Abstract
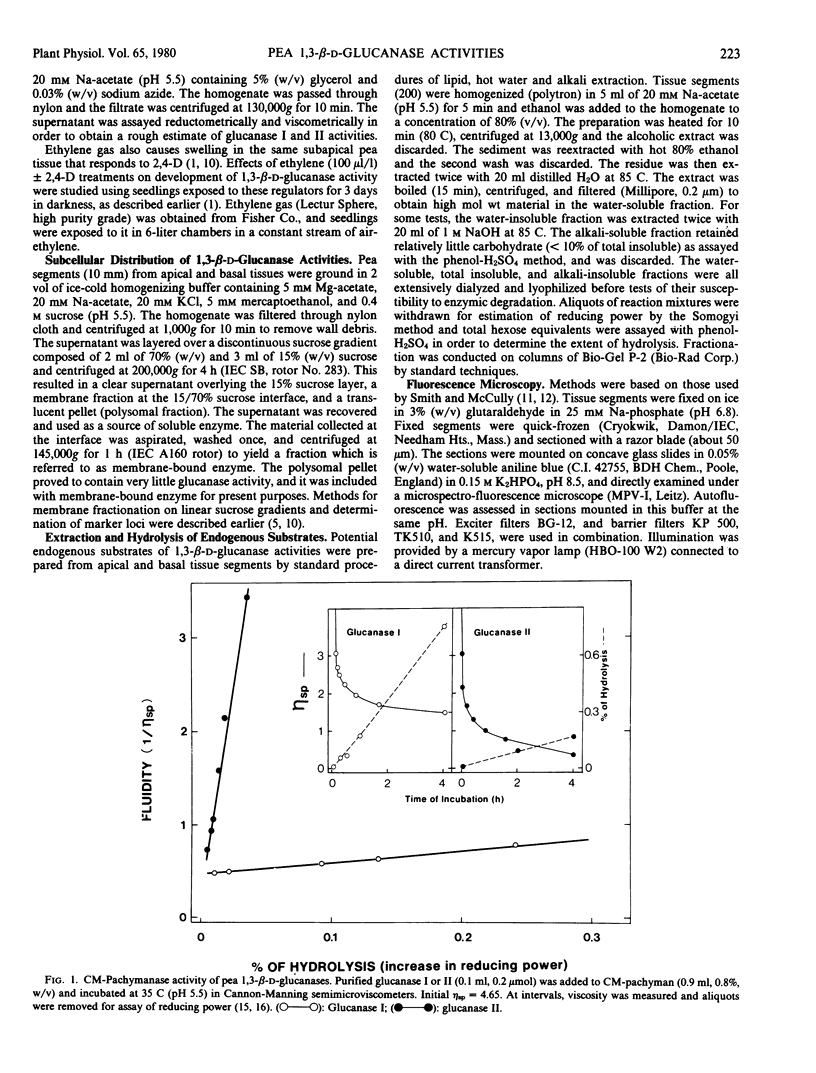
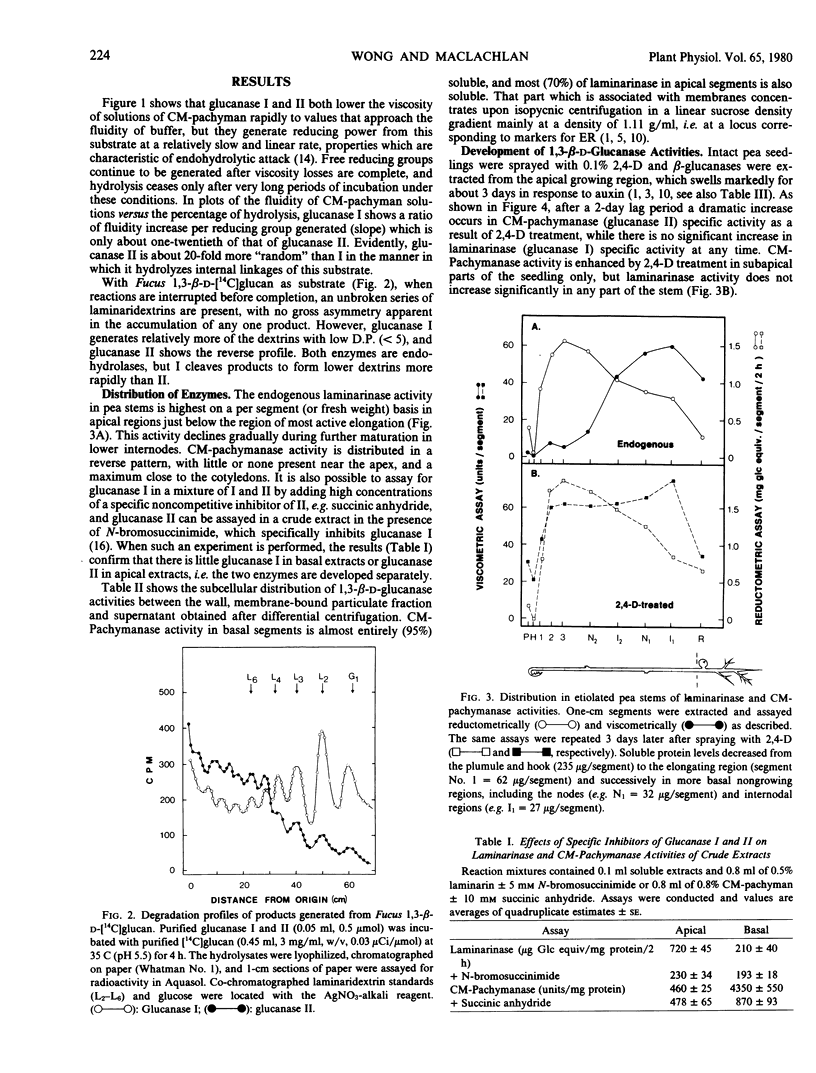
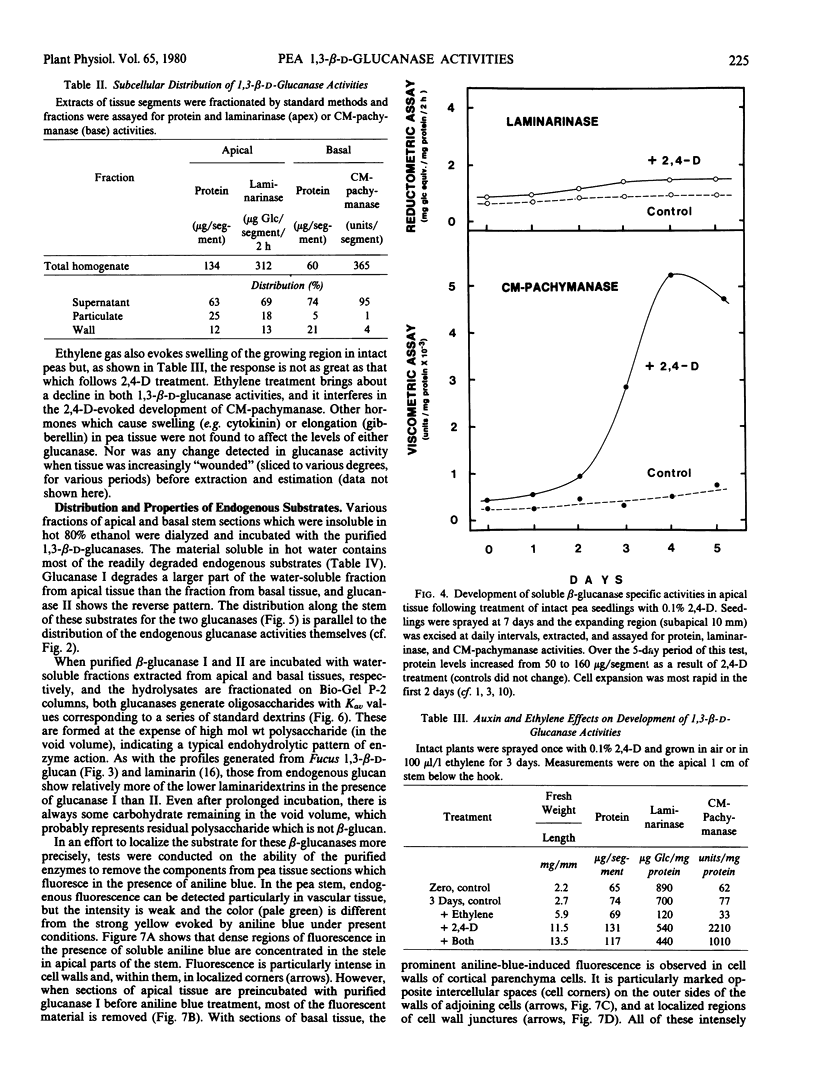
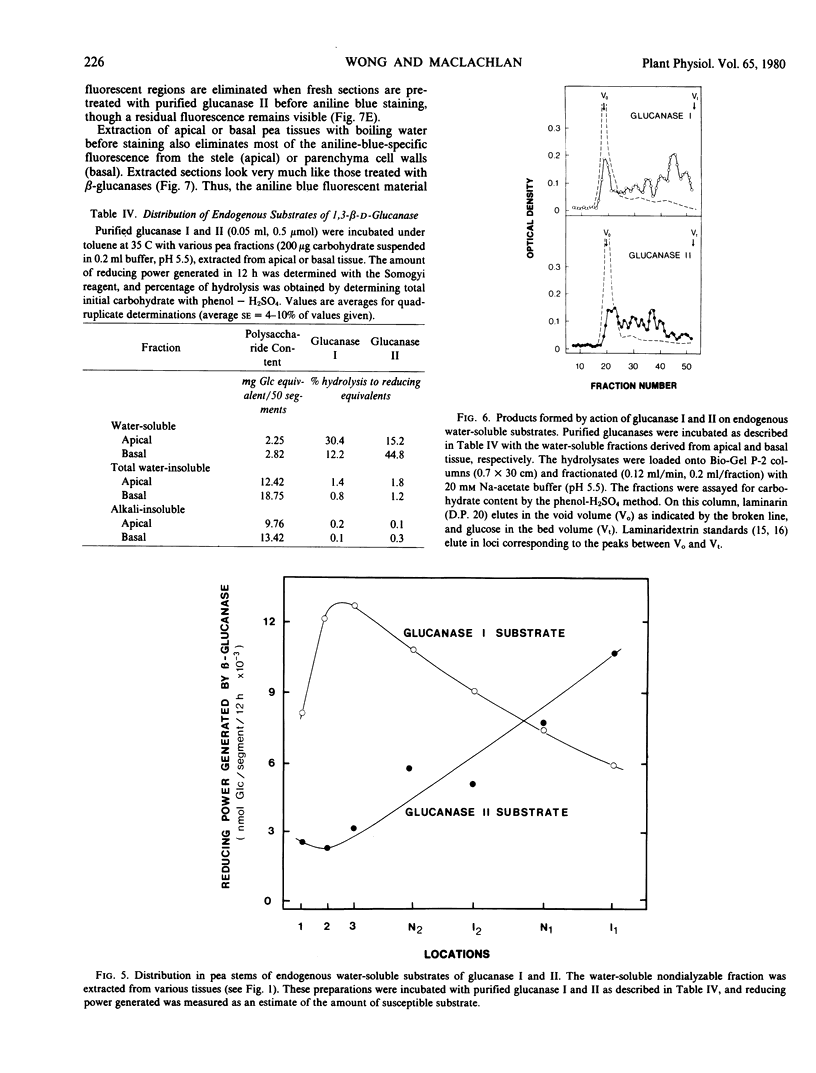
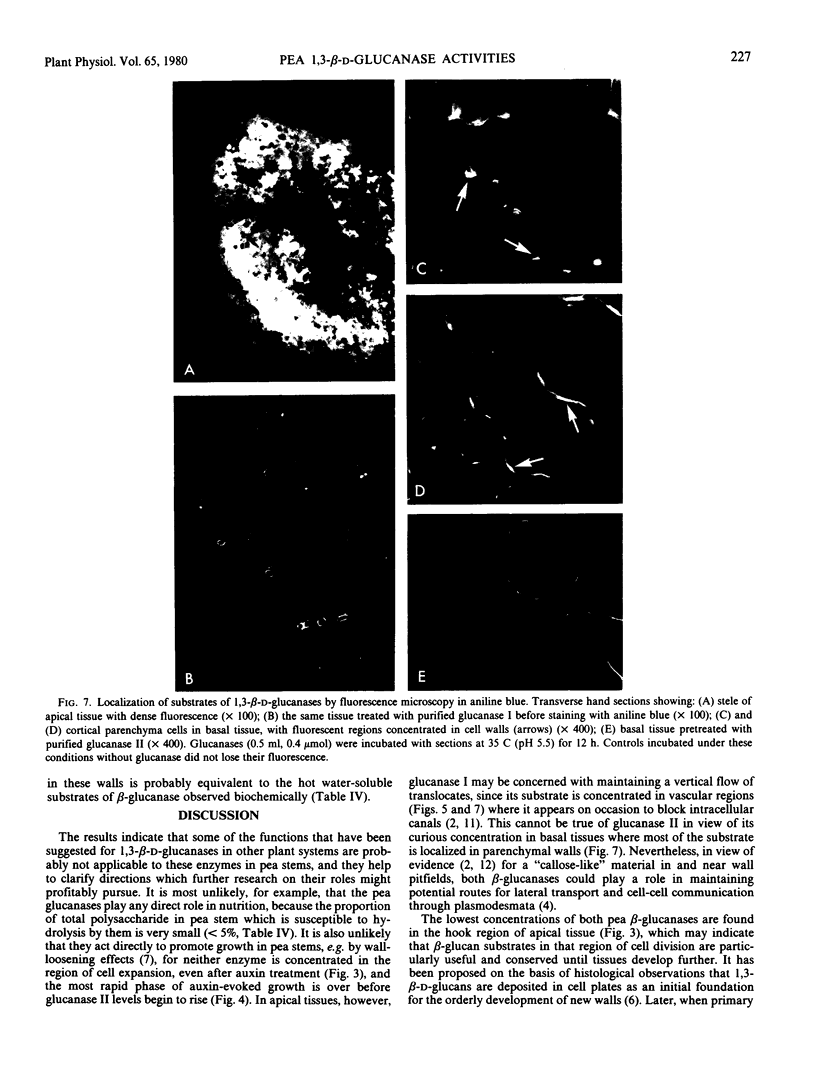
Two endo-1,3,-β-d-glucanases (I and II, EC 3.2.1.6) are present in etiolated peas at opposite ends of the stem. Glucanase I from subapical regions degrades substrates to a series of low molecular weight dextrins, and is most readily assayed reductometrically (e.g. as laminarinase). Glucanase II from basal regions preferentially hydrolyzes internal linkages of long chains, and is most sensitively assayed viscometrically (e.g. as carboxymethylpachymanase). The activity of glucanase II but not I increases greatly near the apex in response to treatment of the tissue with auxin, and ethylene gas suppresses endogenous activities and the auxin response, i.e. levels of these enzymes are under developmental controls which can be regulated. Different natural substrates for the two enzymes were identified primarily in tissue fractions soluble in hot water. Substrates for glucanase I are concentrated in apical regions, as is the enzyme itself, and those for glucanase II are in basal regions, implying that enzymes and substrates are normally in separate cellular compartments. Tissue sections stained with aniline blue for β-glucan show enhanced fluorescence in cell walls, and most of this can be removed either by hot water or the appropriate purified β-glucanase. The enzymes are not likely to function directly in promoting nutrition or growth in peas, but they could help, following secretion, to maintain channels for communication and translocation through cell walls.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Datko A. H., Maclachlan G. A. Indoleacetic Acid and the synthesis of glucanases and pectic enzymes. Plant Physiol. 1968 May;43(5):735–742. doi: 10.1104/pp.43.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr M., Bailey D. S., MacLachlan G. Subcellular distribution of membrane-bound glycosyltransferases from pea stems. Eur J Biochem. 1979 Jul;97(2):445–453. doi: 10.1111/j.1432-1033.1979.tb13132.x. [DOI] [PubMed] [Google Scholar]
- Matile P., Cortat M., Wiemken A., Frey-Wyssling A. Isolation of glucanase-containing particles from budding Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1971 Mar;68(3):636–640. doi: 10.1073/pnas.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatrano R. S., Stevens P. T. Cell wall assembly in fucus zygotes: I. Characterization of the polysaccharide components. Plant Physiol. 1976 Aug;58(2):224–231. doi: 10.1104/pp.58.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble beta-1,4-glucan (cellulose) synthetase activities. J Cell Biol. 1975 Mar;64(3):557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
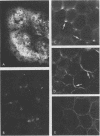
- Smith M. M., McCully M. E. Enhancing aniline blue fluorescent staining of cell wall structures. Stain Technol. 1978 Mar;53(2):79–85. doi: 10.3109/10520297809111446. [DOI] [PubMed] [Google Scholar]
- Wong Y. S., Fincher G. B., Maclachlan G. A. Kinetic properties and substrate specificities of two cellulases from auxin-treated pea epicotyls. J Biol Chem. 1977 Feb 25;252(4):1402–1407. [PubMed] [Google Scholar]