Abstract
Bisphenol A (BPA), an endocrine disruptor used in consumer products, may perturb thyroid function. Prenatal BPA exposure may have sex-specific effects on thyroid hormones (THs). Our objectives were to investigate whether maternal urinary BPA concentrations during pregnancy were associated with THs in maternal or cord serum, and whether these associations differed by newborn sex or maternal iodine status. We measured urinary BPA concentrations at 16 and 26 weeks gestation among pregnant women in the HOME Study (2003–2006, Cincinnati, Ohio). Thyroid stimulating hormone (TSH) and free and total thyroxine (T4) and triiodothyronine (T3) were measured in maternal serum at 16 weeks (n=181) and cord serum at delivery (n=249). Associations between BPA concentrations and maternal or cord serum TH levels were estimated by multivariable linear regression. Mean maternal urinary BPA was not associated with cord THs in all newborns, but a 10-fold increase in mean BPA was associated with lower cord TSH in girls (percent change= −36.0%; 95% confidence interval (CI): −58.4, −1.7%), but not boys (7.8%; 95% CI: −28.5, 62.7%; p-for-effect modification=0.09). We observed no significant associations between 16-week BPA and THs in maternal or cord serum, but 26-week maternal BPA was inversely associated with TSH in girls (−42.9%; 95% CI: −59.9, −18.5%), but not boys (7.6%; 95% CI: −17.3, 40.2%; p-for-effect modification=0.005) at birth. The inverse BPA-TSH relation among girls was stronger, but less precise, among iodine deficient versus sufficient mothers. Prenatal BPA exposure may reduce TSH among newborn girls, particularly when exposure occurs later in gestation.
Keywords: Bisphenol A, Thyroid hormones, Thyroid stimulating hormone, Thyroxine, triiodothyronine, Pregnant women, Newborns, Endocrine Disruptors, Epidemiology
1. Introduction
Bisphenol A (BPA) is a synthetic chemical used as a monomer in polycarbonate plastics and epoxy resins. Virtually every pregnant woman and child in the United States (US) is exposed to BPA (Calafat et al., 2008; Woodruff et al., 2011). Interference of BPA with the action of thyroid hormones (THs) during critical developmental stages may adversely affect neurobehavioral outcomes (Boas et al., 2012; Henrichs et al., 2013). BPA may interfere with TH action by interacting directly with the TH receptor, affecting the normal delivery of TH to target cells, or altering the metabolism of THs, including thyroid stimulating hormone (TSH), free and total thyroxine (FT4, TT4), or free and total triiodothyronine (FT3, TT3). Prenatal THs are essential for normal brain development. Shifts in the distribution of maternal or newborn TH levels that are not associated with acute clinical sequelae could have population-level impacts on child neurodevelopment. A number of studies have shown that variations in maternal T4 or TSH levels during gestation are associated with reduced cognitive abilities and increased risk of behavior problems in children (Ghassabian et al., 2011; Henrichs et al., 2013; Julvez et al., 2013).
Few experimental or epidemiological studies have examined the effect of BPA exposure on the thyroid axis. In vitro and rodent studies report inconsistent effects (Nieminen et al., 2002a; Nieminen et al., 2002b; Xu et al., 2007; Zoeller et al., 2005) and epidemiological studies, which have predominately been cross-sectional, have also been equivocal. Two studies from China reported that occupational exposure to BPA exposure was associated with elevated FT3 and FT4 and reduced TSH concentrations (Wang et al., 2012; Wang et al., 2013). A study in US adult men found no associations with FT3, FT4, and TSH (Meeker et al., 2010), whereas, a nationally representative cross-sectional study in the US reported that BPA concentrations were associated with lower TT4 (Meeker and Ferguson, 2011). Prenatal urinary BPA concentrations were associated with lower TT4 in pregnant women and reduced TSH in their male newborns in a pregnancy cohort study from California (Chevrier et al., 2013). To address this knowledge gap, we sought to determine if maternal urinary BPA concentrations during pregnancy were associated with thyroid hormones in women or their newborns, and to assess whether newborn sex modified these associations. Additionally, we explored whether maternal iodine status was an effect modifier of these associations.
2. Materials and methods
2.1 Study Setting
The Health Outcomes and Measures of the Environment (HOME) Study is a prospective pregnancy and birth cohort in the greater Cincinnati, Ohio metropolitan area that was designed to investigate the impact of exposures to common environmental chemicals on child health and development. At baseline, women were eligible to participate if they were pregnant (16±3 weeks gestation), >18 years old, English speakers, living in a home built before 1978, intending to continue prenatal care and deliver at a HOME Study-affiliated obstetric practice, and had no history of HIV infection. Women taking medication for seizure or thyroid disorders were not eligible to participate. Women were enrolled in the study between March 2003 and January 2006. Of 1,263 eligible women, 37% enrolled (n=468) and 83% (n=389) were followed through live birth of a singleton infant. We excluded one mother-newborn pair in which the woman had a urinary BPA concentration of 583 µg/g creatinine (Sathyanarayana et al., 2011), orders of magnitude higher than the median (2.7µg/g creatinine) among these participants. The Institutional Review Boards of Cincinnati Children’s Hospital Medical Center, the Centers for Disease Control and Prevention (CDC), and all delivery hospitals approved the study protocol. All mothers provided written informed consent before enrollment in the study.
2.2 BPA assessment in maternal urine
Women provided two spot urine samples directly into polypropylene specimen cups at an average of 16.0 (range=13.0–20.9) and 26.5 (range=23.1–34.6) weeks of gestation. Urine was stored at or below −20°C until samples were analyzed for total BPA (conjugated plus free) using online solid phase extraction coupled to high performance liquid chromatography-isotope dilution tandem mass spectrometry(Ye et al., 2005). Four quality control (QC) samples were analyzed in each analytic run. For 31 analytical batches analyzed in a period of one year, the coefficients of variation were 6.9–9.2% for the low-concentration QC samples (~2.8 µg/L), and 3.4–7.6% for the high-concentration QC samples (~9.7 µg/L). The limit of detection (LOD) was 0.4 µg/L; concentrations below the LOD were given a value of LOD/√2. All study protocols included appropriate measures to prevent BPA contamination during collection, storage, and analysis of urine. Urinary creatinine concentrations were measured using enzymatic methods and urinary BPA concentrations were creatinine-standardized (µg/g creatinine) to account for individual variability in urine dilution. The creatinine-standardized BPA concentrations were log10-transformed, and the mean of the log10-transformed BPA concentrations from the urine collected at 16 and 26 weeks gestation was calculated.
2.3 Serum thyroid hormone and antibody concentrations
Maternal blood was collected at approximately 16 weeks gestation, and venous cord blood was collected at delivery. Serum was separated from clotted blood and stored at −80 °C until analysis. THs (TSH, TT4, TT3, FT4, and FT3), and thyroid antibodies [thyroid peroxidase (TPOAb) and thyroglobulin antibodies (TgAb)] were measured in maternal and cord sera at the Department of Laboratory Medicine at the University of Washington clinical chemistry laboratories using an Access2 automated clinical immunoassay analyzer (Beckman Coulter Inc., Fullerton, CA). The coefficient of variation (CV) for the thyroid hormone assays ranged from <1.0% to 10% (Supplemental Material, Table S1).
2.4 Covariate data
During the second trimester, demographic, socioeconomic, perinatal, and behavioral factors, as well as reproductive and medical histories, were collected using a computer-assisted questionnaire administered by trained research staff. Delivery method and newborn sex were abstracted from neonatal medical records. To account for previously observed associations between polychlorinated biphenyls (PCBs) and THs (Chevrier et al., 2007), we measured PCB-153 concentrations in maternal serum samples collected at 16 weeks gestation (Sjodin et al., 2014). Tobacco smoke exposure at 16 weeks was assessed using serum cotinine, a sensitive and specific biomarker of secondhand and active tobacco smoke exposure, using previously described methods (Bernert et al., 2009). Urinary iodine was measured in maternal urine collected at 26 (n=228) or 16 weeks (n=8) with an Agilent 7500× Inductively Coupled Plasma-Mass Spectrometer (ICP-MS) (Caldwell et al., 2003). The LOD was 0.5 µg I/L, and the average CV for all QC specimens was ≤10%. Maternal urinary iodine was creatinine-standardized and women were categorized as iodine sufficient (≥150 µg I/g creatinine) or deficient (<150 µg I/g creatinine) (Ghassabian et al., 2014).
2.5 Statistical Analysis
We examined the distribution of average maternal urinary BPA, maternal serum TSH, and newborn cord serum TSH concentrations across categories of maternal sociodemographic, behavioral, and perinatal factors. TSH was natural log-transformed because its distribution was right-skewed. T4 and T3 were expressed on the arithmetic scale. We fit 3-knot, restricted-cubic splines to assess model linearity assumptions for linear regression (Desquilbet and Mariotti, 2010). We observed approximately linear relationships between log10-transformed BPA and THs (data not shown). We used multivariable linear regression to estimate the adjusted differences in THs with each 10-fold increase in urinary BPA concentrations. For the analyses of THs in mothers, only urinary BPA collected at 16 weeks was considered because the 26 week urine was collected later in pregnancy than the assessment of THs. For the analyses of THs in newborns, average BPA and the individual measures (16 or 26 week) of maternal urinary BPA were considered. The regression coefficients were used to characterize the percent change in TSH or the mean change in T3 and T4 for each 10-fold increase in BPA concentration.
We adjusted for the following variables based on a priori knowledge of their potential associations with exposure and/or outcome (Calafat et al., 2008; Chevrier et al., 2007; Chevrier et al., 2013; Herbstman et al., 2008; Meeker et al., 2010): maternal age at delivery, race/ethnicity, parity, serum cotinine concentrations, alcohol consumption, prenatal vitamin use, and log10-transformed maternal serum PCB-153 concentrations. Models examining maternal THs also included adjustment for household income, and gestational week of serum/urine collection. For the analyses of cord THs we also adjusted for maternal education, infant sex, gestational age at delivery, and mode of delivery (Herbstman et al., 2008). Because prior research suggests that sex may modify the association between gestational BPA exposure and THs (Chevrier et al., 2013), we ran additional models that included a product interaction term between maternal urinary BPA and infant sex when analyzing cord serum THs. We considered p-values <0.10 for interaction terms to be indicative of effect modification.
We conducted an exploratory analysis examining effect modification by maternal iodine status. We also created a model including all variables from the final multivariable model plus iodine deficiency (≥150 µg I/g creatinine vs. <150 µg I/g creatinine) and product terms for BPA × infant sex, BPA × iodine deficiency, infant sex × iodine deficiency, and a three-way term for BPA × infant sex × iodine deficiency.
We additionally conducted a series of sensitivity analyses to evaluate the robustness of our results. We assessed for potential confounding by maternal iodine status by adjusting for maternal urinary iodine concentrations. We re-ran the final models using maternal urinary BPA concentrations without creatinine-standardization to determine whether our method for accounting for urine dilution altered our results. We also examined whether compromised thyroid function modifies the association between BPA and THs (Webster et al., 2014). Very few women had clinically significant elevations in thyroid antibody [TPOAb >9.0 IU/mL (n=15); TgAb >2.0 IU/mL (n=5)]. Therefore, we dichotomized TPOAb at the median level and TgAb as detected versus not detected. Product terms of maternal urinary BPA and thyroid antibodies (dichotomous) were added to linear regression models and considered significant if p<0.10. Stata version 13.0 (StataCorp, College Station, TX) and SAS version 9.3 (SAS Institute, Inc., Cary, NC) were used for statistical analyses.
3. Results
3.1 Participant characteristics
The maternal analysis included 181 women who had complete covariate information and both maternal urinary BPA and serum TH concentrations (collected at ~16 weeks gestation). The newborn analysis included 249 infants who had complete covariate information, cord serum TH concentrations, and whose mothers had at least one urinary BPA measurement. One woman provided a urine sample only at 16 weeks, 11 women provided samples only at 26 weeks, and 237 women provided samples at both time points. Mothers in the study were primarily 25–35 years old (65%), non-Hispanic white (67%), and married (73%). Most women had an annual household income from $40,000–80,000 (36%), education equivalent to or greater than a bachelor’s degree (56%), and private health insurance (79%). During pregnancy, the majority of women reported that they regularly took prenatal vitamins (88%) and did not consume alcohol (55%); only 10% of women were active smokers during pregnancy. Fewer than half of the women were nulliparous at enrollment (44%) and most delivered their newborns vaginally (73%) (Table 1).
Table 1.
Maternal and newborn characteristics of participants in the Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2006
| Covariate | n(%) | Urinary BPAa Median(IQR) |
Newborn TSHb Median(IQR) |
n(%) | Maternal TSHc Median(IQR) |
|
|---|---|---|---|---|---|---|
| Overall | 249(100) | 2.2(1.5–3.4) | 7.1(5.1–10.1) | 181(100) | 1.3(0.9–2.0) | |
| Maternal age (years) | ||||||
| <25 | 45(18) | 2.7(1.7–3.7) | 6.4(4.9–8.3) | 33(18) | 1.1(0.7–1.8) | |
| 25–35 | 161(65) | 2.2(1.5–3.4) | 7.5(5.4–10.7) | 112(62) | 1.3(0.8–2.0) | |
| >35 | 43(17) | 2.0(1.6–3.0) | 6.5(4.4–7.6) | 36(20) | 1.5(1.3–2.2) | |
| Race/ethnicity | ||||||
| Non-Hispanic White | 166(67) | 2.1(1.6–3.5) | 7.4(5.4–10.7) | 116(64) | 1.4(0.9–2.1) | |
| Black | 66(27) | 2.3(1.5–3.2) | 6.4(4.4–7.9) | 49(27) | 1.2(0.8–1.7) | |
| Other | 17(7) | 2.6(1.4–4.0) | 7.4(4.4–9.3) | 16(9) | 1.6(0.8–2.3) | |
| Marital Status | ||||||
| Married | 182(73) | 2.1(1.5–3.4) | 7.3(5.3–10.3) | 128(71) | 1.4(0.9–2.1) | |
| Unmarried, cohabiting | 25(10) | 2.7(1.6–3.8) | 7.4(5.9–9.9) | 20(11) | 1.4(0.9–1.8) | |
| Unmarried, living alone | 42(17) | 2.3(1.7–3.2) | 5.8(4.1–7.7) | 33(18) | 1.1(0.6–1.6) | |
| Household Income ($/year) | ||||||
| <20,000 | 43(17) | 2.7(1.7–3.8) | 6.4(4.1–9.7) | 37(20) | 1.1(0.7–1.6) | |
| 20–<40,000 | 40(16) | 2.3(1.8–3.3) | 6.5(5.3–8.9) | 28(16) | 1.6(1.0–2.0) | |
| 40–80,000 | 89(36) | 2.1(1.5–3.2) | 7.4(5.8–10.1) | 62(34) | 1.3(0.8–2.1) | |
| >80,000 | 77(31) | 2.0(1.4–3.4) | 7.6(5.3–11.6) | 54(30) | 1.5(1.0–2.3) | |
| Education | ||||||
| Less than high school | 22(9) | 3.1(1.7–3.9) | 7.0(4.2–10.3) | 20(11) | 1.2(0.6–1.7) | |
| High school or some college | 88(35) | 2.2(1.6–3.3) | 6.9(5.1–8.4) | 55(30) | 1.3(0.8–1.9) | |
| Bachelors or more | 139(56) | 2.1(1.5–3.4) | 7.4(5.3–11.3) | 106(59) | 1.4(0.9–2.2) | |
| Insurance | ||||||
| Private | 196(79) | 2.1(1.5–3.4) | 7.4(5.5–10.6) | 138(76) | 1.4(0.9–2.1) | |
| Public/uninsured | 53(21) | 2.7(1.7–3.8) | 6.4(4.1–7.8) | 43(24) | 1.1(0.7–1.6) | |
| Prenatal vitaminsd | ||||||
| Regular use | 218(88) | 2.2(1.5–3.4) | 7.3(5.4–10.1) | 158(87) | 1.3(0.9–2.0) | |
| Never/infrequent use | 31(12) | 2.2(1.7–3.5) | 5.5(4.2–7.6) | 23(13) | 1.3(1.0–2.0) | |
| Alcohol consumptione | ||||||
| Any | 112(45) | 2.1(1.5–3.4) | 7.5(5.4–11.1) | 80(44) | 1.5(1.0–2.1) | |
| None | 137(55) | 2.2(1.6–3.4) | 7.0(5.1–9.8) | 101(56) | 1.3(0.8–2.0) | |
| Serum cotinine (ng/mL)f | ||||||
| <0.015 (Unexposed) | 77(31) | 2.0(1.4–3.0) | 7.6(5.6–10.9) | 57(32) | 1.5(1.1–2.3) | |
| 0.015–3 (Secondhand) | 148(59) | 2.3(1.7–3.7) | 7.1(5.0–10.1) | 104(57) | 1.2(0.8–1.9) | |
| >3 (Active smoker) | 24(10) | 2.5(1.5–3.5) | 6.0(4.3–7.2) | 20(11) | 1.4(0.7–1.7) | |
| Maternal BMI (kg/m2)g | ||||||
| <24.9 (Underweight-normal) | 102(41) | 2.3(1.6–3.4) | 6.5(5.2–9.4) | 82(45) | 1.5(1.0–2.1) | |
| 25.0–29.9 (Overweight) | 82(33) | 2.0(1.5–3.4) | 7.8(5.5–12.5) | 39(22) | 1.6(1.2–2.3) | |
| ≥30 (Obese) | 56(22) | 2.2(1.4–3.3) | 7.2(4.6–9.1) | 52(29) | 1.0(0.6–1.7) | |
| Mode of delivery | ||||||
| Vaginal delivery | 182(73) | 2.3(1.6–3.5) | 7.2(4.9–10.3) | 127(70) | 1.3(0.9–2.0) | |
| Cesarean section | 67(27) | 2.0(1.4–3.1) | 7.1(5.6–9.0) | 54(30) | 1.3(0.9–2.1) | |
| Parity | ||||||
| Nulliparous | 110(44) | 2.2(1.5–3.5) | 7.5(5.4–11.6) | 87(48) | 1.6(1.0–2.1) | |
| Primiparous | 78(31) | 2.1(1.6–2.8) | 7.2(5.0–10.2) | 55(30) | 1.3(0.9–2.2) | |
| Multiparous | 61(25) | 2.3(1.5–3.5) | 6.5(4.7–7.7) | 39(22) | 1.1(0.7–1.5) | |
| Newborn Sex | ||||||
| Female | 135(54) | 2.2(1.5–3.5) | 6.7(4.4–9.9) | 98(54) | 1.3(0.9–2.1) | |
| Male | 114(46) | 2.2(1.5–3.4) | 7.5(5.8–10.1) | 83(46) | 1.3(0.9–2.0) | |
| Polychlorinated Biphenyl-153 | ||||||
| Low(<9.2 ng/g lipid) | 82(33) | 2.2(1.5–3.5) | 7.5(5.2–10.3) | 51(28) | 1.5(1.0–2.1) | |
| Middle (9.2–14.0 ng/g lipid) | 80(32) | 2.2(1.4–3.5) | 7.0(5.0–10.0) | 59(32) | 1.3(0.7–2.1) | |
| High (>14.0 ng/g lipid) | 87(35) | 2.2(1.7–3.4) | 7.0(5.3–10.1) | 72(40) | 1.3(0.8–2.0) | |
| Maternal urinary iodineh | ||||||
| Sufficient (≥150 µg/g Cr) | 137(55) | 2.2(1.5–3.5) | 7.2(5.3–11.0) | 101(56) | 1.4(0.9–2.0) | |
| Deficient (<150 µg/g Cr) | 56(22) | 2.2(1.6–2.8) | 7.2(5.1–8.4) | 48(27) | 1.2(0.7–2.0) | |
BMI=Body Mass Index, BPA=Bisphenol A, Cr=Creatinine, IQR=Interquartile range, and TSH=thyroid stimulating hormone
Median level of the average of maternal urinary creatinine-standardized bisphenol A collected at 16 and 26 gestational weeks (µg/g Cr)
Concentration of thyroid stimulating hormone (mIU/L) in cord serum collected at delivery for newborns included in the final multivariate analysis
Concentration of thyroid stimulating hormone (mIU/L) in maternal serum collected at 16 weeks gestation for women included in the final multivariate analysis
Use of prenatal vitamins at least weekly during pregnancy versus less frequent use
Serum cotinine at 16 weeks gestation estimated tobacco smoke exposure during pregnancy
Self-reported maternal consumption of alcohol during pregnancy
Maternal body mass index at 16 weeks gestation, (n=9 (4%) missing from newborn analytic sample; n=8 (4%) missing from maternal analytic sample)
Maternal urinary iodine, (n=56 (22%) missing from newborn analytic sample; n=32 (17%) missing from maternal analytic sample)
Women included in the final analyses were more likely to be >25 years old, non-Hispanic white, married, highly educated (>bachelor’s degree), overweight at enrollment (>25 kg/m2), taking prenatal vitamins, or have a household income >$20,000/year (Supplemental Material, Table S2).The geometric mean of 16 week urinary BPA was similar for women excluded from and included (1.9 vs. 2.1 µg/g creatinine) in the maternal analyses. Among newborns excluded from and included in the final analyses, the geometric mean of their mothers’ average maternal urinary BPA concentrations was also similar (2.5 vs. 2.4 µg/g creatinine).
3.2 Maternal urinary BPA concentrations and maternal and newborn thyroid hormones
Median maternal urinary BPA concentrations were relatively similar at 16 and 26 weeks gestation (Table 2). Very few mothers (n=13, 7.2%) had TSH serum levels suggestive of subclinical or clinical hypothyroidism (>3.0 mIU/L) (Shields et al., 2013); likewise, few newborns (n=11, 4.4%) had cord blood TSH levels indicative of insufficient thyroid function (>20 mIU/L) (Manglik et al., 2005).
Table 2.
Maternal urinary bisphenol A and maternal and cord sera thyroid hormone concentrations in the Health Outcomes and Measures of the Environment Study, Cincinnati, Ohio, 2003–2006
| Biomarker | n | Maternal Analysis GM (95% CI) Mean ± SD |
n | Newborn Analysis GM (95% CI) Mean ± SD |
|---|---|---|---|---|
| Average BPA (µg/g Cr)a | --- | 249 | 2.4(2.2, 2.6) | |
| 16 week BPA (µg/g Cr)b | 181 | 2.1(1.8, 2.4) | 248 | 2.0(1.8, 2.2) |
| 26 week BPA (µg/g Cr)b | --- | 238 | 2.3(2.1, 2.5) | |
| TSH (mIU/L) | 181 | 1.2(1.1, 1.4) | 249 | 7.2(6.7, 7.8) |
| TT4 (µg/dL) | 181 | 10.3 ± 1.9 | 245 | 9.6 ± 1.8 |
| TT3 (ng/dL) | 181 | 160 ± 24 | 249 | 52 ± 19 |
| FT4 (ng/dL) | 181 | 0.7 ± 0.1 | 249 | 1.0 ± 0.2 |
| FT3 (pg/mL) | 181 | 3.2 ± 0.3 | 247 | 1.7 ± 0.3 |
BPA=Bisphenol A, CI=Confidence interval, Cr=creatinine FT3=free triiodothyronine, FT4=free thyroxine, GM=geometric mean, TSH=thyroid stimulating hormone, TT3=total triiodothyronine, and TT4=total thyroxine
The average of the creatinine-standardized bisphenol A from maternal urine collected at the 16 and 26 week visits
Creatinine-standardized bisphenol A in maternal urine
In adjusted analyses, we did not observe any significant associations between maternal urinary BPA and serum TH concentrations at 16 weeks gestation (Table 3). Likewise, we did not identify any notable associations between maternal urinary BPA concentrations (average, 16 week, or 26 week) and newborn THs when both sexes were combined (Table 3). In contrast, we observed inverse associations between BPA and TSH and suggestive positive relations between BPA and TT3 among female newborns for both average and 26 week urinary BPA (Table 3). Among female newborns, each 10-fold increase in average maternal urinary BPA was associated with decreased cord TSH (percent change=−36.0%; 95% confidence interval [CI]: −58.4, −1.7%) (Table 3); no association was observed among males (7.8%; 95% CI: −28.5, 62.7%; p for effect modification=0.09) (Table 3). In analyses of individual BPA measures, maternal urinary BPA concentrations at 16 weeks were not associated with TSH (Table 3). However, 26 week maternal urinary BPA was associated with reduced TSH in female (−42.9%; 95% CI:−59.9, −18.5%), but not male newborns (7.6%; 95% CI:−17.3, 40.2%; p for effect modification=0.005) (Table 3). Unadjusted analyses yielded a similar pattern of results (Supplemental Material, Table S3).
Table 3.
Difference or percent change in thyroid hormone concentrations in maternal (n=181) and cord sera (n=249) with 10-fold increase in maternal urinary BPA in the Health Outcomes and Measures of the Environment Study
| BPA Measurement |
Mothers Estimate (95% CI)a |
All Newborns Estimate (95% CI)b |
Female Newborns Estimate (95% CI)c |
Male Newborns Estimate (95% CI)c |
p-valued |
|---|---|---|---|---|---|
| Averagee | |||||
| % Change in TSH f | --- | −19.4 (−40.3, 8.9) | −36.0 (−58.4, −1.7) ** | 7.8 (−28.5, 62.7) | 0.09 * |
| TT4 (µg/dL) | --- | 0.12 (−0.82, 1.05) | 0.51 (−0.62, 1.64) | −0.36 (−1.90, 1.16) | 0.37 |
| TT3 (ng/dL) | --- | 3.97 (−6.52, 14.45) | 11.89 (−1.08, 24.87) * | −6.13 (−23.37, 11.12) | 0.11 |
| FT4 (ng/dL) | --- | −0.03 (−0.11, 0.04) | −0.06 (−0.17, 0.05) | 0.002 (−0.11, 0.12) | 0.44 |
| FT3 (pg/mL) | --- | −0.06 (−0.23, 0.10) | 0.04 (−0.15, 0.22) | −0.18 (−0.46, 0.11) | 0.24 |
| 16 weeksg | |||||
| % Change in TSH f | 5.6 (−19.1, 37.9) | −4.6 (−24.4, 20.5) | −7.9 (−32.3, 25.2) | 0.6 (−30.6, 46.0) | 0.72 |
| TT4 (µg/dL) | −0.14 (−1.00, 0.71) | −0.31 (−0.91, 0.29) | −0.004 (−0.71, 0.70) | −0.77 (−1.79, 0.25) | 0.23 |
| TT3 (ng/dL) | 2.74 (−7.14, 12.62) | −1.39 (−8.63, 5.84) | 2.69 (−5.89, 11.27) | −7.39 (−19.75, 4.97) | 0.20 |
| FT4 (ng/dL) | −0.001 (−0.03, 0.03) | −0.03 (−0.08, 0.02) | −0.04 (−0.10, 0.03) | −0.02 (−0.10, 0.06) | 0.75 |
| FT3 (pg/mL) | 0.02 (−0.10, 0.15) | −0.06 (−0.18, 0.06) | 0.002 (−0.13, 0.13) | −0.14 (−0.35, 0.06) | 0.24 |
| 26 weeks g | |||||
| % Change in TSH f | --- | −19.9 (−36.6, 1.2) * | −42.9 (−59.9, −18.5) ** | 7.6 (−17.3, 40.2) | 0.005** |
| TT4 (µg/dL) | --- | 0.51 (−0.27, 1.28) | 0.73 (−0.43, 1.89) | 0.33 (−0.67, 1.33) | 0.60 |
| TT3 (ng/dL) | --- | 6.25 (−1.80, 14.29) | 12.79 (−0.41, 26.00) * | 0.41 (−9.14, 9.99) | 0.14 |
| FT4 (ng/dL) | --- | −0.003 (−0.07, 0.06) | −0.03 (−0.14, 0.08) | 0.02 (−0.05, 0.09) | 0.46 |
| FT3 (pg/mL) | --- | −0.004 (−0.13, 0.12) | 0.05 (−0.14, 0.24) | −0.05 (−0.22, 0.12) | 0.46 |
BPA=bisphenol A, CI=Confidence interval, FT4=free thyroxine, TSH=thyroid stimulating hormone, TT3=total triiodothyronine, and TT4=total thyroxine
p<0.10
p<0.05
Adjusted for maternal age, race, income, alcohol consumption, serum cotinine at 16 weeks gestation, parity, prenatal vitamin use, Log10-PCB 153, and gestational week at urine/serum collection
Adjusted for maternal age, race, education, alcohol consumption, serum cotinine at 16 weeks gestation, parity, prenatal vitamin use, Log10-PCB 153, delivery by Cesarean section, gestational week at delivery, and newborn sex
Adjusted for maternal age, race, education, alcohol consumption, serum cotinine at 16 weeks gestation, parity, prenatal vitamin use, Log10-PCB 153, delivery by Cesarean section, gestational week at delivery, and newborn sex, and including an interaction term for maternal bisphenol A and newborn sex in the model
p-value for the maternal bisphenol A and newborn sex interaction term
The average of the log10-transformed creatinine-standardized bisphenol A from maternal urine collected at the 16 and 26 week visits
Percent change calculated as eβ−1×100, where β=coefficient from the multivariable model
Log10-transformed creatinine-standardized bisphenol A in maternal urine
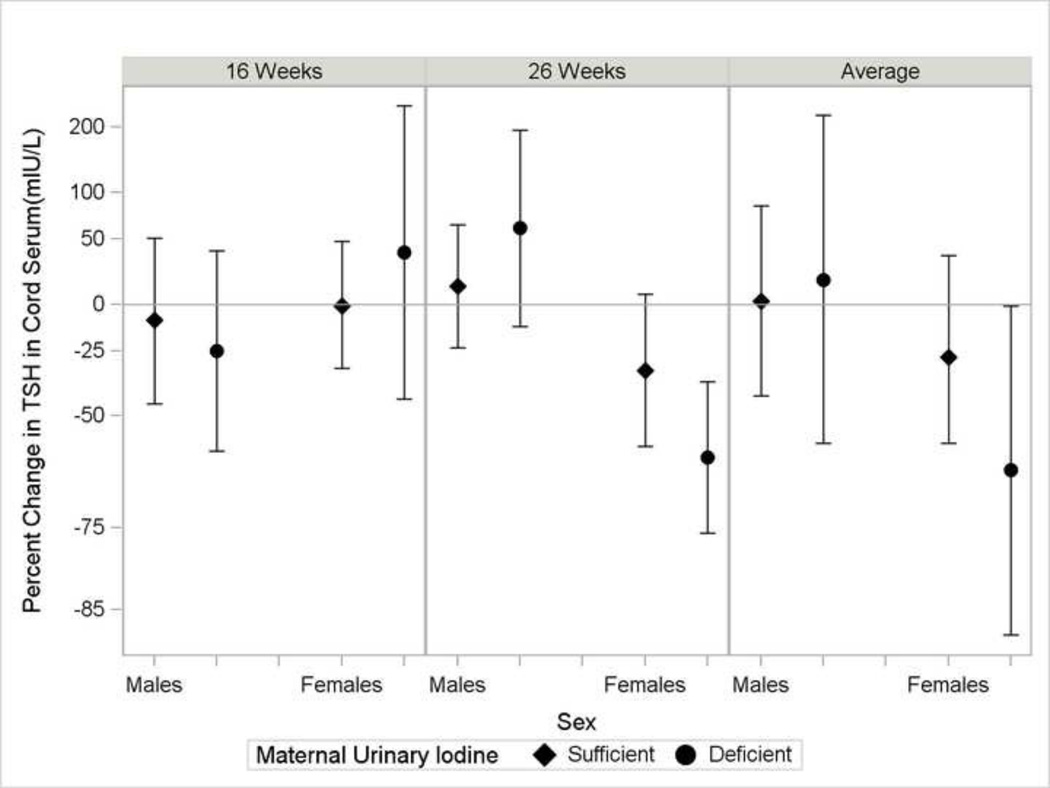
In our exploratory analysis, we observed no evidence of effect modification of the relations between BPA and THs by iodine status among mothers or all newborns (Supplemental Material, Table S4). There was some suggestion that the inverse relation between BPA and TSH was strongest among female newborns with iodine deficient mothers (p-for-effect modification=0.11 of the BPA-TSH relation by iodine deficiency among girls) (Figure 1, Supplemental Material, Table S5).
Figure 1.
Percent change in thyroid stimulating hormone concentrations in cord serum (n=193) with 10-fold increase in maternal urinary BPA in the Health Outcomes and Measures of the Environment Study by newborn sex and iodine deficiency. Sufficient iodine was defined by maternal urinary iodine ≥150 µg/g creatinine (diamond marker) and deficient iodine as maternal urinary iodine <150 µg/g creatinine (circle marker). Percent change calculated as eβ −1×100, where β=coefficient from a multivariable linear regression model including adjustment for maternal age, race, education, alcohol consumption, smoking, parity, prenatal vitamin use, Log10-polychlorinated biphenyl 153, delivery by Cesarean section, gestational week at delivery, newborn sex, iodine insufficiency, and product terms for bisphenol A by newborn sex, bisphenol A × iodine deficiency, newborn sex × iodine deficiency, and a bisphenol A × newborn sex × iodine deficiency. From left to right, the panels represent estimates from log10-transformed creatinine-standardized bisphenol A in maternal urine at 16 weeks, 26 weeks, and the average of the log10-transformed creatinine-standardized bisphenol A from maternal urine collected at the 16 and 26 week visits urine
3.3 Sensitivity analyses
In general, estimates obtained in the sensitivity analyses were similar to those described above. Adjustment for iodine deficiency did not substantially alter the observed pattern of associations (Supplemental Material, Table S6). Our results were similar when we used non-creatinine standardized BPA measurements (Supplemental Material, Table S7). Thyroid antibody status did not modify the associations between maternal urinary BPA and maternal serum THs at 16 weeks or average maternal urinary BPA and cord serum THs (Supplemental Material, Table S8 & Table S9).
4. Discussion
In the present study, we observed an inverse association between prenatal BPA exposure and TSH among female newborns, but not males. The association among female newborns was stronger for the more temporally proximal maternal urinary BPA concentration from 26 weeks gestation. We did not observe notable associations between prenatal BPA measures and maternal serum TH concentrations or with cord serum concentrations of T4 or T3.
In contrast with our study results, a prior study of pregnant women enrolled in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort observed an inverse association between urinary BPA and maternal TT4 serum levels during pregnancy (Chevrier et al., 2013). Results from studies of BPA and THs in non-pregnant adults have been inconsistent, with some observing elevated FT3 and FT4 and lowered TSH (Wang et al., 2012; Wang et al., 2013), and others observing reductions in TT4 (Meeker and Ferguson, 2011), or no association (Meeker et al., 2010).
In the only prior study of prenatal BPA exposure and newborn TSH, Chevrier et al. observed an inverse association between urinary BPA and TSH among male newborns (Chevrier et al., 2013), whereas we only observed an inverse association among female newborns. For each doubling of average maternal urinary BPA (across 1st, 2nd, and 3rd trimester urine samples), Chevrier et al. observed a ~10% reduction in TSH among male but not female newborns (Chevrier et al., 2013). Discrepant results between these studies could be due to differences in the racial, ethnic, or socioeconomic makeup of participants, BPA levels [median maternal urinary BPA (IQR) – CHAMACOS: 1.2 µg/g creatinine (0.8–1.9) versus HOME Study 2.2 µg/g creatinine (1.5–3.4)], co-exposures such as pesticides, or timing and method of outcome measurements (i.e., heel stick blood spot at a median of 21 hours in CHAMACOS versus cord blood in the current study).
Further, assessment of maternal dietary iodine using a questionnaire to assess did not appear to modify the associations between BPA and either maternal or newborn THs in prior work (Chevrier et al., 2013). We did not see evidence of effect modification by maternal iodine status among mothers or newborns with both sexes combined. However, our exploratory analysis suggested that gestational BPA exposure might have the greatest adverse influence among female newborns with iodine deficient mothers. A relatively small number of female newborns had mothers with iodine deficiency in our analytic sample (n=24), and thus our statistical power was substantially reduced when analyzing this subgroup.
Because excess levels of TT3 may influence the negative feedback loop of the hypothalamic-pituitary-thyroid axis, resulting in less production of TSH (Melmed and Williams, 2011), the pattern of reduced TSH among female newborns with greater prenatal BPA exposure in our study is consistent with the observed positive association between maternal urinary BPA and TT3 concentrations among female newborns. In general, women have a higher prevalence of thyroid disorders than men (Fortunato et al., 2014). Although the responsible underlying mechanisms are not well understood, it is hypothesized that disruption of gonadal hormones, particularly estrogen, and gender-related differences in the thyroid redox environment may be involved in the pathogenesis of thyroid disorders among women (Fortunato et al., 2014). Purported differences in regulation of oxidative stress for the male versus female thyroid have some support in animal models (Fortunato et al., 2013). We speculate that such differences, if further elucidated, may extend to the female fetal thyroid and account for sexually dimorphic sensitivity of the thyroid to environmental chemicals. However, rodent studies specifically assessing the effect of prenatal BPA exposure on thyroid hormones have been inconsistent, with one study suggesting that BPA affects thyroid hormones among male pups only (Xu et al., 2007), another reporting similar thyroid hormone responses to BPA in both male and female pups (Zoeller et al., 2005), and another observing no effect of BPA on thyroid function for male or female pups (Kobayashi et al., 2005).
Similar to the associations observed by Chevrier et al. (2013), the relation observed in our study between BPA and newborn THs was stronger when the most temporally proximal exposure measurement (26 week BPA) was used in the models. Chevrier et al. (2013) observed that the association between BPA and newborn TSH levels among male newborns was strongest when maternal urinary BPA concentration was measured in the third trimester versus the second, or first trimester. The stronger relation between 26-week BPA compared to 16 week BPA in the present study lends additional support to the possibility that BPA exposure later in gestation has greater influence on newborn TSH. Alternatively, rather than suggesting a window of susceptibility, this may reflect a transient effect of BPA on the thyroid axis, made more apparent by the shorter period of time between BPA measurements near 26 weeks gestation and cord blood THs at delivery (Chevrier et al., 2013).
At birth, a surge of TSH occurs, which subsequently leads to increased T4 and T3 production (Melmed and Williams, 2011). It is unknown whether the lower levels of TSH that we observed among female newborns with greater prenatal BPA exposure translates into meaningful reductions in subsequent T4 or leads to clinical or subclinical hypothyroidism. However, we previously observed a suggestive association between BPA concentrations in our population and infant hypotonia (Yolton et al., 2011), which is a symptom of hypothyroidism (Rastogi and LaFranchi, 2010). In addition, we previously observed that increasing prenatal urinary BPA concentrations were associated with more anxious and hyperactive behaviors among 2–3 year old girls, but not boys (Braun et al., 2011b; Braun et al., 2009). A prior rodent study found that gestational hypothyroidism induced learning deficits and hyperactivity in females, but not males (Friedhoff et al., 2000). This suggests that TH disruption is a potential mediator of the BPA-behavior associations we previously observed that appear to be specific to girls. Interestingly, the CHAMACOS study observed that higher prenatal exposure to BPA was associate with increased anxiety among male children at age 7 (Harley et al., 2013), echoing the same potential relation between lower neonatal TSH and health later in childhood. Further research is necessary to confirm this hypothesis.
Our study had several strengths, including a prospective design, rich covariate data, exclusion of women who had thyroid disorders or were taking any thyroid medication and up to two measures of urinary BPA concentrations during the latter two-thirds of pregnancy. Due to the short biological half-life of BPA, the average of multiple spot urine samples may be a better reflection of BPA exposure over time than a single sample (Braun et al., 2011a). Likewise, our study has limitations worth noting. Although we were able to account for exposure to PCBs, we did not adjust for all known environmental thyroid disruptors (e.g., perchlorate). Furthermore, we may also have residual bias due to exposure misclassification of BPA, which is a non-persistent compound with episodic exposures. If present, such exposure misclassification is likely to be non-differential and would attenuate the observed associations towards the null, suggesting that we may have missed associations and that the true associations between BPA and THs may be stronger than those reported in our study. Similarly, physiologic changes during late pregnancy can increase renal clearance of chemicals (Costantine, 2014) such that urinary levels of BPA may have been overestimated at 26 weeks gestation in our study. However, exposure measurement error due to pharmacokinetic changes in the elimination of BPA in later pregnancy is also unlikely to be differentially associated with cord serum TH concentrations. Day-to-day variability in urinary iodine concentrations may have resulted in some misclassification of women’s iodine status (Busnardo et al., 2006). Also, our statistical power was reduced when we examined for effect modification by child sex and maternal iodine deficiency, and by the smaller sample size for the analysis of maternal THs (n=181). Finally, the clinical implications of small reductions in TSH at birth are not certain. However, over 10% of US children are affected by learning disabilities, autism spectrum disorders, affective disorders, and ADHD (Boyle et al., 2011; CDC, 2012; Costello et al., 2003), and disruption of prenatal THs may play a role in their etiology (Gilbert et al., 2012). These disorders have profound impacts on the health and well-being of children and their families and carry substantial financial costs (Leibson et al., 2001), making the identification of modifiable environmental risk factors for thyroid dysfunction a critical research priority.
5. Conclusions
We observed an inverse relation between maternal urinary BPA concentration and cord blood TSH concentrations among female but not male newborns. The association between maternal urinary BPA and cord blood TSH among female newborns was strongest in BPA measures collected later in pregnancy, which may suggest a window of susceptibility or a transient effect of BPA on TH function. Our findings raise the possibility that disturbance of the thyroid axis may be a mechanism by which BPA could be related to child neurobehavioral outcomes.
Supplementary Material
Highlights.
-
□
Examined associations of BPA with thyroid hormones in pregnant women and newborns
-
□
Assessed effect modification of BPA-thyroid hormone associations by newborn sex
-
□
Greater BPA related to decreased thyroid stimulating hormone in girls’ cord serum
-
□
Results may suggest window of susceptibility to BPA in later gestation
-
□
BPA potentially has greatest adverse effect on girls with iodine deficient mothers
Acknowledgements
This research was supported by R00-ES020346, R01-ES020349, P01-ES11261, P01ES022832-02, and RD-83544201. The authors thank the HOME Study personnel for their skillful assistance and acknowledge the Centers for Disease Control and Prevention (CDC) laboratory staff who performed the measurements of BPA, creatinine, PCBs, and cotinine
Dr. Lanphear has served as an expert witness and a consultant to the California Attorney General’s Office for the plaintiffs in a public nuisance case related to childhood lead poisoning, but he has not personally received any compensation for these services. Dr. Lanphear has also served as a paid consultant on a US Environmental Protection Agency research study related to childhood lead poisoning. Dr. Braun received financial compensation for conducting a re-analysis of a study of child lead exposure for the plaintiffs in a public nuisance case related to childhood lead poisoning.
Abbreviations
- BMI
body mass index
- BPA
bisphenol A
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CHAMACOS
Center for the Health Assessment of Mothers and Children of Salinas
- Cr
creatinine
- CV
coefficient of variation
- FT3
free triiodothyronine
- FT4
free thyroxine
- GM
geometric mean
- HOME
Health Outcomes and Measures of the Environment
- I
iodine
- ICP-MS
inductively coupled plasma-mass spectrometer
- IQR
interquartile range
- LOD
limit of detection
- PCB
polychlorinated biphenyl
- QC
quality control
- TgAb
thyroglobulin antibody
- TH
thyroid hormone
- TPOAb
thyroid peroxidase antibody
- TSH
thyroid stimulating hormone
- TT3
total triiodothyronine
- TT4
total thyroxine
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest to declare.
Competing Financial Interests: The authors have no competing financial interests.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure: None of these activities are directly related to the present study.
References
- Bernert JT, et al. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res. 2009;11:1458–1466. doi: 10.1093/ntr/ntp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, et al. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355:240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Boyle CA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- Braun JM, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011a;119:131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011b;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnardo B, et al. Restricted intraindividual urinary iodine concentration variability in nonfasting subjects. Eur J Clin Nutr. 2006;60:421–425. doi: 10.1038/sj.ejcn.1602334. [DOI] [PubMed] [Google Scholar]
- Calafat AM, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KL, et al. Use of inductively coupled plasma mass spectrometry to measure urinary iodine in NHANES 2000: comparison with previous method. Clin Chem. 2003;49:1019–1021. doi: 10.1373/49.6.1019. [DOI] [PubMed] [Google Scholar]
- CDC. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. doi:(ISSN 1545-8636) [PubMed] [Google Scholar]
- Chevrier J, et al. Associations between prenatal exposure to polychlorinated biphenyls and neonatal thyroid-stimulating hormone levels in a Mexican-American population, Salinas Valley, California. Environ Health Perspect. 2007;115:1490–1496. doi: 10.1289/ehp.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, et al. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect. 2013;121:138–144. doi: 10.1289/ehp.1205092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. doi: 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, et al. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- Fortunato RS, et al. Sexual dimorphism of thyroid reactive oxygen species production due to higher NADPH oxidase 4 expression in female thyroid glands. Thyroid: Official Journal of the American Thyroid Association. 2013;23:111–119. doi: 10.1089/thy.2012.0142. [DOI] [PubMed] [Google Scholar]
- Fortunato RS, et al. Sexual dimorphism and thyroid dysfunction: a matter of oxidative stress? J Endocrinol. 2014 doi: 10.1530/JOE-13-0588. [DOI] [PubMed] [Google Scholar]
- Friedhoff AJ, et al. Role of maternal biochemistry in fetal brain development: effect of maternal thyroidectomy on behaviour and biogenic amine metabolism in rat progeny. Int J Neuropsychopharmacol. 2000;3:89–97. doi: 10.1017/S1461145700001863. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, et al. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatr Res. 2011;69:454–459. doi: 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, et al. Maternal urinary iodine concentration in pregnancy and children's cognition: results from a population-based birth cohort in an iodine-sufficient area. BMJ Open. 2014;4:e005520. doi: 10.1136/bmjopen-2014-005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, et al. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33:842–852. doi: 10.1016/j.neuro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Harley KG, et al. Prenatal and Early Childhood Bisphenol A Concentrations and Behavior in School-Aged Children. Environmental research. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichs J, et al. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clinical Endocrinology. 2013;79:152–162. doi: 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- Herbstman J, et al. Maternal, infant, and delivery factors associated with neonatal thyroid hormone status. Thyroid. 2008;18:67–76. doi: 10.1089/thy.2007.0180. [DOI] [PubMed] [Google Scholar]
- Julvez J, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology. 2013;24:150–157. doi: 10.1097/EDE.0b013e318276ccd3. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, et al. Effects of in utero and lactational exposure to bisphenol A on thyroid status in F1 rat offspring. Ind Health. 2005;43:685–690. doi: 10.2486/indhealth.43.685. [DOI] [PubMed] [Google Scholar]
- Leibson CL, et al. Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. JAMA. 2001;285:60–66. doi: 10.1001/jama.285.1.60. [DOI] [PubMed] [Google Scholar]
- Manglik AK, et al. Umbilical cord blood TSH levels in term neonates: a screening tool for congenital hypothyroidism. Indian Pediatr. 2005;42:1029–1032. doi:(ISSN 0019-6061) [PubMed] [Google Scholar]
- Meeker JD, et al. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. 2010;44:1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ Health Perspect. 2011;119:1396–1402. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S, Williams RH. Williams textbook of endocrinology. Philadelphia: Elsevier/Saunders; 2011. [Google Scholar]
- Nieminen P, et al. In vivo effects of bisphenol A on the polecat (mustela putorius) J Toxicol Environ Health A. 2002a;65:933–945. doi: 10.1080/00984100290071063. [DOI] [PubMed] [Google Scholar]
- Nieminen P, et al. Bisphenol A affects endocrine physiology and biotransformation enzyme activities of the field vole (Microtus agrestis) Gen Comp Endocrinol. 2002b;126:183–189. doi: 10.1006/gcen.2002.7792. [DOI] [PubMed] [Google Scholar]
- Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17. doi: 10.1186/1750-1172-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, et al. Case report: high prenatal bisphenol a exposure and infant neonatal neurobehavior. Environ Health Perspect. 2011;119:1170–1175. doi: 10.1289/ehp.1003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields BM, et al. Five-year follow-up for women with subclinical hypothyroidism in pregnancy. J Clin Endocrinol Metab. 2013;98:E1941–E1945. doi: 10.1210/jc.2013-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, et al. Polybrominated Diphenyl Ethers, Polychlorinated Biphenyls, and Persistent Pesticides in Serum from the National Health and Nutrition Examination Survey: 2003–2008. Environmental Science & Technology. 2014;48:753–760. doi: 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, et al. High urinary bisphenol A concentrations in workers and possible laboratory abnormalities. Occup Environ Med. 2012;69:679–684. doi: 10.1136/oemed-2011-100529. [DOI] [PubMed] [Google Scholar]
- Wang T, et al. Urinary bisphenol a concentration and thyroid function in Chinese adults. Epidemiology. 2013;24:295–302. doi: 10.1097/EDE.0b013e318280e02f. [DOI] [PubMed] [Google Scholar]
- Webster GM, et al. Associations between Perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: A population-based cohort study. Environmental Research. 2014;133:338–347. doi: 10.1016/j.envres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, et al. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, et al. Perinatal bisphenol A affects the behavior and SRC-1 expression of male pups but does not influence on the thyroid hormone receptors and its responsive gene. Neurosci Res. 2007;58:149–155. doi: 10.1016/j.neures.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Ye X, et al. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Yolton K, et al. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol. 2011;33:558–566. doi: 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, et al. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.