Abstract
Purpose
Several properties of ocular tissue make fixation for light microscopy problematic. Because the eye is spherical, immersion fixation necessarily results in a temporal gradient of fixation, with surfaces fixing more rapidly and thoroughly than interior structures. The problem is compounded by the fact that the layers of the eye wall are compositionally quite different, resulting in different degrees of fixation-induced shrinkage and distortion. Collectively, these result in non-uniform preservation, as well as buckling and/or retinal detachment. This gradient problem is most acute for the lens, where the density of proteins can delay fixation of the central lens for days, and where the fixation gradient parallels the age gradient of lens cells, which complicates data interpretation. Our goal was to identify a simple method for minimizing some of the problems arising from immersion fixation, which avoided covalent modification of antigens, retained high quality structure, and maintained tissue in a state that is amenable to common cytochemical techniques.
Methods
A simple and inexpensive derivative of the freeze-substitution approach was developed and compared to fixation by immersion in formalin. Preservation of structure, immunoreactivity, GFP and tdTomato fluorescence, lectin reactivity, outer segment auto fluorescence, Click-iT chemistry, compatibility with in situ hybdrdization, and the ability to rehydrate eyes after fixation by freeze substitution for subsequent cryo sectioning were assessed.
Results
An inexpensive and simple variant of the freeze substitution approach provides excellent structural preservation for light microscopy, and essentially eliminates ocular buckling, retinal detachment, and outer segment auto-fluorescence, without covalent modification of tissue antigens. The approach shows a notable improvement in preservation of immunoreactivity. TdTomato intrinsic fluorescence is also preserved, as is compatibility with in situ hybridization, lectin labeling, and the Click-iT chemistry approach to labeling the thymidine analog EdU. On the negative side, this approach dramatically reduced intrinsic GFP fluorescence.
Conclusions
A simple, cost-effective derivative of the freeze substitution process is described that is of particular value in the study of rodent or other small eyes, where fixation gradients, globe buckling, retinal detachment, differential shrinkage, autofluorescence, and tissue immunoreactivity have been problematic.
Introduction
Sectioned tissue offers a powerful substrate for many cell biologic approaches, such as immunocytochemistry, in situ hybridization, and the identification of dividing cells. However, these approaches usually require striking a balance between efforts required to preserve structure and the structural artifacts and experimental limitations imparted by those efforts. Covalent modification of epitopes by aldehyde fixatives is a prime example of how efforts to preserve structure impact antigenicity. Further, protocols that work well for an approach such as in situ hybridization do not necessarily translate into optimal conditions for another approach such as immunocytochemistry. A less common but legitimate concern is the fixation gradient that occurs when tissues are fixed by immersion: tissues at the surface will fix more rapidly and more extensively than those in the center of the tissue.
We were motivated to address the issue of fixation gradients by our studies of the rodent ocular lens. The lens is a roughly spherical structure that grows throughout the life of the organism by the addition of new layers to its surface (lens structure and development reviewed in [1]). The net result is an onion-like structure, consisting of hundreds to thousands of concentric shells, each consisting of a single generation of differentiated lens fiber cells about 3 microns thick. Like growth rings of a tree, the shells are arranged in a perfect chronological order: oldest at the center, youngest at the periphery. Also like tree growth rings, no layers of lens cells are lost with age. This means that there is a perfect chronological gradient of cells from surface to center, with recently differentiated layers at the surface and cells generated in utero at the center.
In an effort to optimize its role as an optical element, the lens is also avascular. Further, only the first 50–100 or so layers of lens fiber cells (of the several thousand present in the human lens) retain organelles. This means that the majority of fiber cells in the adult lens spend a lifetime without any of the functions imparted by organelles, including mitosis, translation, and transcription, making them a superb model for cellular aging. Finally, the lens must elevate its index of refraction well above that of the surrounding aqueous media, and does so by elevating cytoplasmic protein concentrations to a level higher than those of any other cell type. These structural features of the lens combine to create a problem for microscopic study. The lens is not uniformly fixed by perfusion because it lacks blood vessels. Lacking any connective tissue, the lens is too soft and gelatinous to dissect in the fresh state without introducing serious shear and distortion. Lens protein levels are so high that chemical immersion fixation creates a barrier that impedes further fixative penetration. Studies using 3H-formaldehyde showed that, while the lens surface was thoroughly fixed in a matter of hours, the center of the lens was not equally fixed even after immersion for days [2]. Thus, immersion fixation creates a fixation gradient that parallels the gradient in fiber cell age, creating questions of whether studies that compare surface cells to deeper cells are reflecting the age gradient or fixation gradient, or some combination.
While these issues are pronounced in the lens, they are valid concerns for any structure that is stratified from surface-to-center, and which is fixed by immersion. These would include tissues, such as eyes, brain, bone, kidneys, adrenals, etc. It also raises a question of sampling: if a large homogenous tissue such as the liver is fixed by immersion, will there be a difference between tissue harvested from the surface and that harvested from the center? In many cases, the differences may be unimportant, but an absence of differences cannot be assumed. To minimize these effects, we adapted a well established approach, freeze substitution [3], to the rodent eye, using inexpensive, readily available equipment.
Methods
Tissue processing
Mouse eyes were rapidly frozen by immersion in dry ice-cooled liquid propane. For a propane source and injector, we used a propane torch kit (~$15.00) that is used for joining copper plumbing (Figure 1A), and which is available at hardware stores (Ace 14.1-oz Propane Torch Kit with brass head). The diffuser sleeve at the tip of the torch was removed, revealing the pinhole outlet. As a vessel to hold the liquid propane, we used a “retired” aluminum PCR block, buried in crushed dry ice (Figure 1B). Tape was used to cover 1 well, (volume 6 ml) to prevent atmospheric water from condensing inside the chilled block. After the block was equilibrated at dry ice temperature, the tape was removed. In a chemical hood, and well away from flames or electrical devices that are switching on/off (propane is combustible), liquid propane was slowly delivered to the well (Figure 1B), until the well was nearly full. We selected propane because it is inexpensive, readily available, remains liquid at dry ice temperatures, and does not boil like liquid nitrogen when room-temperature tissues are immersed, thereby facilitating freezing. The propane tank is inverted to ensure a liquid jet, and gently injected into the chilled PCR block (Figure 1B). We then allowed about 15 min for the liquid propane to reach dry ice temperatures. In parallel, we chilled a 25 ml glass vial containing 20 ml of 97% methanol and 3% acetic acid (M-AA) to dry ice temperatures. We chose this solvent because it is a common “denaturing fix” used in routine histology, and it remains liquid at dry ice temperatures. The ratio of solvent:tissue water probably has some lower limit, but we have established that 20 ml solvent for each pair of mouse eyes worked well.
Figure 1.

Propane source and injector. A: Propane canisters in a variety of sizes and shapes are readily available as camping stove/barbecue fuel, and for use in copper pipe joinery. The brass torch adaptor screwed to the top was specifically for copper pipe use, and is available at most hardware stores. The diffuser was unscrewed from the tip of the torch to reveal the pinhole outlet. B: The inverted propane tank will eject liquid propane that remains liquid as it hits the dry ice–chilled PCR blocks.
Mice were euthanized by dry CO2 inhalation, as approved by UCD IAUCUC #17903. Eyes were rapidly enucleated, picked up by grasping a strand of extraocular connective tissue with fine-tipped forceps, immersed rapidly in the chilled propane for 1 min, and then rapidly transferred to the chilled M-AA. The goal was to instantly preserve structure and stop biologic degradation through rapid freezing, then more gradually fix the tissue by freeze substitution of M-AA for ice. To achieve this, the vial with eyes was placed at −80 °C for 48 h. Because the ice dissolves in the −80 °C solvent, the tissue remains frozen while the liquid solvent replaces the ice and fixes the tissue. After 48 h, the vial was warmed stepwise by 4 h stops at −40 °C, −20 °C, and then room temperature for 48 h. After 48 h at room temperature, the M-AA was replaced with three changes of 100% ethanol, for 15 min each, then processed into paraffin using routine protocols. Specifically, eyes were moved from 100% ethanol though two 15-min changes of 100% xylene, then three 45-min changes of 60 °C paraffin. Sections 4 microns in thickness were harvested using a Leica RM2125Rt microtome.
To test the contribution of some of the variables involved in the freeze substitution approach on both structure and immunocytochemical sensitivity, we explored the impact of several variants in both the solvent composition, and the freezing regimen, with all eyes ultimately processed into paraffin. All images presented are from paraffin sections.
1) To determine if acetic acid was necessary, we tried methanol only (M).
2) To determine if the length of time of freeze substitution was critical, we compared freeze substitution for 48 h (M-AA 48 −80) and 1 week (M-AA 1W),
3) To determine if the time of room temperature exposure to the acidic fixative was essential or detrimental, we exchanged the −80 M-AA for −80 ethanol prior to warming (M-AA −80Eth) and compared this to tissue allowed to remain in room temperature M-AA for 48 h before processing (M-AA 48RT).
4) Because acetone is a common histologic fixative, we substituted acetone instead for M-AA in the M-AA 48 regimen (Ac).
5) Because frozen sectioning is generally superior to paraffin processing for immunoreactivity, we tested whether tissue could be fixed by freeze substitution to preserve structure, then rehydrated for subsequent processing for frozen sections. To accomplish this, we used the M-AA 48 −80 regimen, then after 2 h at room temperature (RT), M-AA was exchanged with 100% ethanol. The tissue was then very slowly rehydrated. Rehydration was achieved by sequential exchanges with ethanol:water that progressed from 95:5 ethanol:water to 100% water in 5% steps, at 20 min each. To control all other variables, this tissue was then dehydrated again and processed into paraffin (M-AA H20).
6) To determine if liquid propane was essential, eyes were frozen by immersion directly into −80 M-AA
7) These variants were compared to eyes fixed by immersion in RT 10% neutral buffered formalin (Sigma HT5014) for 4 and 24 h (formalin).
EdU labeling
To determine whether freeze substitution was compatible with the EdU Click-iT approach to the labeling of dividing cells, we injected EdU at 10ugm/gm bodyweight 24 h before sacrifice [4]. We used the Click-iT® EdU Alexa Fluor® 488 Imaging Kit (Life Technologies, catalog number C10337), and followed the manufacturer’s directions. Tissue was then subsequently labeled by immunocytochemistry as described below.
Mouse strains
Two-month-old C57Bl6 mice were used in all studies except the GFP and TdTomato studies. The GFP/tdTomato mice, genetically engineered to express GFP and tdTomato under control of the Prox 1 promoter, were obtained from the UC Davis Mutant Mouse Regional Resource Center. Both tdTomato (Cat #036531-UCD) and EGFP (Cat # 031,006-UCD) are in the FVB/N-Crl:CD1(ICR) hybrid background.
Antibodies
To assess the impact of freeze substitution on the preservation of immunoreactivity, six different polyclonal antibody preparations were used to compare the freeze substitution routines to formalin immersion fixation. Antibodies included: affinity-purified rabbit anti-vimentin (vim) [5], affinity-purified rabbit anti-periplakin (Ppl) [6], affinity-purified anti-CP49 (CP49) [5], rabbit anti p57 Kip 2 (p57 Kip2; Abcam Cat# ab4058, gift from Nadean Brown, UC Davis, Davis, CA), rabbit anti-gamma-tubulin (gift of Ken Beck, UC Davis), and chicken anti-plectin prepared in-house from recombinant plectin fragment (plectin). Sections were de-paraffinized, blocked with tris-buffered-saline with 0.1% Tween-20 (TBS-T), and 5% powdered milk. To remove any aggregates, antibodies were diluted in a blocker, then spun in a tabletop microfuge at 15 K for 20 min. Labeling was conducted for 4 h, followed by three 15-min washes in a large volume of TBS-T. Secondary antibodies (Life Technologies: Alexafluor 488 goat anti-rabbit, Cat# A-11008; Alexafluor 555 goat anti-rabbit, Cat# A-21428; Alexafluor 488 goat anti-chicken antibodies, Cat# A11039) were applied at 1:1000 in the blocker and incubated at RT for 4 h, then washed as above. After a final wash in distilled water, coverslips were applied with the ProLong Gold anti-fade reagent (Life Technologies #P10144), which included DAPI for nuclear localization.
Image capture
Images were captured with a Nikon Eclipse 800, equipped with a Q Imaging Exi Aqua camera and Chroma Technology filter sets (red, Cat# 49,005; green, Cat# 49,002). For each experimental group, we determined the upper and lower levels of signal intensity, then selected an exposure time that just registered a signal for the lowest-intensity sample, and held the exposure constant for that group.
In situ hybridization
In situ hybridization was conducted with RNAscope® 2.0 HD Assay (red) probes for mouse bfsp2 (CP49) prepared by Advanced Cell Diagnostics. Tissue was processed per the manufacturer’s instructions. To test whether lectin labeling was significantly affected, sections were labeled with Alexa Fluor 488-wheat germ agglutinin (WGA; Life Technologies, Cat# W11261).
Results
Structural preservation
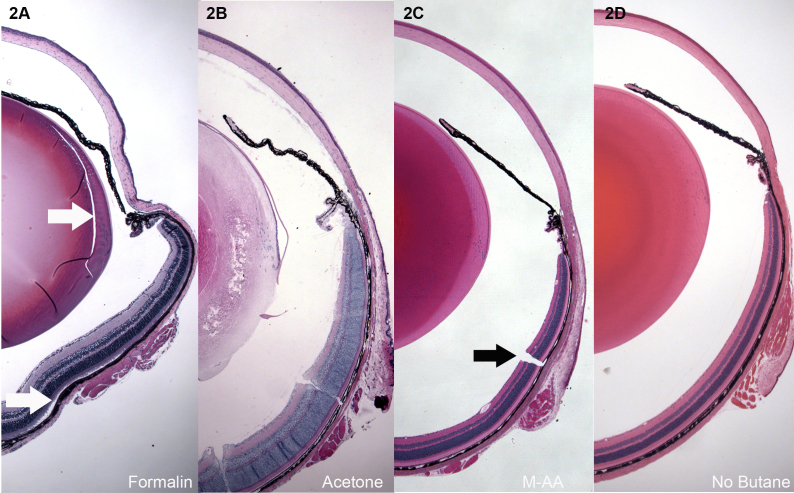
Figure 2A-D presents low-magnification overviews of mouse eyes fixed by immersion in 10% formalin for 24 h (Figure 2A), freeze substituted with acetone (Figure 2B), freeze substituted with methanol:acetic acid (Figure 2C), and freeze substituted by direct immersion into −80 M-AA (no butane, Figure 2D). The formalin-fixed sample (Figure 2A) shows shrinkage/distortion in all ocular tissues, as well as areas of tissue splitting and retinal detachment (white arrows, Figure 2A). Acetone freeze substitution (Figure 2B) was unacceptable for most of the eye, but the cornea was well preserved.
Figure 2.
Low magnification overviews of H&E-stained paraffin sections of mouse eyes. A: Immersion in 10% neutral buffered formalin for 24 h. B: Freeze substitution in acetone. C: Freeze substitution in 97% methanol 3% acetic acid (M-AA). D: Same as in C, but skipping the butane freezing step, and freezing instead by direct immersion in −80 M-AA. Formalin-fixed eyes consistently showed shrinkage and buckling, and splitting caused by shear (white arrow, lens Figure 1A) as tissue fixed and shrank. Retinal detachment was common as well (white arrow, A). Acetone fixation (B) was good for the cornea, but not for most other ocular structures. Panel C shows tissue preserved by freeze substitution in methanol, or methanol:acetic acid. This level of preservation was typical of all the different routines described in Materials and Methods, but only the M-AA −80 is shown. Panel D shows that good quality can be achieved without the butane step, though, as shown in Figure 3, Figure 4, and Figure 5, some loss of quality can be seen on closer inspection. Higher magnification views of the retina, cornea, and lens are shown from each of the different freeze substitution routines in subsequent images. With the exception of the not-uncommon freezing cracks (black arrow C), the overall relationships of ocular structures are consistently well preserved, and lacking in buckling and retinal detachment.
As described in M&M, several variants of the methanol-based freeze substitution routine were explored, and the results from each were comparable. To determine if liquid butane freezing was critical, samples were processed by freezing in −80 butane (Figure 2C) or by immersion directly into −80 M-AA. Ocular buckling, retinal detachment, and shrinkage tears were essentially nonexistent in all freeze substitution-preserved eyes. The single biggest issue for the freeze substitution approach was the occasional crack (arrow Figure 2C) resulting from the freezing process. The fact that similar results were obtained for all the variants of the methanol-based freeze substitution described in M&M suggests that there is likely considerable latitude in the process.
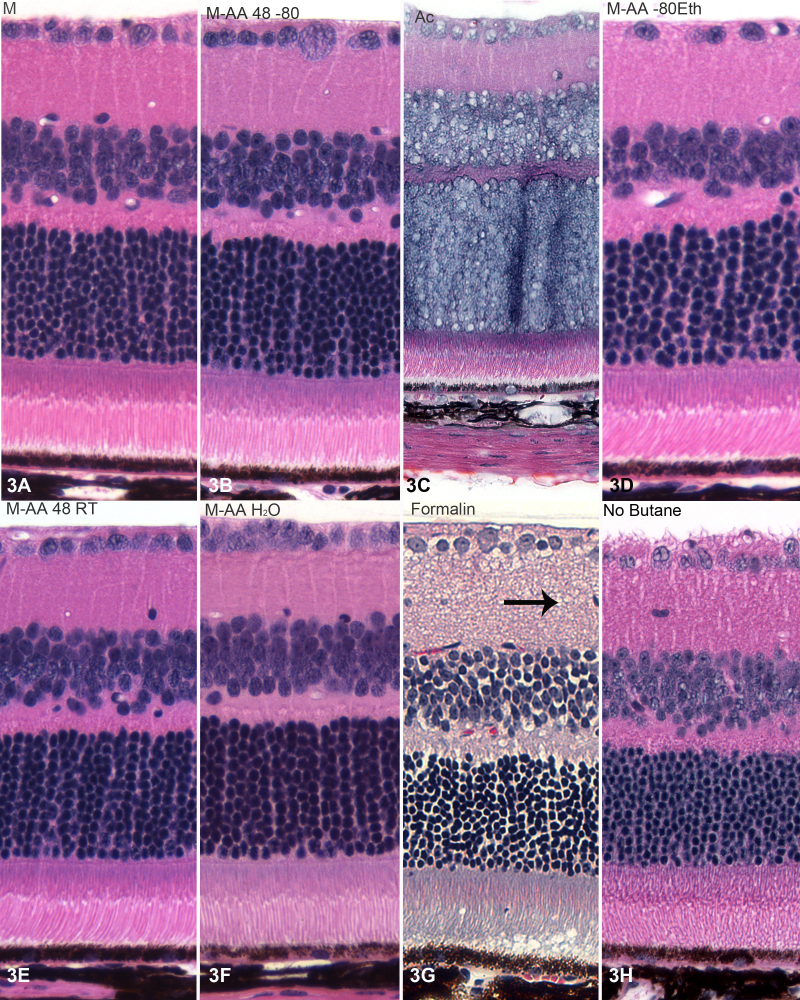
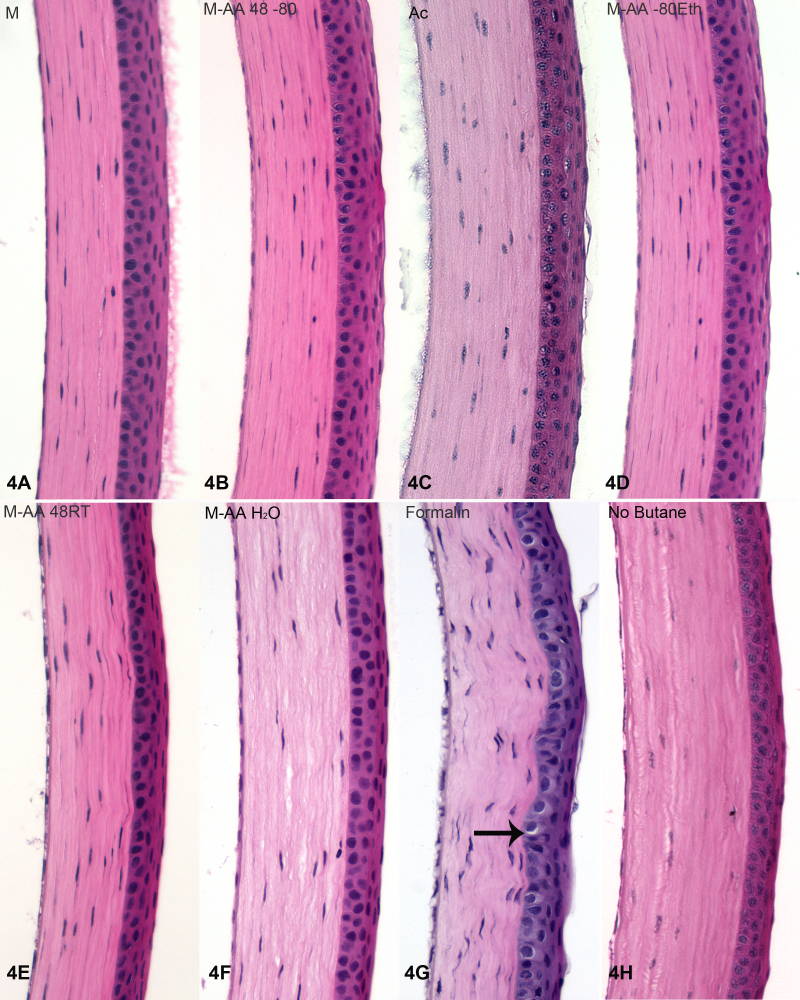
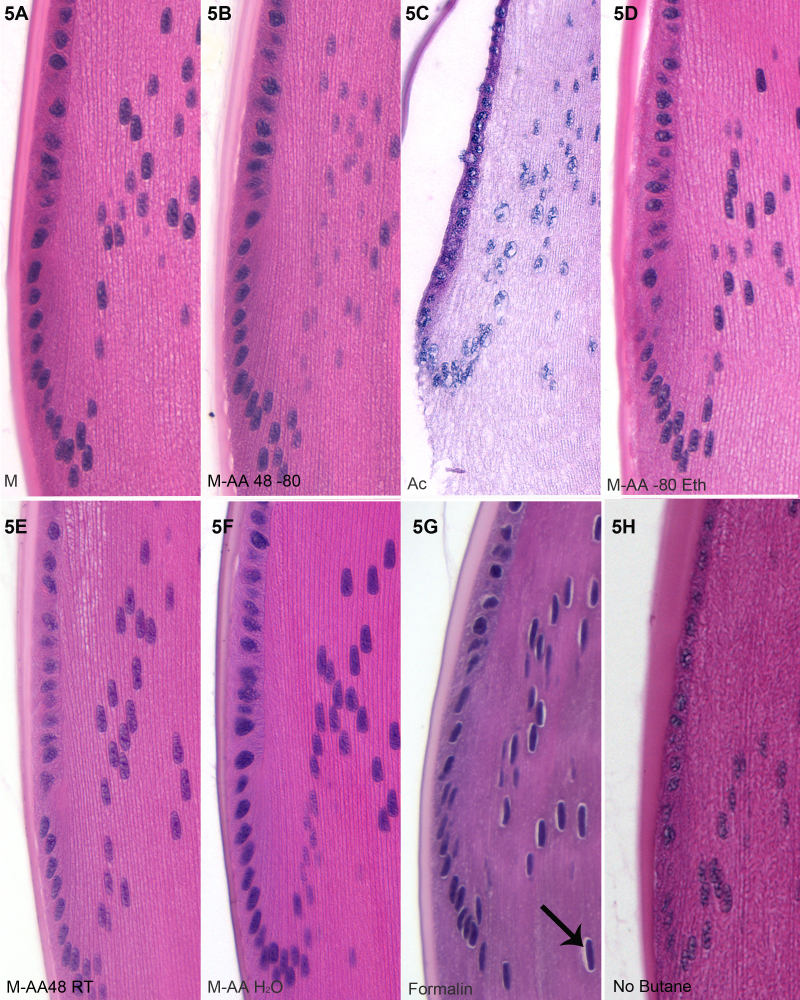
Figure 3, Figure 4, and Figure 5 present higher magnification views of the retina (Figure 3), cornea (Figure 4), and lens (Figure 5) from each of the different fixation protocols. As noted above, acetone produced unacceptable preservation in both the retina (Figure 3C) and lens (Figure 5C), though the cornea (Figure 4C) was acceptably preserved. Formalin immersion consistently resulted in evidence of shrinkage, both at the gross level (shrinkage tears, white arrows, Figure 2A) and waviness of the epithelial-stromal interface (Figure 4G), and at the cellular level (e.g., gaps around nuclei, arrows, Figure 3G, Figure 4G, Figure 5G). All of the variants of freeze substitution produced consistently high-quality preservation of all ocular structures when compared to formalin preservation, with the exception of the no-butane group (3 h, 4 h, and 5 h). The no-butane protocol produced very good quality overall structure (2 h), but there was some loss of quality at higher magnification views, though it was at least comparable to formalin immersion.
Figure 3.
H&E-stained sections of retinas. Tissue fixed by freeze substitution with acetone (C) yielded what appears to be the worst overall quality of preservation, though formalin immersion (G) also reveals much evidence of shrinkage and “vacuolation.” Tissues processed through the different freeze substitution routines show little evidence of shrinkage artifacts, and exhibit good fine details, such as the outer limiting membrane, and good outer segment preservation.
Figure 4.
H&E stained corneas. Examples from each fixation routine are shown, labeled according to the key in Materials and Methods, and as in Figure 3. Acetone preservation was reasonably good for the cornea (C). The shrinkage and distortion caused by formalin (G) is evident as a nonlinear interface between the corneal epithelium and stroma. Also common to formalin fixation is perinuclear shrinkage (arrow G). In the rehydrated tissue (F), there is some evidence of separation in the corneal stroma. As this was not seen in either the retina (Figure 3F) or lens (Figure 5F), this likely results from the presence of a strongly anionic and hygroscopic matrix that is uniquely high in the cornea and absent from the other tissues.
Figure 5.
H&E stained lens bow regions. Examples of each fixation routine are presented, labeled as described in Materials and Methods. Acetone fixation (C) has a clearly negative effect on the lens, with capsular separation and severe shrinkage. As seen in D, inconsistent separation between capsule and lens mass can occur, though this was not common. Formalin induces some lens shrinkage and the emergence of perinuclear shrinkage (black arrow, G).
Because cryostat-sectioned material is generally thought to be the best means of retaining immunoreactivity, we tested whether eyes fixed by freeze substitution could then be rehydrated without significant impact on structure. This would allow for the rapid immobilization achieved by freezing, but also the immunoreactivity of cryosections. Figure 3F, Figure 4F, and Figure 5F show retina, cornea and lens that were rehydrated in this manner, then processed into paraffin so that only one variable (the step of rehydration) was changed. Had we conducted cryosectioning, we would have been unable to discriminate between damage caused by rehydration and damage caused by preparation for cryosectioning. In the rehydrated tissue, the retina and lens looked good, but the cornea showed some separation in the corneal stroma (Figure 4F). This may represent swelling during rehydration caused by the highly anionic and hygroscopic corneal extracellular matrix. We observed that rapid rehydration did result in shrinkage/swelling damage, so a slow rehydration is critical. The contrast between the quality of acetone preservation of the cornea (Figure 4C) and the lens (Figure 5C) underscore how the compositional difference of tissues affects fixation outcomes.
Preservation of immunoreactivity
To assess the impact of freeze substitution on overall preservation of immunoreactivity, and to determine if there are differences among different freeze substitution routines, we compared formalin fixation to several variants of the freeze substitution approach. Acetone fixation was so poor that no further studies were conducted on acetone-fixed tissue. The goal in applying these different antibodies was not to address the biology of the antigen, but to simply assess the relative degree of immunoreactivity in the different preparations. Because there can be considerable differences in how specific antibodies/antigens are affected by fixation, we present data from several polyclonal preparations to different antigens, to determine if there is a generalizable pattern.
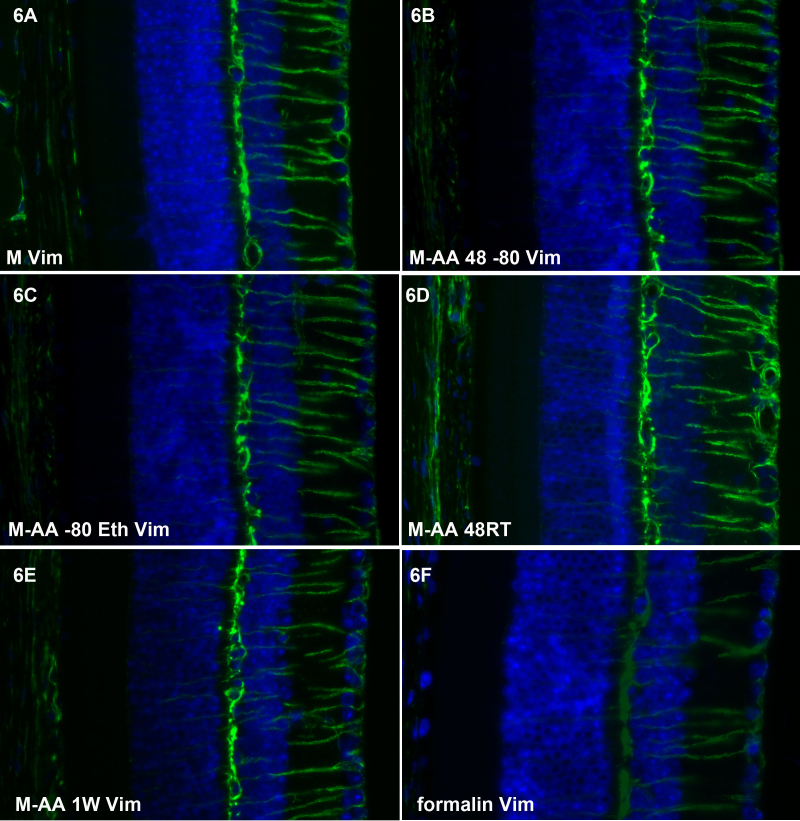
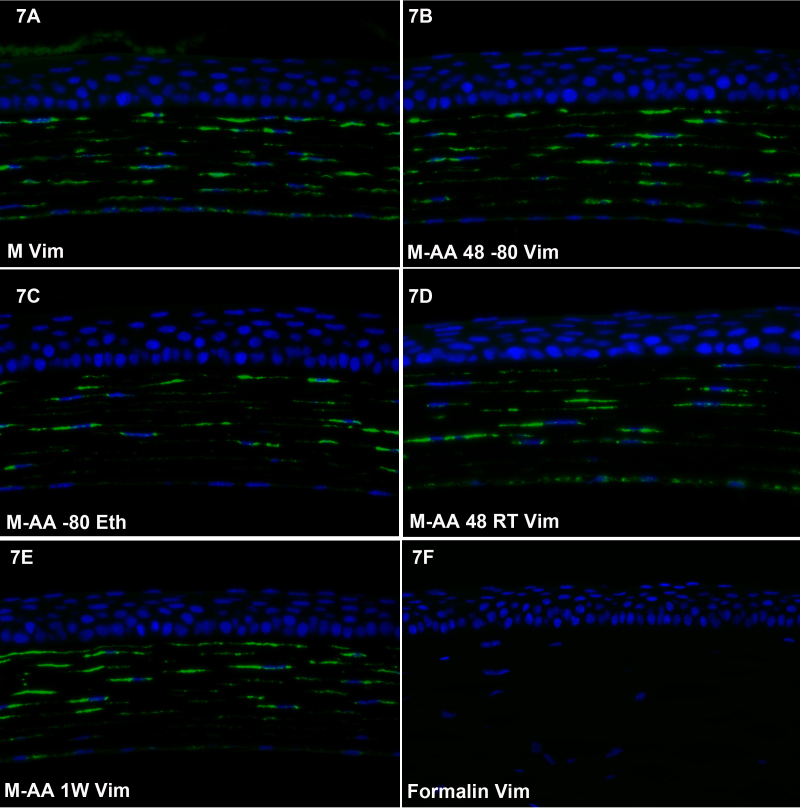
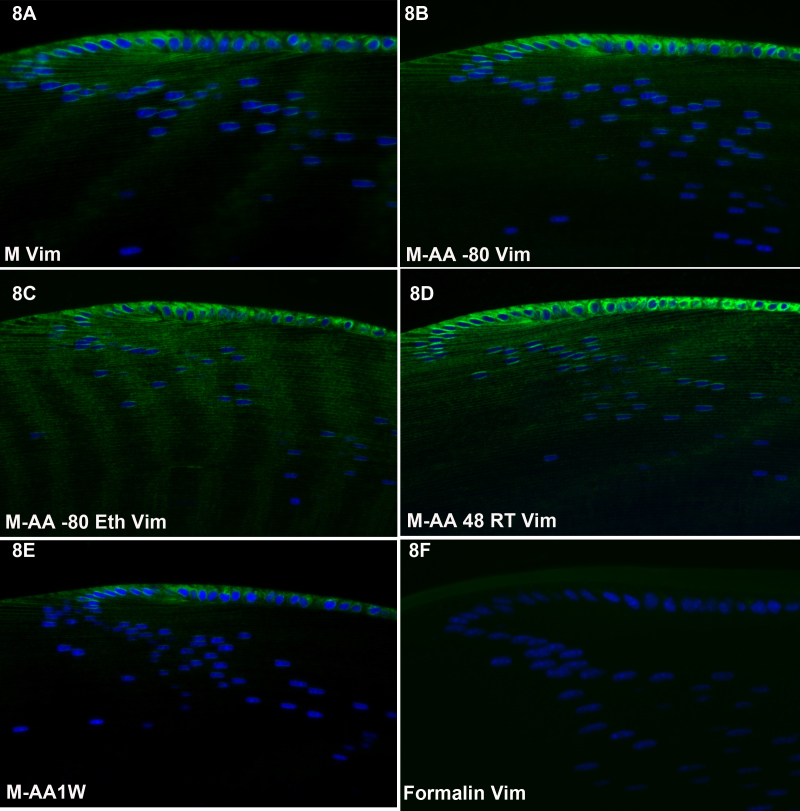
Figure 6, Figure 7, and Figure 8 show the results of labeling with antibodies to vimentin, a Type III intermediate filament protein, in the retina (Figure 6), cornea (Figure 7) and lens (Figure 8). Though vimentin is a common IF protein, it is not present in all cell types (e.g., the corneal epithelium lacks vimentin, while corneal keratocytes express it), providing a good internal control for non-specificity. Each of the freeze substitution variants (Figure 6A-E, Figure 7A-E, 8 and Figure 8A-E) was compared to formalin-preserved tissue (Figure 6F, Figure 7F, and Figure 8F). All of the freeze-substituted samples showed a greater preservation of immunoreactivity than formalin-fixed samples. Of all antibodies tested, however, the disparity between freeze-substituted and formalin-fixed tissues was less with anti-vimentin labeling of the retina than in any other tissue or antibody we tested. The M-AA 48RT (Figure 6D), here and in some other samples, appears somewhat better in retention of immunoreactivity. In the cornea (Figure 7) there appears to be slightly better retention of reactivity in the corneal endothelium in Figure 7A and again in Figure 7D compared to other routines. In sections of the lens (Figure 8), there was more variability between different routines than was seen in the retina and cornea, but again, all freeze-substituted variants (Figure 8A-E) retained better immunoreactivity than formalin-fixed tissue. As in Figure 6 and Figure 7, the M-AA 48 RT appears strongest. The M-AA 1W appears to be the least reactive of the freeze-substituted tissues using the vimentin antibodies, but is still an improvement over formalin.
Figure 6.
Retinal labeling with anti-vimentin antibody. All of the freeze substituted samples (A-E) show a higher level of immunoreactivity than the formalin-fixed sample (F). Of all the antibodies tested, however, the disparity between freeze-substituted and formalin-fixed tissues was less with anti-vimentin labeling of the retina than in any other tissue or antibody we tested. The M-AA 48RT (D) here, and in some other samples, appears to be somewhat better in the retention of immunoreactivity.
Figure 7.
Corneal labeling with anti-vimentin antibody. Freeze substitution variants (A-E) all yielded greater immunoreactivity than the formalin-fixed tissue. There appears to be slightly better retention of reactivity in the corneal endothelium in A and again in D.
Figure 8.
Lens labeling with anti-vimentin antibody. There was more variability between different freeze substitution routines in vimentin labeling of the lens than was evident in other tissues, but again, all freeze-substituted variants (A-E) retained better activity than in formalin. As in Figure 6 and Figure 7, the M-AA 48 RT appears strongest. The M-AA 1W appears to be the least reactive of the freeze-substituted tissues.
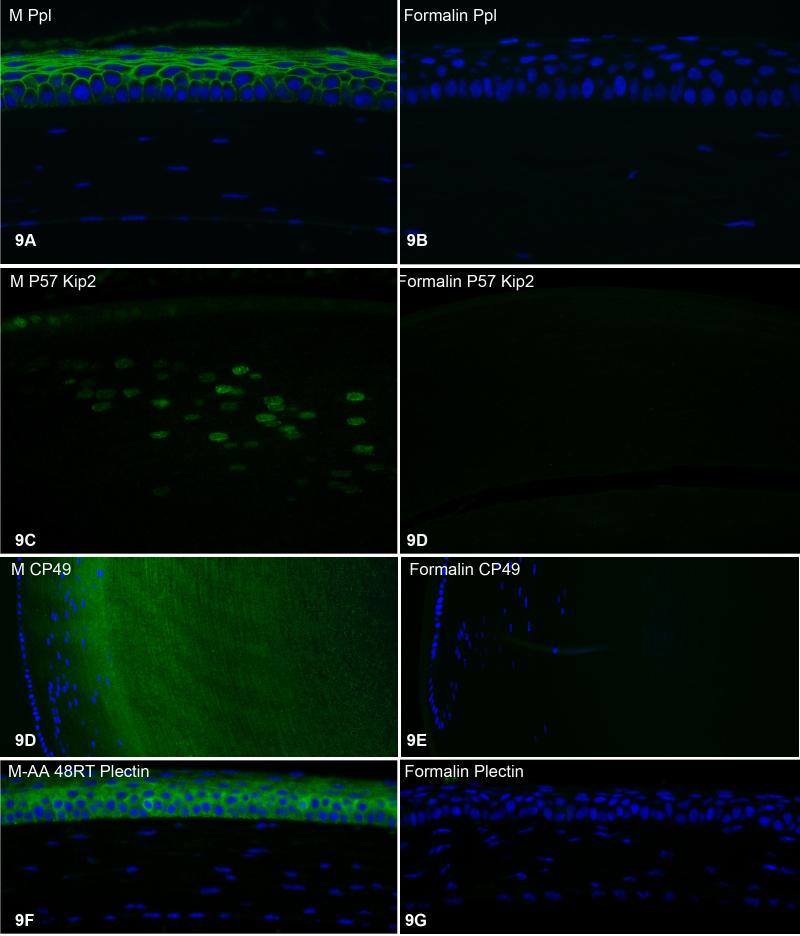
Figure 9 compares the labeling of freeze-substituted tissue to formalin-fixed tissue using four different additional polyclonal antibodies: anti-periplakin (Ppl, in cornea, Figure 9A-B); anti-P57 Kip2 (P57 Kip 2, in lens, Figure 9C-D); anti-CP49, a lens-specific intermediate filament protein (CP49, in lens, Figure 9E-F); and anti-plectin (plectin, in cornea, Figure 9F-G). In all cases, the retention of immunoreactivity was substantially greater in the freeze-substituted tissue than in formalin-fixed tissue. Similar immunolabeling was conducted on all of the freeze-substitution variants described in Material and Methods, and the results were the same: No matter which variant of the freeze-substitution process was used, the retention of immunoreactivity was substantially greater than that seen in formalin-fixed tissue (not shown). Any differences between the freeze-substitution variants were minor and not consistent; i.e., no variant was consistently better/worse.
Figure 9.
Labeling with four additional polyclonal antibodies. A, C, D, and F were all prepared by freeze substitution; B, D, E, and G were all prepared by immersion in formalin. Figure 9A-B: anti-periplakin labeling of the cornea. Figure 9C-D:anti-P57 Kip2 labeling of the lens bow region. Figure 9D-E: anti-CP49 labeling of the lens bow region. Figure 9F-G: anti-plectin labeling of the cornea.
Fluorescent Proteins tdTomato and GFP
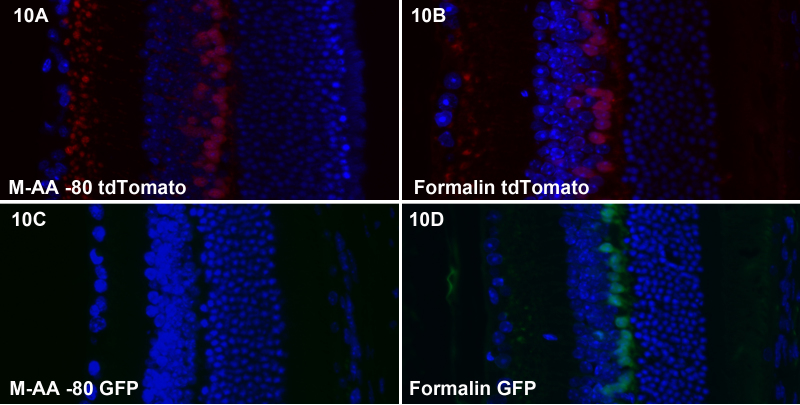
We tested freeze substitution and formalin for their impact on the intrinsic fluorescence of tdTomato and GFP, two of the many fluorescent protein tools available. Figure 10A-B show that the intrinsic fluorescence of tdTomato is not substantially different between freeze substitution and formalin fixation. In contrast, Figure 10C-D demonstrate that freeze substitution essentially eliminates intrinsic GFP fluorescence.
Figure 10.
Retention of tdTomato and GFP intrinsic fluorescence. A-B compares freeze substitution (A) and formalin (B) for impact on tdTomato intrinsic fluorescence, while panels C and D compare GFP fluorescence. There was little obvious difference for tdTomato fluorescence, while GFP fluorescence is essentially eliminated by freeze substitution.
Autofluorescence
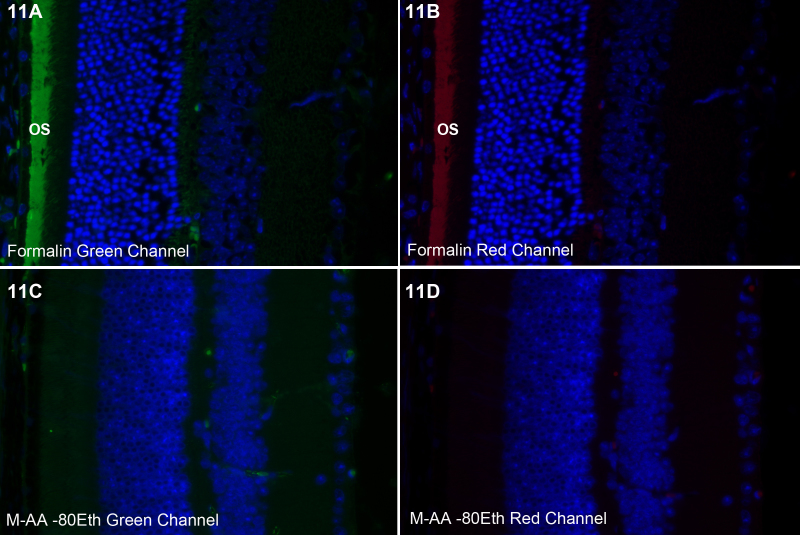
Formalin-fixed retinas exhibit a broad-spectrum autofluorescence in the photoreceptor outer segments (os). This can be seen in Figure 11A-B which show fluorescence in both green and red channels. The use of freeze substitution essentially eliminates this, as can be seen in Figure 11C-D, which were exposed for the same time as Figure 11A-B in both red and green channels.
Figure 11.
Photoreceptor outer segment autofluorescence. Formalin-fixed tissue exhibits a broad autofluorescence in both the green (A) and red (B) channels in the outer segments (os). Preservation by freeze substitution eliminated this autofluorescence (C-D).
EdU, Lectin labeling and in situ hybridization
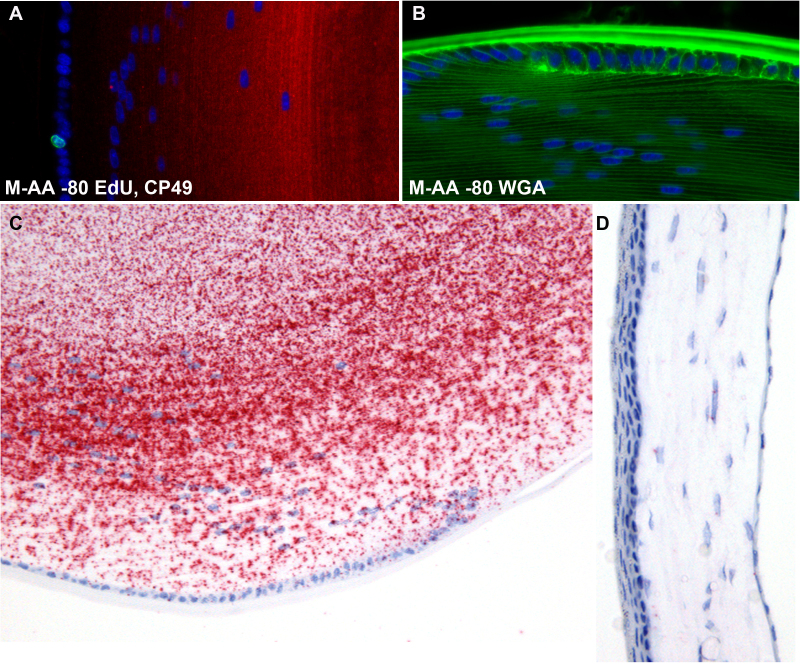
Figure 12A demonstrates that the Click-iT labeling of dividing cells using the thymidine analog EdU (green nucleus) can be conducted readily on freeze-substituted tissue, and that the same tissue can be subsequently processed for immunocytochemistry (CP49, red). Figure 12B shows robust labeling of the lens with Wheat Germ Agglutinin conjugated to Alexa Fluor 488. This non-confocal image also underscores the quality of tissue preservation achieved with freeze substitution, as it shows the high degree of spatial resolution between parallel fiber cell membranes separated by very short distances. Figure 12C-D demonstrate that freeze substitution is fully compatible with in situ hybridization; i.e., the process did not result in substantial degradation of mRNA. Though we did not directly compare freeze substitution to formalin, Figure 12C shows a robust labeling of mRNA for the lens-specific protein CP49. Because CP49 is not known to be expressed in the cornea, the cornea from that same section serves as an internal control and exhibits a very low level of background signal due to non-specific binding of ISH probe (Figure 12D).
Figure 12.
EdU and lectin labeling, in situ hybdridization. Panel A demonstrates that the freeze substitution approach does not negatively affect the utility of the Click-iT chemistry approach to the identification of dividing cells using the thymidine analog EdU. A single, Alexa fluor 488-labeled nucleus (green) can be seen in the lens epithelium. The slide was subsequently labeled with antibodies to CP49 (red), as in Figure 9. Panel B shows that the labeling intensity of fluorescent lectins (WGA) is not negatively affected by the freeze substitution preservation. To determine whether the freeze substitution methodology interfered with the capacity to conduct in situ hybridization, we probed de-paraffinized sections from M-AA −80 samples with probes for the lens-specific CP49 mRNA. While no direct comparison was made with formalin-fixed tissue, Panel C clearly shows a robust signal, while the internal control from the same section (cornea, Panel D) shows very low levels of background binding of ISH probe to CP49. The tissue processing steps required for the in situ hybridization process introduced some loss of tissue structure, as can be seen in the slightly buckled cornea.
Discussion
The motivation for conducting this study was derived from the difficulties and caveats associated with common approaches used to preserve small eyes, and lenses in particular, for routine cytochemical approaches. We were especially concerned with the problems that may be induced by the gradient of fixation: surface structures fixing at rates far different from interior structures, a problem that is acute in efforts to preserve the lens. Using 3H-formaldheyde and autoradiography, Blankenship et al. demonstrated that formaldehyde does not reach the nuclear region of the mouse lens even after 24 h of immersion [2]. This barrier to formaldehyde penetration is likely due to the crosslinking of the exceptionally high levels of protein that are unique to the fiber cell. This gradient in fixation means that immunolabeling of sections of lenses fixed by formaldehyde immersion will be comparing heavily fixed cortex to unfixed or—at best—lightly fixed central lens. This raises the possibility that any cytochemical differences seen between the cortex and nucleus may not be due to the age of the cell or expression levels, but instead to artifactual differences created by the fixation gradient. At a broader level, immersion fixation is prone to causing ocular buckling and retinal detachment as well. Finally, at a general level, fixatives that produce covalent modification can be catastrophic to monoclonal antibodies, or “polyclonal” antibodies to peptides or very small epitopes, which are commonly de facto monoclonal antibodies.
Freeze substitution is a common and well used method. In its most sophisticated applications, it can require elaborate equipment and great expense, and yield tissue that is beautifully preserved even at the ultrastructural level. We adapted the approach in a way that is inexpensive, uses readily accessible items, and yields structural preservation at the light microscope level that is comparable or better to that achieved with formalin. There is likely considerable latitude in the specifics of how the tissue is processed. In this report, we sought to abide by general freeze-substitution principles that have been refined for ultrastructural work [7-9] and adapt them in a simple, cost-effective way for light microscopy of the small eye. Among these principles are a) the rapid immobilization that is a function of the speed of freezing and b) subsequent “fixation” while the tissue is still frozen. Different freezing media, freezing/substituting regimens, and fixatives have been employed in the literature, and are reviewed in the text by Humbel (Figure 9). In this report, we compare the impact of modifying several parameters in the freeze substitution process, and show that most produce comparable results. Because −40 freezers may not be as common as −80, we also tried skipping this step in the warmup phase, and saw no obvious differences in structure (not shown). While the overall structure of the eye was good when tissue was frozen by direct immersion in −80 M-AA (no butane, Figure 2), there appeared to be some loss of quality on closer examination (no butane, Figure 3, Figure 4, and Figure 5). This loss may be an acceptable trade for the convenience of skipping the butane step.
One of the most common applications of sectioned material is immunocytochemistry. While every antibody-antigen combination will have a different sensitivity to fixation and embedding approaches, the data presented here suggest a generalizable conclusion that freeze substitution is likely to be significantly better at preserving immuoreactivity than formalin. This conclusion is suggested by the data that show that several different polyclonal antibodies to different antigens all showed substantially better immunoreactivity in all tissues preserved by freeze substitution than could be achieved by immersion in formalin. Since freeze substitution avoids covalent modification of epitopes, it would seem likely that the success rate would be higher for monoclonal and peptide-specific antibodies as well. Note that no efforts at antigen retrieval were included in this study.
The data presented here are all derived from tissues that were subsequently processed for paraffin sectioning, a technique that yields good structure but likely reduces sensitivity when compared to cryostat-generated sections. To establish whether the benefits of freeze substitution could be combined with the increased sensitivity of cryostat sections, we freeze-substituted the eye, then returned it to room temperature. After equilibration in 100% ethanol, we gradually exchanged ethanol for water. The eye could not be moved from the −80 freezer directly into the cryostat because the eye was equilibrated in methanol-acetic acid that remains liquid even at −80 °C. This precluded cryosectioning until the organic solvent was replaced with an aqueous one. If the rehydration process is slow, the transition from organic solvent to an aqueous environment can be achieved with a minimal impact on architecture, as shown in Figure 3, Figure 4, and Figure 5. Tissue can then be processed for cryostat sectioning using conventional routines. We did not cryosection the rehydrated eye because we would have then been comparing the structure of cryosections to paraffin sections, and would not have been able to ascertain whether any damage was caused by rehydration or by cryosectioning (two variables). Instead, we processed the rehydrated tissue into paraffin so that the only variable was the rehydration.
As noted in Results, the freeze-substitution regimen eliminates the intrinsic fluorescence of GFP. This can be overcome if necessary by using immunocytochemistry to localize the GFP. In contrast, tdTomato fluorescence was at least as good as that seen in formaldehyde-fixed tissue, but these results suggest that the use of each fluorescent protein must be checked on a case-by-case basis.
In situ hybridization was also tested on freeze-substituted tissue, though a direct comparison to formalin-fixed tissue was not attempted. The resulting images indicate a robust localization of the mRNA for CP49, a lens-specific cytoskeletal protein, and very low background in the cornea, the internal control tissue that does not express CP49. While we did not directly compare the relative sensitivities of formalin and freeze substitution, we can conclude that the freeze-substitution process does not appear to have a significantly negative impact on mRNA.
Acknowledgments
Funding provided by NEI RO1 EY08747, NEI Core facilities grant P30EY012576, and unrestricted funds provided by UC Davis to PGF.
References
- 1.Lovicu FJ, Robinson ML. Development of the ocular lens. 2004, Cambridge, UK; New York: Cambridge University Press. xv, 398 p. [Google Scholar]
- 2.Blankenship T, Bradshaw L, Shibata B, Fitzgerald P. Structural specializations emerging late in mouse lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2007;48:3269–76. doi: 10.1167/iovs.07-0109. [DOI] [PubMed] [Google Scholar]
- 3.Shiurba R. Freeze-substitution: origins and applications. Int Rev Cytol. 2001;206:45–96. doi: 10.1016/s0074-7696(01)06019-3. [DOI] [PubMed] [Google Scholar]
- 4.Wiley LA, Shui YB, Beebe DC. Visualizing lens epithelial cell proliferation in whole lenses. Mol Vis. 2010;16:1253–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Alizadeh A Clark JI, Seeberger T, Hess J, Blankenship T, Spicer A, FitzGerald PG. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest Ophthalmol Vis Sci. 2002;43:3722–7. [PubMed] [Google Scholar]
- 6.Yoon KH, FitzGerald PG. Periplakin interactions with lens intermediate and beaded filaments. Invest Ophthalmol Vis Sci. 2009;50:1283–9. doi: 10.1167/iovs.08-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echlin P. (1992) Low-Temperature Microscopy and Analysis. Plenum Press, New York. [Google Scholar]
- 8.Gilkey JC, Staehelin LA. Advances in ultrarapid freezing for the preservation of cellular ultrastructure. J Electron Microsc Tech. 1986;3:177–210. [Google Scholar]
- 9.Humbel B. (2009) Freeze substitution. Handbook of Cryo-Preparation Methods for Electron Microscopy (ed. by A. Cavalier, D. Spehner & B. Humbel), pp. 319–341. CRC Press, Boca Raton, Florida. [Google Scholar]