. [DOI] [PubMed] [Google Scholar]
- 57.Estevez R, Camps M, Rojas AM, Testar X, Deves R, Hediger MA, Zorzano A, Palacin M. The amino acid transport system y+L/4F2hc is a heteromultimeric complex. FASEB J. 1998;12(13):1319–1329. doi: 10.1096/fasebj.12.13.1319. [DOI] [PubMed] [Google Scholar]
- 58.Torrents D, Estevez R, Pineda M, Fernandez E, Lloberas J, Shi YB, Zorzano A, Palacin M. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J Biol Chem. 1998;273(49):32437–32445. doi: 10.1074/jbc.273.49.32437. [DOI] [PubMed] [Google Scholar]
- 59.Fukasawa Y, Segawa H, Kim JY, Chairoungdua A, Kim DK, Matsuo H, Cha SH, Endou H, Kanai Y. Identification and characterization of a Na(+)-independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral D- and L-amino acids. J Biol Chem. 2000;275(13):9690–9698. doi: 10.1074/jbc.275.13.9690. [DOI] [PubMed] [Google Scholar]
- 60.Nakauchi J, Matsuo H, Kim DK, Goto A, Chairoungdua A, Cha SH, Inatomi J, Shiokawa Y, Yamaguchi K, Saito I, et al. Cloning and characterization of a human brain Na(+)-independent transporter for small neutral amino acids that transports d-serine with high affinity. Neurosci Lett. 2000;287(3):231–235. doi: 10.1016/S0304-3940(00)01169-1. [DOI] [PubMed] [Google Scholar]
- 61.Fenczik CA, Zent R, Dellos M, Calderwood DA, Satriano J, Kelly C, Ginsberg MH. Distinct domains of CD98hc regulate integrins and amino acid transport. J Biol Chem. 2001;276(12):8746–8752. doi: 10.1074/jbc.M011239200. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura E, Sato M, Yang H, Miyagawa F, Harasaki M, Tomita K, Matsuoka S, Noma A, Iwai K, Minato N. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem. 1999;274(5):3009–3016. doi: 10.1074/jbc.274.5.3009. [DOI] [PubMed] [Google Scholar]
- 63.Broer A, Friedrich B, Wagner CA, Fillon S, Ganapathy V, Lang F, Broer S. Association of 4F2hc with light chains LAT1, LAT2 or y+LAT2 requires different domains. Biochem J. 2001;355(Pt 3):725–731. doi: 10.1042/bj3550725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Charrier L, Gewirtz A, Sitaraman S, Merlin D. CD98 and intracellular adhesion molecule I regulate the activity of amino acid transporter LAT-2 in polarized intestinal epithelia. J Biol Chem. 2003;278(26):23672–23677. doi: 10.1074/jbc.M302777200. [DOI] [PubMed] [Google Scholar]
- 65.Chubb S, Kingsland AL, Broer A, Broer S. Mutation of the 4F2 heavy-chain carboxy terminus causes y+LAT2 light-chain dysfunction. Mol Membr Biol. 2006;23(3):255–267. doi: 10.1080/09687860600652968. [DOI] [PubMed] [Google Scholar]
- 66.Simmons D, Makgoba MW, Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- 67.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137(1):245–254. [PubMed] [Google Scholar]
- 68.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390(6655):81–85. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- 69.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-S. [DOI] [PubMed] [Google Scholar]
- 70.Albelda SM. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993;68(1):4–17. [PubMed] [Google Scholar]
- 71.Zent R, Fenczik CA, Calderwood DA, Liu S, Dellos M, Ginsberg MH. Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. J Biol Chem. 2000;275(7):5059–5064. doi: 10.1074/jbc.275.7.5059. [DOI] [PubMed] [Google Scholar]
- 72.Henderson NC, Collis EA, Mackinnon AC, Simpson KJ, Haslett C, Zent R, Ginsberg M, Sethi T. CD98hc (SLC3A2) interaction with beta 1 integrins is required for transformation. J Biol Chem. 2004;279(52):54731–54741. doi: 10.1074/jbc.M408700200. [DOI] [PubMed] [Google Scholar]
- 73.Feral CC, Nishiya N, Fenczik CA, Stuhlmann H, Slepak M, Ginsberg MH. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci USA. 2005;102(2):355–360. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feral CC, Zijlstra A, Tkachenko E, Prager G, Gardel ML, Slepak M, Ginsberg MH. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J Cell Biol. 2007;178(4):701–711. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prager GW, Feral CC, Kim C, Han J, Ginsberg MH. CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J Biol Chem. 2007;282(33):24477–24484. doi: 10.1074/jbc.M702877200. [DOI] [PubMed] [Google Scholar]
- 76.Lemaitre G, Stella A, Feteira J, Baldeschi C, Vaigot P, Martin MT, Monsarrat B, Waksman G. CD98hc (SLC3A2) is a key regulator of keratinocyte adhesion. J Dermatol Sci. 2011;61(3):169–179. doi: 10.1016/j.jdermsci.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 77.Higuchi S, Tabata N, Tajima M, Ito M, Tsurudome M, Sudo A, Uchida A, Ito Y. Induction of human osteoclast-like cells by treatment of blood monocytes with anti-fusion regulatory protein-1/CD98 monoclonal antibodies. J Bone Miner Res. 1998;13(1):44–49. doi: 10.1359/jbmr.1998.13.1.44. [DOI] [PubMed] [Google Scholar]
- 78.Suga K, Katagiri K, Kinashi T, Harazaki M, Iizuka T, Hattori M, Minato N. CD98 induces LFA-1-mediated cell adhesion in lymphoid cells via activation of Rap1. FEBS Lett. 2001;489(2–3):249–253. doi: 10.1016/S0014-5793(00)02222-5. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen HT, Dalmasso G, Yan Y, Obertone TS, Sitaraman SV, Merlin D. Ecto-phosphorylation of CD98 regulates cell–cell interactions. PLoS One. 2008;3(12):e3895. doi: 10.1371/journal.pone.0003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Redegeld FA, Caldwell CC, Sitkovsky MV. Ecto-protein kinases: ecto-domain phosphorylation as a novel target for pharmacological manipulation? Trends Pharmacol Sci. 1999;20(11):453–459. doi: 10.1016/S0165-6147(99)01399-1. [DOI] [PubMed] [Google Scholar]
- 81.Tsumura H, Suzuki N, Saito H, Kawano M, Otake S, Kozuka Y, Komada H, Tsurudome M, Ito Y. The targeted disruption of the CD98 gene results in embryonic lethality. Biochem Biophys Res Commun. 2003;308(4):847–851. doi: 10.1016/S0006-291X(03)01473-6. [DOI] [PubMed] [Google Scholar]
- 82.Sato Y, Heimeier RA, Li C, Deng C, Shi YB. Extracellular domain of CD98hc is required for early murine development. Cell Biosci. 2011;1(1):7. doi: 10.1186/2045-3701-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by alpha3beta1 integrin and regulated by insulin-like growth factor-1 and CD98. J Biol Chem. 1999;274(16):11408–11416. doi: 10.1074/jbc.274.16.11408. [DOI] [PubMed] [Google Scholar]
- 84.Collier-Hyams LS, Neish AS. Innate immune relationship between commensal flora and the mammalian intestinal epithelium. Cell Mol Life Sci. 2005;62(12):1339–1348. doi: 10.1007/s00018-005-5038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chillaron J, Roca R, Valencia A, Zorzano A, Palacin M. Heteromeric amino acid transporters: biochemistry, genetics, and physiology. Am J Physiol Renal Physiol. 2001;281(6):F995–F1018. doi: 10.1152/ajprenal.2001.281.6.F995. [DOI] [PubMed] [Google Scholar]
- 86.Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem Biophys Res Commun. 1999;262(3):720–725. doi: 10.1006/bbrc.1999.1051. [DOI] [PubMed] [Google Scholar]
- 87.Shishido T, Uno S, Kamohara M, Tsuneoka-Suzuki T, Hashimoto Y, Enomoto T, Masuko T. Transformation of BALB3T3 cells caused by over-expression of rat CD98 heavy chain (HC) requires its association with light chain: mis-sense mutation in a cysteine residue of CD98HC eliminates its transforming activity. Int J Cancer. 2000;87(3):311–316. doi: 10.1002/1097-0215(20000801)87:3<311::AID-IJC1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 88.Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci USA. 1995;92(10):4482–4486. doi: 10.1073/pnas.92.10.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 90.Diaz LA., Jr Friedman AW, He X, Kuick RD, Hanash SM, Fox DA: Monocyte-dependent regulation of T lymphocyte activation through CD98. Int Immunol. 1997;9(9):1221–1231. doi: 10.1093/intimm/9.9.1221. [DOI] [PubMed] [Google Scholar]
- 91.Nakao M, Kubo K, Hara A, Hirohashi N, Futagami E, Shichijo S, Sagawa K, Itoh K. A monoclonal antibody (H227) recognizing a new epitope of 4F2 molecular complex associated with T cell activation. Cell Immunol. 1993;152(1):226–233. doi: 10.1006/cimm.1993.1282. [DOI] [PubMed] [Google Scholar]
- 92.Freidman AW, Diaz LA, Jr, Moore S, Schaller J, Fox DA. The human 4F2 antigen: evidence for cryptic and noncryptic epitopes and for a role of 4F2 in human T lymphocyte activation. Cell Immunol. 1994;154(1):253–263. doi: 10.1006/cimm.1994.1075. [DOI] [PubMed] [Google Scholar]
- 93.Warren AP, Patel K, Miyamoto Y, Wygant JN, Woodside DG, McIntyre BW. Convergence between CD98 and integrin-mediated T-lymphocyte co-stimulation. Immunology. 2000;99(1):62–68. doi: 10.1046/j.1365-2567.2000.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyamoto YJ, Mitchell JS, McIntyre BW. Physical association and functional interaction between beta1 integrin and CD98 on human T lymphocytes. Mol Immunol. 2003;39(12):739–751. doi: 10.1016/S0161-5890(02)00255-9. [DOI] [PubMed] [Google Scholar]
- 95.Komada H, Imai A, Hattori E, Ito M, Tsumura H, Onoda T, Kuramochi M, Tani M, Yamamoto K, Yamane M, et al. Possible activation of murine T lymphocyte through CD98 is independent of interleukin 2/interleukin 2 receptor system. Biomed Res. 2006;27(2):61–67. doi: 10.2220/biomedres.27.61. [DOI] [PubMed] [Google Scholar]
- 96.Melchior A, Denys A, Deligny A, Mazurier J, Allain F. Cyclophilin B induces integrin-mediated cell adhesion by a mechanism involving CD98-dependent activation of protein kinase C-delta and p44/42 mitogen-activated protein kinases. Exp Cell Res. 2008;314(3):616–628. doi: 10.1016/j.yexcr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 97.Abraham RT. Mammalian target of rapamycin: immunosuppressive drugs uncover a novel pathway of cytokine receptor signaling. Curr Opin Immunol. 1998;10(3):330–336. doi: 10.1016/S0952-7915(98)80172-6. [DOI] [PubMed] [Google Scholar]
- 98.Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19(3):162–172. doi: 10.1016/j.smim.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 100.Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12(5):563–568. doi: 10.1016/S0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 101.Dong S, Hughes RC. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen) Glycoconj J. 1997;14(2):267–274. doi: 10.1023/A:1018554124545. [DOI] [PubMed] [Google Scholar]
- 102.Dalton P, Christian HC, Redman CW, Sargent IL, Boyd CA. Membrane trafficking of CD98 and its ligand galectin 3 in BeWo cells—implication for placental cell fusion. FEBS J. 2007;274(11):2715–2727. doi: 10.1111/j.1742-4658.2007.05806.x. [DOI] [PubMed] [Google Scholar]
- 103.Sundblad V, Croci DO, Rabinovich GA. Regulated expression of galectin-3, a multifunctional glycan-binding protein, in haematopoietic and non-haematopoietic tissues. Histol Histopathol. 2011;26(2):247–265. doi: 10.14670/HH-26.247. [DOI] [PubMed] [Google Scholar]