Abstract
Given the continued growth in the number of persons with cancer in the United States, the primary prevention of cancer remains an urgent public health priority. As the field of cancer prevention continues to mature and scientific knowledge evolves, it is imperative to challenge the status quo and embrace new approaches to cancer prevention. In this commentary, we summarize recent trends and some of the scientific advances that have been made over the past few decades regarding the complex process of cancer development and the interaction of individual and social risk factors. We examine some of the assumptions and terminology that have characterized cancer prevention approaches for more than a quarter century and the impact of these assumptions and our use of terminology. We propose that it is possible for today’s youth to experience lower cancer incidence rates as adults compared with previous generations. To accomplish this goal, a more transdisciplinary and multifaceted approach is needed, adapted as appropriate for different populations and stages of life. The greatest improvements in cancer prevention may occur as a result of innovative, multilevel interventions that build on the expanding scientific evidence base.
Keywords: Cancer prevention, Cancer causes, Modifiable risk factor, Social determinants, Cancer incidence trends, Cancer continuum, Lifestyle
“It may be hard for an egg to turn into a bird: it would be a jolly sight harder for it to learn to fly while remaining an egg. We are like eggs at present. And you cannot go on indefinitely being just an ordinary, decent egg. We must be hatched or go bad.”
—C. S. Lewis
Prevention has been the top cancer control objective for more than a quarter century, but the promise of prevention remains largely unfulfilled [1]. In cancer control, different approaches exist for cancer prevention [2]. Efforts to detect cancer early or to reduce second cancers among cancer survivors are termed secondary prevention [3]. Primary prevention is the appropriate term for efforts to reduce the incidence of disease. Primary prevention is a traditional focus of public health, and it is the topic of this article. Research during the past several decades has vastly changed our understanding of cancer biology and the complex interaction of risk factors at both the individual and societal levels [4]. We argue that new approaches are needed to address cancer prevention in public health that incorporate different perspectives and challenge the status quo.
Background
The National Cancer Act of 1971 is now more than 40 years old [5]. During the past four decades, our understanding of cancer risk factors has advanced substantially as has our ability to detect and treat cancer [6,7]. Despite these advances, each year about 1.5 million people in the United States are told they have cancer [8]. Although incidence rates for many cancers have declined since the 1970s, most notablya decrease in lung cancer incidence in the past decade corresponding with reduced smoking prevalence [9], incidence rates have increased during the past decade for all childhood cancers and for some types of adult cancer, including melanoma, cancers of the pancreas, liver, thyroid, and kidney, and certain types of esophageal and oropharyngeal cancers [10–14]. Figure 1 shows U.S. trends in total cancer incidence rates from 1973 to 2009 and illustrates how cancers with increasing incidence rates have offset cancers with decreasing rates and contributed to an overall increase in total cancer incidence during this period. Some researchers attribute the increases in certain cancers, in part, to the rising prevalence of obesity and increased detection of early-stage tumors [12]. In general, however, the reasons for the observed increases are not entirely known [13]. In addition, because the U.S. population is aging, the total number of incident cancer cases in the United States will continue to grow unless we can reduce incidence rates considerably [15].
Figure 1.
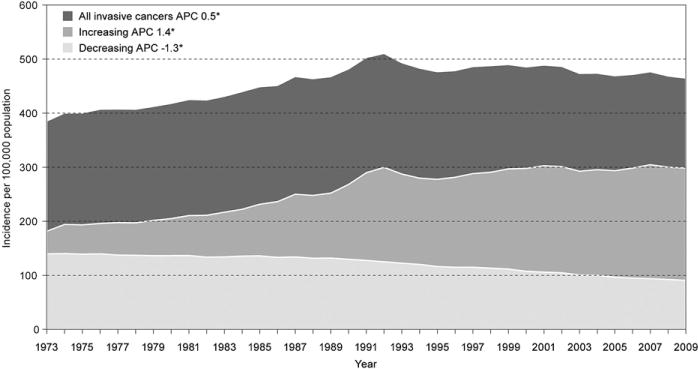
Trends in invasive cancer rates, 1973–2009. APC = average annual percentage change, 1973–2009. *Statistical significance. Cancers defined as increasing or decreasing in incidence were those with a statistically significant APC during 1973–2009. Data from National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, Available at: http://seer.cancer.gov/. Accessed February 23, 2013.
Theories of Cancer Causation
Broadly speaking, cancer is the result of multiple alterations in the processes that control cell proliferation, invasion, and spread. Nearly all cancers result from multiple factors that influence these processes over an extended time. In their recent update on the hallmarks of cancer, Hanahan and Weinberg [16] describe the multistep development of human tumors and the current understanding of the complex biology of cancer. Although the biology of cancer is still not completely understood, present scientific findings point to multiple cellular pathways by which different cancer risk factors could affect the multistep evolution of normal cells into cancer cells during a person’s lifespan.
Many physical and chemical substances that cause cancer in humans act through genetic changes that lead to downstream changes in RNA and protein processing [17]. The most well-studied and common genetic alterations in cancer include mutations in oncogenes and tumor-suppressor genes. Normal cells have multiple independent mechanisms that regulate cell growth and differentiation. For cancer to begin and spread, several separate events need to occur to override these regulating mechanisms. The number of events required to cause cancer is unknown and probably varies by cancer type, but modeling studies suggest that some cancers (e.g., lung, breast, colorectal) may require five or six steps [18].
Along with a greater understanding of the origin of cancer has also come a greater awareness of the heterogeneity of cancer. What was once understood and treated as a single disease is now thought to comprise distinct types, each of which may have different etiologies and different options for prevention. Breast cancer is a good example. Recent work has divided breast cancer into five intrinsic subtypes. One of these subtypes (basal-like) was characterized by up-regulation of certain proliferation genes and nonexpression of the estrogen receptor (ER), progesterone receptor (PR), and HER-2 receptor [19,20]. A related clinical phenotype—the triple-negative cancer (negative for the ER, PR, and HER-2 receptors)—has received much recent attention. Basal-like or triple negative tumors are typically more aggressive, tend to occur in women younger than 40 years of age, and have a worse prognosis than tumors expressing these receptor markers [21,22]. Given their phenotypic differences, researchers have proposed that basal-like or triple negative tumors may have a different etiology from other subtypes of breast cancer. For example, a recent analysis of pooled data found that obesity and reproductive factors (e.g., nulliparity, increasing age at first childbirth) were associated with increased risks for ER+/PR+ breast cancer but not triple-negative cancer [23].
The Interaction of Genes and Environment
As the genomic changes that lead to cancer have become better understood, so too has the importance of the interaction between genes and the environment in cancer development. For example, gene–environment interactions are thought to explain why some smokers get lung cancer, but most do not [24]. Tobacco smoke contains numerous known carcinogens including polycyclic aromatic hydrocarbons, N-nitrosamines, aromatic amines, aldehydes, benzene, and butadiene. Polymorphisms in genes that metabolize tobacco byproducts (e.g., CYP gene superfamily and glutathione S-transferases) may explain differences in people’s risk for tobacco-related cancers [25]. Similarly, risk for alcohol-related cancers may be related to variation in genes that metabolize alcohol (e.g., ADH1 family, ALDH2, CYP family, MTHFR) [26,27].
Genetic association and gene–environment studies hold great potential for understanding cancer etiology. However, good study design and methodological rigor are of paramount importance. To assess these associations and interactions appropriately and accurately, studies must be large, replicable, and well-powered with appropriate case and control selection criteria [28].
Applying what we learn about gene–environment interactions to preventive interventions highlights an important distinction between individual and population-based strategies for prevention. More than 25 years ago, Rose described the differences between preventing high-risk individuals from getting cancer and reducing the incidence of cancer in a large population [29]. The goal of selecting high-risk individuals for preventive activities shares some features with research in genomics and its promises of delivering personalized prevention—the goal being to identify individuals with predictive genetic markers of susceptibility. A parallel process is the development of statistical risk prediction models—the most well-known being the Gail model for predicting risk for developing breast cancer [30]. However, even well-established models lack accuracy in predicting future disease in individuals [31,32]. Furthermore, applying a high-risk prevention strategy to the average-risk population is challenging and costly. It requires that everyone in a population be screened to determine which individuals are at high risk. Among other challenges to the individual strategy, some high-risk individuals may not have access to health care or be motivated to seek preventive care and therefore may not be reached. Another problem with the high-risk individual strategy is that it focuses only on susceptible persons and does not alter the underlying causes of disease for the population at large [29]. From what we learned through studies of human genes that predispose people to cancer, a large majority of the population may be at intermediate risk for the disease [33].
Attributable Risk Estimates
More than three decades ago, two eminent British epidemiologists, Sir Richard Doll and Sir Richard Peto, were commissioned by the Office of Technology Assessment, U.S. Congress, to review the evidence on ways to avoid cancer and to quantify reductions in death rates that could be achieved by preventive measures taken during the next one to two decades. Because cancer incidence data were not available at that time, Doll and Peto examined variations in cancer deaths among adults aged 35–64 in different geographic areas and some epidemiologic study results. In their report on the causes of cancer, Doll and Peto [34] estimated that 25%–40% of cancer-related deaths could be attributed to tobacco use (best estimate 30%), 10%–70% could be attributed to poor diet (best estimate 35%), and a much smaller percentage could be attributed to occupation (4%), pollution (2%), and other factors or class of factors.
Many leading cancer control investigators characterize the Doll and Peto report as a landmark article [35–37]. In 1996, the Harvard Center for Cancer Prevention updated the report [38] and concurred with the Doll and Peto best estimates for most risk factors. The most significant difference between the 1981 report and the updated version is that the 35% estimate of risk attributed to poor diet is now attributed to a combination of poor diet or obesity (30%) and sedentary lifestyle (5%). In the Harvard update, the estimates were presented as point estimates without ranges and the sum of all estimates totaled exactly 100%. Many authoritative institutions, including the Institute of Medicine [1], the American Cancer Society [39], and the World Health Organization [40], cite these estimates or close approximations to emphasize the importance of tobacco use and poor diet relative to other risk factors for cancer.
Many epidemiologists, however, criticize the methods of Doll and Peto and their estimates of attributable proportions [41,42]. The editors of Modern Epidemiology [43] used the Doll and Peto 1981 report to illustrate the inappropriate interpretation of attributable fractions. Because cancer has multiple causes that interact with each other at different points in life, the sum of attributable fractions of causes for cancer is not 100%; instead, the sum is infinite [43]. This important caveat about attributable fractions for cancer is often not recognized, and pie charts and tables showing the causes of cancers commonly add neatly to 100% [37,38,44,45].
The attributable fraction is a tool that epidemiologists can use to make study results relevant for public health policy or to garner resources [37,46]. Others argue that when diseases have multiple causes (e.g., cancer), attributable fractions are meaningless and should not be used to rank individual causes [42,47]. From a cross-discipline perspective, ranking cancer causes in order of importance can be counterproductive because it fosters competition between disciplines rather than collaboration. One might expect that different disciplines would compete to claim a bigger piece of the pie, when increasingly, the value of working across disciplines to gain new knowledge and find new ways of solving problems is being recognized [48].
Environmental health scientists criticize the low attributable fractions assigned to environmental and occupational exposures on many grounds: for example, the fractions are based on unverified assumptions and exclude experimental evidence [41,42]. In addition, Doll’s undisclosed consultancy work for the chemical industry led some to question whether he had a conflict of interest [49]. After hearing presentations from many leading experts on environmental cancer risks, the recent President’s Cancer Panel on reducing environmental cancer risk concluded that the widely quoted estimates of Doll and Peto were “woefully out of date” and that the true burden of environmentally induced cancer had been “grossly underestimated” [50].
The simplistic ranking of broad classes of risk factors can no longer be characterized as consistent with current scientific evidence and may actually impede the advancement of scientific knowledge across different disciplines. Because most cancers are multifactorial with multiple etiologic pathways, prevention may be possible by focusing on several different factors at multiple periods during the lifespan.
Clinical Dimensions of Cancer Prevention
Since the mid-1970s, some health care professionals include various aspects of cancer control in the term cancer control continuum: prevention, detection, diagnosis, treatment, and survivorship [51,52]. This term is very similar to the term continuum of cancer care, which describes the delivery of health care during all phases of illness from diagnosis to death [53]. Including cancer prevention in the cancer control continuum expands the traditional focus of cancer care beyond diagnosis and treatment.
Because cancer prevention is linked with other components of the medical care system through this cancer control continuum, preventive health services have prominence, such as screening for breast or colon cancer, counseling about tobacco cessation, vaccination against infectious agents that increase risk for cancer (e.g., human papillomavirus, hepatitis B), and chemoprevention (e.g., Tamoxifen, Raloxifene). The health care system, however, is less likely to influence cancer incidence than to influence morbidity and survival [52]. Many prevention activities occur outside of the health care system, such as policy interventions and environmental changes [2]. In addition, including disease prevention on a continuum with the diagnosis and treatment of disease creates a paradox: if successful, cancer prevention would halt the subsequent phases and thus invalidate the term continuum.
Definition of Modifiable Risk Factor
Disease prevention is based on the premise that some risk factors can be modified or controlled [54]. Cancer risk factors are often measured at the individual level, and many individual risk factors (e.g., sex, age, genetic inheritance, sometimes education and income) are regarded as fixed, not modifiable. Modifiable or avoidable risk factors for cancer are typically dichotomized as either lifestyle or environmental. Lifestyle is often used as an adjective to characterize individual behaviors, such as tobacco smoking, poor diet, and physical inactivity [55]. The term lifestyle implies individual volition. Environment can have many meanings [56], but environmental risk factor usually refers to exposure to carcinogenic substances and is generally understood as something over which the individual has little control. During the past 40 years, this dichotomy between lifestyle and environment created distinct areas of research and practice for cancer prevention.
In recent years, transdisciplinary approaches to disease control attempt to integrate bodies of knowledge and practice from avariety of disciplines to solve problems [48]. Rosenfield described this approach as transcending the limits of individual disciplines and providing “a systematic, comprehensive, theoretical framework for the definition and analysis of the social, economic, political, environmental, and institutional factors influencing human health and well-being” [57]. For example, Hiatt and Breen described a transdisciplinary science approach to social determinants that operate at the societal level, the health care system level, the individual-behavior or psychological level, and the biological level [52]. The societal level includes factors such as environmental contamination, conditions that are unsafe for physical activity, and food deserts where people have little access to fruits and vegetables (a factor that disproportionately affects low socioeconomic [SES] populations). Tobacco use is a good example of a risk factor that is both behavioral and social, each requiring different control programs. Among adolescents, community-based interventions (e.g., increasing the price of tobacco, mass media campaigns to counter tobacco industry advertising in combination with other interventions, restricting minors’ access to tobacco products) are effective strategies for reducing the proportion of adolescents who begin using tobacco [58]. To reduce tobacco use among older people, tobacco control switches from preventing tobacco use to helping tobacco users to quit, because most new smokers are younger than 18 years old [59].
Social Determinants of Cancer
The distinction between lifestyle and environmental factors is becoming increasingly blurred as researchers turn their attention to the social and environmental determinants of health-related behaviors [60,61]. Several influential groups call for emphasizing social determinants of health, because health is strongly correlated with educational level and SES [62–64]. Freudenberg [65] proposes that corporate practices have a dominant influence on health-related behaviors by influencing the social, physical, and policy factors that shape individual decisions. As with other health outcomes, many cancers disproportionately affect people of color and people of low SES [64]. Breast and skin cancer are notable exceptions, but when those cancer types are diagnosed in low-SES patients, they generally have poor survival rates [39]. Income inequality is strongly correlated with excess preventable mortality [66]. The “upstream” social determinants of disease and health are the neighborhood conditions, environmental exposures, social and occupational opportunities, and personal resources that create the context within which health decisions are made and health behaviors are carried out. The “downstream” consequences of such social determinants are damages to organ systems or genes [52].
One approach that attempts to reconcile individual and population level characteristics is multilevel analysis [67,68]. This modeling technique allows researchers to identify characteristics of the social structure and ecology of neighborhoods, which may lead to better designed community interventions [69]. However, using regression models to investigate complex chains of relationships between social level and individual characteristics is methodologically challenging. Ecological and individual level characteristics rarely exist apart from each other [70]. Usually, the way in which a social group is organized has multiple influences on individual health. The converse is also true: individuals can affect how their society is organized in multiple ways. Another challenge is that the relationship between any one characteristic (e.g., gender, race) and disease can be more or less important depending on the history of the community [71]. That is, each social group is unique with respect to its experiences over time, and these group experiences become part of all group members’ experience whether or not they actually lived those experiences [72]. The recognition that society-, individual-, and gene-level characteristics operate together, and possibly synergistically, to influence illness and health is an important shift from the traditional, individual-based or strictly environmental models of risk factors and disease.
Despite the recognized importance of this shift, however, the model of multiple levels of influence on health outcomes comes under some criticism. Krieger criticized the language of “levels” and the distinction between “proximal” and “distal” causes and between “upstream” and “downstream” factors as conflating levels with causal strength. Changes in “distal” or “upstream” factors do not have to work through all intervening levels and are not limited to a distant, weak effect but can directly and strongly affect cancer risk for individuals [73]. For example, interventions at the social and policy level can directly affect the health of individuals, as in the case of environmental regulations that reduce individuals’ exposure to toxic contamination.
When to Take Action
Approaches to cancer prevention have included harm reduction (reducing exposure to known causes of cancer), clinical interventions (vaccines, chemoprevention), and health promotion (promoting behaviors that are associated with reduced cancer risk) [2]. The threshold of evidence needed to take action may vary depending on the type of preventive approach. The U.S. Preventive Services Task Force requires the strongest levels of evidence before making recommendations on clinical preventive services for the general population [74]. Also, the Guide to Community Preventive Services looks for methodologically strong studies when conducting reviews of effective interventions in communities [75].
When the focus is on reducing harm from a particular exposure, the available scientific evidence rarely includes controlled clinical trials or controlled interventions in community settings. The President’s Cancer Panel Report highlighted the vast number of chemicals that the public is routinely exposed to that may be carcinogenic, but are yet to be adequately researched [50]. Although much more research needs to be conducted on chemical exposures as well as in other realms of cancer prevention in order to have actionable evidence, scientists, policy makers and public health organizations must consider the thresholds of evidence that they deem necessary to warrant intervening on potentially harmful or protective factors. Issues surrounding the level of evidence necessary for action have been encountered in many realms of prevention science [76–83]. When the goal is to determine if sufficient evidence exists to justify taking precautionary action, the results of observational and epidemiologic studies and animal models should be taken into consideration and a different threshold of evidence may be appropriate. By better defining the levels of evidence necessary to implement different types of interventions to prevent cancer, this research may be able to become more targeted, generate more actionable results, and shorten the 17-year lag from research to practice [84].
During the past 40 years, our approaches to cancer prevention had limited success, whereas scientific understanding of the complex process of cancer development has advanced to provide new insights into causation and prevention. New approaches to cancer prevention must use this expanded scientific knowledge and our understanding of the interplay between various cancer risk factors at multiple levels within a particular social and historical setting. The labeling of exposures or risk factors as either lifestyle or environmental and rank ordering of risk factors shapes our approach to prevention, may limit opportunities for change, and is not consistent with current scientific knowledge. Prevention practices might benefit from an examination of exposures usually termed environmental to see how behaviors and actions by individuals influence personal exposures, environmental degradation, and societal changes. Likewise, a better understanding is needed on how social, economic, and environmental circumstances can influence, support or limit “lifestyle” and other behaviors at different stages of life. The recently released National Prevention Strategy aims to increase the number of Americans who are healthy at every stage of life by integrating actions across multiple settings and presents an approach that is highly relevant for cancer prevention [85].
We believe that it is possible for today’s youth, as they grow older, to experience lower cancer incidence rates than previous generations. We need to adopt a more transdisciplinary approach to prevention [48], adapted as appropriate for different stages of life. In the future, the greatest improvements in cancer prevention might occur as a result of innovative, multilevel interventions that build on the expanding base of scientific evidence. The articles in this special issue of the Journal of Adolescent Health elaborate further on preadolescence and adolescence as a special period of vulnerability [86–93]. Several articles describe innovative approaches and practical challenges to cancer prevention at this stage of life [94–99]. Collectively, these articles suggest several promising opportunities and directions for cancer prevention.
Acknowledgments
The authors thank Lisa C. Richardson, M.D., M.P.H., for reviewing and providing constructive comments on this manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publication of this article was supported by the Centers for Disease Control and Prevention. All authors are federal government employees, and the preparation of this manuscript was entirely funded by the U.S. government.
Footnotes
The authors report no potential conflicts of interest.
References
- 1.Curry SJ, Byers T, Hewitt M, et al. Fulfilling the potential of cancer prevention and early detection. Washington, DC: Institute of Medicine; 2003. [PubMed] [Google Scholar]
- 2.Frieden T, Myers JE, Krauskopf M, et al. A public health approach to winning the war against cancer. Oncologist. 2008;13:1306–13. doi: 10.1634/theoncologist.2008-0157. [DOI] [PubMed] [Google Scholar]
- 3.Porta M, editor. A Dictionary of Epidemiology. New York, NY: Oxford University Press; 2008. [Google Scholar]
- 4.Varmus H. The new era in cancer research. Science. 2006;312:1162–5. doi: 10.1126/science.1126758. [DOI] [PubMed] [Google Scholar]
- 5.Senate Bill 1828, PL 92-218. 1971. The National Cancer Act of 1971. [Google Scholar]
- 6.Schottenfeld D, Fraumeni J, editors. Cancer Epidemiology and Prevention. 3. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 7.Gaspar S, Thun M. Progress in the war on cancer. JAMA. 2010;303:1084–5. doi: 10.1001/jama.2010.284. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Cancer Statistics Working Group. United States cancer statistics: 1999–2009 incidence and mortality web-based Report. U.S Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. Available at: www.cdc.gov/uscs. [Google Scholar]
- 9.Centers for Disease Control and Prevention. State-specific trends in lung cancer incidence and smoking—United States, 1999–2008. MMWR. 2011;60:1243–7. [PubMed] [Google Scholar]
- 10.Li J, German R, King J, et al. Recent trends in prostate cancer testing and incidence among men under age of 50. Cancer Epidemiol. 2012;36:122–7. doi: 10.1016/j.canep.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998–2003. Int J Cancer. 2008;123:1422–8. doi: 10.1002/ijc.23691. [DOI] [PubMed] [Google Scholar]
- 12.Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–66. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62:118–28. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17–25. e11–13. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg R. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg RA. The biology of cancer. New York, NY: GS Garland Science; 2007. [Google Scholar]
- 18.Karakosta A, Golias C, Charalabopoulos A, et al. Genetic models of human cancer as a multistep process. Paradigm models of colorectal cancer, breast cancer, and chronic myelogenous and acute lymphoblastic leukaemia. J Exp Clin Cancer Res. 2005;24:505–14. [PubMed] [Google Scholar]
- 19.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 20.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 22.Rakha EA, El-Rehim DA, Paish C, et al. Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. Eur J Cancer. 2006;42:3149–56. doi: 10.1016/j.ejca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–63. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz AG. Lung cancer: Family history matters. Chest. 2006;130:936–7. doi: 10.1378/chest.130.4.936. [DOI] [PubMed] [Google Scholar]
- 25.Taioli E. Gene-environment interaction in tobacco-related cancers. Carcinogenesis. 2008;29:1467–74. doi: 10.1093/carcin/bgn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Druesne-Pecollo N, Tehard B, Mallet Y, et al. Alcohol and genetic polymorphisms: Effect on risk of alcohol-related cancer. Lancet Oncol. 2009;10:173–80. doi: 10.1016/S1470-2045(09)70019-1. [DOI] [PubMed] [Google Scholar]
- 27.Herceg Z, Vaissiere T. Epigenetic mechanisms and cancer: An interface between the environment and the genome. Epigenetics. 2011;6:804–19. doi: 10.4161/epi.6.7.16262. [DOI] [PubMed] [Google Scholar]
- 28.Thomas D. Gene–environment-wide association studies: Emerging approaches. Nat Rev Genet. 2010;11:259–72. doi: 10.1038/nrg2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–8. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 30.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 31.Rockhill B. Individual risk prediction and population-wide disease prevention. Epidemiol Rev. 2000;22:176–80. doi: 10.1093/oxfordjournals.epirev.a018017. [DOI] [PubMed] [Google Scholar]
- 32.Rockhill B. Theorizing about causes at the individual level while estimating effects at the population level. Epidemiology. 2005;16:124–9. doi: 10.1097/01.ede.0000147111.46244.41. [DOI] [PubMed] [Google Scholar]
- 33.Cazier JB, Tomlinson I. General lessons from large-scale studies to identify human cancer predisposition genes. J Pathol. 2010;220:255–62. doi: 10.1002/path.2650. [DOI] [PubMed] [Google Scholar]
- 34.Doll R, Peto R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–308. [PubMed] [Google Scholar]
- 35.Lee IM, Gaziano JM, Buring JE. Vitamin E in the prevention of prostate cancer: Where are we today? J Natl Cancer Inst. 2006;98:225–7. doi: 10.1093/jnci/djj066. [DOI] [PubMed] [Google Scholar]
- 36.Samet J, Speizer F. Sir Richard Doll, 1912–2005. Am J Epidemiol. 2006;164:95–100. [Google Scholar]
- 37.Colditz GA, Sellers TA, Trapido E. EpidemiologydIdentifying the causes and preventability of cancer? Nat Rev Cancer. 2006;6:75–83. doi: 10.1038/nrc1784. [DOI] [PubMed] [Google Scholar]
- 38.Harvard Center for Cancer Prevention. Causes of human cancer: Summary. Cancer Causes Control. 1996;7:S55–8. [Google Scholar]
- 39.Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 40.Stewart BW, Kleihues, editors. World Cancer Report. Lyon, France: IARC Press; 2003. [Google Scholar]
- 41.Tomatis L, Huff J, Hertz-Picciotto I, et al. Avoided and avoidable risks of cancer. Carcinogenesis. 1997;18:97–105. doi: 10.1093/carcin/18.1.97. [DOI] [PubMed] [Google Scholar]
- 42.Saracci R, Vineis P. Disease proportions attributable to environment. Environ Health. 2007;6:38. doi: 10.1186/1476-069X-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothman K, Greenland S, Lash T. Modern epidemiology. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 44.Mackay J, Jemal A, Lee N, et al. The cancer atlas. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 45.Wolin K, Carson K, Colditz G. Obesity and cancer. Oncologist. 2010;15:556–65. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steenland K, Armstrong B. An overview of methods for calculating the burden of disease due to specific risk factors. Epidemiology. 2006;17:512–9. doi: 10.1097/01.ede.0000229155.05644.43. [DOI] [PubMed] [Google Scholar]
- 47.Levine B. What does the population attributable fraction mean? Prev Chronic Dis [serial online] 2007;4:A14. Available at: http://www.cdc.gov/pcd/issues/2007/jan/06_0091.htm. [PMC free article] [PubMed] [Google Scholar]
- 48.Gehlert S. Turning disciplinary knowledge into solutions. J Adolesc Health. 2013;52(suppl 5):S98–102. doi: 10.1016/j.jadohealth.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tweedale G. Hero or villain? Sir Richard Doll and occupational cancer. Int J Occup Environ Health. 2007;13:233–5. doi: 10.1179/oeh.2007.13.2.233. [DOI] [PubMed] [Google Scholar]
- 50.President’s Cancer Panel. Reducing environmental cancer risk: What we can do now. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2010. 2008–2009 annual report. [Google Scholar]
- 51.National Cancer Institute. Cancer control continuum. Available at: http://cancercontrol.cancer.gov/od/continuum.html.
- 52.Hiatt RA, Breen N. The social determinants of cancer: A challenge for transdisciplinary science. Am J Prev Med. 2008;35:S141–50. doi: 10.1016/j.amepre.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Cancer Institute. Dictionary of cancer terms, continuum of care. Available at: http://www.cancer.gov/dictionary?cdrid=561395.
- 54.Goodman SN, Samet JM. Cause and cancer epidemiology. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2006. pp. 3–9. [Google Scholar]
- 55.Probst-Hensch N, Künzli N. Commentary: Preventing noncommunicable diseases—beyond lifestyle. Epidemiology. 2012;23:181–3. doi: 10.1097/EDE.0b013e318246031d. [DOI] [PubMed] [Google Scholar]
- 56.McGuinn L, Ghazarian A, Ellison G, et al. Cancer and the environment: Definitions and misconceptions. Environ Res. 2012;112:230–4. doi: 10.1016/j.envres.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenfield PL. The potential of transdisciplinary research for sustaining and extending linkages between the health and social sciences. Social Sci Med. 1992;35:1343–57. doi: 10.1016/0277-9536(92)90038-r. [DOI] [PubMed] [Google Scholar]
- 58.Zaza S, Briss P, Harris K, editors. The Guide to Community Preventive Services. New York, NY: Oxford University Press; 2005. [Google Scholar]
- 59.Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies; 2009. [Google Scholar]
- 60.Frieden TR. A framework for public health action: The Health Impact Pyramid. Am J Public Health. 2010;100:590–5. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.U.S. Department of Health and Human Services. Healthy People 2020: Improving the health of Americans. Available at: www.healthypeople.gov.
- 62.Braveman PA, Egerter SA, Mockenhaupt RE. Broadening the focus: The need to address the social determinants of health. Am J Prev Med. 2011;40(1 Suppl 1):S4–18. doi: 10.1016/j.amepre.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Commission on Social Determinants of Health. Final Report of the Commission on Social Determinants of Health. Geneva: World Health Organization; 2008. Closing the gap in a generation: Health equity through action on the social determinants of health. [Google Scholar]
- 64.Centers for Disease Control and Prevention. CDC health disparities and inequalities reporteUnited States, 2011. MMWR. 2011;60(Suppl):1–124. [PubMed] [Google Scholar]
- 65.Freudenberg N. The manufacture of lifestyle: The role of corporations in unhealthy living. J Public Health Pol. 2012;33:244–56. doi: 10.1057/jphp.2011.60. [DOI] [PubMed] [Google Scholar]
- 66.Kawachi I, Kennedy BP. Health and social cohesion: Why care about income inequality? BMJ. 1997;314:1037–40. doi: 10.1136/bmj.314.7086.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diez Roux A. Multilevel analysis in public health research. Ann Rev Public Health. 2000;21:171–92. doi: 10.1146/annurev.publhealth.21.1.171. [DOI] [PubMed] [Google Scholar]
- 68.Diez Roux A. The study of group-level factors in epidemiology: Rethinking variables, study designs, and analytical approaches. Epidemiol Rev. 2004;26:104–11. doi: 10.1093/epirev/mxh006. [DOI] [PubMed] [Google Scholar]
- 69.Pickett K, Pearl M. Multilevel analyses of neighborhood socioeconomic context and health outcomes: A critical review. J Epidemiol Community Health. 2001;55:111–22. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wing S. Workshop on Critical Theory in Epidemiology. Salvador, Brazil: Departmento d Medicina Preventiva, Universidad Federal de Bahia; 1993. Concepts in modern epidemiology: Population, risk dose response and confounding. [Google Scholar]
- 71.Kunitz S. Sex, race and social role—History and the social determinants of health. Int J Epidemiol. 2007;36:3–10. doi: 10.1093/ije/dyl296. [DOI] [PubMed] [Google Scholar]
- 72.Krieger N. Embodiment: A conceptual glossary for epidemiology. J Epidemiol Community Health. 2005;59:350–5. doi: 10.1136/jech.2004.024562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krieger N. Proximal, distal, and the politics of causation: What’s level got to do with it? Am J Public Health. 2008;98:221–30. doi: 10.2105/AJPH.2007.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.U.S. Preventive Services Task Force. Grade definitions after July 2012. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.
- 75.Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive servicesdMethods. Am J Prev Med. 2000;18:35–43. doi: 10.1016/s0749-3797(99)00119-1. [DOI] [PubMed] [Google Scholar]
- 76.Blumberg J, Heaney RP, Huncharek M, et al. Evidence-based criteria in the nutritional context. Nutrit Rev. 2010;68:478–84. doi: 10.1111/j.1753-4887.2010.00307.x. [DOI] [PubMed] [Google Scholar]
- 77.Victoria CG, Habicht JP, Bryce J. Evidence-Based public health: Moving beyond randomized trials. Am J Public Health. 2004;94:400–5. doi: 10.2105/ajph.94.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hertz-Picciotto I. Epidemiology and quantitative risk assessment: A bridge from science to policy. Am J Public Health. 1995;85:484–91. doi: 10.2105/ajph.85.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stayner L. Silica and lung cancer: When is enough evidence enough? Epidemiology. 2007;18:23–4. doi: 10.1097/01.ede.0000249538.78415.58. [DOI] [PubMed] [Google Scholar]
- 80.Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. 2007;28:413–33. doi: 10.1146/annurev.publhealth.28.021406.144145. [DOI] [PubMed] [Google Scholar]
- 81.Brownson RC, Gurney JG, Garland HL. Evidence-based decision making in public health. J Public Health Manage and Pract. 1999;5:86–97. doi: 10.1097/00124784-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 82.McQueen DV. Strengthening the evidence base for health promotion. Health Promot Int. 2010;16:261–7. doi: 10.1093/heapro/16.3.261. [DOI] [PubMed] [Google Scholar]
- 83.Treadwell JR, Singh S, Talati R, et al. AHRQ Publication No. 11-EHC046-EF. Rockville, MD: Agency for Healthcare Research and Quality; Jun, 2011. A framework for “best evidence” approaches in systematic reviews—Methods research report. Available at: http://www.effectivehealthcare.ahrq.gov/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid706. [PubMed] [Google Scholar]
- 84.Balas EA, Boren SA. Managing clinical knowledge for health care improvement. In: Van Bemmel JH, McCray AT, editors. Yearbook of Medical Informatics: Patient Centered Systems. Stuttgart, Germany: Schattauer; 2000. pp. 65–70. [PubMed] [Google Scholar]
- 85.National Prevention Council. National prevention strategy. Washington, DC: U.S. Department of Health and Human Services, Office of the Surgeon General; 2011. [Google Scholar]
- 86.Biro FM, Deardorff J. Identifying opportunities for cancer prevention during preadolescence and adolescence: Puberty as a window of susceptibility. J Adolesc Health. 2013;52(suppl 5):S15–20. doi: 10.1016/j.jadohealth.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bushkin-Bedient S, Carpenter DO. Exposure to chemicals and radiation during childhood and risk for cancer later in life. J Adolesc Health. 2013;52(suppl 5):S21–9. doi: 10.1016/j.jadohealth.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 88.Mahabir S. Association between diet during preadolescence and adolescence and risk for breast cancer during adulthood. J Adolesc Health. 2013;52(suppl 5):S30–5. doi: 10.1016/j.jadohealth.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frazier AL, Rosenberg SM. Pre-adolescent and adolescent risk factors for benign breast disease. J Adolesc Health. 2013;52(suppl 5):S36–40. doi: 10.1016/j.jadohealth.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santelli J, Sivaramakrishnan K, Edelstein Z, et al. Adolescent risk taking, cancer risk and lifecourse approaches to prevention. J Adolesc Health. 2013;52(suppl 5):S41–4. doi: 10.1016/j.jadohealth.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 91.Dube SR, Arrazola R, Lee J, et al. Pro-tobacco influences and susceptibility to smoking cigarettes among middle and high school students–United States, 2011. J Adolesc Health. 2013;52(suppl 5):S45–51. doi: 10.1016/j.jadohealth.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holman DM, Watson M. Correlates of intentional tanning among adolescents in the united states: A systematic review of the literature. J Adolesc Health. 2013;52(suppl 5):S52–9. doi: 10.1016/j.jadohealth.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holman D, Rodriguez J, Peipins L, et al. Highlights from a workshop on opportunities for cancer prevention during pre-adolescence and adolescence. J Adolesc Health. 2013;52(suppl 5):S8–14. doi: 10.1016/j.jadohealth.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomas TL, Strickland O, Diclemente R, et al. An opportunity for cancer prevention during preadolescence and adolescence: Stopping human papillomavirus (HPV)-related cancer through HPV vaccination. J Adolesc Health. 2013;52(suppl 5):S60–8. doi: 10.1016/j.jadohealth.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morales-Campos DY, Markham C, Peskin MF, et al. Hispanic mothers’ and high school girls’ perceptions of cervical cancer, human papilloma virus, and the human papilloma virus vaccine. J Adolesc Health. 2013;52(suppl 5):S69–75. doi: 10.1016/j.jadohealth.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 96.Lazovich D, Choi K, Rolnick C, et al. An intervention to decrease adolescent indoor tanning: A multi-method pilot study. J Adolesc Health. 2013;52(suppl 5):S76–82. doi: 10.1016/j.jadohealth.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Strunin L, Wulach L, Yang GL, et al. Preventing cancer: A community-based program for youths in public housing. J Adolesc Health. 2013;52:S83–8. doi: 10.1016/j.jadohealth.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 98.Morse LL. Let schools do it! Helping schools find a role in cancer prevention. J Adolesc Health. 2013;52(suppl 5):S89–92. doi: 10.1016/j.jadohealth.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 99.Cost NG, Applegate KE. Image Gently: A campaign to reduce children’s and adolescents’ risk for cancer during adulthood. J Adolesc Health. 2013;52(suppl 5):S93–7. doi: 10.1016/j.jadohealth.2013.03.006. [DOI] [PubMed] [Google Scholar]


