Abstract
Heart disease is the leading cause of death in humans, and myocarditis is one predominant cause of heart failure in young adults. Patients affected with myocarditis can develop dilated cardiomyopathy (DCM), a common reason for heart transplantation, which to date is the only viable option for combatting DCM. Myocarditis/DCM patients show antibodies to coxsackievirus B (CVB)3 and cardiac antigens, suggesting a role for CVB-mediated autoimmunity in the disease pathogenesis; however, a direct causal link remains to be determined clinically. Experimentally, myocarditis can be induced in susceptible strains of mice using the human isolates of CVB3, and the disease pathogenesis of postinfectious myocarditis resembles that of human disease, making the observations made in animals relevant to humans. In this review, we discuss the complex nature of CVB3-induced myocarditis as it relates to the damage caused by both the virus and the host's response to infection. Based on recent data we obtained in the mouse model of CVB3 infection, we provide evidence to suggest that CVB3 infection accompanies the generation of cardiac myosin-specific CD4 T cells that can transfer the disease to naïve recipients. The therapeutic implications of these observations are also discussed.
Keywords: Heart, viral myocarditis, coxsackievirus, autoimmunity
1. Introduction
Dilated cardiomyopathy (DCM), a myocardial disease that commonly results in congestive heart failure, is a challenging clinical entity with no known cure or specific causes, and it is a major indication for heart transplantation. The five-year survival rate of patients with DCM is less than 50% [1]. DCM can develop in individuals affected with myocarditis, for which various non-infectious and infectious agents have been implicated. Prominent among the infectious causes for myocarditis are enteroviruses like coxsackievirus B3 (CVB), a bona fide pathogen of the cardiovascular system. In the U.S., approximately five million enteroviral infections are attributed to CVB1-5. A proportion of these (12%) may have myocardial involvement in which CVB1, CVB3 and CVB5 serotypes are commonly implicated [2, 3]. Serologically, CVB3-reactive antibodies are found in about 50% of DCM patients, while enteroviral genomic material can be detected in up to 70% [4-8], suggesting that CVB3 infection is an important environmental predisposing factor for the development of DCM. In this review, we discuss the mechanisms related to the initial damage caused by the virus and how such damage can later be precipitated by the host's response to infection, leading to the establishment of self-destructive (autoimmune) phenomena and their implications for therapy in those affected.
2. Virus life cycle
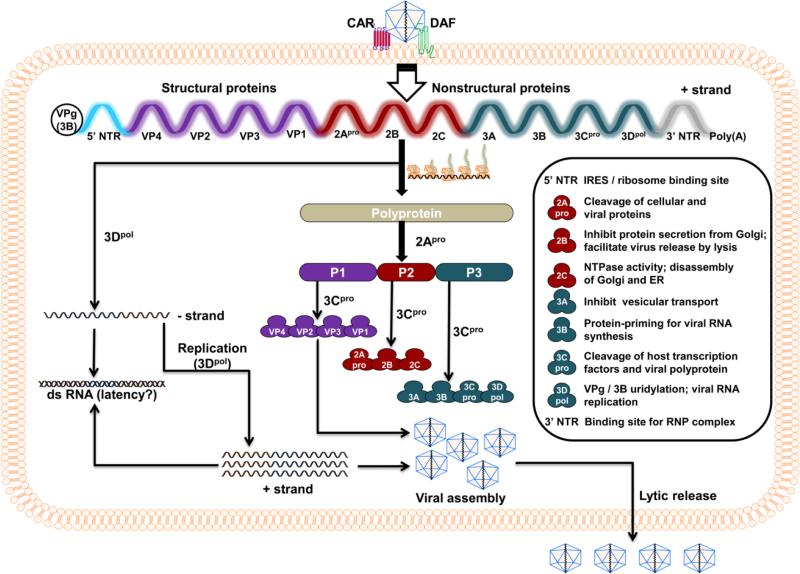
Coxsackievirus, a member of the genus enterovirus, is a positive-sense single-stranded RNA virus belonging to the Picornaviridae family [9, 10]. Six serotypes have been identified (CVB1 to 6) and our focus is CVB3. The CVB3 viral genome consists of 7400 bases, and a single open reading frame flanked by 5’ and 3’ non-translated regions (NTRs) at the termini. Additionally, multiple secondary stem-loop structures can be formed in the 5’ NTR, which is known to harbor molecular determinants of viral pathogenicity [11, 12]. However, for replication of the viral genome, both the 5’ and 3’ NTRs can act as binding sites for a viral genome-linked protein (VPg), also called 3B [13, 14]. The viral genome encodes for a large polyprotein, which is proteolytically cleaved to produce structural and nonstructural (NS) proteins (Fig. 1; [15]. While structural proteins are required for virus assembly, NS proteins mediate the processing of viral polyprotein and replication of the viral genome [15-17]. The CVB3 genome lacks a 5’ 7-methyl guanosine cap structure, which is typically seen in most eukaryotic and many positive-sense viral RNAs and is needed to facilitate translation [18, 19]. Instead, the 5’ NTR, which accounts for 10% of the viral genome (742 out of 7400 nucleotides [nts]), contains an internal ribosome entry site (IRES) and mediates translation of positive-sense viral RNAs [20, 21].
Fig. 1. The life cycle of CVB3.
Virus entry into the target cells first requires interaction with DAF, which facilitates viral attachment to CAR, leading to internalization of the virus in the cytoplasm. After uncoating, the positive-sense single-stranded RNA genome is translated to yield a large single polyprotein. This process requires binding of ribosomes to IRES. The polyprotein is then proteolytically cleaved to generate structural (P1 cluster: VP1 to VP4) and NS (P2 cluster: 2Apro, 2B and 2C; P3 cluster: 3A, 3B, 3Cpro and 3Dpol) proteins by 2Apro and 3Cpro, two viral proteases. While the structural proteins, also called capsid proteins, contribute to the conformation of the virus, NS proteins mediate a variety of functions as indicated in the inset. During viral replication, 3Dpol plays a critical role in the formation of negative and positive strands of viral genomes, which can be paired to form dsRNA as an intermediate stage. The progeny virus containing a single-strand positive-sense RNA genome and structural proteins is finally released through cell lysis.
For any productive infection, viruses have to enter host cells, multiply, and release progeny of infectious virions from the infected cells. The usual target tissues for CVB3 are heart and pancreas, although other organs such as brain, prostate, testis, liver, lung, and intestine can be infected [15, 22, 23]. Virus entry into the target tissues is mediated by two receptors: decay accelerating factor (DAF/CD55) and coxsackievirus and adenovirus receptor (CAR; Fig. 1) [24, 25]. Most tissues express DAF, a glycosyl-phosphatidylinositol-anchored membrane protein. The initial attachment of the virus occurs first through DAF, resulting in the rearrangement of cytoskeletal actin that involves activation of Abl and Fyn kinases [25]. This process facilitates movement of CVB3 along the apical surface of the cell membrane, which provides access to CAR in the tight junctions of epithelial cells [26, 27]. In contrast to DAF, CAR acts as an internalization receptor in the target cells, where virus interacts with CAR's two extracellular Ig domains, D1 and D2 [24]. This interaction triggers Fyn-mediated phosphorylation of caveolin-1, leading to endocytosis of the virus [26, 27] and subsequent uncoating of the RNA genome (positive-strand) into the cytoplasm.
The positive-strand RNA translates into a large polyprotein by a 5’ cap-independent mechanism, whereby the IRES region of 5’ NTR acts as a ‘ribosome landing pad’ [20, 21]. The polyprotein is then proteolytically cleaved by two viral proteases – 2A protease (pro) and 3Cpro – to generate three protein clusters, P1, P2, and P3, through cis-cleavage [28]. These protein clusters undergo a series of trans-cleavage steps to yield individual proteins: P1 – structural or capsid proteins (viral protein [VP]1, VP2, VP3, and VP4); P2 – 2Apro, 2B, 2C; and P3 – 3A, 3B, 3Cpro, and 3D polymerase (pol) [Fig. 1;[28, 29]. In the meantime, 2Apro and 3Cpro can shut off host protein synthesis by their proteolytic activity, allowing the virus to take control of infected cells. For example, 2Apro cleaves eukaryotic initiation factor (EIF)-4γ, poly (A) binding protein, and cytoskeletal dystrophin, in addition to triggering the caspase-3-dependent apoptotic pathway [30-32]. Similarly, 3Cpro also can cleave host transcription factors (TATA-binding protein, cAMP response element binding protein, octamer-binding transcription factor), and Bax and Bid proteins, resulting in sequestration of mitochondrial cytochrome c into cytoplasm and activation of the apoptotic pathway [33-36]. While both 2B and 3A proteins inhibit vesicular transportation from the Golgi complex and exocytosis of cellular proteins, protein 2C causes disassembly of the Golgi complex and the endoplasmic reticulum [ER; [37-39]. The 3Dpol (RNA-dependent-RNA polymerase) generated from the initial translation adds two uridine residues to VPg by a process called uridylation, thereby acting as a primer for the synthesis of negative-strand RNA from the poly (A) tail [40, 41]. The negative strand then acts as a template for multiple copies of positive-strand viral genome to be synthesized by 3Dpol; the positive strands are then packaged with the structural proteins and form infectious virions. The viral protein 2B, also called viroporin, oligomerizes and enters the cell membrane to form channels through which virions are released by cell lysis [42].
During the replicative cycle of the virus, however, dsRNAs can be generated utilizing the negative- and positive-strand viral genomes [43]. But the viral 2C protein, exhibiting nucleoside triphosphatase (NTPase) activity, dissociates dsRNA by unwinding [44, 45]. Variants of CVB3 with lower cytolytic activity can persist in the host as dsRNAs [43], but their identity as infectious viral particles has not been reported. Whether enteroviral RNAs like CVB3 present in DCM patients as reported in the literature [46-48] represent dsRNAs also remains to be investigated.
3. Pathogenesis of CVB3 infection
Studies from various experimental mouse models of CVB3 infections suggest that the disease course of myocarditis assumes two distinct stages that occur in continuum [49, 50]: the acute phase (14-18 days postinfection), in which infectious virus is present, causing damage to cardiomyocytes; and the chronic phase (beyond 18 days postinfection), in which inflammation persists, although the extent of virus replication is much reduced due to selection of a defective virus [50, 51]. This is consistent with the observation that infectious CVB3 cannot be isolated in cardiac tissues from patients with DCM [52, 53]. To understand the immune pathogenesis of viral myocarditis, numerous mouse models have been developed involving the use of various strains of CVB3 [54-58]; Table 1].
Table 1.
List of agents tested in various mouse models of CVB3 infection
| Agent | Outcome | Ref. |
|---|---|---|
| Anti-viral agents | ||
| 2-(3,4-dichlorophenoxy)-5-nitrobenzonitrile | Reduced viral titers; suppressed myocardial inflammatory foci | [142] |
| CVB3 3C protease inhibitor | Inhibited viral replication; attenuated myocardial inflammation and fibrosis | [143] |
| Histone deacetylase inhibitors | Suppressed viral replication in vitro | [144] |
| Recombinant CAR4/7 | Suppressed CVB3 infection; myocardial inflammation was aggravated, likely as a secondary event | [145] |
| Ribavirin, and recombinant IFN-α | Inhibited myocardial viral replication; reduced myocardial damage | [146] |
| Short hairpin (Sh) RNA specific to 2B gene | Improved survival rate, reduced viral titers; attenuated tissue damage | [147] |
| Sh RNA to 2C protein | Reduced viral titers, suppressed myocarditis, and proinflammatory cytokine production | [148] |
| Soluble CAR-Fc fusion protein | Reduced viral infection, myocardial damage, and inflammation | [149] |
| Soluble viral traps (CAR-DAF-Fc) | Attenuated viral infection, myocardial inflammation, and fibrosis | [150] |
| Pharmacological agents/herbal compounds | ||
| 20 (S)-protopanaxtriol | Antiviral effects in vitro and reduced disease severity | [151] |
| α-bromo-4-chlorocinnamaldehyde | Reduced disease severity and viral titers; inhibited nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) activation | [152] |
| β1 blocker (NO-metoprolol) | Cardioprotective | [153] |
| Aqueous extract of Spatholobus suberectus Dunn | Reduced viral titers and suppressed disease severity | [154] |
| Astragaloside IV | Decreased viral titers, necrosis and cellular infiltrations; attenuated myocardial fibrosis via inhibition of transforming growth factor beta -β1 signaling | [155] |
| Calycosin-7-O-P-D-glucopyranoside from Astragalus membranaceus var. Mongholicu | Improved survival rate; alleviated cardiac damage and reduced virus titers in the heart | [156] |
| Curcumin | Attenuated disease severity by inhibiting Phosphoinositide 3-kinase (PI3K)/Akt/NF-kB signaling | [157] |
| Phyllaemblicin B, extract from Phyllanthus emblica | Reduced viral titers; inhibited virus-mediated apoptosis and cardiac muscle damage | [158] |
| Immunological agents | ||
| Adoptive transfer of Treg cells | Protected against disease through IL-10 production | [159] |
| Anti-4-1BBL | Reduced cardiac damage and inflammation | [160] |
| Anti-B7.1 and anti-B7.2 | Anti-B7.1 prolonged the survivability of myocarditic mice; anti-B7.2 abrogated the protective effect of anti-B7.1 | [161] |
| Anti-FAS | Reduced caspase-3 expression, viral replication and cardiomyocyte apoptosis and myocardial injury | [162] |
| Atorvastatin | Attenuated myocardial injury by enhancing the expression of gap junction proteins | [163] |
| CD40-Ig fusion protein | Relieved myocardial injury and inhibited viral replication | [164] |
| CpG nucleotides | Inhibited viral infection | [165] |
| CTLA-4-Ig fusion protein | Reduced mortality and IFN-γ production | [166] |
| CXCL10 | Inhibited viral replication by recruiting NK cells | [167] |
| Galectin-9 | Ameliorated the disease by decreasing Th1 cytokines and promoting Treg and Th2 phenotypes | [168] |
| IFN-β and IFN-α 2 | Mediated anti-viral effects and protected from disease | [169] |
| IL-1 receptor antagonist encoded in plasmid DNA | Attenuated inflammation | [170] |
| IL-17 receptor A delivered through adenoviral vector | Decreased mortality by downregulating production of inflammatory mediators | [171] |
| IL-35 | Protected from disease via reduced IL-17 production | [172] |
| IL-4 | Improved cardiac function by promoting anti-inflammatory effects and downregulation of matrix metalloproteinases | [173] |
| IL-4 gene therapy | Protected from disease through Th2 polarization | [174] |
| miR-21 | Conferred resistance by inhibiting programmed cell death 4 expression | [175] |
| Nasal cardiac myosin or OX40 blockade | Ameliorated disease by enhancing Treg and IL-10 production | [129] |
| Plasmid DNA encoding soluble TNF receptor-Fc | Attenuated inflammation and myocardial fibrosis | [176] |
| Polyclonal Ig therapy | Protected from disease | [177] |
| Proteasome inhibitors | Reduced myocardial damage | |
| Recombinant CVB3/IFN-γ | Conferred protection | [178] |
| Thrombospondin-2 | Inhibited cardiac inflammation | [179] |
| TNF-α-induced protein 3 | Alleviated the disease by inhibiting NF-kB signaling | [180] |
| Truncated monocyte chemoattractant protein-1 | Ameliorated the disease via reduced infiltrations | [181] |
| α-Galactosylceramide | Protected from disease by modulating inflammatory cytokines and anti-vial immune responses | [182] |
3.1.Virus-induced cardiac damage
CVB3 replication can induce myocardial injury through apoptosis and necrosis of cardiomyocytes. The NS proteins generated from translation of the viral genome can significantly affect the structure and functions of cellular proteins (Fig. 2). Some of these include:
Fig 2. Pathogenic mechanism of CVB3-induced cardiac damage.
In cardiomyocytes infected with CVB3, the viral genome is translated to yield several NS viral proteins, whose main functions are highlighted in the inset.
Shutdown of host proteins and cleavage of transcription factors
Notably, 2Apro protein cleaves cytoskeletal dystrophin, and dystrophin-associated glycoproteins such as α-sarcoglycan, β-dystroglycan, and extracelluar laminin-2 [30, 59, 60]. Dystrophin is critical for connecting with the contractile protein F-actin within the cardiomyocytes [61, 62]. Dystrophin deficiency may be relevant to human disease because patients affected with familial DCM show lack of dystrophin production [61, 62]. Likewise, viral protease 3Cpro can alter translation of cellular proteins by cleaving transcription factors such as TATA-binding protein, cAMP responsive element, and octamer-binding protein [34-36].
Cell cycle arrest
Arrest in cell cycle progression has been reported in CVB3 infection, due to reduced synthesis or proteasome-mediated degradation of cyclin-D1 following the degradation of EIF-4γ by viral protease 2Apro [63].
Inhibition of vesicular transport
By blocking the transportation of proteins from the ER to the Golgi complex, viral protein 3A can inhibit exocytosis of secretory cellular proteins [37, 38].
Apoptosis
Cardiomyocytes infected with CVB3 can show DNA fragmentation and apoptotic bodies as early as nine hours postinfection, and viral proteases (2Apro and 3Cpro) are believed to mediate this process [64, 65].
Cell lysis
Being a cytolytic virus, CVB3 can injure the cell membrane, leading to lysis of cells by increasing the membrane permeability and pore formation [42, 66].
While the above mechanisms point to the possibility that the virus can directly damage cardiomyocytes as long as it is present in the tissues during acute infections, the pathomechanisms underlying the persistence of inflammation during the later stages of the disease process in the absence of detectable infectious viral particles remain elusive. Nonetheless, CVB3 RNA can be detected in the chronic stages in infected animals through 21 days postinfection, and the viral genomes may contain deletions of 7 to 49 nucleotides in their 5’ termini [51]. Likewise, viral genomic material containing 5’ terminal deletions was detected from heart tissue from a fatal case of enterovirus-associated myocarditis [67]. Whether such defective viruses can reactivate and contribute to tissue damage like that caused by wild type virus has not been proved as viruses with 5’ terminal deletions were non-cytolytic. However, evidence suggests that the defective virus can replicate in cell culture; mice inoculated with this virus showed the presence of viral RNA for up to 25 weeks, but their hearts or pancreases were normal [51]. Thus, the notion of immune-mediated damage is gaining more attention in explaining the disease pathology in the chronic stages.
3.2. Immune pathogenesis
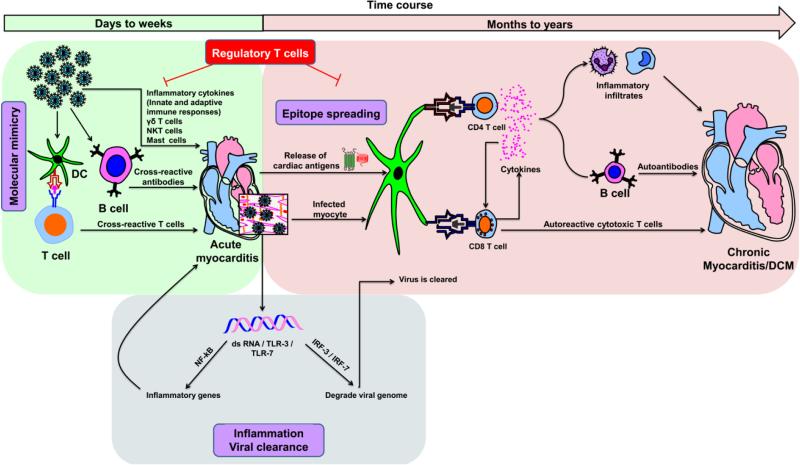
As CVB3 injures cardiomyocytes, the tissue destruction can be potentiated by inflammatory cytokines (interleukin [IL]-1, IL-6 and tumor necrosis factor [TNF]-α) produced by cells of the innate (e.g., macrophages) and adaptive (antiviral, T helper [Th]1 and Th17 cytokines) immune systems (Fig. 3). Toll-like receptors (TLRs) can influence the severity of CVB3-induced myocarditis: mice deficient for TLR-3 developed more severe myocarditis than their wild type littermates, whereas TLR-4-deficient mice developed less severe myocarditis [68, 69]. Furthermore, expression of TLRs can occur gender dependently: elevated expression of TLR-4 in the macrophages of male mice contributed to mortalities in CVB3-infected animals, but the underlying mechanisms are unknown [70, 71]. In contrast, the roles played by certain cellular components of the innate immune system, such as gamma delta (γδ) T cells and natural killer (NK)-T cells, are more complex, in that γδ T cells and NK-T cells perform opposing functions (Fig. 3). Vγ4+ γδ T cells promote CVB3-induced myocarditis by killing regulatory T (Treg) cells through CD1d-mediated lysis and support interferon (IFN)-γ- production by CD4 T cells. NK-T cells, however, favor generation of Treg cells and dampen the inflammatory response triggered by γδ T cells. γδ T cells also can induce cardiac damage by killing the virus-infected CD1d-expressing cardiomyocytes by Fas/Fas-L pathway [72-74], while NK cells are protective in CVB3-induced myocarditis: mice depleted of NK cells showed increased viral titers and exacerbated myocarditis [75, 76]. Recent studies indicate that CVB3 infection can lead to degranulation of mast cells within 6 hours postinfection and to production of proinflammatory cytokines (IL-1, IL-6, IL-8, TNF-α, granulocyte macrophage-colony stimulating factor and other molecules (histamine, heparin, and proteases). These mediators have the potential to precipitate the severity of viral myocarditis [77].
Fig. 3. Proposed mechanisms of inflammatory heart disease.
Acute myocarditis can result from an overt immune response to exposure to cardiotropic pathogens, and cardiac damage can be due to the production of inflammatory cytokines of innate and adaptive immune systems. On one hand, although transitory, dsRNAs produced during the replication cycle of CVB3 can bind TLR-3 or TLR-7 and promote inflammatory response through the activation of NFkB. On the other, dsRNAs can mediate viral clearance by degrading the viral genome by activating IRF-3 / IRF-7 pathways. Various innate immune cell types, such as γδ T cells, NK-T cells and mast cells, can influence the disease-outcome. While γδ T cells and mast cells promote cardiac damage, NK-T cells can dampen such a response. Exposure to environmental microbes that carry mimicry sequences for cardiac antigens can lead to the generation of pathogenic cross-reactive T cell and antibody responses. Nonetheless, once cardiac damage sets in, intracellular proteins (e.g., Myhc-α) that were previously invisible to the immune system can be released as a result of epitope spreading, leading to their uptake by APCs (e.g., DCs) and inducing CD4 T cell responses. Alternatively, APCs can engulf infected cardiomyocytes and induce both CD4 and/or CD8 T cell responses via cross-priming. Upon activation, antigen-sensitized CD4 T cells secrete Th1 and Th17 cytokines that favor heart autoimmunity by activating macrophages and promoting neutrophil infiltration, thereby aggravating cardiac damage while providing help to CD8 T cells and B cells via cytokines and chemokines. CD8 T cells can contribute to myocarditis in two ways: 1) secretion of cytokines similar to those secreted by CD4 T cells with identical consequences; and 2) killing of cardiomyocytes infected with cardiotropic pathogens (e.g., CVB3) via MHC class I-dependent pathway. In the meantime, pathogen-specific T cells, neutralizing antibodies and antiviral cytokines such as IFN-α, IFN-β can be generated, which facilitate elimination of the microbe. Nonetheless, the newly generated cardiac-reactive CD4 and CD8 T cells can persist and contribute to chronic inflammation. In these scenarios, however, presence of efficient Treg cells can dampen autoimmune responses, as they are highly critical for maintenance of self-tolerance.
The relevance of the molecular mimicry hypothesis in the causation of CVB3-induced myocarditis also has been tested, but direct evidence is lacking (Fig. 3). Anti-streptococcal antibodies and anti-streptococcal T cells were shown to bind CVB3 and cardiac myosin; these responses were shown to be pathogenic [78-84]. Similarly, the phenomenon of epitope spreading also may be relevant to the pathogenesis of viral myocarditis, because pathogens like CVB3 that primarily infect hearts can lead to secondary generation of autoimmune responses resulting from release of self-antigens due to cardiac injury (Fig. 3). Either the newly released antigens are taken up by the resident antigen-presenting cells (APCs) (e.g., dendritic cells, DCs), or APCs can engulf cardiomyocytes infected with an infectious agent, leading to induction of pathogenic CD4 Th and/or cytolytic T lymphocyte (CTL) responses by cross-priming. Similarly, under the influence of Th cytokines, B cells sensitized with self-antigens can precipitate cardiac damage by producing pathogenic autoantibodies (Fig. 3). But in these scenarios, presence of efficient Treg cells can dampen inflammatory responses. For example, adoptive transfer of Treg cells prior to infection with CVB3 protects mice from developing myocarditis [85], pointing to a possibility that autoimmune responses accompany CVB3 infection.
In support of autoimmune theory, autoantibodies to various cardiac antigens – such as cardiac myosin heavy chain (Myhc)-α, adenine nucleotide translocator 1 (ANT), β-adrenergic receptor-1 (BAR), branched chain α-ketoacid dehydrogenase (BCKD), cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase (CATP), laminin, and the muscarinic receptor – have been detected in patients with myocarditis and DCM, including experimental CVB3 myocarditis models [86-93]. But the pathological significance of these autoantibodies continues to be uncertain. Evidence for the generation of autoreactive T cells in CVB3 infection was first provided based on the finding that the CTLs obtained from Balb/C mice infected with CVB3 lysed the cardiomyocytes, and the T cells transferred the disease into naïve animals, but their antigen-specificity was not known [56, 94]. To delineate the autoimmune mechanisms in CVB3 myocarditis, we recently created major histocompatibility complex (MHC) class II dextramers (the new generation MHC class II tetramers) for Myhc-α 334-352, permitting us to determine the antigen-specificity of autoreactive CD4 T cells that might be generated in infected mice [55, 95]. Myhc-α has been recognized as a major autoantigen candidate in heart autoimmunity, and CD4 T cells sensitized with Myhc-α or its immunodominant epitopes induce autoimmune myocarditis, the phenotype of which resembles postviral myocarditis in humans [96, 97]. For example, Myhc-α 334-352 and Myhc-α 614-643 induce lymphocytic myocarditis in A/J and Balb/c mice, respectively [98, 99]. Using Myhc-α 334-352 dextramers, we demonstrated that A/J mice infected with CVB3 showed generation of Myhc-α-specific CD4 T cells and that antigen-specific CD4 T cells infiltrate hearts in infected mice [55]. More importantly, using adoptive transfer protocols, we showed that autoreactive CD4 T cells generated in mice infected with CVB can transfer myocarditis to naïve mice. The pathological changes observed in these mice were restricted to hearts, as pancreases were normal [55]. In addition, T cells sensitized with irrelevant antigen and virus did not induce disease. Furthermore, by proving that the naïve repertoire does not contain a significant proportion of Myhc-α-reactive T cells, we concluded that generation of the Myhc-α-reactive T cells was secondary to the damage caused primarily by the virus [55]. We proposed that when intracellular proteins like Myhc-α are released as a consequence of the cardiomyocytolytic effect of viruses, they become visible to the immune system, leading to the induction of pathogenic autoimmune responses. This may be the reason inflammation persists in the absence of recoverable infectious virus in chronically infected mice. Similar observations are made in human DCM patients, who exhibit the signature of CVB infections, but actively replicating virions are rarely detected in cardiac tissues [100].
4. Antiviral immune responses and viral clearance
Experimentally, the two phases of CVB3 infection (acute and chronic) occur in continuum, but the infectious virus particles cannot be detected in mice beyond 14 to 18 days postinfection; furthermore, successful viral clearance may require the participation of both B cells and T cells [50, 57, 94, 101-103]. The importance of antibodies in the attenuation of viral myocarditis is demonstrated by the observation that animals infected with CVB3 could generate strong virus-specific neutralizing antibodies, and that B cell-deficient mice were unable to resolve CVB3 infection and showed elevated viral titers [102, 104, 105]. Sex hormones appear to influence the disease outcome. Male Balb/C mice infected with CVB3 showed robust disease-inducing IgG2a and IFN-γ-producing CD4 T cells (Th1-type), as opposed to females, in which dominant IgG1 and IL-4-producing CD4 T cells (Th2-type) favored disease resistance [106]. Previously, severity of CVB3-induced myocarditis was shown to be relatively high in mice deficient for CD8, but the disease was attenuated in mice deficient for CD4 [103, 107]. Conversely, mice deficient for both CD8 and CD4 were protected, suggesting that both cell types modulate the disease outcome [103].
The data available on the roles played by Th cytokines, however, are conflicting. For example, IFN-γ-knockout mice infected with CVB3 were protected from myocarditis, while reconstitution with recombinant IFN-γ restored disease susceptibility [108]. Similarly, IL-12Rβ1-deficient mice infected with CVB3 showed decreased viral replication and reduced inflammation in the hearts, whereas IFN-γ-deficiency exacerbated viral replication [69]. In other studies, however, IL-12 was found to be protective in CVB3-induced myocarditis by increasing the production of IFN-γ [109]. Furthermore, non-obese diabetic mice transgenically expressing IFN-γ specifically in the pancreas were found to be protected from CVB3 infection [110]. In contrast, IL-4-deficient C57Bl/6 and IL-13-knockout Balb/C mice developed severe myocarditis with prolonged viral persistence, suggesting that Th2 cytokines promote disease protection in CVB3 infection [111, 112]. Recent studies indicate that IL-17 produced by Th17 cells is a critical mediator of CVB3 pathogenesis, as IL-17 levels were elevated in both acute and chronic phases of viral infection [113-115]. Furthermore, blockade of IL-17 using neutralizing antibodies led to reduction in myocardial damage as well as viral replication [115, 116]. The question of whether induction of robust IL-17 responses coincides with viral clearance requires additional studies. However, neutralization of IL-22 in IL-17A-deficient mice decreased the severity of myocarditis and enhanced viral replication, raising the question whether Th17 and Th22 cells display differential effects on CVB3 pathogenesis because IL-22 can be secreted by both subsets [117].
Finally, formation of dsRNAs, although transitory, can interact with TLR-3, and TLR-7 and induce synthesis of type I interferons (IFN-α and IFN-β) by activating interferon regulatory transcription factor (IRF)-3 and IRF-7 pathways [Fig. 3; [118, 119]. IFNs can then activate two host proteins – RNA-dependent protein kinase and 2’ 5’-oligoadenylate synthetase – resulting in the synthesis of EIF-2α and ribonuclease (RNase) L [120]. While EIF-2α inhibits viral replication, RNase L promotes degradation of the viral genome, but both can trigger apoptosis [121-124]. In addition, EIF-2α can induce the transcription of apoptotic genes by enhancing the synthesis of activating transcription factor-4. Similarly, RNase L can activate c-Jun-NH2-terminal kinase and promote the transcription of apoptotic genes and/or activation of the mitochondrial death pathway [121, 122]. Thus, apoptosis of infected cells is considered to be one of the important mechanisms of viral clearance. Consistent with this notion, it has been recently demonstrated that selective ablation of type I IFN receptor in cardiomyocytes leads to exacerbation of myocarditis likely due to a delay in the clearance of virus [125].
5. Therapeutic implications
Various therapeutic strategies have been developed in the medical management of patients with myocarditis/DCM. These include the use of antiviral compounds, interferons, intravenous immunoglobulin therapy, and immunosuppressive agents such as corticosteroids, and azathioprine [Table 1; [126-128]. None of these therapeutic agents, however, have proven to be effective in preventing the disease progression nor are these generally recommended for all patients regardless of the stage of disease. Similarly, no clear consensus has emerged for the use of immunosuppressive drugs because the supporting data are lacking. These judgmental limitations are in part due to the practical difficulties to differentiate patients with or without infectious origin in the clinical settings. Thus, for these groups of patients, the use of antiviral compounds and immunosuppressive drugs has been suggested as therapeutic modalities [126-128].
Experimentally, the use of animal models offers the advantage of testing the efficacy of therapeutics under defined conditions. To this end, as exemplified in Table 1, a variety of compounds such as antiviral drugs, small molecules, and natural medicines have been shown to attenuate the severity of acute CVB3 myocarditis through multiple mechanisms. Likewise, immunologically, costimulatory molecules, such as B7, CD40 and cytotoxic T-lymphocyte antigen (CTLA)-4, have been successfully targeted for therapy, in addition to the use of cytokines (Table 1). The observations made in these animal models are relevant to CVB3 infections in humans because human isolates of CVB3 induce disease in mice with comparable pathologies, and the disease patterns also are similar, in that the disease course assumes two stages [50, 57]. While the acute phase is accompanied by multiplication of virus and cardiac inflammation, the chronic phase is devoid of actively multiplying viruses, yet inflammation continues to persist. In these circumstances, autoimmunity becomes a prime suspect. Our recent data showing the appearance of pathogenic Myhc-α-reactive T cells in mice infected with CVB3 is the first direct evidence to prove that autoimmunity can be an important component of CVB3 pathogenesis. Whether such an outcome is possible in humans remains to be investigated. If testing reveals this hypothesis to be true, it may provide a basis for the use of immune suppressive therapy in DCM patients who test positive for CVB3. Additionally, it also may create avenues to develop other immunologic strategies, like antigen-specific T cell therapies, to dampen autoimmune responses. Two such examples are the use of altered peptide ligands and induction of tolerance by administering soluble antigens via oral or nasal routes [129-132]. As a proof of principle for the latter approach, mice tolerized with Myhc-α and later infected with CVB3 have shown decreased severity of myocarditis, an effect mediated through T cells but not antibodies [129]. Thus, in the long term, we propose that antigen-specific T cell-based therapies may be a viable alternative to the use of chemotherapy and heart transplantations for myocarditis/DCM patients.
Conversely, it may be possible to prevent the occurrence of CVB3-mediated myocarditis through vaccination, but no commercial vaccines currently are available for use in humans. Nevertheless, experimentally, various vaccination strategies, such as the use of CVB3 virus-like particles, attenuated viral strains, and priming and boosting with CVB3 DNA and recombinant viral protein, respectively, have been shown to effectively confer varying degrees of protection [133-136].
CVB3, a bona fide pathogen of cardiovascular system, is ubiquitously present in the environment, making it possible that most humans may have a chance of exposure to this virus at some point in their lifetime. Although, the disease induced with CVB3, in particular, myocarditis may go unnoticed, but chronically, few of these individuals may develop DCM. Due to the lack of effective chemotherapy options, heart transplantation has become a critical necessity for patients with end-stage disease. For such patients in whom no infectious virions are present in cardiac biopsies, it becomes difficult to justify the use of antiviral drugs. The pathogenesis of CVB3 infection is complex in that tissue destruction involves the mediation of both the virus, through its lytic properties, and the host, via immune-mediated damage, importantly through the induction of pathogenic autoreactive T cell responses, as we have recently demonstrated in the mouse model. Thus, we have begun to provide answers for previously unanswered questions about why inflammation persists in the absence of detectable infectious virions, which if otherwise present, would be expected to continue to damage the cardiac tissue. Our data may support the notion that the immune-mediated damage is superimposed on the initial tissue destruction caused by the virus, but once autoimmune response sets in, the disease process becomes perpetual, at which stage, immune-suppressive therapies may become the only therapeutic option. Finally, it is to be noted that enteroviral infections have been shown to be associated with various other chronic disease conditions, such as Type I diabetes, coeliac disease, and asthma [137-141]. Whether their pathogeneses involve the mechanisms similar to myocarditis triggered by CVB3 may be a worthy area for further investigations.
Highlights.
Coxsackievirus B3 (CVB3) is a common suspect in patients with chronic myocarditis/DCM
Autoimmunity is suspected, but a direct causal link remains elusive clinically
Damage caused by autoreactive T cells may be a key mechanism in chronic viral infections
Acknowledgements
This work was supported by the National Institutes of Health (HL114669). CM is a recipient of a postdoctoral research fellowship grant awarded by the Myocarditis Foundation, NJ.
List of abbreviations
- ANT
adenine nucleotide translocator 1
- APCs
antigen-presenting cells
- BAR
β-adrenergic receptor-1
- BCKD
branched chain α-ketoacid dehydrogenase
- CAR
coxsackievirus and adenovirus receptor
- CATP
cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase
- CTL
cytolytic T lymphocyte
- CTLA
cytotoxic T-lymphocyte antigen
- CVB
coxsackievirus B
- DAF
decay accelerating factor
- DCM
dilated cardiomyopathy
- DCs
dendritic cells
- EIF
eukaryotic initiation factor
- IL
interleukin
- IRES
internal ribosome entry site
- IRF
interferon regulatory transcription factor
- MHC
major histocompatibility complex
- Myhc
cardiac myosin heavy chain
- NK
natural killer
- NS
nonstructural
- NTPase
nucleoside triphosphatase
- NTRs
non-translated regions
- nts
nucleotides
- RNase
ribonuclease
- Sh
short hairpin
- Th
T helper
- TLR
Toll-like receptors
- TNF
tumor necrosis factor
- Treg
regulatory T
- VPg
viral genome-linked protein
- γδ
gamma delta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876–90. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 2.Henke A, Jarasch N, Martin U, Zell R, Wutzler P. Characterization of the protective capability of a recombinant coxsackievirus B3 variant expressing interferon-gamma. Viral Immunol. 2008;21:38–48. doi: 10.1089/vim.2007.0077. [DOI] [PubMed] [Google Scholar]
- 3.Kim KS, Hufnagel G, Chapman NM, Tracy S. The group B coxsackieviruses and myocarditis. Rev Med Virol. 2001;11:355–68. doi: 10.1002/rmv.326. [DOI] [PubMed] [Google Scholar]
- 4.Archard LC, Bowles NE, Cunningham L, Freeke CA, Olsen EG, Rose ML, et al. Molecular probes for detection of persisting enterovirus infection of human heart and their prognostic value. Eur Heart J. 1991;12(Suppl D):56–9. doi: 10.1093/eurheartj/12.suppl_d.56. [DOI] [PubMed] [Google Scholar]
- 5.Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95–114. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–93. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 7.Martino T, Liu P, Sole MJ. Human Enterovirus infections. ASM; Washington, DC: 1995. Enteroviral myocarditis and dialted cardiomyopathy: a review of clinical and experimental studies. [Google Scholar]
- 8.Chapman NM, Kim KS. Persistent coxsackievirus infection: enterovirus persistence in chronic myocarditis and dilated cardiomyopathy. Curr Top Microbiol Immunol. 2008;323:275–92. doi: 10.1007/978-3-540-75546-3_13. [DOI] [PubMed] [Google Scholar]
- 9.Hyypia T, Kallajoki M, Maaronen M, Stanway G, Kandolf R, Auvinen P, et al. Pathogenetic differences between coxsackie A and B virus infections in newborn mice. Virus Res. 1993;27:71–8. doi: 10.1016/0168-1702(93)90113-2. [DOI] [PubMed] [Google Scholar]
- 10.Paque RE, Gauntt CJ, Nealon TJ. Assessment of cell-mediated immunity against coxsackievirus B3-induced myocarditis in a primate model (Papio papio). Infect Immun. 1981;31:470–9. doi: 10.1128/iai.31.1.470-479.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracy S, Liu HL, Chapman NM. Coxsackievirus B3: primary structure of the 5' non-coding and capsid protein-coding regions of the genome. Virus Res. 1985;3:263–70. doi: 10.1016/0168-1702(85)90050-4. [DOI] [PubMed] [Google Scholar]
- 12.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–80. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 13.Flanegan JB, Petterson RF, Ambros V, Hewlett NJ, Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977;74:961–5. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YF, Nomoto A, Detjen BM, Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977;74:59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–55. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson MF, Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968;61:77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wimmer E, Hellen CU, Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee AK. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980;44:175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, et al. Field's Virology. Lippincott Williams & Wilkins (LWW); 2007. [Google Scholar]
- 20.Jang SK, Krausslich HG, Nicklin MJ, Duke GM, Palmenberg AC, Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988;62:2636–43. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–5. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 22.Bergelson JM, Krithivas A, Celi L, Droguett G, Horwitz MS, Wickham T, et al. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–9. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997;94:3352–6. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y, Chipman PR, Howitt J, Bator CM, Whitt MA, Baker TS, et al. Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor. Nat Struct Biol. 2001;8:874–8. doi: 10.1038/nsb1001-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shenoy-Scaria AM, Kwong J, Fujita T, Olszowy MW, Shaw AS, Lublin DM. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J Immunol. 1992;149:3535–41. [PubMed] [Google Scholar]
- 26.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–31. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 27.Marchant D, Si X, Luo H, McManus B, Yang D. The impact of CVB3 infection on host cell biology. Curr Top Microbiol Immunol. 2008;323:177–98. doi: 10.1007/978-3-540-75546-3_8. [DOI] [PubMed] [Google Scholar]
- 28.Palmenberg AC. Proteolytic processing of picornaviral polyprotein. Annu Rev Microbiol. 1990;44:603–23. doi: 10.1146/annurev.mi.44.100190.003131. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DA, Smith WW, Ferre RA, Condon B, Budahazi G, Sisson W, et al. Structure of human rhinovirus 3C protease reveals a trypsin-like polypeptide fold, RNA-binding site, and means for cleaving precursor polyprotein. Cell. 1994;77:761–71. doi: 10.1016/0092-8674(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 30.Badorff C, Lee GH, Lamphear BJ, Martone ME, Campbell KP, Rhoads RE, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–6. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 31.Ehrenfeld E. Poliovirus-induced inhibition of host-cell protein synthesis. Cell. 1982;28:435–6. doi: 10.1016/0092-8674(82)90195-7. [DOI] [PubMed] [Google Scholar]
- 32.Ventoso I, MacMillan SE, Hershey JW, Carrasco L. Poliovirus 2A proteinase cleaves directly the eIF-4G subunit of eIF-4F complex. FEBS Lett. 1998;435:79–83. doi: 10.1016/s0014-5793(98)01027-8. [DOI] [PubMed] [Google Scholar]
- 33.Chau DH, Yuan J, Zhang H, Cheung P, Lim T, Liu Z, et al. Coxsackievirus B3 proteases 2A and 3C induce apoptotic cell death through mitochondrial injury and cleavage of eIF4GI but not DAP5/p97/NAT1. Apoptosis. 2007;12:513–24. doi: 10.1007/s10495-006-0013-0. [DOI] [PubMed] [Google Scholar]
- 34.Clark ME, Lieberman PM, Berk AJ, Dasgupta A. Direct cleavage of human TATA-binding protein by poliovirus protease 3C in vivo and in vitro. Mol Cell Biol. 1993;13:1232–7. doi: 10.1128/mcb.13.2.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yalamanchili P, Datta U, Dasgupta A. Inhibition of host cell transcription by poliovirus: cleavage of transcription factor CREB by poliovirus-encoded protease 3Cpro. J Virol. 1997;71:1220–6. doi: 10.1128/jvi.71.2.1220-1226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yalamanchili P, Weidman K, Dasgupta A. Cleavage of transcriptional activator Oct-1 by poliovirus encoded protease 3Cpro. Virology. 1997;239:176–85. doi: 10.1006/viro.1997.8862. [DOI] [PubMed] [Google Scholar]
- 37.Doedens JR, Giddings TH, Jr., Kirkegaard K. Inhibition of endoplasmic reticulum-to-Golgi traffic by poliovirus protein 3A: genetic and ultrastructural analysis. J Virol. 1997;71:9054–64. doi: 10.1128/jvi.71.12.9054-9064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doedens JR, Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995;14:894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandoval IV, Carrasco L. Poliovirus infection and expression of the poliovirus protein 2B provoke the disassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. J Virol. 1997;71:4679–93. doi: 10.1128/jvi.71.6.4679-4693.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui T, Sankar S, Porter AG. Binding of encephalomyocarditis virus RNA polymerase to the 3'-noncoding region of the viral RNA is specific and requires the 3'-poly(A) tail. J Biol Chem. 1993;268:26093–8. [PubMed] [Google Scholar]
- 41.Paul AV, van Boom JH, Filippov D, Wimmer E. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature. 1998;393:280–4. doi: 10.1038/30529. [DOI] [PubMed] [Google Scholar]
- 42.van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, et al. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–32. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tam PE, Messner RP. Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J Virol. 1999;73:10113–21. doi: 10.1128/jvi.73.12.10113-10121.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorbalenya AE, Koonin EV, Donchenko AP, Blinov VM. A conserved NTP-motif in putative helicases. Nature. 1988;333:22. doi: 10.1038/333022a0. [DOI] [PubMed] [Google Scholar]
- 45.Klein M, Eggers HJ, Nelsen-Salz B. Echovirus 9 strain barty non-structural protein 2C has NTPase activity. Virus Res. 1999;65:155–60. doi: 10.1016/s0168-1702(99)00110-0. [DOI] [PubMed] [Google Scholar]
- 46.Andreoletti L, Bourlet T, Moukassa D, Rey L, Hot D, Li Y, et al. Enteroviruses can persist with or without active viral replication in cardiac tissue of patients with end-stage ischemic or dilated cardiomyopathy. J Infect Dis. 2000;182:1222–7. doi: 10.1086/315818. [DOI] [PubMed] [Google Scholar]
- 47.Andreoletti L, Hober D, Decoene C, Copin MC, Lobert PE, Dewilde A, et al. Detection of enteroviral RNA by polymerase chain reaction in endomyocardial tissue of patients with chronic cardiac diseases. J Med Virol. 1996;48:53–9. doi: 10.1002/(SICI)1096-9071(199601)48:1<53::AID-JMV9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 48.Kuethe F, Sigusch HH, Hilbig K, Tresselt C, Gluck B, Egerer R, et al. Detection of viral genome in the myocardium: lack of prognostic and functional relevance in patients with acute dilated cardiomyopathy. Am Heart J. 2007;153:850–8. doi: 10.1016/j.ahj.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Lerner AM, Wilson FM. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- 50.Rose NR, Wolfgram LJ, Herskowitz A, Beisel KW. Postinfectious autoimmunity: two distinct phases of coxsackievirus B3-induced myocarditis. Ann N Y Acad Sci. 1986;475:146–56. doi: 10.1111/j.1749-6632.1986.tb20864.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim KS, Tracy S, Tapprich W, Bailey J, Lee CK, Kim K, et al. 5'-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J Virol. 2005;79:7024–41. doi: 10.1128/JVI.79.11.7024-7041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pauschinger M, Phan MD, Doerner A, Kuehl U, Schwimmbeck PL, Poller W, et al. Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation. 1999;99:889–95. doi: 10.1161/01.cir.99.7.889. [DOI] [PubMed] [Google Scholar]
- 53.Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapman NM, Tu Z, Tracy S, Gauntt CJ. An infectious cDNA copy of the genome of a non-cardiovirulent coxsackievirus B3 strain: its complete sequence analysis and comparison to the genomes of cardiovirulent coxsackieviruses. Archives of virology. 1994;135:115–30. doi: 10.1007/BF01309769. [DOI] [PubMed] [Google Scholar]
- 55.Gangaplara A, Massilamany C, Brown DM, Delhon G, Pattnaik AK, Chapman N, et al. Coxsackievirus B3 infection leads to the generation of cardiac myosin heavy chain-alpha-reactive CD4 T cells in A/J mice. Clin Immunol. 2012;144:237–49. doi: 10.1016/j.clim.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Huber SA, Lodge PA. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984;116:21–9. [PMC free article] [PubMed] [Google Scholar]
- 57.Rose NR, Hill SL. The pathogenesis of postinfectious myocarditis. Clin Immunol Immunopathol. 1996;80:S92–9. doi: 10.1006/clin.1996.0146. [DOI] [PubMed] [Google Scholar]
- 58.Tracy S, Hofling K, Pirruccello S, Lane PH, Reyna SM, Gauntt CJ. Group B coxsackievirus myocarditis and pancreatitis: connection between viral virulence phenotypes in mice. J Med Virol. 2000;62:70–81. doi: 10.1002/1096-9071(200009)62:1<70::aid-jmv11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 59.Kramer B, Huber M, Kern C, Klingel K, Kandolf R, Selinka HC. Chinese hamster ovary cells are non-permissive towards infection with coxsackievirus B3 despite functional virus-receptor interactions. Virus Res. 1997;48:149–56. doi: 10.1016/s0168-1702(96)01438-4. [DOI] [PubMed] [Google Scholar]
- 60.Straub V, Campbell KP. Muscular dystrophies and the dystrophin-glycoprotein complex. Curr Opin Neurol. 1997;10:168–75. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 61.Beggs AH. Dystrophinopathy, the expanding phenotype. Dystrophin abnormalities in X-linked dilated cardiomyopathy. Circulation. 1997;95:2344–7. doi: 10.1161/01.cir.95.10.2344. [DOI] [PubMed] [Google Scholar]
- 62.Muntoni F, Cau M, Ganau A, Congiu R, Arvedi G, Mateddu A, et al. Brief report: deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy. N Engl J Med. 1993;329:921–5. doi: 10.1056/NEJM199309233291304. [DOI] [PubMed] [Google Scholar]
- 63.Luo H, Zhang J, Dastvan F, Yanagawa B, Reidy MA, Zhang HM, et al. Ubiquitin-dependent proteolysis of cyclin D1 is associated with coxsackievirus-induced cell growth arrest. J Virol. 2003;77:1–9. doi: 10.1128/JVI.77.1.1-9.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carthy CM, Granville DJ, Watson KA, Anderson DR, Wilson JE, Yang D, et al. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J Virol. 1998;72:7669–75. doi: 10.1128/jvi.72.9.7669-7675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carthy CM, Yanagawa B, Luo H, Granville DJ, Yang D, Cheung P, et al. Bcl-2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology. 2003;313:147–57. doi: 10.1016/s0042-6822(03)00242-3. [DOI] [PubMed] [Google Scholar]
- 66.Van kuppeveld FJ, Melchers WJ, Kirkegaard K, Doedens JR. Structure-function analysis of coxsackie B3 virus protein 2B. Virology. 1997;227:111–8. doi: 10.1006/viro.1996.8320. [DOI] [PubMed] [Google Scholar]
- 67.Chapman NM, Kim KS, Drescher KM, Oka K, Tracy S. 5' terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart. Virology. 2008;375:480–91. doi: 10.1016/j.virol.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abston ED, Coronado MJ, Bucek A, Bedja D, Shin J, Kim JB, et al. Th2 regulation of viral myocarditis in mice: different roles for TLR3 versus TRIF in progression to chronic disease. Clin Dev Immunol. 2012;2012:129486. doi: 10.1155/2012/129486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, et al. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170:4731–7. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- 70.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–4. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 71.Roberts BJ, Moussawi M, Huber SA. Sex differences in TLR2 and TLR4 expression and their effect on coxsackievirus-induced autoimmune myocarditis. Exp Mol Pathol. 2012 doi: 10.1016/j.yexmp.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huber SA. Depletion of gammadelta+ T cells increases CD4+ FoxP3 (T regulatory) cell response in coxsackievirus B3-induced myocarditis. Immunology. 2009;127:567–76. doi: 10.1111/j.1365-2567.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu W, Huber SA. Cross-talk between cd1d-restricted nkt cells and gammadelta cells in t regulatory cell response. Virol J. 2011;8:32. doi: 10.1186/1743-422X-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu W, Li S, Tian W, Li W, Zhang Z. Immunoregulatory effects of alpha-GalCer in a murine model of autoimmune myocarditis. Exp Mol Pathol. 2011;91:636–42. doi: 10.1016/j.yexmp.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 75.Gauntt CJ, Godeny EK, Lutton CW, Fernandes G. Role of natural killer cells in experimental murine myocarditis. Springer Semin Immunopathol. 1989;11:51–9. doi: 10.1007/BF00197084. [DOI] [PubMed] [Google Scholar]
- 76.Vella C, Festenstein H. Coxsackievirus B4 infection of the mouse pancreas: the role of natural killer cells in the control of virus replication and resistance to infection. J Gen Virol. 1992;73(Pt 6):1379–86. doi: 10.1099/0022-1317-73-6-1379. [DOI] [PubMed] [Google Scholar]
- 77.Fairweather D, Frisancho-Kiss S, Gatewood S, Njoku D, Steele R, Barrett M, et al. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37:131–45. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 78.Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med. 1999;341:2068–74. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- 79.Cunningham MW. T cell mimicry in inflammatory heart disease. Mol Immunol. 2004;40:1121–7. doi: 10.1016/j.molimm.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 80.Cunningham MW. Turning point in myocarditis. Circ Res. 2009;105:403–5. doi: 10.1161/CIRCRESAHA.109.205195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cunningham MW, Antone SM, Gulizia JM, McManus BM, Fischetti VA, Gauntt CJ. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci U S A. 1992;89:1320–4. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guilherme L, Kalil J, Cunningham M. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity. 2006;39:31–9. doi: 10.1080/08916930500484674. [DOI] [PubMed] [Google Scholar]
- 83.Root-Bernstein R, Vonck J, Podufaly A. Antigenic complementarity between coxsackie virus and streptococcus in the induction of rheumatic heart disease and autoimmune myocarditis. Autoimmunity. 2009;42:1–16. doi: 10.1080/08916930802208540. [DOI] [PubMed] [Google Scholar]
- 84.Swerlick RA, Cunningham MW, Hall NK. Monoclonal antibodies cross-reactive with group A streptococci and normal and psoriatic human skin. J Invest Dermatol. 1986;87:367–71. doi: 10.1111/1523-1747.ep12524838. [DOI] [PubMed] [Google Scholar]
- 85.Shi Y, Fukuoka M, Li G, Liu Y, Chen M, Konviser M, et al. Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor beta-coxsackie-adenovirus receptor pathway. Circulation. 2010;121:2624–34. doi: 10.1161/CIRCULATIONAHA.109.893248. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–40. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- 87.Mascaro-Blanco A, Alvarez K, Yu X, Lindenfeld J, Olansky L, Lyons T, et al. Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies. Autoimmunity. 2008;41:442–53. doi: 10.1080/08916930802031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neu N, Beisel KW, Traystman MD, Rose NR, Craig SW. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987;138:2488–92. [PubMed] [Google Scholar]
- 89.Neumann DA, Burek CL, Baughman KL, Rose NR, Herskowitz A. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol. 1990;16:839–46. doi: 10.1016/s0735-1097(10)80331-6. [DOI] [PubMed] [Google Scholar]
- 90.Neumann DA, Lane JR, LaFond-Walker A, Allen GS, Wulff SM, Herskowitz A, et al. Heart-specific autoantibodies can be eluted from the hearts of Coxsackievirus B3-infected mice. Clin Exp Immunol. 1991;86:405–12. doi: 10.1111/j.1365-2249.1991.tb02945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neumann DA, Rose NR, Ansari AA, Herskowitz A. Induction of multiple heart autoantibodies in mice with coxsackievirus B3- and cardiac myosin-induced autoimmune myocarditis. J Immunol. 1994;152:343–50. [PubMed] [Google Scholar]
- 92.Schulze K, Becker BF, Schauer R, Schultheiss HP. Antibodies to ADP-ATP carrier--an autoantigen in myocarditis and dilated cardiomyopathy--impair cardiac function. Circulation. 1990;81:959–69. doi: 10.1161/01.cir.81.3.959. [DOI] [PubMed] [Google Scholar]
- 93.Schulze K, Witzenbichler B, Christmann C, Schultheiss HP. Disturbance of myocardial energy metabolism in experimental virus myocarditis by antibodies against the adenine nucleotide translocator. Cardiovasc Res. 1999;44:91–100. doi: 10.1016/s0008-6363(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 94.Huber SA, Job LP. Differences in cytolytic T cell response of BALB/c mice infected with myocarditic and non-myocarditic strains of coxsackievirus group B, type 3. Infect Immun. 1983;39:1419–27. doi: 10.1128/iai.39.3.1419-1427.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Massilamany C, Upadhyaya B, Gangaplara A, Kuszynski C, Reddy J. Detection of autoreactive CD4 T cells using major histocompatibility complex class II dextramers. BMC Immunol. 2011;12:40. doi: 10.1186/1471-2172-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alvarez FL, Neu N, Rose NR, Craig SW, Beisel KW. Heart-specific autoantibodies induced by Coxsackievirus B3: identification of heart autoantigens. Clin Immunol Immunopathol. 1987;43:129–39. doi: 10.1016/0090-1229(87)90164-4. [DOI] [PubMed] [Google Scholar]
- 97.Rose NR, Herskowitz A, Neumann DA, Neu N. Autoimmune myocarditis: a paradigm of post-infection autoimmune disease. Immunol Today. 1988;9:117–20. doi: 10.1016/0167-5699(88)91282-0. [DOI] [PubMed] [Google Scholar]
- 98.Donermeyer DL, Beisel KW, Allen PM, Smith SC. Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart. J Exp Med. 1995;182:1291–300. doi: 10.1084/jem.182.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pummerer CL, Luze K, Grassl G, Bachmaier K, Offner F, Burrell SK, et al. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97:2057–62. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gebhard JR, Perry CM, Harkins S, Lane T, Mena I, Asensio VC, et al. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am J Pathol. 1998;153:417–28. doi: 10.1016/S0002-9440(10)65585-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huber S, Polgar J, Moraska A, Cunningham M, Schwimmbeck P, Schultheiss P. T lymphocyte responses in CVB3-induced murine myocarditis. Scand J Infect Dis Suppl. 1993;88:67–78. [PubMed] [Google Scholar]
- 102.Mena I, Perry CM, Harkins S, Rodriguez F, Gebhard J, Whitton JL. The role of B lymphocytes in coxsackievirus B3 infection. Am J Pathol. 1999;155:1205–15. doi: 10.1016/S0002-9440(10)65223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Opavsky MA, Penninger J, Aitken K, Wen WH, Dawood F, Mak T, et al. Susceptibility to myocarditis is dependent on the response of alphabeta T lymphocytes to coxsackieviral infection. Circ Res. 1999;85:551–8. doi: 10.1161/01.res.85.6.551. [DOI] [PubMed] [Google Scholar]
- 104.Dorries R, ter Meulen V. Specificity of IgM antibodies in acute human coxsackievirus B infections, analysed by indirect solid phase enzyme immunoassay and immunoblot technique. J Gen Virol. 1983;64(Pt 1):159–67. doi: 10.1099/0022-1317-64-1-159. [DOI] [PubMed] [Google Scholar]
- 105.Haarmann CM, Schwimmbeck PL, Mertens T, Schultheiss HP, Strauer BE. Identification of serotype-specific and nonserotype-specific B-cell epitopes of coxsackie B virus using synthetic peptides. Virology. 1994;200:381–9. doi: 10.1006/viro.1994.1202. [DOI] [PubMed] [Google Scholar]
- 106.Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–32. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Henke A, Huber S, Stelzner A, Whitton JL. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995;69:6720–8. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huber S, Sartini D. T cells expressing the Vgamma1 T-cell receptor enhance virus-neutralizing antibody response during coxsackievirus B3 infection of BALB/c mice: differences in male and female mice. Viral Immunol. 2005;18:730–9. doi: 10.1089/vim.2005.18.730. [DOI] [PubMed] [Google Scholar]
- 109.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Steele RA, et al. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005;174:261–9. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- 110.Horwitz MS, La Cava A, Fine C, Rodriguez E, Ilic A, Sarvetnick N. Pancreatic expression of interferon-gamma protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat Med. 2000;6:693–7. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- 111.Cihakova D, Barin JG, Afanasyeva M, Kimura M, Fairweather D, Berg M, et al. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am J Pathol. 2008;172:1195–208. doi: 10.2353/ajpath.2008.070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leipner C, Grun K, Borchers M, Stelzner A. The outcome of coxsackievirus B3-(CVB3-) induced myocarditis is influenced by the cellular immune status. Herz. 2000;25:245–8. doi: 10.1007/s000590050014. [DOI] [PubMed] [Google Scholar]
- 113.Yang F, Wu WF, Yan YL, Pang Y, Kong Q, Huang YL. Expression of IL-23/Th17 pathway in a murine model of Coxsackie virus B3-induced viral myocarditis. Virol J. 2011;8:301. doi: 10.1186/1743-422X-8-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yuan J, Yu M, Lin QW, Cao AL, Yu X, Dong JH, et al. Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol. 2010;185:4004–10. doi: 10.4049/jimmunol.1001718. [DOI] [PubMed] [Google Scholar]
- 115.Yuan J, Yu M, Lin QW, Cao AL, Yu X, Dong JH, et al. Neutralization of IL-17 inhibits the production of anti-ANT autoantibodies in CVB3-induced acute viral myocarditis. Int Immunopharmacol. 2010;10:272–6. doi: 10.1016/j.intimp.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 116.Fan Y, Weifeng W, Yuluan Y, Qing K, Yu P, Yanlan H. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of coxsackievirus b3-induced viral myocarditis reduces myocardium inflammation. Virol J. 2011;8:17. doi: 10.1186/1743-422X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kong Q, Xue Y, Wu W, Yang F, Liu Y, Gao M, et al. IL-22 exacerbates the severity of CVB3-induced acute viral myocarditis in IL-17A-deficient mice. Mol Med Rep. 2013;7:1329–35. doi: 10.3892/mmr.2013.1323. [DOI] [PubMed] [Google Scholar]
- 118.Conzelmann KK. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J Virol. 2005;79:5241–8. doi: 10.1128/JVI.79.9.5241-5248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gribaudo G, Lembo D, Cavallo G, Landolfo S, Lengyel P. Interferon action: binding of viral RNA to the 40-kilodalton 2'-5'-oligoadenylate synthetase in interferon-treated HeLa cells infected with encephalomyocarditis virus. J Virol. 1991;65:1748–57. doi: 10.1128/jvi.65.4.1748-1757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oritani K, Kanakura Y. IFN-zeta/ limitin: a member of type I IFN with mild lympho-myelosuppression. J Cell Mol Med. 2005;9:244–54. doi: 10.1111/j.1582-4934.2005.tb00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dai R, Yan D, Li J, Chen S, Liu Y, Chen R, et al. Activation of PKR/eIF2alpha signaling cascade is associated with dihydrotestosterone-induced cell cycle arrest and apoptosis in human liver cells. J Cell Biochem. 2012;113:1800–8. doi: 10.1002/jcb.24051. [DOI] [PubMed] [Google Scholar]
- 122.Li G, Xiang Y, Sabapathy K, Silverman RH. An apoptotic signaling pathway in the interferon antiviral response mediated by RNase L and c-Jun NH2-terminal kinase. J Biol Chem. 2004;279:1123–31. doi: 10.1074/jbc.M305893200. [DOI] [PubMed] [Google Scholar]
- 123.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Talloczy Z, Jiang W, Virgin HWt, Leib DA, Scheuner D, Kaufman RJ, et al. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci U S A. 2002;99:190–5. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Althof N, Harkins S, Kemball CC, Flynn CT, Alirezaei M, Whitton JL. In vivo ablation of type I interferon receptor from cardiomyocytes delays coxsackieviral clearance and accelerates myocardial disease. J Virol. 2014;88:5087–99. doi: 10.1128/JVI.00184-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brunetti L, DeSantis ER. Treatment of viral myocarditis caused by coxsackievirus B. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2008;65:132–7. doi: 10.2146/ajhp060586. [DOI] [PubMed] [Google Scholar]
- 127.Camargo PR, Okay TS, Yamamoto L, Del Negro GM, Lopes AA. Myocarditis in children and detection of viruses in myocardial tissue: implications for immunosuppressive therapy. Int J Cardiol. 2011;148:204–8. doi: 10.1016/j.ijcard.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 128.Maisch B, Hufnagel G, Kolsch S, Funck R, Richter A, Rupp H, et al. Treatment of inflammatory dilated cardiomyopathy and (peri)myocarditis with immunosuppression and i.v. immunoglobulins. Herz. 2004;29:624–36. doi: 10.1007/s00059-004-2628-7. [DOI] [PubMed] [Google Scholar]
- 129.Fousteri G, Dave A, Morin B, Omid S, Croft M, von Herrath MG. Nasal cardiac myosin peptide treatment and OX40 blockade protect mice from acute and chronic virally-induced myocarditis. J Autoimmun. 2011;36:210–20. doi: 10.1016/j.jaut.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Godsel LM, Leon JS, Wang K, Fornek JL, Molteni A, Engman DM. Captopril prevents experimental autoimmune myocarditis. J Immunol. 2003;171:346–52. doi: 10.4049/jimmunol.171.1.346. [DOI] [PubMed] [Google Scholar]
- 131.Gonnella PA, Waldner H, Del Nido PJ, McGowan FX. Inhibition of experimental autoimmune myocarditis: peripheral deletion of TcR Vbeta 8.1, 8.2+ CD4+ T cells in TLR-4 deficient mice. J Autoimmun. 2008;31:180–7. doi: 10.1016/j.jaut.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y, Afanasyeva M, Hill SL, Kaya Z, Rose NR. Nasal administration of cardiac myosin suppresses autoimmune myocarditis in mice. J Am Coll Cardiol. 2000;36:1992–9. doi: 10.1016/s0735-1097(00)00939-6. [DOI] [PubMed] [Google Scholar]
- 133.Zhang H, Morgan-Capner P, Latif N, Pandolfino YA, Fan W, Dunn MJ, et al. Coxsackievirus B3-induced myocarditis. Characterization of stable attenuated variants that protect against infection with the cardiovirulent wild-type strain. Am J Pathol. 1997;150:2197–207. [PMC free article] [PubMed] [Google Scholar]
- 134.Lan J, Gao Z, Xiong H, Chuai X, Jin Y, Li J, et al. Generation of protective immune responses against coxsackievirus B3 challenge by DNA prime-protein boost vaccination. Vaccine. 2011;29:6894–902. doi: 10.1016/j.vaccine.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 135.Zhang L, Parham NJ, Zhang F, Aasa-Chapman M, Gould EA, Zhang H. Vaccination with coxsackievirus B3 virus-like particles elicits humoral immune response and protects mice against myocarditis. Vaccine. 2012;30:2301–8. doi: 10.1016/j.vaccine.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 136.Koho T, Koivunen MR, Oikarinen S, Kummola L, Makinen S, Mahonen AJ, et al. Coxsackievirus B3 VLPs purified by ion exchange chromatography elicit strong immune responses in mice. Antiviral Res. 2014;104:93–101. doi: 10.1016/j.antiviral.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 137.Hober D, Alidjinou EK. Enteroviral pathogenesis of type 1 diabetes: queries and answers. Current opinion in infectious diseases. 2013;26:263–9. doi: 10.1097/QCO.0b013e3283608300. [DOI] [PubMed] [Google Scholar]
- 138.Sarmiento L, Galvan JA, Cabrera-Rode E, Aira L, Correa C, Sariego S, et al. Type 1 diabetes associated and tissue transglutaminase autoantibodies in patients without type 1 diabetes and coeliac disease with confirmed viral infections. J Med Virol. 2012;84:1049–53. doi: 10.1002/jmv.23305. [DOI] [PubMed] [Google Scholar]
- 139.Oikarinen M, Tauriainen S, Oikarinen S, Honkanen T, Collin P, Rantala I, et al. Type 1 diabetes is associated with enterovirus infection in gut mucosa. Diabetes. 2012;61:687–91. doi: 10.2337/db11-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsukagoshi H, Ishioka T, Noda M, Kozawa K, Kimura H. Molecular epidemiology of respiratory viruses in virus-induced asthma. Frontiers in microbiology. 2013;4:278. doi: 10.3389/fmicb.2013.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Joao Silva M, Ferraz C, Pissarra S, Cardoso MJ, Simoes J, Bonito Vitor A. Role of viruses and atypical bacteria in asthma exacerbations among children in Oporto (Portugal). Allergologia et immunopathologia. 2007;35:4–9. doi: 10.1157/13099088. [DOI] [PubMed] [Google Scholar]
- 142.Padalko E, Verbeken E, De Clercq E, Neyts J. Inhibition of coxsackie B3 virus induced myocarditis in mice by 2-(3,4-dichlorophenoxy)-5-nitrobenzonitrile. J Med Virol. 2004;72:263–7. doi: 10.1002/jmv.10570. [DOI] [PubMed] [Google Scholar]
- 143.Yun SH, Lee WG, Kim YC, Ju ES, Lim BK, Choi JO, et al. Antiviral activity of coxsackievirus B3 3C protease inhibitor in experimental murine myocarditis. J Infect Dis. 2012;205:491–7. doi: 10.1093/infdis/jir745. [DOI] [PubMed] [Google Scholar]
- 144.Shim SH, Park JH, Ye MB, Nam JH. Histone deacetylase inhibitors suppress coxsackievirus B3 growth in vitro and myocarditis induced in mice. Acta virologica. 2013;57:462–6. doi: 10.4149/av_2013_04_462. [DOI] [PubMed] [Google Scholar]
- 145.Dorner A, Grunert HP, Lindig V, Chandrasekharan K, Fechner H, Knowlton KU, et al. Treatment of coxsackievirus-B3-infected BALB/c mice with the soluble coxsackie adenovirus receptor CAR4/7 aggravates cardiac injury. J Mol Med (Berl) 2006;84:842–51. doi: 10.1007/s00109-006-0076-y. [DOI] [PubMed] [Google Scholar]
- 146.Okada I, Matsumori A, Matoba Y, Tominaga M, Yamada T, Kawai C. Combination treatment with ribavirin and interferon for coxsackievirus B3 replication. The Journal of laboratory and clinical medicine. 1992;120:569–73. [PubMed] [Google Scholar]
- 147.Yao H, Zhang Y, He F, Wang C, Xiao Z, Zou J, et al. Short hairpin RNA targeting 2B gene of coxsackievirus B3 exhibits potential antiviral effects both in vitro and in vivo. BMC Infect Dis. 2012;12:177. doi: 10.1186/1471-2334-12-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim YJ, Ahn J, Jeung SY, Kim DS, Na HN, Cho YJ, et al. Recombinant lentivirus-delivered short hairpin RNAs targeted to conserved coxsackievirus sequences protect against viral myocarditis and improve survival rate in an animal model. Virus genes. 2008;36:141–6. doi: 10.1007/s11262-007-0192-y. [DOI] [PubMed] [Google Scholar]
- 149.Pinkert S, Westermann D, Wang X, Klingel K, Dorner A, Savvatis K, et al. Prevention of cardiac dysfunction in acute coxsackievirus B3 cardiomyopathy by inducible expression of a soluble coxsackievirus-adenovirus receptor. Circulation. 2009;120:2358–66. doi: 10.1161/CIRCULATIONAHA.108.845339. [DOI] [PubMed] [Google Scholar]
- 150.Lim BK, Choi JH, Nam JH, Gil CO, Shin JO, Yun SH, et al. Virus receptor trap neutralizes coxsackievirus in experimental murine viral myocarditis. Cardiovasc Res. 2006;71:517–26. doi: 10.1016/j.cardiores.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 151.Wang X, Wang Y, Ren Z, Qian C, Li Y, Wang Q, et al. Protective effects of 20(s)-protopanaxtriol on viral myocarditis infected by coxsackievirus B3. Pathobiology. 2012;79:285–9. doi: 10.1159/000331229. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Y, Cao W, Xie YH, Yang Q, Li XQ, Liu XX, et al. The comparison of alpha-bromo-4-chlorocinnamaldehyde and cinnamaldehyde on coxsackie virus B3-induced myocarditis and their mechanisms. Int Immunopharmacol. 2012;14:107–13. doi: 10.1016/j.intimp.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 153.Gluck B, Dahlke K, Zell R, Krumbholz A, Decker M, Lehmann J, et al. Cardioprotective effect of NO-metoprolol in murine coxsackievirus B3-induced myocarditis. J Med Virol. 2010;82:2043–52. doi: 10.1002/jmv.21928. [DOI] [PubMed] [Google Scholar]
- 154.Pang J, Guo JP, Jin M, Chen ZQ, Wang XW, Li JW. Antiviral effects of aqueous extract from Spatholobus suberectus Dunn. against coxsackievirus B3 in mice. Chinese journal of integrative medicine. 2011;17:764–9. doi: 10.1007/s11655-011-0642-1. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y, Zhu H, Huang C, Cui X, Gao Y, Huang Y, et al. Astragaloside IV exerts antiviral effects against coxsackievirus B3 by upregulating interferon-gamma. Journal of cardiovascular pharmacology. 2006;47:190–5. doi: 10.1097/01.fjc.0000199683.43448.64. [DOI] [PubMed] [Google Scholar]
- 156.Zhu H, Zhang Y, Ye G, Li Z, Zhou P, Huang C. In vivo and in vitro antiviral activities of calycosin-7-O-beta-D-glucopyranoside against coxsackie virus B3. Biological & pharmaceutical bulletin. 2009;32:68–73. doi: 10.1248/bpb.32.68. [DOI] [PubMed] [Google Scholar]
- 157.Song Y, Ge W, Cai H, Zhang H. Curcumin protects mice from coxsackievirus B3-induced myocarditis by inhibiting the phosphatidylinositol 3 kinase/Akt/nuclear factor-kappaB pathway. Journal of cardiovascular pharmacology and therapeutics. 2013;18:560–9. doi: 10.1177/1074248413503044. [DOI] [PubMed] [Google Scholar]
- 158.Wang YF, Wang XY, Ren Z, Qian CW, Li YC, Kaio K, et al. Phyllaemblicin B inhibits Coxsackie virus B3 induced apoptosis and myocarditis. Antiviral Res. 2009;84:150–8. doi: 10.1016/j.antiviral.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 159.Cao Y, Xu W, Xiong S. Adoptive transfer of regulatory T cells protects against Coxsackievirus B3-induced cardiac fibrosis. PLoS One. 2013;8:e74955. doi: 10.1371/journal.pone.0074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cheung CT, Deisher TA, Luo H, Yanagawa B, Bonigut S, Samra A, et al. Neutralizing anti-4-1BBL treatment improves cardiac function in viral myocarditis. Lab Invest. 2007;87:651–61. doi: 10.1038/labinvest.3700563. [DOI] [PubMed] [Google Scholar]
- 161.Seko Y, Takahashi N, Yagita H, Okumura K, Azuma M, Yazaki Y. Effects of in vivo administration of anti-B7-1/B7-2 monoclonal antibodies on the survival of mice with chronic ongoing myocarditis caused by Coxsackievirus B3. J Pathol. 1999;188:107–12. doi: 10.1002/(SICI)1096-9896(199905)188:1<107::AID-PATH319>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 162.Chang H, Han B, Han XZ. [The mechanisms responsible for the therapeutic effects of anti-Fas ligand antibody on viral myocarditis in mice]. Zhonghua er ke za zhi Chinese journal of pediatrics. 2005;43:920–4. [PubMed] [Google Scholar]
- 163.Zhang A, Zhang H, Wu S. Immunomodulation by atorvastatin upregulates expression of gap junction proteins in coxsackievirus B3 (CVB3)-induced myocarditis. Inflamm Res. 2010;59:255–62. doi: 10.1007/s00011-009-0093-8. [DOI] [PubMed] [Google Scholar]
- 164.Bo H, Zhenhu L, Lijian Z. Blocking the CD40-CD40L interaction by CD40-Ig reduces disease progress in murine myocarditis induced by CVB3. Cardiovasc Pathol. 2010;19:371–6. doi: 10.1016/j.carpath.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 165.Zhao T, Wu X, Song D, Fang M, Guo S, Zhang P, et al. Effect of prophylactically applied CpG ODN on the development of myocarditis in mice infected with Coxsackievirus B3. Int Immunopharmacol. 2012;14:665–73. doi: 10.1016/j.intimp.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 166.Han B, Jiang H, Liu Z, Zhang Y, Zhao L, Lu K, et al. CTLA4-Ig relieves inflammation in murine models of coxsackievirus B3-induced myocarditis. Can J Cardiol. 2012;28:239–44. doi: 10.1016/j.cjca.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 167.Yuan J, Liu Z, Lim T, Zhang H, He J, Walker E, et al. CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis. Circ Res. 2009;104:628–38. doi: 10.1161/CIRCRESAHA.108.192179. [DOI] [PubMed] [Google Scholar]
- 168.Lv K, Xu W, Wang C, Niki T, Hirashima M, Xiong S. Galectin-9 administration ameliorates CVB3 induced myocarditis by promoting the proliferation of regulatory T cells and alternatively activated Th2 cells. Clin Immunol. 2011;140:92–101. doi: 10.1016/j.clim.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 169.Wang YX, da Cunha V, Vincelette J, White K, Velichko S, Xu Y, et al. Antiviral and myocyte protective effects of murine interferon-beta and -{alpha}2 in coxsackievirus B3-induced myocarditis and epicarditis in Balb/c mice. Am J Physiol Heart Circ Physiol. 2007;293:H69–76. doi: 10.1152/ajpheart.00154.2007. [DOI] [PubMed] [Google Scholar]
- 170.Lim BK, Choe SC, Shin JO, Ho SH, Kim JM, Yu SS, et al. Local expression of interleukin-1 receptor antagonist by plasmid DNA improves mortality and decreases myocardial inflammation in experimental coxsackieviral myocarditis. Circulation. 2002;105:1278–81. [PubMed] [Google Scholar]
- 171.Xie Y, Li M, Wang X, Zhang X, Peng T, Yang Y, et al. In vivo delivery of adenoviral vector containing interleukin-17 receptor a reduces cardiac remodeling and improves myocardial function in viral myocarditis leading to dilated cardiomyopathy. PLoS One. 2013;8:e72158. doi: 10.1371/journal.pone.0072158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hu Y, Dong C, Yue Y, Xiong S. In vivo delivery of interleukin-35 relieves coxsackievirus-B3-induced viral myocarditis by inhibiting Th17 cells. Archives of virology. 2014 doi: 10.1007/s00705-014-2098-z. [DOI] [PubMed] [Google Scholar]
- 173.Li J, Leschka S, Rutschow S, Schwimmbeck PL, Husmann L, Noutsias M, et al. Immunomodulation by interleukin-4 suppresses matrix metalloproteinases and improves cardiac function in murine myocarditis. Eur J Pharmacol. 2007;554:60–8. doi: 10.1016/j.ejphar.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 174.Jiang Z, Xu W, Li K, Yue Y, Xu L, Ye F, et al. Remission of CVB3-induced viral myocarditis by in vivo Th2 polarization via hydrodynamics-based interleukin-4 gene transfer. J Gene Med. 2008;10:918–29. doi: 10.1002/jgm.1215. [DOI] [PubMed] [Google Scholar]
- 175.He J, Yue Y, Dong C, Xiong S. MiR-21 confers resistance against CVB3-induced myocarditis by inhibiting PDCD4-mediated apoptosis. Clinical and investigative medicine Medecine clinique et experimentale. 2013;36:E103–11. doi: 10.25011/cim.v36i2.19573. [DOI] [PubMed] [Google Scholar]
- 176.Kim JM, Lim BK, Ho SH, Yun SH, Shin JO, Park EM, et al. TNFR-Fc fusion protein expressed by in vivo electroporation improves survival rates and myocardial injury in coxsackievirus induced murine myocarditis. Biochem Biophys Res Commun. 2006;344:765–71. doi: 10.1016/j.bbrc.2006.03.170. [DOI] [PubMed] [Google Scholar]
- 177.Weller AH, Hall M, Huber SA. Polyclonal immunoglobulin therapy protects against cardiac damage in experimental coxsackievirus-induced myocarditis. Eur Heart J. 1992;13:115–9. doi: 10.1093/oxfordjournals.eurheartj.a060030. [DOI] [PubMed] [Google Scholar]
- 178.Gao G, Zhang J, Si X, Wong J, Cheung C, McManus B, et al. Proteasome inhibition attenuates coxsackievirus-induced myocardial damage in mice. Am J Physiol Heart Circ Physiol. 2008;295:H401–8. doi: 10.1152/ajpheart.00292.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Papageorgiou AP, Swinnen M, Vanhoutte D, VandenDriessche T, Chuah M, Lindner D, et al. Thrombospondin-2 prevents cardiac injury and dysfunction in viral myocarditis through the activation of regulatory T-cells. Cardiovasc Res. 2012;94:115–24. doi: 10.1093/cvr/cvs077. [DOI] [PubMed] [Google Scholar]
- 180.Gui J, Yue Y, Chen R, Xu W, Xiong S. A20 (TNFAIP3) alleviates CVB3-induced myocarditis via inhibiting NF-kappaB signaling. PLoS One. 2012;7:e46515. doi: 10.1371/journal.pone.0046515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shen Y, Zhang FQ, Wei X. Truncated monocyte chemoattractant protein-1 can alleviate cardiac injury in mice with viral myocarditis via infiltration of mononuclear cells. Microbiol Immunol. 2014;58:195–201. doi: 10.1111/1348-0421.12130. [DOI] [PubMed] [Google Scholar]
- 182.Wu CY, Feng Y, Qian GC, Wu JH, Luo J, Wang Y, et al. alpha-Galactosylceramide protects mice from lethal Coxsackievirus B3 infection and subsequent myocarditis. Clin Exp Immunol. 2010;162:178–87. doi: 10.1111/j.1365-2249.2010.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]